Abstract
Through evolution, plants have developed a myriad of strategies to adapt to environmental perturbations. Using 3 Arabidopsis ecotypes in conjunction with various transgenic and mutant lines, we provide evidence that wounding and drought differentially alter the metabolic signatures derived from the 2 main competing oxylipin-pathway branches, namely the JA and its precursor 12-OPDA produced by Allene oxide synthase (AOS) branch, and aldehydes and corresponding alcohols generated by Hydroperoxide lyase (HPL) branch. Specifically, we show that wounding induces production of both HPL and AOS-derived metabolites whereas, drought stress only elicits production of hexenal but suppresses hexenol, and further uncouples the conversion of 12-OPDA to JA. This finding led to uncovering of 12-OPDA as a functional convergence point of oxylipin and ABA pathways to control stomatal aperture in plant adaptive responses to drought. In addition, using transgenic lines overexpressing plastidial and extraplastidial HPL enzyme establish the strong interdependence of AOS- and HPL-branch pathways, and the importance of this linkage in tailoring plant adaptive responses to the nature of perturbations.
Keywords: Oxylipin pathway, allene oxide synthase (AOS), hydroperoxidelyase (HPL), 12-OPDA, abiotic stress, drought
Polyunsaturated fatty acids (PUFA) are central to the structural integrity of biological membranes and serve as precursors for active metabolites responsible for modulation of a multitude of signal transduction pathways evoked by environmental stimuli. Indeed, a prime consequence of environmental stresses is alteration of membrane lipid composition and the de novo synthesis of biologically active compounds called “oxylipins,” derivatives of oxygenated PUFAs. Among the oxylipin pathways, the enzymes allene oxide synthase (AOS) and hydroperoxidelyase (HPL) are the two major branches that compete for the same substrates and are critical plant stress response pathways.1-3
The AOS pathway produces 12-oxo-phytodienoic acid (12-OPDA) and jasmonic acid (JA). JA biosynthesis begins in plastids, and 12-OPDA is the final product of the plastid-localized part of the pathway.4,5 12-OPDA is then translocated to the peroxisome where it is reduced by 12-OPDA reductase 3 (OPR3), and subsequently activated by CoA ester prior to undergoing three rounds of β−oxidation to form JA.6-8 12-OPDA is not only a metabolic intermediate but also a signaling molecule with both overlapping and distinct functions from JA, best evidenced from studies on Arabidopsis opr3, a mutant that accumulates 12-OPDA but is deficient in JA.5 For example, JA and 12-OPDA both induce expression of stress genes, but profiles of induced genes only partially overlap, and many genes are induced only by one of the two metabolites.9,10
The HPL branch of the oxylipin pathway produces aldehydes and corresponding alcohols. One or more HPL genes encode the first enzyme in the pathway, differing in their substrate specificity and subcellular localization, suggesting their diverse function(s) in tailoring plant responses to an specific stress.11 Our recent findings lend further support to this notion by establishing that the three rice HPLs (HPL1 through HPL3)have distinct subcellular localization. We have specifically shown that HPL2 is an ER associated enzymes whereas HPL3 is plastid localized.12 Importantly, Col-0 transgenic lines overexpressing HPL2 and HPL3 enzymes independently, display distinct patterns of AOS- and HPL-derived metabolites in response to different stresses.12 In particular, subcellular localization of HPL drastically alters jasmonates signature in response to specific stresses. That is, overexpression of plastidial HPL3 reduces the stress inducible levels of both 12-OPDA and JA as compared with the WT plants. This suggests that overexpression of the plastidial HPL leads to channeling of the substrate pool away from AOS branch pathway. By contrast, transgenic lines overexpressing extraplastidial HPL2 or engineered HPL3 that lack a plastidial transit peptide, display an equal or higher stress-inducible levels of 12-OPDA and JA as compared with the WT plants. These data indicate that the extraplastidial localization of HPL enhances activation of the AOS-pathway, directly or indirectly by HPL-derived metabolite. Moreover, the results clearly confirm the interdependence of AOS- and HPL- branch pathways, and raises the question of the governing biochemical and molecular mechanism(s) by which extraplastidial HPL enzyme modulate jasmonates levels.
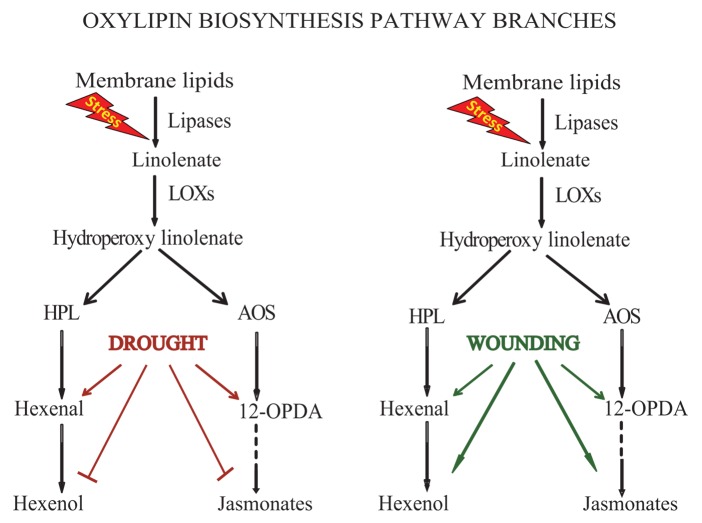
Additionally, we have established the importance of fine turning of HPL and AOS pathway metabolites in tailoring plant adaptive responses to a diverse range of perturbations. Using the HPL overexpressing lines together with various mutants of AOS pathway genes we have demonstrated that wounding induces production of the HPL-derived metabolites, hexenal and hexenol, and AOS-derived compounds, namely 12-OPDA and JA (Fig. 1). By contrast, drought stress only induces production of hexenal and 12-OPDA, suppresses hexenol and maintains JA at the basal levels (Fig. 1). Further studies established that drought-mediated induction of 12-OPDA is accompanied by a reduction of the stomatal aperture independently from the classical drought responsive hormone ABA, as evidenced by the regulatory function of 12-OPDA in ABA-deficient mutant aba2–1. Not surprisingly, we also established that higher 12-OPDA levels correlates with lower stomatal conductance and transpiration rate, and hence elevated plant drought tolerance. Using agricultural crops, tomato and Brassica napus, confirmed the potency of 12-OPDA as regulator of stomatal aperture functioning most effectively in cooperation with ABA.
