Abstract
Lamins are the main components of the metazoan lamina, and while the organization of the nuclear lamina of metazoans and plants is similar, there are apparently no genes encoding lamins or most lamin-binding proteins in plants. Thus, the plant lamina is not lamin-based and the proteins that form this structure are still to be characterized. Members of the plant NMCP/LINC/CRWN protein family share the typical tripartite structure of lamins, although the 2 exhibit no sequence similarity. However, given the many similarities between NMCP/LINC/CRWN proteins and lamins (structural organization, position of conserved regions, sub-nuclear distribution, solubility, and pattern of expression), these proteins are good candidates to carry out the functions of lamins in plants. Moreover, functional analysis of NMCP/LINC mutants has revealed their involvement in maintaining nuclear size and shape, another activity fulfilled by lamins. This review summarizes the current understanding of NMCP/LINC proteins and discusses future studies that will be required to demonstrate definitively that these proteins are plant analogs of lamins.
Keywords: LINC proteins, NMCP proteins, CRWN proteins, plant nuclear envelope, nuclear size, plant lamina, lamins
The presence of a fibrous lamina underlying the nuclear envelope that binds to the nuclear pore complex (NPC) was first revealed by TEM in invertebrates in the 1950s (protozoa, gregarines, and annelids),1-4 and later in vertebrates.5 Yet, it was not until the 1970s that this structure was isolated from rat liver nuclei6 and its main polypeptides identified.7 These polypeptides, now known as lamins, have since been characterized extensively and shown to be restricted to metazoans.8 However, 2 lamin-like proteins were recently identified in unicellular eukaryotes: the Dictyostelium NE81 protein is considered to be an evolutionary precursor of lamins,9,10 while Trypanosoma NUP1 is an unrelated long coiled-coil protein with lamin-like functions, participating in the regulation of nuclear shape and structure, chromatin organization, and the distribution of NPCs.11 Lamins not only provide mechanical support to the nucleus and the nuclear envelope (NE) and promote the association between the nucleoskeleton (NSK) and cytoskeleton (CSK), but they are also involved in a multitude of nuclear functions such as chromatin organization, gene regulation, signaling, and DNA repair.12-14
The plant nuclear lamina was described by TEM in isolated NSKs in the early 1990s,15-20 and subsequently, a number of the similarities between the plant and vertebrate lamina were defined by field emission scanning electron microscopy (feSEM) of whole nuclei.21 Fully sequenced plant genomes lack genes encoding lamins22,23 or lamin-binding proteins, with the exception of the SUN-domain proteins.22,24 Given the crucial role of lamins in nuclear and cellular functions,12-14 the fact that plants possess a non-lamin-based lamina has raised certain interest, and considerable research effort has been dedicated to the characterization of the proteins that compose the plant lamina. In this review, we summarize the current understanding of nuclear matrix constituent proteins (NMCPs)/little nuclei proteins (LINCs)/crowded nucleus proteins (CRWN), the main candidates to fulfil lamin-like functions in plants, and we compare and contrast these proteins with the well-characterized lamins, the main components of the metazoan lamina. In addition, we discuss the main functions attributed to NMCPs and the evidence that must be accumulated in future studies to definitively consider these proteins as analogs of plant lamins.
Organization and composition of the metazoan lamina
The nuclear lamina is a complex protein meshwork attached to the inner nuclear membrane (INM) and the nucleoplasmic ring of NPCs.25,26 The metazoan lamina consists of a polymeric layer of lamins that belong to the intermediate filament protein superfamily, numerous transmembrane lamin-binding proteins that anchor the lamina to the INM, and chromatin-associated factors.14,27 The nuclear lamina not only provides support for the NE, NPCs, and chromatin anchoring sites but it is also involved in linking the NSK to the CSK, and in regulating signaling and gene activity.25 The ultra-structural organization of the lamina has been well characterized in amphibian oocytes, and it consists of 10-nm lamin filaments arranged in a regular orthogonal pattern. By contrast, more irregular filamentous networks have been observed in somatic cells.25,26
Lamins, the building blocks of the lamina
Lamins are the main components of the nuclear lamina.7 Sequence comparisons and those of exon/intron patterns indicate that lamins are the founding members of the IF protein family.8 Based on their structure, expression pattern, mitotic behavior, and biochemical characteristics, lamins have been classified into 2 types (A and B).13,14 Most invertebrates have a single lamin gene encoding a type B lamin,8,28 while vertebrates possess 4 lamin genes: LMNB1, LMNB2, LIII (sometimes called XLMNB3), and LMNA that encodes lamins A and C. The LIII gene has been lost in mammals, and moreover, mammals possess an additional type A lamin, lamin C, which is produced by alternative splicing of lamin A transcripts.8 The tail domain of lamin A contains a unique 90-amino acid segment not found in type B lamins, probably due to the insertion of a new exon in the last intron of a type B progenitor gene. Lamin A interacts with numerous nuclear proteins, and it is involved in multiple nuclear and cellular functions, as witnessed by the broad spectrum of human diseases caused by LMNA gene mutations.29
Lamins have the conserved tripartite structure typical of IF proteins, consisting of a coiled-coil central rod domain that contains 4 coils (1A, 1B, 2A, and 2B), each separated from one another by 3 short linkers (L1, L12, and L2). The rod domain is flanked by a short N-terminal domain that contains a conserved phosphorylation site for cdk1,8 which is involved in head-to-tail polymerization, and a longer C-terminal tail domain containing a second conserved cdk1 phosphorylation site required for mitotic lamin depolymerisation.13 In addition, there is a nuclear localization signal (NLS) located between the C-end of the rod domain and the highly conserved IgG fold, as well as a C-terminal CAAX box (Fig. 2).12,30
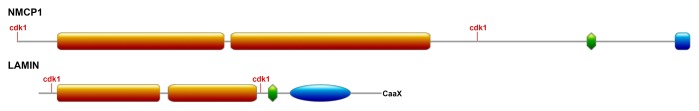
Figure 2. Comparison of the structure of NMCP proteins and lamins. Both have a tripartite structure with a central coiled-coil domain (orange boxes) flanked by cdk1 phosphorylation sites and a tail domain with an NLS (green boxes) and a conserved C-terminus (blue box in the case of the NMCPs and CaaX in the case of lamins). NMCP proteins lack the IgG fold typical of lamins (blue oval).
