Abstract
Elevated atmospheric CO2 concentration is a serious global environmental problem. Elevated CO2 affects plant growth by changing primary metabolism, closely related to carbon (C) and nitrogen (N) availability. Under sufficient N conditions, plant growth is dramatically promoted by elevated CO2. When N availability is limited, however, elevated CO2 disrupts the balance between cellular C and N (C/N). Disruption of the C/N balance is regarded as an important factor in plant growth defects. Here we highlight the regulation of senescence in higher plants by atmospheric CO2 and N, and the physiological function of C/N-related ubiquitin ligase ATL31 under condition of elevated CO2. We also provide an overview of the ubiquitin ligases and related enzymes involved in regulating senescence in plants.
Keywords: C/N balance, carbon metabolism, nutrient, ubiquitin ligase, biomass
Plant Growth Regulation in Response to CO2/N Balance
Nutrient availability, in particular the availability of carbon (C) and nitrogen (N), is important in the regulation of plant metabolism and development. In addition to their independent utilization, the ratio of C to N metabolites in the cell, referred to as the C/N balance, is also important for the regulation of plant growth.1,2 In nature, the availability of C and N changes in response to environmental conditions, such as atmospheric CO2, light availability, diurnal cycles, seasonal effects, rainfall and factors influencing microbial activity.3,4,5,6 Since atmospheric CO2 is the sole C source for plants in nature, the cellular C/N balance is affected by the balance between atmospheric CO2 and the available N amount taken up from soil (CO2/N). The increasing concentration of atmospheric CO2, however, is a serious environmental problem worldwide, causing not only elevated temperatures but having major ecological consequences, such as changes in plant growth.7,8,9 Increases in atmospheric CO2 concentration are generally thought to promote plant growth, since higher amounts of CO2 can provide more sugars to plants as energy sources. Under certain conditions, however, elevated CO2 may reduce plant growth by altering the primary metabolism of plants.10,11 This, in turn, can disrupt the cellular C/N balance, resulting in plant growth defects. Elevated atmosphere CO2 concentration can cause excess carbohydrates to accumulate in plants,12,13 altering nitrogen metabolism and partitioning in plants.10,11 In addition, elevated CO2 concentration can inhibit the assimilation of nitrate, the predominant N source in soils, causing N depletion and inhibiting plant growth.11,14 Thus, elevations in atmospheric CO2 concentration alter the C/N balance, increasing C and reducing N, repressing the expression of photosynthesis-related genes and promoting the expression of stress-responsive genes. These alterations also affect root architecture and biomass allocation between roots and shoots. Conditions of elevated CO2 and limited N have been reported to promote lateral root formation, a process mediated by sugars and the phytohormone auxin signaling pathway.15
Our recent study demonstrated that conditions of elevated CO2 combined with limited N promote the progression of plant senescence, such as leaf yellowing and anthocyanin accumulation.16 Under these conditions, the expression of the senescence regulator WRKY53 and genes that respond to N-starvation, including cytosolic glutamine synthase (GS1.4) and high affinity nitrate transporter (NRT2.4),17,18,19 were upregulated compared with their levels in plants grown under normal CO2/N conditions. The senescence phenotype and the upregulation of senescence-related genes were not observed in plants grown at elevated CO2/normal N or normal CO2/limited N conditions, indicating that the senescence phenotype was not due to either CO2 or N level alone, but was dependent upon the CO2/N balance.16 According to previous studies, the amounts of several sugars were found to increase, while the amounts of nitrogen compounds decreased, in senescent leaves.20,21,22,23 Taken together, these results indicated that elevated CO2 and limited N availability mutually affect each other, markedly disrupting the cellular C/N balance and leading to the promotion of leaf senescence in mature plants.