Figure 1. Simplified model of differential response of oxylipin pathway branches to wounding and drought stress.
In summary, this report not only recognizes 12-OPDA as a new player in plant drought tolerance, but ushers in a new area of research focused on the mechanism by which drought signaling uncouples conversion of 12-OPDA to JA.
Glossary
Abbreviations:
- LOXs
lipoxygenases
- AOS
allene oxide synthase
- 12-OPDA
12-oxo-phytodienoic acid
- HPL
hydroperoxidelyase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Institute of Health (R01GM107311) and National Science Foundation (IOS-1036491) grants awarded to KD.
References
- 1.Chehab EW, Kaspi R, Savchenko T, Rowe H, Negre-Zakharov F, Kliebenstein D, Dehesh K. . Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS One 2008; 3:e1904; http://dx.doi.org/ 10.1371/journal.pone.0001904; PMID: 18382679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feussner I, Wasternack C. . The lipoxygenase pathway. Annu Rev Plant Biol 2002; 53:275 - 97; http://dx.doi.org/ 10.1146/annurev.arplant.53.100301.135248; PMID: 12221977 [DOI] [PubMed] [Google Scholar]
- 3.Howe GA, Schilmiller AL. . Oxylipin metabolism in response to stress. Curr Opin Plant Biol 2002; 5:230 - 6; http://dx.doi.org/ 10.1016/S1369-5266(02)00250-9; PMID: 11960741 [DOI] [PubMed] [Google Scholar]
- 4.Schaller A, Stintzi A. . Enzymes in jasmonate biosynthesis - structure, function, regulation. Phytochemistry 2009; 70:1532 - 8; http://dx.doi.org/ 10.1016/j.phytochem.2009.07.032; PMID: 19703696 [DOI] [PubMed] [Google Scholar]
- 5.Stintzi A, Browse J. . The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci U S A 2000; 97:10625 - 30; http://dx.doi.org/ 10.1073/pnas.190264497; PMID: 10973494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kienow L, Schneider K, Bartsch M, Stuible HP, Weng H, Miersch O, Wasternack C, Kombrink E. . Jasmonates meet fatty acids: functional analysis of a new acyl-coenzyme A synthetase family from Arabidopsis thaliana.. J Exp Bot 2008; 59:403 - 19; http://dx.doi.org/ 10.1093/jxb/erm325; PMID: 18267944 [DOI] [PubMed] [Google Scholar]
- 7.Koo AJ, Chung HS, Kobayashi Y, Howe GA. . Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J Biol Chem 2006; 281:33511 - 20; http://dx.doi.org/ 10.1074/jbc.M607854200; PMID: 16963437 [DOI] [PubMed] [Google Scholar]
- 8.Schaller F, Biesgen C, Müssig C, Altmann T, Weiler EW. . 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 2000; 210:979 - 84; http://dx.doi.org/ 10.1007/s004250050706; PMID: 10872231 [DOI] [PubMed] [Google Scholar]
- 9.Ribot C, Zimmerli C, Farmer EE, Reymond P, Poirier Y. . Induction of the Arabidopsis PHO1;H10 gene by 12-oxo-phytodienoic acid but not jasmonic acid via a CORONATINE INSENSITIVE1-dependent pathway. Plant Physiol 2008; 147:696 - 706; http://dx.doi.org/ 10.1104/pp.108.119321; PMID: 18434606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, et al. . 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 2005; 139:1268 - 83; http://dx.doi.org/ 10.1104/pp.105.067058; PMID: 16258017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chehab EW, Raman G, Walley JW, Perea JV, Banu G, Theg S, Dehesh K. . Rice HYDROPEROXIDE LYASES with unique expression patterns generate distinct aldehyde signatures in Arabidopsis. Plant Physiol 2006; 141:121 - 34; http://dx.doi.org/ 10.1104/pp.106.078592; PMID: 16531481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savchenko T, Kolla VA, Wang CQ, Nasafi Z, Hicks DR, Phadungchob B, Chehab WE, Brandizzi F, Froehlich J, Dehesh K. . Functional convergence of oxylipin and abscisic Acid pathways controls stomatal closure in response to drought. Plant Physiol 2014; 164:1151 - 60; http://dx.doi.org/ 10.1104/pp.113.234310; PMID: 24429214 [DOI] [PMC free article] [PubMed] [Google Scholar]