The expression of lamin genes is developmentally regulated, and while lamin B2 is constitutively expressed in somatic cells, the expression of lamin B1 is more restricted. Lamin LIII is the predominant lamin in oocytes and embryos, yet its expression in somatic cells is restricted to a few differentiated tissues. Lamin A is expressed late in development, normally correlating with differentiation.29 Except for lamin C, all lamins are expressed as prelamins, and they undergo highly regulated and extensive post-translational modification of the C-terminal CAAX box via cysteine farnesylation, which is followed by proteolytic cleavage of the AAX by a prenyl protease and carboxymethylation of the C-terminal cysteine. B-type lamins remain permanently farnesylated and carboxymethylated, whereas prelamin A undergoes the removal of 15 amino acids from the C-terminus to produce the mature lamin A that lacks these farnesyl and carboxymethyl modifications.12,27
Lamins also undergo other post-translational modifications, such as phosphorylation and sumoylation. The polymerization and mitotic disassembly of lamins is regulated by extensive phosphorylation by cdk1, PKC, PKA, S6-kinaseII, and Akt. Lamins have 12 conserved phosphorylation sites that are involved in mitotic lamin polymerization and that are located in the head and tail domains. Other conserved phosphorylation sites are probably involved in the regulation of conserved functions, while unique phosphorylation sites likely mediate the differential regulation of lamins in distinct tissues.27
The assembly of lamins into the nuclear lamina is a complex multistep process. In vitro reconstitution and structural analysis revealed that the building blocks of lamin polymers are formed by parallel dimerization of the rod domains that then assemble longitudinally to form higher order head-to-tail lamin polar oligomers. Two head-to-tail oligomers then interact laterally to form tetrameric protofilaments, which further assemble to form 10-nm filaments, as successfully assembled in vitro using Ce-lamin (most other lamins form paracrystalline arrays in vitro).13,31 The rod segments play an important role in lamin homodimerization, and in the formation of lateral and longitudinal contacts.32,33 Moreover, mutational analyses suggest that the 2B coil plays a dual role in dimerization and in the interdimer interactions necessary for filament formation.33
Current information about the organization of lamins in vivo is largely based on studies of the amphibian oocyte lamina, which express a single type of lamin (LIII). By contrast, lamin organization in somatic cells remains poorly understood, probably due to the complex interactions of lamins with chromatin and other proteins or nuclear structures. In vivo lamins form an intricate orthogonal meshwork of filaments within the lamina,25,26,34 and they also reside in the nucleoplasm in a less organized state but with much greater mobility than that observed in the lamina.35 Although type A and B lamins form separate filament networks at the nuclear envelope and in the interior of the nucleus, these individual networks interact to varying degrees.26,34,36,37 During the open mitosis of metazoans, type A and B lamins display different assembly and disassembly properties. When the NE is disassembled during late prophase, lamins are depolymerised by mitotic kinases, and while type A lamins are dispersed throughout the cytoplasm, type B lamins remain associated to the nuclear membranes that disperse throughout the endoplasmic reticulum, probably due to their permanent farnesylated state. Type A and B type lamins also undergo spatially and temporally distinct forms of assembly into the nuclear lamina at the end of mitosis. Type B lamins accumulate around decondensing chromosomes to form relatively stable complexes at telophase, while type A lamins are transported into the nucleus at a later stage, after the formation of an intact NE.12
Lamins are involved in many nuclear functions, such as: the regulation of nuclear shape and architecture; the association of the NSK to the CSK; epigenetic modifications; chromatin organization and positioning; DNA replication, repair, and transcription; and cell proliferation and differentiation. They also perform several structural functions including the regulation of the size, shape, and mechanical properties of the nucleus, and they are important for NE stabilization and the incorporation and spacing of NPCs.12-14,30,35,36,38 In addition, lamins participate in the physical connection between the nucleus and the CSK through their interaction with the LINC complex, which is formed by SUN- and KASH-domain proteins and is essential for nuclear positioning and migration, centrosome attachment to the nucleus, meiotic chromosome pairing, and mechanotransduction.39,40 Lamins also act as modulators of transcription through their influence on chromatin structure and organization as a result of direct interactions with either DNA, histones, and/or other chromatin-associated proteins, such as LBR, HP1, and BAF, or their direct or indirect interaction with transcription factors that affect cell proliferation, differentiation, and apoptosis.13,41,42 The direct or indirect interaction of chromatin with lamins also has a strong effect on the epigenetic modification of histones.43,44 The absence of lamins affects the organization of chromosome territories and domains, and a role for lamins in the localization and function of centromeres and telomeres has also been demonstrated.45,46 Lamins localize to replication foci and interact with PCNA, a component of the replication machinery,47 while mutations in lamins can produce genomic instability by compromising DNA repair.48 Together, all the above observations point to lamins as key determinants of nuclear architecture and function.
It is clear from the above that the interaction of lamins with distinct proteins, including structural and regulatory proteins, defines their activity.27,49 Lamin protein partners, mainly those of lamin A, have been studied extensively.14,27,49 The proteins that interact with lamin A are involved in different nuclear activities, and they include components of the NSK and NPCs, such as lamins B1 and B2, actin (lamins have 2 actin binding sites in their tail domain),50 nesprin 1α and nesprin 2, lamin companion 1 (LCO1), SUN1 and SUN2, the nucleoporins Nup153 and Nup88, and LEM domain proteins such as LAP2α, MAN1, LEM2, and emerin. Other partners include chromatin-associated proteins such as BAF, PCNA, HP1, and histones, as well as transcription factors like Rb or other proteins involved in transcription and signaling.27 The partners of type B lamins are less well known, although lamin B1 is known to interact with the lamin B receptor (LBR), which contains 8 transmembrane domains, as well as with emerin, MAN1, actin, LCO1 and Nup153, PCNA, and histones.14,27
Some of the best characterized lamin-binding proteins are those that share the LEM domain, a motif of about 45 residues that folds as 2 α-helices and binds BAF, a mobile lamin-binding protein that interacts with histones.49 Most LEM proteins are integral proteins of the INM and have 1 or 2 transmembrane domains, while some have additional domains that bind DNA or chromatin-binding proteins. All LEM domain proteins bind type A and/or B lamins through a direct interaction with the IgG fold in their tail domains.14 Emerin binds many other proteins in addition to lamins, including: structural proteins (e.g., nesprin1α, nesprin2β, actin, nuclear myosin c, and nuclear αII-spectrin); other INM proteins (e.g., MAN1, LUMA); proteins involved in signaling, transcription, and mRNA splicing; and BAF.51 Emerin forms several multiprotein complexes, some of which contain mainly architectural components and others containing chromatin and gene regulators.49,51 LAP2α and LCO1 bind lamin A and form three-dimensional scaffolds in the nuclear interior.35 Lamins, LEM proteins, BAF, and probably other INM proteins form a complex multiprotein network involved in anchoring chromatin to the NE.49 These functions and interactions of LEM proteins, lamins, and BAF are strongly conserved in metazoans, emphasizing their fundamental roles in the nucleus.