Ubiquitin Ligase ATL31 Regulates Senescence When the CO2/N is Disrupted
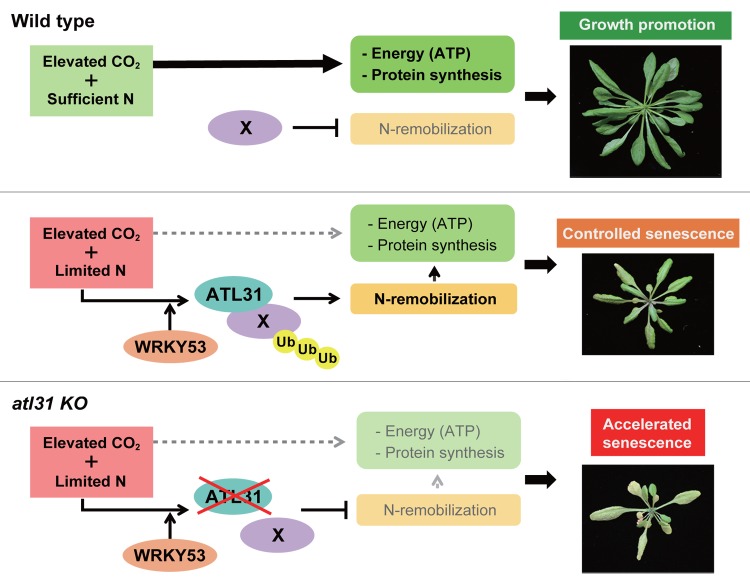
We previously identified ATL31 as a novel C/N regulator in Arabidopsis. In the post-germination stage, ATL31-overexpressing plants (ATL31 OX) were insensitive to the high C/low N stress medium containing excess sugar and limited nitrogen (300 mM glucose/0.1 mM nitrogen), whereas an atl31 loss-of-function mutant (atl31 KO) showed a hypersensitive phenotype.24 ATL31 is a member of the plant-specific ubiquitin ligase ATL family, which is comprised of proteins that contain a transmembrane-like hydrophobic region at the N-terminus, a region rich in basic amino acids, a RING-H2 type zinc finger domain and a non-conserved C-terminal region.25,26 Subsequent analysis revealed that ATL31 targets 14–3-3 proteins for ubiquitination and degradation, which regulates the post-germinative growth of Arabidopsis plant in response to C/N status.27 In evaluating the physiological function of ATL31 under different atmospheric CO2 and N conditions, we found that the promotion of senescence under conditions of elevated CO2 and limited N was suppressed in ATL31 OX but enhanced in atl31 KO plants.16 Moreover, under these conditions, the expression of ATL31 was transcriptionally upregulated in senescent leaves. Interestingly, the promoter region of the ATL31 gene contains several putative W-box sequences, a DNA motif directly recognized by WRKY transcription factors.19 Public microarray database analysis also indicated that the expression of ATL31 highly correlated with that of WRKY53, suggesting a close relationship between ATL31 and WRKY53 in plants. Protoplast reporter analysis confirmed that WRKY53 could directly activate ATL31 transcription and that ATL31 mRNA expression was promoted in Arabidopsis plants that overexpress WRKY53.16 These results indicated that ATL31 functions in senescent leaves, that ATL31 negatively regulates senescence progression in response to CO2 and N availability, and that ATL31 is under the control of WRKY53 by regulating C/N signaling and/or metabolism in plants (Fig. 1).
Figure 1.Proposed model of plant growth regulation by elevated CO2 concentration and the role of the ubiquitin ligase ATL31. (Upper panel) When both C and N sources are abundant, organic compounds such as ATP production and protein synthesis are upregulated, promoting vegetative growth and increasing plant biomass. (Middle panel) When N supplies are limited, the C/N balance in plants is disrupted, resulting in high C and low N in plant cells. Under these conditions, plants remobilize cellular organic compound and control the progression of senescence, enabling successful reproductive growth of wild-type Arabidopsis plants. ATL31 is transcriptionally promoted by the transcriptional factor WRKY53 under conditions of elevated CO2 and limited N. This process mediates the control of N remobilization sources via the ubiquitination and degradation of target protein (X) in order to control the progression of senescence. (Lower panel) Plants with an atl31 loss of function mutation (atl31 KO) are unable to adapt to disrupted cellular C/N when CO2 is elevated and N is limited, resulting in accelerated senescence.
