Abstract
Plant nucleotide-binding (NB) and leucine-rich repeat (LRR) receptors mediate effector-triggered immunity. Two major classes of NB-LRR proteins are involved in this process, namely, toll-interleukin receptor (TIR)-NB-LRR and coiled coil (CC)-NB-LRR proteins. Recent reports show that some of the TIR-NB-LRRs and CC-NB-LRRs localize to the cytoplasm and nucleus. Equilibrium between these pools is required for full resistance, suggesting tight regulation of nucleocytoplasmic receptor shuttling. We recently showed that SGT1, a protein that controls NB-LRR receptor stability and activity, facilitates nuclear import of N protein, which is a TIR-NB-LRR receptor. In this addendum, we show that the subcellular localization of Rx, a CC-NB-LRR protein, reflects the positions of SGT1 ectopic variants in the cell. This suggests that SGT1 might have a general role in maintaining the nucleocytoplasmic balance of NB-LRR receptors. We discuss these results in light of differences in the N and Rx systems of effector-triggered immunity.
Keywords: N, NB-LRR, Rx, SGT1, nucleocytoplasmic shuttling, plant disease resistance
Plants have evolved multiple defense mechanisms against viruses that interfere with the infection process at several key stages. Potato plants carrying Rx, which encodes CC-NB-LRR type R protein, establish an extreme resistance (ER) to potato virus X (PVX) and severely attenuate virus multiplication. In resistant tobacco plants, tobacco mosaic virus (TMV) multiplies in inoculated cells and moves intercellularly before triggering a hypersensitive response (HR) mediated by N protein.1 The function and stability of both Rx and N depend on the activity of a chaperone complex containing SGT12,3,4 (Table 1). We recently showed that localization of SGT1 exclusively in the nucleus shifted the cytoplasmic N protein pool toward the nucleus whereas forced cytoplasmic localization of SGT1 did not reduce nuclear N levels.5 Previous reports suggested that Rx trafficking might be regulated by SGT1, because SGT1 silencing impaired nuclear Rx localization.6 This might be explained by decreased Rx stability in the absence of SGT1.2,3 However no significant reduction in steady-state levels of GFP-Rx or 4HA-GFP-Rx was observed in SGT1-silenced plants compared with that in controls.6 We have observed that GFP-like tags may stabilize protein constructs expressed in planta.7 Alternatively, SGT1 might affect the conformation or act directly on translocation of Rx.
Table 1. Summary of properties of two NB-LRR receptors that mediate resistance to viruses. TIR-NB-LRR, toll-interleukin receptor–nucleotide-binding–leucine-rich repeat; CC-NB-LRR, coiled coil–nucleotide-binding–leucine-rich repeat; PVX, potato virus X; TMV, tobacco mosaic virus; HR, hypersensitive response.
| N | Rx | |
|---|---|---|
| Structure | TIR-NB-LRR16 | CC-NB-LRR17 |
| Chaperone complex | Interaction with SGT1-HSP90-RAR1 complex4 SGT1 affects stability8 |
NDa for interaction SGT1 affects stability2,3 |
| Localization | Predominantly nuclear5,18 | Predominantly cytoplasmic6 |
| Ligand recognition | Recognition of the helicase domain of the TMV replicase (p50) in the cytoplasm or nucleus5,18 | Recognition of PVX coat protein exclusively in cytoplasm6 |
| Signaling | Oligomerization,8 conformational change18,19 | Conformational change,20 oligomerization? |
| Interaction with transcription factors | SPL621 | NDa |