Other well-characterized lamin partners are the SUN-domain proteins of the INM, proteins that form trimers that interact with 3 KASH domain proteins of the ONM in the lumen of the NE, forming LINC complexes.39 SUN proteins are conserved in eukaryotes, including yeast and plants,52,53 and they are characterized by their C-terminal SUN domain, a 120 residue motif involved in binding the KASH domain, and a nearby coiled-coil domain that mediates trimerization.40,54 SUN domain proteins bind to lamins through a direct interaction with their nucleoplasmic N-terminal domain.55
The plant lamina
The presence of a peripheral layer similar to the metazoan lamina was identified by TEM in the NSK and nucleus of both dicot and monocot plants in the early 1990s.15-19,56 A more recent feSEM study of the nucleus revealed the presence of a plant lamina attached to the INM and linked to the nucleoplasmic ring of NPCs, with a highly organized filamentous structure similar to that of the metazoan nuclear lamina.21,57 Plants lack orthologs of lamins,22,23 as well as most lamin-binding proteins except for the SUN proteins,52,58 although Nup136 is a functional analog of metazoan lamin binding protein, Nup153.59 The similar organization of the lamina and the fulfilment of the main activities of lamins in the plant nucleus suggest that plants express proteins that functionally replace lamins, and that probably share their structural and functional properties rather than sequence similarity, as described for NUP1 in Trypanosoma.11
Since the first description of a plant lamina, a few insoluble proteins have been identified in this structure, mainly by immunological methods.20,60 Some of these proteins are immunologically related to vertebrate lamins, and are of similar sizes, with comparable pI values and nuclear distributions.16,17,19,61 These include the NIF group of proteins, which not only exhibit the aforementioned similarities with lamins, but they also form 6–12-nm filaments in vitro.16,61 Unfortunately the sequence of these proteins remains unavailable to compare them to lamins.
As indicated above, the best candidates to fulfill the functions of lamins in plants are NMCPs, which have a predicted secondary structure similar to that of lamins and thus, should be able to dimerize and form filaments. NMCP1 was first described in 1993 in carrot as a residual protein of the nuclear matrix with a pI value similar to that of lamins but a much higher molecular mass.18 This protein was later shown to have a predicted tripartite structure with a central coiled-coil domain similar to that of lamins,56 and to assemble and disassemble in mitosis like lamins.62 Four homologs of carrot NMCP1 were subsequently identified in a genome-wide search for coiled-coil proteins in Arabidopsis thaliana.23 These 4 A. thaliana genes were named LINC (little nuclei) 1 to 4 after the phenotype of their corresponding mutants.63 This term is somewhat misleading, as it is already used to describe the linker of the NSK to CSK complex of the NE.40 Accordingly, it was proposed to change this term to CRWN (crowded nucleus), after another phenotype of the mutants,24 although this could add further confusion to the field and in our opinion, the original term NMCP (nuclear matrix constituent proteins) should be used to refer to these proteins. More recently, searches of plant genomes have identified genes encoding other NMCP homologs in many different species, confirming that NMCPs are well conserved in plants.64,65 Mutational analysis in A. thaliana has revealed that NMCP proteins participate in some nuclear functions mediated by lamins in metazoans, such as the regulation of nuclear size and shape.63,66 Although NMCPs do not share strong sequence similarity with lamins, their predicted structure and sub-nuclear distribution suggest that they may participate in the formation of the plant lamina. This observation, along with their demonstrated role in regulating nuclear shape and size, make these proteins good candidates to be lamin analogs in plants.
The NMCP protein family
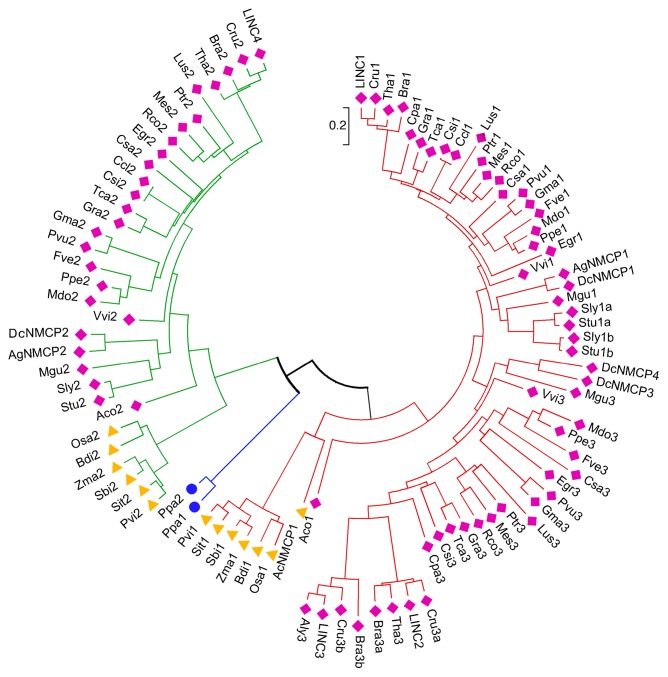
The NMCP protein family has been characterized using bioinformatic and biochemical approaches, as well as with molecular biology tools. Members of this family share a high degree of sequence similarity, and they have been identified in all land plants (Embryophytes) analyzed, including a moss (Physcomitrella patens) and vascular plants (Tracheophyte), although they appear to be absent from single cell plants (Volvox carteri and the unicellular algue Chlamydomonas reinhardtii). However, these proteins are not conserved in metazoans, yeast, or bacteria.65 Based on sequence similarities, structural analogies and phylogenetic relationships, and in agreement with a prior study that analyzed a small number of sequences, an exhaustive analysis of 97 sequences from 37 plant genomes (Table S1) recently classified NMCP proteins into 2 clusters: NMCP1 and NMCP2 (Fig. 1).64,65 All plants carry 1 NMCP2 gene, and while monocots have 1 NMCP1 gene, dicots carry several NMCP1 genes: 1 NMCP1 gene and an additional gene encoding other NMCP1-related proteins named NMCP3. A. thaliana carries 4 genes, LINC1–4.23,63 LINC1 is an ortholog of NMCP1, whereas LINC2 and LINC3 are classified as NMCP3-type genes and LINC4 is a NMCP2 protein (Fig. 1).64,65 Like A. thaliana, some other dicots (Dacus carota, Capsella rubella, and Brassica rapa) express 2 NMCP3-type proteins, while Solanum tuberosum and Solanum lycopersicum contain 2 NMCP1-type but no NMCP3-type proteins (Fig. 1).
Figure 1. Evolutionary relationships of NMCP proteins. Sequences classified as NMCP1 (NMCP1 and NMCP3) are marked in red and those classified as NMCP2 in green. The 2 proteins of Physcomitrella patens are in blue. Dicotyledon species are represented by cian rhombi, monocotyledons by yellow triangles, and mosses by blue circles.