Senescence is Regulated by Post-Translational Ubiquitin Modification
In addition to ATL31, several ubiquitin ligases and enzymes related to ubiquitin modification have been reported to function in regulating senescence in Arabidopsis (Table 1). A member of the HECT ubiquitin-protein ligase (UPL) family, UPL5, was identified as a ubiquitin ligase targeting the senescence regulator WRKY53 in Arabidopsis.28 WRKY53 is a transcription factor that promotes the expression of several senescence-associated genes (SAGs) and other transcription factors.19 UPL5 interacts with WRKY53 in cytoplasm and was able to ubiquitinate WRKY53 in vitro. UPL5 overexpression decreased the amounts of WRKY53, whereas a loss-of-function mutant of UPL5 promoted senescence, in a manner similar to that of a WRKY53 overexpressor. Taken together, these findings indicated that UPL5 negatively regulates senescence progression via WRKY53 degradation. Although the detailed regulatory mechanisms underlying these protein-protein interactions remain unclear, the expression of UPL5 and WRKY53 mRNAs were regulated reciprocally by hydrogen peroxide, jasmonic acid and plant development, suggesting that WRKY53 activity is regulated at the transcriptional and post-transcriptional levels.28
Table 1. Ubiquitin ligases and related enzymes involved in senescence regulation.
| Protein name | Type | Function | Substrate | Ref |
| UPL5 | HECT E3 | Repression of SAGs transcription | WRKY53 | 28 |
| SAUL1 | U-box E3 | Inhibition of ABA biosynthesis | AAO3 | 29 |
| NLA | RING E3 | Adaptation to low N and Pi homeostasis |
PHT1s | 32, 35 |
| ATL31 | RING E3 | Adaptation to disrupted CO2/N | 14–3-3/? | 16, 27 |
| PHO2/UBC24 | E2 | Pi homeostasis | PHT1s | 33 |
| AMSH1 | MPN+ DUB | Membrane trafficking and autophagic degradation | PIN2* | 37 |
AMSH1 is thought to target multiple cargo proteins on the endosome, including the autophagosome
SENESCENCE-ASSOCIATED UBIQUITIN LIGASE (SAUL1) is a U-box type ubiquitin ligase that prevents premature senescence in Arabidopsis.29 SAUL1 was identified as one of the U-box proteins transcriptionally regulated by ABA treatment. A SAUL1 loss of function mutant showed an earlier senescence phenotype than wild-type, with the levels of ABA increased in the mutant. Assessments of the stability of ABA biosynthesizing enzymes showed that SAUL1 directly regulates AAO3 degradation in plants.29 The AAO3 gene encodes the aldehyde oxidase isoform that catalyzes the last step in ABA biosynthesis, suggesting that SAUL1 functions in senescence via the ABA pathway.
Ubiquitin ligases responsive to nitrogen nutrient conditions have also been reported to affect senescence progression in Arabidopsis, a process designated NITROGEN LIMITATION ADAPTAION (NLA).30 An nla mutant showed a delay in senescence phenotype under limited nitrogen conditions, affecting the accumulation of inorganic phosphate (Pi) in plants.31 NLA is a RING-type ubiquitin ligase carrying an SPX domain.30 Recently, the NLA was shown to interact directly with a member of the phosphate transporter (PHT1) family at plasma membrane and to ubiquitinate this protein.32 The amounts of PHT1 protein were increased in nla mutants, resulting in the overaccumulation of Pi. In addition, the ubiquitination of PHT1s by NLA was reported to triggers clathrin-dependent endocytosis followed by endosomal sorting to vacuoles.32 The E2 enzyme UBC24, designated as phosphate 2 (PHO2) was also reported to localize to the plasma membrane and to regulate the stability of PHT1s.33 Intriguingly, NLA and PHO2 are the targets of the microRNAs miR827 and miR399, respectively, both of which are induced by Pi starvation, suggesting that feedback regulation of the amounts of PHT1 are mediated by NLA and PHO2 activity in response to Pi availability.34 In addition, NLA was shown to regulate the stability of PT2, a member of the PHT1 family, by proteasomal degradation following poly-ubiquitination with PHO2.35 Further studies are expected to reveal the complex mechanisms by which ubiquitin-mediated transporter regulation modulates Pi homeostasis and senescence.
