Abstract
The obligate biotrophic protist Plasmodiophora brassicae causes worldwide devastating losses on Brassica crops. Among these are oilseed rape, vegetable brassicas, and turnips. However, the fact that Arabidopsis thaliana is a good host for P. brassicae, has boosted research on the molecular interaction using the resources available for this model plant. Due to the uncontrolled growth of infected host root tissues the disease has been coined “clubroot.” Consequently, during the last years, alterations in host hormone metabolisms have been described. Influencing the hormonal balance leads to aberrant growth responses in the clubbed roots. The discussion presented in the following will focus on growth promoting hormones, mainly auxins, with the interaction to other growth associated hormonal signaling pathways, such as cytokinins and brassinosteroids.
Keywords: Arabidopsis thaliana, auxin, biotrophic protist, clubroot disease, Plasmodiophora brassicae
The clubroot disease is caused by the obligate biotrophic protist Plasmodiophora brassicae and affects culturable brassicas worldwide.1 The disease symptoms result in enlarged root systems, the so called “clubroots” and stunted to wilted upper plant parts due to nutrient and water losses during later disease stages.1 Plant hormones have been associate with the disease progression in the roots.
While the biosynthesis of the plant hormone indole-3-acetic acid (IAA) has been studied during the clubroot disease for already some time,1 it was only recently shown that transcripts encoding auxin conjugate synthetases (GH3 protein family) were strongly differentially regulated during the clubroot disease.2 The GH3 proteins conjugate various substrates (i.e., IAA and other auxins, as well as jasmonic, salicylic, and benzoic acids) to a broad spectrum of amino acids.3-5 GH3 proteins capable to conjugate IAA were thought to be involved in the regulation of auxin homeostasis, but they also play roles in plant pathogen interactions and during abiotic stress responses.6 Jahn et al.2 found that genes encoding the auxin conjugating enzymes, which are partially also auxin inducible,7 were upregulated during the clubroot disease. Extracting data for GH3 gene expression from a recently published cell-type specific microarray8 corroborated these results (Fig. 1). In this research Laser Capture Microdissection was used to enrich specific cell populations from P. brassicae-infected roots harboring different developmental stages of the pathogen.8 These included small and large plasmodia, the main metabolically active structures of the protist within the host cells. The cell populations were transcriptionally compared with control roots. A role for the GH3 proteins in auxin homeostasis in clubroots can be postulated also based on these results (Fig. 1). While the genes for auxin conjugate synthetases were not regulated at all at an early time point (2 weeks after inoculation) in cells harboring small plasmodia of the pathogen, the majority of GH3 genes was differentially expressed (mainly upregulated) in already hypertrophied cells, where it can be assumed that auxin levels are high. The upregulation of auxin amino acid conjugate synthesis can be interpreted as means of the plant to control disease symptoms to some extent. In line of these findings, the nitrilase protein of Arabidopsis, involved in IAA biosynthesis, has been localized also in cells harboring large plasmodia by immunohistochemical methods.9
Figure 1. Relative GH3 gene expression data extracted from microarray data8 representing transcirptome profiles in a cell-type specific manner. The samples were from roots 14 and 21 d after inoculation and included small (SP) and large plasmodia (LP)-containing cells. The 2 GH3 genes investigated in more detail in Jahn et al.2 are highlighted in red. The cartoons indicate the respective cell type. The zones and size of plasmodia are the same as in Figure 3. The genes are not sorted by their consecutive number, but grouped by substrate they convert to amino acid conjugates.