In vascular plants, NMCPs evolved from 2 genes: the NMCP1 and NMCP2 progenitors. The 2 P. patens NMCPs evolved from a common NMCP progenitor gene and are included in the NMCP2 cluster, suggesting that the archetypal NMCP progenitor was an NMCP2 protein (Fig. 1).65
All LINC genes are expressed in whole A. thaliana plants.66 The expression profile of LINC1–4 genes show that they are co-expressed with genes encoding proteins involved in the cell cycle, DNA processing and transcription.63 Microarray data from root tissues shows that A. thaliana LINC1, LINC4, and LINC3 are expressed most strongly, whereas LINC2 is generally expressed more weakly. The expression of NMCP/LINC proteins is developmentally regulated. LINC1 is strongly expressed in meristems, and less so between the elongation and the differentiated root zones, correlating with the pattern of expression of LINC1-GFP from its native promoter.67-70 The expression of LINC2 and LINC3 also decreases from the meristem to the differentiated zone, with a particularly steep decrease in LINC2 expression between the meristematic and the elongation zones. The expression of LINC4 decreases in the elongation zone but increases slightly again in the differentiated zone.68-70 NMCP1 western blots and the distribution of LINC1-GFP protein support these results and demonstrate that NMCP1/LINC1 is abundant in both proliferating and quiescent meristems, although it accumulates in much smaller amounts in the cells of the mature zone.65,67 The expression profile of NMCP1/LINC1 resembles that of lamin B1, which is abundant in proliferating and quiescent meristematic cells but is weakly expressed in differentiated cells.71-73
NMCP proteins have a tripartite structure with a central coiled-coil rod domain, and non-coiled head and tail domains (Fig. 2).56,63-65 Most NMCPs contain 2 coiled coils, separated by a linker of about 20 residues, which form a central rod domain that is predicted to dimerize. Short linkers have also been predicted to reside inside the coiled-coil segments. The length of the rod domain is conserved in NMCPs, as are the positions of the linkers in the NMCP1 and NMCP2 proteins.65 In addition, both termini of the NMCP coiled-coil domain are conserved in all NMCPs, suggesting that the structure of the rod domain is well conserved across the NMCP family and that it plays an important role in oligomerization. The NMCPs in Physcomitrella patens have a longer sequence than other NMCP proteins, and they contain a long insert in the rod domain. This insertion results in a unique distribution of coiled-coils and altered linker positions in Ppa proteins (Ciska et al., unpublished).
The general organization of coiled coils in lamins and NMCPs is similar, although the rod domain of NMCPs is twice as long as that of lamins (Fig. 2). NMCPs exhibit a high degree of sequence similarity in the rod domain, which contains 5 highly conserved regions at each end and within the second coil, just before the second linker. Another conserved region includes the linker separating the 2 coils, and it is conserved in all NMCPs except for those in P. patens.65 Lamins exhibit a similar distribution of conserved motifs, and those located at either end of the coiled-coil domain are prime candidates to mediate the head-to-tail associations.32 The analogous structures, and the location of conserved motifs in the rod domain of NMCPs and lamins, suggest a similar mechanism of oligomerization and protofilament formation. This hypothesis is supported by the presence of consensus sequences recognized by cyclin dependent kinase (cdk1) and protein kinase (PKC)74 at either side of the rod domain.
NMCPs also contain several highly conserved motifs in the less conserved tail domain, including a NLS and NMCP-specific regions.65 A conserved region close to the NLS in NMCP1 proteins (RYNLRR), along with the NLS and the N-terminal region of the protein, is required for proper localization of the protein to the periphery of the nucleus in carrot (Masuda et al. unpublished). This region also contains a 5-amino acid stretch that is identical to a specific region of lamin A (EYNLRSRT)8 and that probably serves as an actin-binding site.50 Point mutations in this sequence in lamins cause severe laminopathies, suggesting an important role for this actin-binding site in lamin A.75 Like lamins, most NMCPs contain a predicted NLS in the tail domain, although the position and sequence is only conserved in NMCP1-type proteins. Few sequences lack a predicted NLS, but 2 such sequences (AgNMCP2 and DcNMCP2) localize to the nucleus, to which they are probably directed via an alternative pathway.64
NMCPs lack the C-terminal CAAX box typical of lamins, although the C-terminus of most members contains a highly conserved region that does not appear to be involved in NE association (Masuda et al., unpublished). The C-terminal conserved region is present in NMCP1 clusters and in monocot NMCP2, although it is absent in dicot NMCP2,64,65 which coincides with the appearance of NMCP3 proteins and suggests that this new protein class fulfils some of the functions of NMCP2. This region is preceded by a stretch of acidic amino acids that is also found at the end of the tail domain of vertebrate lamins.76
While the predicted molecular weights of NMCPs from dicots and monocots are similar (130–140 kDa for NMCP1 and 110–120 kDa for NMCP2), the mobility of the endogenous proteins varies across species,64,65 probably due to post-translational modifications. In some monocots, the MW of the endogenous proteins is lower than predicted, suggesting the involvement of alternative splicing or proteolytic cleavage. It is also possible that the differences in NMCP size are also present at the transcript level. NMCP genes may encode multiple transcripts, and while an NMCP1 protein in Sorghum bicolor (Sbi04 g030240.1) is predicted to contain 1156 amino acids, for example, a protein product of the alternative transcript is predicted to lack 134 C-terminal amino acids (Sbi04 g030240.3).
Like lamins, NMCP proteins are predominantly distributed in the nuclear lamina, although they are also found in the nucleoplasm, as demonstrated by immunofluorescence confocal microscopy, immuno-TEM, and the expression of YFP/GFP-LINC proteins. These observations suggest that the proteins are not only involved in nuclear functions associated with the lamina, but also in those mediated by internal lamins such as transcription, cell cycle progression, differentiation, and chromatin organization.35 A predominant distribution at the nuclear periphery has been reported for carrot and celery NMCP1 and NMCP2,56,64 and A. thaliana LINC1 and LINC4.63,66 A. thaliana LINC2 and LINC3 are exclusively nucleoplasmic,63,66 while onion NMCP1 displays both an internal and peripheral localization.65 The mechanisms that direct the proteins to these nuclear compartments are not yet understood. A very recent study demonstrated that the localization of carrot NMCP1 to the nuclear periphery involves a N-terminal 141 amino acid stretch, the conserved motif R/Q/HYNLRR/H and the NLS (Masuda et al., unpublished). Nonetheless, the interactions with other proteins necessary for proper localization remain unknown. While immuno-TEM has demonstrated the presence of onion NMCP1 in the NE in close proximity to NPCs,65 feSEM experiments will be necessary to unequivocally confirm that this protein is a component of the filaments that form the plant lamina.21
As demonstrated by immunofluorescence, the interphase distribution of NMCP1 in onion varies in different root cell populations, as does its expression. AcNMCP1 is regularly distributed along the nuclear envelope in meristematic cells, while its distribution in this structure in differentiated cells is discontinuous, with areas depleted of protein. A similar distribution has been reported for Ce-lamin in aging cells of C. elegans.77 In quiescent meristematic nuclei the protein accumulates in aggregates in the nucleoplasm that may reflect sites of stored protein ready for early activation during root germination, as described for similar quiescent structures containing packed nuclear ribonucleoproteins (RNPs) and actin.78,79 The detection of NMCP proteins in NSK fractions18,65,66 confirms that these proteins are insoluble components of the peripheral lamina and that like lamins, they are present in a minor fraction in the internal NSK.