| Other interactors | 14–3-3,22 NRIP123 | RanGAP211-13 |
| Forced nuclear localization | Wild-type-like HR,5 resistance to TMV not tested | No HR established; compromised resistance to PVX6,11 |
| Forced cytoplasmic localization | No HR established; resistance to TMV not tested18 | Wild-type-like HR; slightly compromised resistance to PVX6,11 |
| Domain role in translocation | LRR possibly promotes nuclear localization,5,18 YFP-LRR co-localizes with SGT15 | LRR promotes cytoplasmic localization, CC-domain required for nuclear localization6 |
| SGT1 role in nucleocytoplasmic shuttling | Mediates nuclear import5 | Crucial for nuclear import,6 mediates nuclear import and export (Fig. 1) |
a No data available
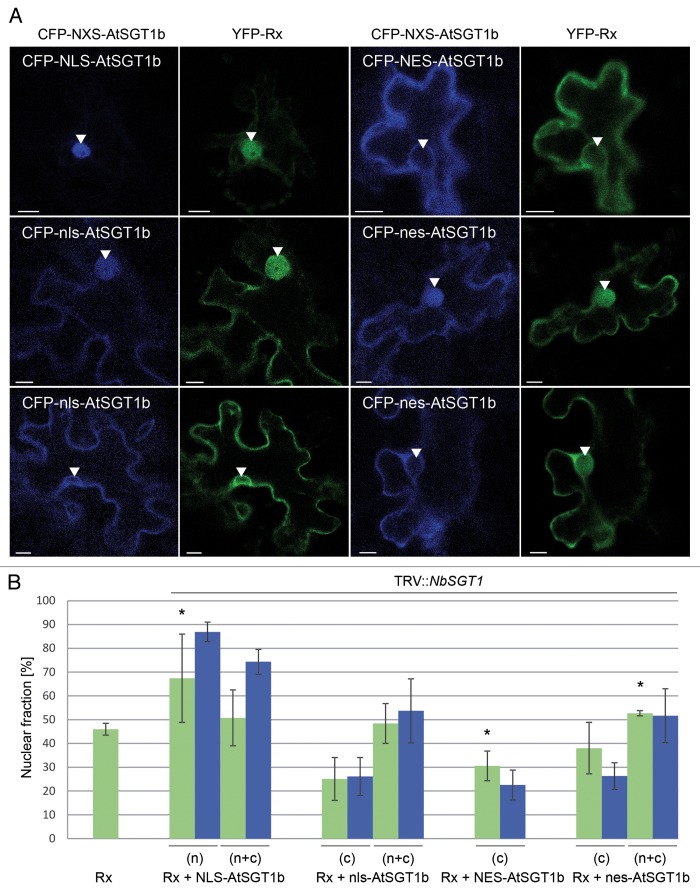
To determine the functional relationship between SGT1 and Rx, we transiently expressed SGT1 variants with forced cytoplasmic or nuclear localization and monitored the effects on cellular Rx distribution. First, endogenous SGT1 was silenced in Nicotiana benthamiana plants using virus-induced gene silencing (VIGS). Subsequently, YFP-Rx was transiently expressed in systemic leaves via bombardment in the presence of AtSGT1b, which carried either nuclear localization signal (NLS; PKKKRKV), nuclear export signal (NES; NELALKLAGLDINK), or mutated versions thereof (nls and nes, respectively). As previously described, NLS-AtSGT1b showed nuclear or nucleocytoplasmic distribution, NES-AtSGT1b was detected predominantly in the cytoplasm, whereas control constructs with the mutated targeting signals in some cells were found in the cytoplasm, but in others were distributed between the nucleus and cytoplasm. The images showed that Rx distribution exactly mirrored that of ectopic AtSGT1b variants (Figure 1A), and this was supported by measurements of relative fluorescence intensities (Figure 1B, Pearson correlation coefficient (r), calculated for cells co-expressing Rx and AtSGT1b, equals 0.8). This suggests that SGT1 facilitates both Rx import into and export from the nucleus, in contrast to that for N protein, which was relocated only toward the nucleus in our experiments.5 In SGT1-silenced plants, N protein has a nucleocytoplasmic distribution pattern similar to that in wild-type plants, which suggests that SGT1 is not essential for nuclear import of N protein but modulates its trafficking.
Figure 1. AtSGT1b subcellular localization determines nucleocytoplasmic partitioning of Rx. (A) Confocal images of representative N. benthamiana leaf epidermal cells transiently co-expressing YFP-Rx with the indicated ectopic constructs of AtSGT1b fused to CFP. (B) Relative percentage of nuclear fractions of Rx and AtSGT1b (fused to fluorescent proteins) shown as a ratio of the fluorescence intensity in the nucleus (IN) to the total fluorescence intensity in the cell, i. e. intensity in the nucleus plus intensity in the cytoplasm (IC); [IN/(IN+IC)]*100. Average percentage of nuclear fluorescence intensities (± SD) was calculated for yellow or cyan fluorescence in the nucleus and cytoplasm, which was determined using ImageJ software, as described previously.15 The cells with nuclear, nucleocytoplasmic or cytoplasmic distribution of AtSGT1b are indicated as (n), (n+c) or (c), respectively. Asterisks indicate that the nuclear fraction of Rx is significantly different from the value for Rx in control plants, as established using Student's t test (P < 0.05).