In addition to attachment of ubiquitin molecule, a recent study found that deubiquitinating enzymes (DUB) also play important roles in regulating senescence. The ASSOCIATED MOLECULE WITH THE SH3 DOMAIN OF STAM (AMSH) proteins are DUBs widely conserved in eukaryotes and are involved in intracellular trafficking through the deubiquitination of Lys-63 type ubiquitinated targets on the endosome, such as receptors and transporters.36 The Arabidopsis AMSH1 protein was found to directly interact with the endosomal complex required for transport-III (ESCRT-III), which is part of the endocytosis machinery, and to mediate autophagic degradation in the dark.37 An AMSH1 loss of function mutant exhibited an early senescence phenotype under dark conditions due to a deficiency in autophagy. The ubiquitin ligase ATL31 localized to the plasma membrane and the endosome is also involved in intracellular trafficking via its interaction with the SNARE protein, SYP121, in response to pathogen attack.38 These findings suggest that the function of ATL31 in endosomal degradation, including in autophagy, be examined under C/N nutrient stress conditions.
Perspectives
Our recent study showed that the ubiquitin ligase ATL31 functions in the progression of leaf senescence in response to CO2 and N availability. Although we previously showed that the 14–3-3 proteins were targets of ATL31 ubiquitination during the post-germination growth stage, the target proteins have not yet been determined in senescence regulation. It is important to clarify whether or not the 14–3-3 proteins are related to senescence regulation, as well as to identify the detailed upstream signaling cascade that modulates ATL31 activity under disrupted CO2/N conditions.
A recent study demonstrated that a sugar metabolite, trehalose 6-phosphate (T6P), is an essential signaling molecule for the initiation of senescence in plants grown in high sugar medium.22 T6P has also been reported to be a regulatory molecule that functions during flowering transition in Arabidopsis.39 Future studies are required to clarify the role of T6P in the C/N signaling cascade.
At present, atmospheric CO2 concentrations are increasing steadily while soil N availability to plants is often limited in nature. Thus, the combination of elevated CO2 and limited N for plants is a serious global problem, affecting crop production and forest maintenance. Clarification of the physiological effects and the detailed molecular mechanism of C/N response would be important for adaptation to the consequences of future increases of atmospheric CO2 concentrations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (No. 24770035) to TS, on Innovation Areas (No. 24114701 and No. 25112501) to JY, and in part by The Akiyama Foundation to TS. LG was supported by the JSPS Invitation Fellowship Program for Research in Japan (n. L-13564) and SA by the Plant Global Education Project from the Nara Institute of Science and Technology (2013–2014).