NMCPs associate to the NE during interphase but they have a distinct spatial and temporal distribution when this structure disassembles during mitosis.62,64,66 All NMCPs disassemble in prometaphase after NE breakdown but they subsequently behave distinctly. NMCP1/LINC1 proteins accumulate on segregating chromosomes during anaphase, although carrot and celery NMCP1 first associate with the mitotic spindle, and then they are finally incorporated into the NE during telophase. NMCP2, LINC4, LINC2, and LINC3 disperse throughout the cytoplasm, and they are then incorporated into the surface of the chromosome and NE envelope following different pathways and at different times during telophase. Accordingly, NMCP2 associates to cytoplasmic nuclear membrane-derived vesicles while LINC4 binds to punctuate structures in the cytoplasm before relocating to the chromosome surface during telophase.62,64,66 The pathway responsible for the assembly of the NMCP1 and LINC1 proteins during mitosis differs from that of lamins, which do not associate to segregating chromosomes,12 suggesting that NMCPs and lamins are subject to distinct assembly processes in the NE.
The functions of NMCPs remain largely unknown. Although quadruple LINC mutants are not viable, indicating that NMCP/LINC proteins participate in essential processes, single, double, and even some triple mutants are viable.24 The phenotypic effects of LINC mutations are not as severe as those caused by lamin mutations that produce a broad spectrum of laminopathies14,29 and they mainly involve plant dwarfism, as well as reduced cellular and nuclear size.63,66,67 Furthermore, single mutants do not produce abnormal phenotypes at the whole plant level, indicating a degree of complementation between different LINC proteins.63,66
Disrupting the cytoskeleton by latrunculin B and /or propyzamide treatment has no effect on plant nuclear morphology, indicating that it is maintained by intranuclear factors and not by the CSK.66 While the underlying molecular mechanisms are unknown, several studies have implicated NMCP/LINC proteins in the regulation of nuclear shape and size,63,66,80 a role also played by lamins.14 LINC mutations result in a decrease in nuclear size and alterations in the shape of differentiated nuclei,63,66,67 while nuclear size is increased by LINC4 overexpression.66 Analyses of mutants have revealed that although all LINCs are involved in the regulation of nuclear shape and size to different extents, LINC1 and LINC4 play predominant non-redundant roles.63,66,67 LINC1 is mainly expressed in meristematic tissues, but it is required to achieve a differentiated nuclear shape, and it has been proposed to participate in a key differentiation step after nuclear formation.63,67 Downregulation of Ce-lamin expression in C. elegans embryos also results in misshapen nuclei.81 Also, mutations inside the rod domain which disrupt the lamin filament formation sometimes show the same phenotype.82,83 Changes in the nuclear shape are observed in some lamin mutations in a developmental- and tissue-specific manner83 as occurs in A. thaliana linc mutants.66,67 Both phenotypes could be caused by the disruption of the proper formation of the lamina, which disrupts regulation of nuclear shape. The increase in nuclear size that occurs during seed germination is also dependent on LINC1 and LINC2 proteins.80 A variety of mechanisms regulating the nuclear size have been described in various experimental systems, but the nuclear import and NE proteins, especially lamins, seem to be the key factors involved,84,85 just like the LINC/NMCP proteins are in A. thaliana.66,67,80
Other nuclear proteins reported to affect nuclear shape in plants include the SUN domain proteins,54,86 the KASH-like domain WIP proteins,54 the WIT proteins,87 and Nup136, a functional homolog of animal Nup15359 producing a similar phenotype to that of linc1linc2 mutants. All that suggests that the plant proteins forming the nucleocytoplasmic linker: SUNs, WIPs and WITs87 and Nup136 interact with NMCP/LINC proteins and act in concert to regulate nuclear morphology, similar to the way in which animal lamins interact with SUN proteins and Nup153 to regulate nuclear shape.12
The role of NMCP proteins in chromatin organization remains unclear. NMCP proteins do not affect DNA content as all linc mutants have normal ploidy levels,63,66 although double linc1linc2 mutations affect the organization of heterochromatin, as witnessed by a significant decrease in the number of chromocenters, probably due to coalescence.63 Nevertheless, similar changes in the relative heterochromatin fraction and the distribution of heterochromatic regions (a centromeric 180 bp repeat, pericentromeric subtelomeric 45s rDNA repeats, and pericentromeric sequences) are observed during germination in linc1/linc2 mutants and in the wild types,80,88 indicating that LINC1 and LINC2 proteins do not participate in the control of heterochromatin compaction. Functional LINC3 and LINC4 proteins may complement the loss of some NMCP functions, and thus, the involvement of LINC proteins in these functions cannot be completely ruled out. Further analysis of the nuclear organization in mutants, including those carrying mutations in other LINC/NMCP genes, is required to verify the role of NMCP proteins in chromatin organization.
A key function of lamins in animals is to regulate nuclear positioning and movement, processes that are mediated by the interaction of type A lamins with SUN and KASH proteins to form the LINC complex, and which requires the Samp1 protein to stabilize the binding of SUNs to lamins, as well as cytoplasmic actin.89 In plants, nuclear movement in response to blue light is mediated by phototropin2, and it involves thick actin filaments that associate to the nucleus.90 The nuclear components involved in this interaction remained unknown. However, a new type of nucleocytoplasmic linker consisting of a Myosin Xl-i motor that binds to both the actin CSK and the WIT proteins in the outer nuclear membrane that in turn interact with the SUN-WIP bridge has been described that is involved in rapid and long distance nuclear movement in response to environmental stimuli.87 Analyses of single and double linc1/4 and linc2/3 mutants have ruled out a role of NMCP proteins in blue light-induced nuclear movement and positioning, although protein complementation cannot be completely discounted.66 These results, along with the revelation that the SUN and KASH-like protein WIP are not required for nuclear movement in developing root hairs,54,86 strongly suggest that the organization of the bridges between the NSK and CSK, as well as the mechanisms regulating nuclear movement in plants, differ from those of animals.
Analysis of the binding partners of NMCPs will contribute to our understanding of their functions, as well as to the composition and organization of the protein networks that form the NE and NSK in plants. As discussed above, the functional analysis of mutants suggests that NMCPs interact with some proteins whose metazoan analogs are lamin-binding proteins,27 such as SUN54,86 and Nup136.59 As yet, no NMCP partners have been unequivocally identified, although preliminary results suggest that NMCP and SUN proteins may interact.24 The identification of NMCP-binding proteins in the INM, and NPCs such as SUN proteins, NUA and actin, would explain the association of NMCPs to these structures and is an interesting issue that deserves further investigation.