These results may reflect the involvement of Rx and N receptors in distinct resistance responses to viral infection (i.e., ER and HR), in which either the cytosolic or nuclear receptor pool plays a predominant role. Another scenario that cannot be excluded is that, in addition to homodimers composed of full-length N protein,8 two N forms (e.g., full-length and truncated) encoded by alternatively spliced transcripts,9 or two truncated forms could associate as other types of hetero- or homo-dimers, respectively. This would add significant system complexity because the different complexes might have different degrees of sensitivity to SGT1 regulation.
In summary, Rx and N belong to different classes of plant NB-LRR receptors, and confer distinct types of resistance to viral infection, which include ER and HR, respectively. However, recent results5,6 and Figure 1 show that nucleocytoplasmic receptor shuttling might be regulated in both systems by SGT1 in the LRR-dependent manner (Table 1). This reveals a novel role of SGT1 in effector recognition by NB-LRR receptors, in addition to its role in the control of steady-state levels and activities of the receptors.10 We proposed that partitioning of the receptors can be finely tuned by phosphorylation of SGT1,5 which might establish another surveillance system. However, the exact mode of SGT1 action in the translocation process remains to be elucidated.
This model does not exclude that the proper equilibrium between nuclear and cytoplasmic receptor pools can be maintained by other means. Multiple levels of regulation might provide specificity for each pathosystem. For example, the observation that the cytoplasmic Rx pool seems to play a dominant role in potato resistance to PVX6,11 is consistent with the fact that cytoplasmic transport of Rx is also controlled by RanGAP2.11,12,13 We speculate that during tobacco defense response to TMV, N partitioning might be regulated by dynamic association of the full-length N protein with the truncated N form encoded by alternatively spliced transcripts.9 Work is underway to test this proposal.
Due to space constraints, we have not focused on SGT1 role in the folding and stabilization of client proteins required for their nuclear import. This aspect has been recently discussed in the review by Takken and Goverse.14
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from Ministry of Science and Higher Education Republic of Poland to M.K. (No N302 015 31/1618 and NN301 163235) and J.H. (N N301 318039). The fellowship for M.Z. was funded by the Foundation for Polish Science, project MPD/20093/2.
References
- 1.Palukaitis P, Carr JP, Schoelz JE. Plant-virus interactions. Methods Mol Biol. 2008;451:3–19. doi: 10.1007/978-1-59745-102-4_1. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo C, Betsuyaku S, Peart J, Takahashi A, Noël L, Sadanandom A, Casais C, Parker J, Shirasu K. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 2006;25:2007–16. doi: 10.1038/sj.emboj.7601084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botër M, Amigues B, Peart J, Breuer C, Kadota Y, Casais C, Moore G, Kleanthous C, Ochsenbein F, Shirasu K, et al. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell. 2007;19:3791–804. doi: 10.1105/tpc.107.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Burch-Smith T, Schiff M, Feng S, Dinesh-Kumar SP. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J Biol Chem. 2004;279:2101–8. doi: 10.1074/jbc.M310029200. [DOI] [PubMed] [Google Scholar]
- 5.Hoser R, Zurczak M, Lichocka M, Zuzga S, Dadlez M, Samuel MA, Ellis BE, Stuttmann J, Parker JE, Hennig J, et al. Nucleocytoplasmic partitioning of tobacco N receptor is modulated by SGT1. New Phytol. 2013;200:158–71. doi: 10.1111/nph.12347. [DOI] [PubMed] [Google Scholar]