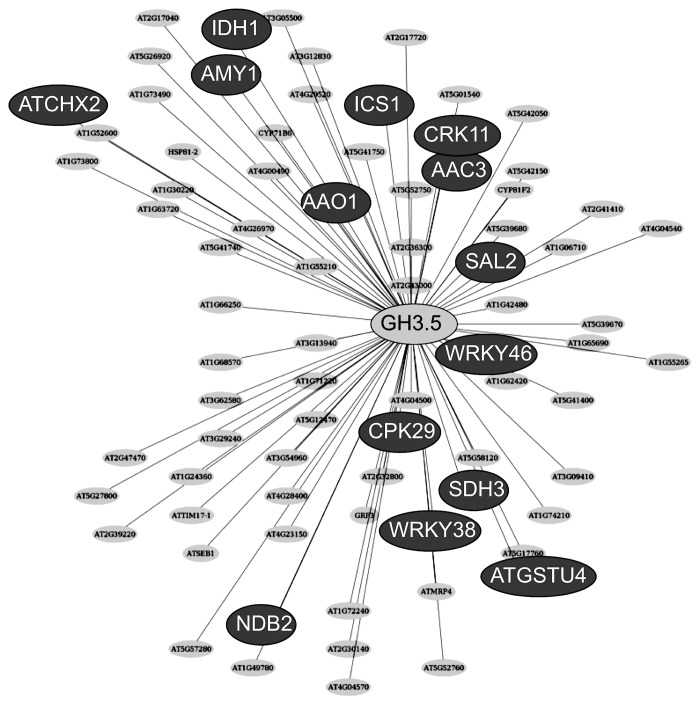
It is interesting to note that GH3.5 shows high expression levels at the second time point (3 weeks after inoculation). Since the protein GH3.5 is also able to conjugate salicylic acid to amino acids10 and since it is involved in the biosynthesis of camalexin,11 its specific regulation warranted further analysis. While single mutants of gh3.5 did not show any altered clubroot phenotype, double mutants in gh3.5gh3.17 were more susceptible to the parasite.2 To find other players involved in GH3.5. function an association map12 was constructed for GH3.5 (Fig. 2). The results of this Network-guided candidate search report show some genes associated with auxin (i.e., AAO1), nutrition, for example the amylase gene AMY1, but several others associated with defense, for example salicylic acid synthesis (ICR1) and WRKY transcription factor genes were found. Also, genes involved in signal transduction (protein kinases CPK29, CRK11) were located in this map. These different pathways reflect the diverse role(s) so far discovered for GH3.5. In addition, some of these genes, for example ATCHX2 or WRKY38, show an upregulation similar to GH3.5 during clubroot development as indicated by microarray data.13 Using such bioinformatic approaches further candidates in the auxin signaling pathway important for clubroot formation can be obtained.
Figure 2. Association map constructed for GH3.5 expression.11 Some genes for which functions are annotated have been highlighted. AAC, ATP:ADP antiporter activity; AAO, aldehyde oxidase; AMY, amylase; CHX, monovalent cation:proton antiporter activity; CPK / CRK protein kinase; GST, glutathione transferase activity; ICS, isochorismate synthase; IDH, isocitrate dehydrogenase; NDB, disulfide oxidoreductase; SAL, inositol or phosphatidylinositol phosphatase activity; SDH, succinate dehydrogenase.
The GH3 gene expression is mediated via the nuclear auxin signaling pathway mediated by the TIR receptor family of F-box proteins.14 It was found that mutants in genes encoding these receptors were more susceptible to P. brassicae,2 indicating that the 2 components TIR and GH3 could act in the same pathway for clubroot formation (see also Fig. 3). In addition, a second auxin receptor seems to play a role in auxin responses, namely ABP115 which was upregulated during the clubroot disease.2 It can be hypothesized that ABP1 is involved in hypertrophy of the host cells via activation of potassium channels, because a K+-channel inhibitor reduced clubroot symptoms.2
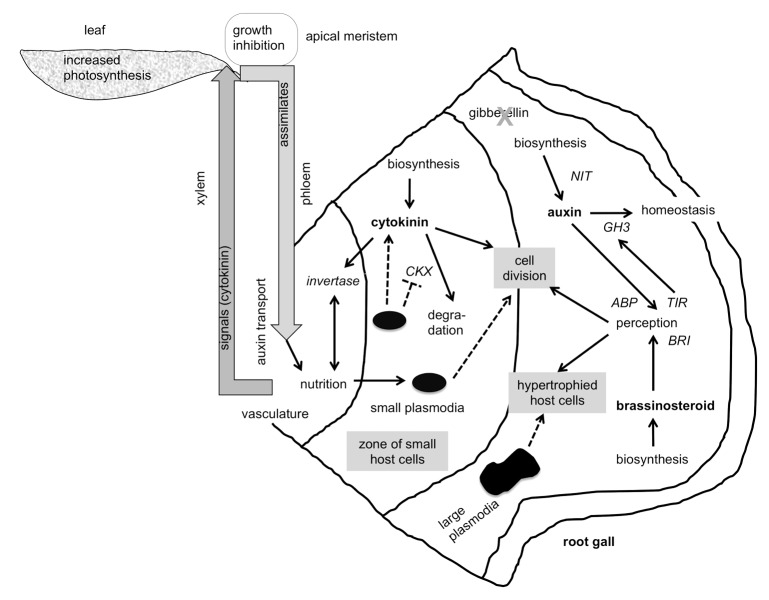
Figure 3. A model on the involvement of growth promoting plant hormones in clubroot development. For further explanations see text. The allocation of the different hormones to the 3 zones has not been experimentally verified. The dashed lines symbolize actions by P. brassicae, the solid lines by the host plant.