References
- 1.Martin T, Oswald O, Graham IA. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiol. 2002;128:472–81. doi: 10.1104/pp.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coruzzi GM, Zhou L. Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects’. Curr Opin Plant Biol. 2001;4:247–53. doi: 10.1016/S1369-5266(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 3.Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30:1126–49. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007;58:2297–306. doi: 10.1093/jxb/erm066. [DOI] [PubMed] [Google Scholar]
- 5.Kiba T, Kudo T, Kojima M, Sakakibara H. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J Exp Bot. 2011;62:1399–409. doi: 10.1093/jxb/erq410. [DOI] [PubMed] [Google Scholar]
- 6.Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39:847–62. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 7.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 8.Hikosaka K, Kinugasa T, Oikawa S, Onoda Y, Hirose T. Effects of elevated CO2 concentration on seed production in C3 annual plants. J Exp Bot. 2011;62:1523–30. doi: 10.1093/jxb/erq401. [DOI] [PubMed] [Google Scholar]
- 9.Knohl A, Veldkamp E. Global change: indirect feedbacks to rising CO2. Nature. 2011;475:177–8. doi: 10.1038/475177a. [DOI] [PubMed] [Google Scholar]
- 10.Takatani N, Ito T, Kiba T, Mori M, Miyamoto T, Maeda S, Omata T. Effects of high CO2 on growth and metabolism of arabidopsis seedlings during growth with a constantly limited supply of nitrogen. Plant Cell Physiol. 2014;55:281–92. doi: 10.1093/pcp/pct186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, Yanagisawa S. Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis. Plant Cell Physiol. 2014;55:306–19. doi: 10.1093/pcp/pct192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore B, Palmquist DE, Seemann JR. Influence of plant growth at high CO2 concentrations on leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase and intracellular distribution of soluble carbohydrates in tobacco, snapdragon, and parsley. Plant Physiol. 1997;115:241–8. doi: 10.1104/pp.115.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng SH, Moore B, Seemann JR. Effects of short- and long-term elevated CO2 on the expression of ribulose-1,5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1998;116:715–23. doi: 10.1104/pp.116.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloom AJ, Burger M, Rubio Asensio JS, Cousins AB. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science. 2010;328:899–903. doi: 10.1126/science.1186440. [DOI] [PubMed] [Google Scholar]
- 15.Hachiya T, Sugiura D, Kojima M, Sato S, Yanagisawa S, Sakakibara H, Terashima I, Noguchi K. High CO2 triggers preferential root growth of Arabidopsis thaliana via two distinct systems under low pH and low N stresses. Plant Cell Physiol. 2014;55:269–80. doi: 10.1093/pcp/pcu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoyama S, Huarancca Reyes T, Guglielminetti L, Lu Y, Morita Y, Sato T, Yamaguchi J. Ubiquitin ligase ATL31 functions in leaf senescence in response to the balance between atmospheric CO2 and nitrogen availability in arabidopsis. Plant Cell Physiol. 2014;55:293–305. doi: 10.1093/pcp/pcu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiba T, Feria-Bourrellier A-B, Lafouge F, Lezhneva L, Boutet-Mercey S, Orsel M, Bréhaut V, Miller A, Daniel-Vedele F, Sakakibara H, et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012;24:245–58. doi: 10.1105/tpc.111.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H. Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J Biol Chem. 2004;279:16598–605. doi: 10.1074/jbc.M313710200. [DOI] [PubMed] [Google Scholar]
- 19.Miao Y, Laun T, Zimmermann P, Zentgraf U. Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol. 2004;55:853–67. doi: 10.1007/s11103-005-2142-1. [DOI] [PubMed] [Google Scholar]
- 20.Pourtau N, Marès M, Purdy S, Quentin N, Ruël A, Wingler A. Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta. 2004;219:765–72. doi: 10.1007/s00425-004-1279-5. [DOI] [PubMed] [Google Scholar]
- 21.Wingler A, Purdy S, MacLean JA, Pourtau N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot. 2006;57:391–9. doi: 10.1093/jxb/eri279. [DOI] [PubMed] [Google Scholar]