- 6.Slootweg E, Roosien J, Spiridon LN, Petrescu AJ, Tameling W, Joosten M, Pomp R, van Schaik C, Dees R, Borst JW, et al. Nucleocytoplasmic distribution is required for activation of resistance by the potato NB-LRR receptor Rx1 and is balanced by its functional domains. Plant Cell. 2010;22:4195–215. doi: 10.1105/tpc.110.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giska F, Lichocka M, Piechocki M, Dadlez M, Schmelzer E, Hennig J, Krzymowska M. Phosphorylation of HopQ1, a type III effector from Pseudomonas syringae, creates a binding site for host 14-3-3 proteins. Plant Physiol. 2013;161:2049–61. doi: 10.1104/pp.112.209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestre P, Baulcombe DC. Elicitor-mediated oligomerization of the tobacco N disease resistance protein. Plant Cell. 2006;18:491–501. doi: 10.1105/tpc.105.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinesh-Kumar SP, Baker BJ. Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc Natl Acad Sci U S A. 2000;97:1908–13. doi: 10.1073/pnas.020367497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadota Y, Shirasu K. The HSP90 complex of plants. Biochim Biophys Acta. 2012;1823:689–97. doi: 10.1016/j.bbamcr.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Tameling WI, Nooijen C, Ludwig N, Böter M, Slootweg E, Goverse A, Shirasu K, Joosten MH. RanGAP2 mediates nucleocytoplasmic partitioning of the NB-LRR immune receptor Rx in the Solanaceae, thereby dictating Rx function. Plant Cell. 2010;22:4176–94. doi: 10.1105/tpc.110.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco MA, Mansoor S, Moffett P. A RanGAP protein physically interacts with the NB-LRR protein Rx, and is required for Rx-mediated viral resistance. Plant J. 2007;52:82–93. doi: 10.1111/j.1365-313X.2007.03213.x. [DOI] [PubMed] [Google Scholar]
- 13.Tameling WI, Baulcombe DC. Physical association of the NB-LRR resistance protein Rx with a Ran GTPase-activating protein is required for extreme resistance to Potato virus X. Plant Cell. 2007;19:1682–94. doi: 10.1105/tpc.107.050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takken FLW, Goverse A. How to build a pathogen detector: structural basis of NB-LRR function. Curr Opin Plant Biol. 2012;15:375–84. doi: 10.1016/j.pbi.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Lichocka M. Biolistic bombardment for co-expression of proteins fused to YFP and mRFP in leaf epidermal cells of Phaseolus vulgarishttp://www.bio-protocol.org/wenzhang.aspx?id=1019#.U00GpVc21zh
- 16.Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell. 1994;78:1101–15. doi: 10.1016/0092-8674(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 17.Bendahmane A, Kanyuka K, Baulcombe DC. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell. 1999;11:781–92. doi: 10.1105/tpc.11.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burch-Smith TM, Schiff M, Caplan JL, Tsao J, Czymmek K, Dinesh-Kumar SP. A novel role for the TIR domain in association with pathogen-derived elicitors. PLoS Biol. 2007;5:e68. doi: 10.1371/journal.pbio.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda H, Yamaguchi Y, Sano H. Direct interaction between the tobacco mosaic virus helicase domain and the ATP-bound resistance protein, N factor during the hypersensitive response in tobacco plants. Plant Mol Biol. 2006;61:31–45. doi: 10.1007/s11103-005-5817-8. [DOI] [PubMed] [Google Scholar]
- 20.Moffett P, Farnham G, Peart J, Baulcombe DC. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 2002;21:4511–9. doi: 10.1093/emboj/cdf453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padmanabhan MS, Ma S, Burch-Smith TM, Czymmek K, Huijser P, Dinesh-Kumar SP. Novel positive regulatory role for the SPL6 transcription factor in the N TIR-NB-LRR receptor-mediated plant innate immunity. PLoS Pathog. 2013;9:e1003235. doi: 10.1371/journal.ppat.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konagaya K-i, Matsushita Y, Kasahara M, Nyunoya H. Members of 14-3-3 protein isoforms interacting with the resistance gene product N and the elicitor of Tobacco mosaic virus. J Gen Plant Pathol. 2004;70:221–31. doi: 10.1007/s10327-003-0113-4. [DOI] [Google Scholar]
- 23.Caplan JL, Mamillapalli P, Burch-Smith TM, Czymmek K, Dinesh-Kumar SP. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell. 2008;132:449–62. doi: 10.1016/j.cell.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]