Future directions and perspectives
Although NMCPs have no sequence similarity with lamins, the 2 proteins share many other features, including their structural organization, sub-nuclear distribution, expression, and function (Table 1), making NMCPs the best candidates to perform the functions of lamins in plants. Further research is required to unequivocally demonstrate whether NMCPs are plant lamin analogs, or just structural components of the plant lamina, and to unravel their functions in the plant NE and nucleus. Key issues to be addressed in future studies include: 1) the localization of NMCPs in the filaments of the lamina by immuno-feSEM; 2) the polymerization of NMCPs in vitro; and the characterization of 3) NMCP binding partners and 4) the functions of NMCPs in the nucleus and cell, as may be determined using multiple mutants.
Table 1. Main features of NMCP proteins and lamins. Similar characteristics are denoted in blue and different ones in red.
| NMCPs | lamins | |
|---|---|---|
| Types |
NMCP1 (monocots 1; dicots 2‒3 genes) NMCP2 (1 gene) |
B-type (invertebrates 1; vertebrates 2‒3 genes) A-type (1 gene in vertebrates and in few invertebrates) |
| Structure | Central coiled-coil rod domain, head and tail Rod domain flanked by cdk1 sites NLS in the tail domain |
Central coiled-coil rod domain, head and tail Rod domain flanked by cdk1 sites NLS in the tail domain |
| Conserved regions | Extremes of the rod domain, linkers and C-terminus | Extremes of the rod domain, linkers and C-terminus |
| Polimerization state | Predicted to dimerize | Dimerize and form filaments |
| MW | Variable (70-200 kDa) | 65-74 kDa |
| pI | Acidic | Acidic (B-type lamins); neutral (A-type) |
| Solubility | NSK component | NSK component |
| Subcellular localization | Lamina, nucleoplasm | Lamina, nucleoplasm |
| Functions | Nuclear shape and size Chromatin organization (?) (decreased number of chromocenters but the distribution of heterochromatic regions not changed) Not involved in light induced nuclear movement |
Nuclear shape and architecture Chromatin organization and positioning Connecting NSK and CSK DNA replication, repair and transcription Cell proliferation and differentiation |
| Genes | Min. 2 in most plants | Min. 3 in vertebrates, 1 in most invertebrates |
| Where? | Multicellular land plants | Metazoan |
Supplementary Material
Acknowledgments
We are grateful to Dr Frantisek Baluska for kindly inviting us to write this review, to Dr Kiyoshi Masuda for kind permission to quote unpublished results, and to Dr Mark Sefton for editorial assistance. We acknowledge support from the Spanish Ministry of Science and Innovation [BFU2010–15900] and the CSIC [PIE 201020E019]. Ciska M was supported by a grant from the Junta de Ampliación de Estudios (JAEPre_08_00012/JAEPre027) and by PIE 201020E019.
Glossary
Abbreviations:
- BAF
barrier to autointegration factor
- CSK
cytoskeleton
- feSEM
field emission scanning electron microscopy
- GFP
green fluorescent protein
- HP1
heterochromatin protein 1
- IF
intermediate filament
- INM
inner nuclear membrane
- LBR
lamin B receptor
- LEM domain
LAP2/Emerin/MAN domain
- LINC complex
linker of the NSK to CSK complex
- LINC proteins
little nuclei proteins
- MW
molecular weight
- NE
nuclear envelope
- NIF
nuclear intermediate filament protein
- NLS
nuclear localization signal
- NMCPs
nuclear matrix constituent proteins
- NPC
nuclear pore complex
- NSK
nucleoskeleton
- NUA
nuclear pore anchor protein
- TEM
transmission electron microscopy
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental materials may be found here: www.landesbioscience.com/journals/psb/article/26669
References
- 1.Frajola WJ, Greider MH, Kostir WJ. Electron microscopy of the nuclear membrane of Amoeba proteus. J Biophys Biochem Cytol. 1956;2:445–8. [PubMed] [Google Scholar]
- 2.Rudzinska MA. Further observations on the fine structure of the macronucleus in Tokophrya infusionum. J Biophys Biochem Cytol. 1956;2:425–30. doi: 10.1083/jcb.2.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beams HW, Tahmisian TN, Devine R, Anderson E. Ultrastructure of the nuclear membrane of a gregarine parasitic in grasshoppers. Exp Cell Res. 1957;13:200–4. doi: 10.1016/0014-4827(57)90071-X. [DOI] [PubMed] [Google Scholar]
- 4.Pappas GD. The fine structure of the nuclear envelope of Amoeba proteus. J Biophys Biochem Cytol. 1956;2:431–4. doi: 10.1083/jcb.2.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawcett DW. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J Anat. 1966;119:129–45. doi: 10.1002/aja.1001190108. [DOI] [PubMed] [Google Scholar]
- 6.Aaronson RP, Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975;72:1007–11. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978;79:546–66. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peter A, Reimer S. Evolution of the lamin protein family: what introns can tell. Nucleus. 2012;3:44–59. doi: 10.4161/nucl.18927. [DOI] [PubMed] [Google Scholar]
- 9.Krüger A, Batsios P, Baumann O, Luckert E, Schwarz H, Stick R, Meyer I, Gräf R. Characterization of NE81, the first lamin-like nucleoskeleton protein in a unicellular organism. Mol Biol Cell. 2012;23:360–70. doi: 10.1091/mbc.E11-07-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batsios P, Peter T, Baumann O, Stick R, Meyer I, Gräf R. A lamin in lower eukaryotes? Nucleus. 2012;3:237–43. doi: 10.4161/nucl.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBois KN, Alsford S, Holden JM, Buisson J, Swiderski M, Bart JM, Ratushny AV, Wan Y, Bastin P, Barry JD, et al. NUP-1 Is a large coiled-coil nucleoskeletal protein in trypanosomes with lamin-like functions. PLoS Biol. 2012;10:e1001287. doi: 10.1371/journal.pbio.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harb Perspect Biol. 2010;2:a000547. doi: 10.1101/cshperspect.a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer TA, Misteli T. The lamin protein family. Genome Biol. 2011;12:222. doi: 10.1186/gb-2011-12-5-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–93. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno Díaz de la Espina S, Barthellemy I, Cerezuela MA. Isolation and ultrastructural characterization of the residual nuclear matrix in a plant cell system. Chromosoma. 1991;100:110–7. doi: 10.1007/BF00418244. [DOI] [Google Scholar]
- 16.Li H, Roux SJ. Casein kinase II protein kinase is bound to lamina-matrix and phosphorylates lamin-like protein in isolated pea nuclei. Proc Natl Acad Sci U S A. 1992;89:8434–8. doi: 10.1073/pnas.89.18.8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mínguez A, Moreno Díaz de la Espina S. Immunological characterization of lamins in the nuclear matrix of onion cells. J Cell Sci. 1993;106:431–9. doi: 10.1242/jcs.106.1.431. [DOI] [PubMed] [Google Scholar]
- 18.Masuda K, Takahashi S, Nomura K, Arimoto M, Inoue M. Residual structure and constituent proteins of the peripheral framework of the cell nucleus in somatic embryos from Daucus carota L. Planta. 1993;191:532–40. doi: 10.1007/BF00195755. [DOI] [Google Scholar]