- 22.Wingler A, Delatte TL, O’Hara LE, Primavesi LF, Jhurreea D, Paul MJ, Schluepmann H. Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiol. 2012;158:1241–51. doi: 10.1104/pp.111.191908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, Balazadeh S, Tohge T, Erban A, Giavalisco P, Kopka J, Mueller-Roeber B, Fernie AR, Hoefgen R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 2013;162:1290–310. doi: 10.1104/pp.113.217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T, Maekawa S, Yasuda S, Sonoda Y, Katoh E, Ichikawa T, Nakazawa M, Seki M, Shinozaki K, Matsui M, et al. CNI1/ATL31, a RING-type ubiquitin ligase that functions in the carbon/nitrogen response for growth phase transition in Arabidopsis seedlings. Plant J. 2009;60:852–64. doi: 10.1111/j.1365-313X.2009.04006.x. [DOI] [PubMed] [Google Scholar]
- 25.Serrano M, Parra S, Alcaraz LD, Guzmán P. The ATL gene family from Arabidopsis thaliana and Oryza sativa comprises a large number of putative ubiquitin ligases of the RING-H2 type. J Mol Evol. 2006;62:434–45. doi: 10.1007/s00239-005-0038-y. [DOI] [PubMed] [Google Scholar]
- 26.Guzmán P. The prolific ATL family of RING-H2 ubiquitin ligases. Plant Signal Behav. 2012;7:1014–21. doi: 10.4161/psb.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Maekawa S, Yasuda S, Domeki Y, Sueyoshi K, Fujiwara M, Fukao Y, Goto DB, Yamaguchi J. Identification of 14-3-3 proteins as a target of ATL31 ubiquitin ligase, a regulator of the C/N response in Arabidopsis. Plant J. 2011;68:137–46. doi: 10.1111/j.1365-313X.2011.04673.x. [DOI] [PubMed] [Google Scholar]
- 28.Miao Y, Zentgraf U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J. 2010;63:179–88. doi: 10.1111/j.1365-313X.2010.04233.x. [DOI] [PubMed] [Google Scholar]
- 29.Raab S, Drechsel G, Zarepour M, Hartung W, Koshiba T, Bittner F, Hoth S. Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J. 2009;59:39–51. doi: 10.1111/j.1365-313X.2009.03846.x. [DOI] [PubMed] [Google Scholar]
- 30.Peng M, Hannam C, Gu H, Bi Y-M, Rothstein SJ. A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J. 2007;50:320–37. doi: 10.1111/j.1365-313X.2007.03050.x. [DOI] [PubMed] [Google Scholar]
- 31.Kant S, Peng M, Rothstein SJ. Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in arabidopsis. PLoS Genet. 2011;7:e1002021. doi: 10.1371/journal.pgen.1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin W-Y, Huang T-K, Chiou T-J. Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell. 2013;25:4061–74. doi: 10.1105/tpc.113.116012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T-Y, Huang T-K, Tseng C-Y, Lai Y-S, Lin S-I, Lin W-Y, Chen J-W, Chiou T-J. PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell. 2012;24:2168–83. doi: 10.1105/tpc.112.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh L-C, Lin S-I, Shih AC-C, Chen J-W, Lin W-Y, Tseng C-Y, Li W-H, Chiou T-J. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009;151:2120–32. doi: 10.1104/pp.109.147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park BS, Seo JS, Chua N-H. NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell. 2014;26:454–64. doi: 10.1105/tpc.113.120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–63. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 37.Katsiarimpa A, Kalinowska K, Anzenberger F, Weis C, Ostertag M, Tsutsumi C, Schwechheimer C, Brunner F, Hückelhoven R, Isono E. The deubiquitinating enzyme AMSH1 and the ESCRT-III subunit VPS2.1 are required for autophagic degradation in Arabidopsis. Plant Cell. 2013;25:2236–52. doi: 10.1105/tpc.113.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maekawa S, Inada N, Yasuda S, Fukao Y, Fujiwara M, Sato T, Yamaguchi J. The carbon/nitrogen regulator ARABIDOPSIS TOXICOS EN LEVADURA31 controls papilla formation in response to powdery mildew fungi penetration by interacting with SYNTAXIN OF PLANTS121 in Arabidopsis. Plant Physiol. 2014;164:879–87. doi: 10.1104/pp.113.230995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science. 2013;339:704–7. doi: 10.1126/science.1230406. [DOI] [PubMed] [Google Scholar]