- 19.Moreno Díaz de la Espina SM. Nuclear matrix isolated from plant cells. Int Rev Cytol. 1995;162B:75–139. doi: 10.1016/s0074-7696(08)62615-7. [DOI] [PubMed] [Google Scholar]
- 20.Moreno Diaz de la Espina S. The plant nucleoskeleton. In: Meier I, ed. Functional organization of the plant nucleus. Berlin, Heidelberg: Springer, 2009:79-100. [Google Scholar]
- 21.Fiserova J, Kiseleva E, Goldberg MW. Nuclear envelope and nuclear pore complex structure and organization in tobacco BY-2 cells. Plant J. 2009;59:243–55. doi: 10.1111/j.1365-313X.2009.03865.x. [DOI] [PubMed] [Google Scholar]
- 22.Mans BJ, Anantharaman V, Aravind L, Koonin EV. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle. 2004;3:1612–37. doi: 10.4161/cc.3.12.1316. [DOI] [PubMed] [Google Scholar]
- 23.Rose A, Manikantan S, Schraegle SJ, Maloy MA, Stahlberg EA, Meier I. Genome-wide identification of Arabidopsis coiled-coil proteins and establishment of the ARABI-COIL database. Plant Physiol. 2004;134:927–39. doi: 10.1104/pp.103.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graumann K, Bass HW, Parry G, SUNrises on the International Plant Nucleus Consortium SEB Salzburg 2012. Nucleus. 2013;4:3–7. doi: 10.4161/nucl.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. J Struct Biol. 2012;177:24–31. doi: 10.1016/j.jsb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci. 2008;121:215–25. doi: 10.1242/jcs.022020. [DOI] [PubMed] [Google Scholar]
- 27.Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma. 2013;122:13–31. doi: 10.1007/s00412-013-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melcer S, Gruenbaum Y, Krohne G. Invertebrate lamins. Exp Cell Res. 2007;313:2157–66. doi: 10.1016/j.yexcr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet. 2012;28:464–71. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–53. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, Aebi U, Herrmann H, Gruenbaum Y, Medalia O. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol. 2009;386:1392–402. doi: 10.1016/j.jmb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Kapinos LE, Schumacher J, Mücke N, Machaidze G, Burkhard P, Aebi U, Strelkov SV, Herrmann H. Characterization of the head-to-tail overlap complexes formed by human lamin A, B1 and B2 “half-minilamin” dimers. J Mol Biol. 2010;396:719–31. doi: 10.1016/j.jmb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Gangemi F, Degano M. Disease-associated mutations in the coil 2B domain of human lamin A/C affect structural properties that mediate dimerization and intermediate filament formation. J Struct Biol. 2013;181:17–28. doi: 10.1016/j.jsb.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg MW, Fiserova J, Huttenlauch I, Stick R. A new model for nuclear lamina organization. Biochem Soc Trans. 2008;36:1339–43. doi: 10.1042/BST0361339. [DOI] [PubMed] [Google Scholar]
- 35.Dechat T, Gesson K, Foisner R. Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb Symp Quant Biol. 2010;75:533–43. doi: 10.1101/sqb.2010.75.018. [DOI] [PubMed] [Google Scholar]
- 36.Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–21. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolb T, Maass K, Hergt M, Aebi U, Herrmann H. Lamin A and lamin C form homodimers and coexist in higher complex forms both in the nucleoplasmic fraction and in the lamina of cultured human cells. Nucleus. 2011;2:425–33. doi: 10.4161/nucl.2.5.17765. [DOI] [PubMed] [Google Scholar]
- 38.Dechat T, Adam SA, Goldman RD. Nuclear lamins and chromatin: when structure meets function. Adv Enzyme Regul. 2009;49:157–66. doi: 10.1016/j.advenzreg.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapley EC, Starr DA. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr Opin Cell Biol. 2013;25:57–62. doi: 10.1016/j.ceb.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–47. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Gräf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–13. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuela N, Bar DZ, Gruenbaum Y. Lamins in development, tissue maintenance and stress. EMBO Rep. 2012;13:1070–8. doi: 10.1038/embor.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 44.Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, van Steensel B. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet. 2006;38:1005–14. doi: 10.1038/ng1852. [DOI] [PubMed] [Google Scholar]
- 45.Galiová G, Bártová E, Raska I, Krejcí J, Kozubek S. Chromatin changes induced by lamin A/C deficiency and the histone deacetylase inhibitor trichostatin A. Eur J Cell Biol. 2008;87:291–303. doi: 10.1016/j.ejcb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28:2414–27. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shumaker DK, Solimando L, Sengupta K, Shimi T, Adam SA, Grunwald A, Strelkov SV, Aebi U, Cardoso MC, Goldman RD. The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J Cell Biol. 2008;181:269–80. doi: 10.1083/jcb.200708155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahen R, Hattori H, Lee M, Sharma P, Jeyasekharan AD, Venkitaraman AR. A-type lamins maintain the positional stability of DNA damage repair foci in mammalian nuclei. PLoS One. 2013;8:e61893. doi: 10.1371/journal.pone.0061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson KL, Foisner R. Lamin-binding Proteins. Cold Spring Harb Perspect Biol. 2010;2:a000554. doi: 10.1101/cshperspect.a000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus. 2010;1:264–72. doi: 10.4161/nucl.1.3.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat Rev Mol Cell Biol. 2011;12:695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- 52.Murphy SP, Simmons CR, Bass HW. Structure and expression of the maize (Zea mays L.) SUN-domain protein gene family: evidence for the existence of two divergent classes of SUN proteins in plants. BMC Plant Biol. 2010;10:269. doi: 10.1186/1471-2229-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Field MC, Horn D, Alsford S, Koreny L, Rout MP. Telomeres, tethers and trypanosomes. Nucleus. 2012;3:478–86. doi: 10.4161/nucl.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Graumann K, Evans DE, Meier I. Novel plant SUN-KASH bridges are involved in RanGAP anchoring and nuclear shape determination. J Cell Biol. 2012;196:203–11. doi: 10.1083/jcb.201108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzur YB, Wilson KL, Gruenbaum Y. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol. 2006;7:782–8. doi: 10.1038/nrm2003. [DOI] [PubMed] [Google Scholar]
- 56.Masuda K, Xu ZJ, Takahashi S, Ito A, Ono M, Nomura K, Inoue M. Peripheral framework of carrot cell nucleus contains a novel protein predicted to exhibit a long α-helical domain. Exp Cell Res. 1997;232:173–81. doi: 10.1006/excr.1997.3531. [DOI] [PubMed] [Google Scholar]
- 57.Fiserova J, Goldberg MW. Relationships at the nuclear envelope: lamins and nuclear pore complexes in animals and plants. Biochem Soc Trans. 2010;38:829–31. doi: 10.1042/BST0380829. [DOI] [PubMed] [Google Scholar]
- 58.Graumann K, Runions J, Evans DE. Characterization of SUN-domain proteins at the higher plant nuclear envelope. Plant J. 2010;61:134–44. doi: 10.1111/j.1365-313X.2009.04038.x. [DOI] [PubMed] [Google Scholar]
- 59.Tamura K, Hara-Nishimura I. Involvement of the nuclear pore complex in morphology of the plant nucleus. Nucleus. 2011;2:168–72. doi: 10.4161/nucl.2.3.16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pérez-Munive C, Blumenthal SS, de la Espina SM. Characterization of a 65 kDa NIF in the nuclear matrix of the monocot Allium cepa that interacts with nuclear spectrin-like proteins. Cell Biol Int. 2012;36:1097–105. doi: 10.1042/CBI20120237. [DOI] [PubMed] [Google Scholar]
- 61.Blumenthal SS, Clark GB, Roux SJ. Biochemical and immunological characterization of pea nuclear intermediate filament proteins. Planta. 2004;218:965–75. doi: 10.1007/s00425-003-1182-5. [DOI] [PubMed] [Google Scholar]
- 62.Masuda K, Haruyama S, Fujino K. Assembly and disassembly of the peripheral architecture of the plant cell nucleus during mitosis. Planta. 1999;210:165–7. doi: 10.1007/s004250050666. [DOI] [PubMed] [Google Scholar]
- 63.Dittmer TA, Stacey NJ, Sugimoto-Shirasu K, Richards EJ. LITTLE NUCLEI genes affecting nuclear morphology in Arabidopsis thaliana. Plant Cell. 2007;19:2793–803. doi: 10.1105/tpc.107.053231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimura Y, Kuroda C, Masuda K. Differential nuclear envelope assembly at the end of mitosis in suspension-cultured Apium graveolens cells. Chromosoma. 2010;119:195–204. doi: 10.1007/s00412-009-0248-y. [DOI] [PubMed] [Google Scholar]
- 65.Ciska M, Masuda K, Moreno Díaz de la Espina S. Lamin-like analogues in plants: the characterization of NMCP1 in Allium cepa. J Exp Bot. 2013;64:1553–64. doi: 10.1093/jxb/ert020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakamoto Y, Takagi S. LITTLE NUCLEI 1 and 4 regulate nuclear morphology in Arabidopsis thaliana. Plant Cell Physiol. 2013;54:622–33. doi: 10.1093/pcp/pct031. [DOI] [PubMed] [Google Scholar]
- 67.Dittmer TA, Richards EJ. Role of LINC proteins in plant nuclear morphology. Plant Signal Behav. 2008;3:485–7. doi: 10.4161/psb.3.7.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. A gene expression map of the Arabidopsis root. Science. 2003;302:1956–60. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- 69.Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–6. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 70.Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–5. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- 71.Lehner CF, Stick R, Eppenberger HM, Nigg EA. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol. 1987;105:577–87. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, Raymond Y, Ramaekers FC. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol. 1997;107:505–17. doi: 10.1007/s004180050138. [DOI] [PubMed] [Google Scholar]
- 73.Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–93. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–49. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- 75.Szeverenyi I, Cassidy AJ, Chung CW, Lee BT, Common JE, Ogg SC, Chen H, Sim SY, Goh WL, Ng KW, et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–60. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 76.Erber A, Riemer D, Hofemeister H, Bovenschulte M, Stick R, Panopoulou G, Lehrach H, Weber K. Characterization of the Hydra lamin and its gene: A molecular phylogeny of metazoan lamins. J Mol Evol. 1999;49:260–71. doi: 10.1007/PL00006548. [DOI] [PubMed] [Google Scholar]
- 77.Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout A, Gruenbaum Y, Liu J. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:16690–5. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui P, Moreno Díaz de la Espina S. Sm and U2B” proteins redistribute to different nuclear domains in dormant and proliferating onion cells. Planta. 2003;217:21–31. doi: 10.1007/s00425-002-0966-3. [DOI] [PubMed] [Google Scholar]
- 79.Cruz JR, Moreno Díaz de la Espina S. Subnuclear compartmentalization and function of actin and nuclear myosin I in plants. Chromosoma. 2009;118:193–207. doi: 10.1007/s00412-008-0188-y. [DOI] [PubMed] [Google Scholar]
- 80.van Zanten M, Koini MA, Geyer R, Liu Y, Brambilla V, Bartels D, Koornneef M, Fransz P, Soppe WJ. Seed maturation in Arabidopsis thaliana is characterized by nuclear size reduction and increased chromatin condensation. Proc Natl Acad Sci U S A. 2011;108:20219–24. doi: 10.1073/pnas.1117726108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J, Rolef Ben-Shahar T, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–47. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schirmer EC, Guan T, Gerace L. Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization. J Cell Biol. 2001;153:479–89. doi: 10.1083/jcb.153.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiesel N, Mattout A, Melcer S, Melamed-Book N, Herrmann H, Medalia O, Aebi U, Gruenbaum Y. Laminopathic mutations interfere with the assembly, localization, and dynamics of nuclear lamins. Proc Natl Acad Sci U S A. 2008;105:180–5. doi: 10.1073/pnas.0708974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edens LJ, White KH, Jevtic P, Li X, Levy DL. Nuclear size regulation: from single cells to development and disease. Trends Cell Biol. 2013;23:151–9. doi: 10.1016/j.tcb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Levy DL, Heald R. Nuclear size is regulated by importin α and Ntf2 in Xenopus. Cell. 2010;143:288–98. doi: 10.1016/j.cell.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oda Y, Fukuda H. Dynamics of Arabidopsis SUN proteins during mitosis and their involvement in nuclear shaping. Plant J. 2011;66:629–41. doi: 10.1111/j.1365-313X.2011.04523.x. [DOI] [PubMed] [Google Scholar]
- 87.Tamura K, Iwabuchi K, Fukao Y, Kondo M, Okamoto K, Ueda H, Nishimura M, Hara-Nishimura I. Myosin XI-i Links the Nuclear Membrane to the Cytoskeleton to Control Nuclear Movement and Shape in Arabidopsis. Curr Biol. 2013;23:1776–81. doi: 10.1016/j.cub.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 88.van Zanten M, Carles A, Li Y, Soppe WJ. Control and consequences of chromatin compaction during seed maturation in Arabidopsis thaliana. Plant Signal Behav. 2012;7:338–41. doi: 10.4161/psb.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borrego-Pinto J, Jegou T, Osorio DS, Auradé F, Gorjánácz M, Koch B, Mattaj IW, Gomes ER. Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci. 2012;125:1099–105. doi: 10.1242/jcs.087049. [DOI] [PubMed] [Google Scholar]
- 90.Iwabuchi K, Minamino R, Takagi S. Actin reorganization underlies phototropin-dependent positioning of nuclei in Arabidopsis leaf cells. Plant Physiol. 2010;152:1309–19. doi: 10.1104/pp.109.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.