A model has been constructed to explain the different signaling pathways that lead to clubroot development (Fig. 3). The infected roots can be divided roughly into 3 zones: 1) The vasculature which provides the connection to the upper plant parts. 2) The zone around the vasculature, where the host cells are still small, but cell divisions already occur and the small plasmodia of the obligate biotrophic protist are present in the cells. Here a strong nutrient exchange has to occur. 3) The zone comprising most of the cortex tissue where cell enlargment (hypertrophy) can be observed and large plasmodia of the pathogen are present (Fig. 3). In this zone also the resting spores develop from the plasmodia (not shown). The pathogen gains nutrition from the leaves, maybe even by activating photosynthesis and assimilate / nutrient transport via the phloem in the first stages.1 Signals for assimilate redirection could be cytokinins (CK). Indications for auxin transport have also been presented,1 but the contribution of IAA transport seems to be important only during early stages of club development. All these metabolic changes could lead to the reduction of growth in the shoot apical meristem (Fig. 3).
The plasmodia are able to synthesize CKs and increase the host CK pool by altering biosynthesis and degradation.1,13 The cytokinins in the clubbed roots also interfere with the sugar metabolism of the host. Invertase can be induced by CK and is necessary for the nutrition of P. brassicae.16 The host cell divisions is also be induced by CKs, but possibly other growth hormones, i.e., IAA and brassinosteroids (BR) play a role here. Recently, it has been shown that BR biosynthesis and signaling is necessary for the development of full size galls.8 This might occur together with IAA preferentially in the zone where large plasmodia can be found (Fig. 3). Interestingly, gibberellins (GA) do not seem to play a role in gall formation, since inhibition of the GA biosynthesis pathway did not alter club size.17 The role of the plasmodia of P. brassicae in this scenario is so far not known and must be subject for further studies.
Glossary
Abbreviations:
- ABP1
auxin binding protein 1
- BR
brassinosteroid
- CK
cytokinin
- GA
gibberellin
- IAA
indole-3-acetic acid
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ludwig-Müller J, Prinsen E, Rolfe S, Scholes J. . Metabolism and plant hormone action during the clubroot disease. J Plant Growth Regul 2009; 28:229 - 44; http://dx.doi.org/ 10.1007/s00344-009-9089-4 [DOI] [Google Scholar]
- 2.Jahn L, Mucha S, Bergmann S, Horn C, Siemens J, Staswick P, Steffens B, Ludwig-Müller J. . The clubroot pathogen (Plasmodiophora brassicae) influences auxin signaling to regulate auxin homeostasis. Plants 2013; 2:726 - 49; http://dx.doi.org/ 10.3390/plants2040726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okrent RA, Brooks MD, Wildermuth MC. . Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J Biol Chem 2009; 284:9742 - 54; http://dx.doi.org/ 10.1074/jbc.M806662200; PMID: 19189963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staswick PE, Tiryaki I. . The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004; 16:2117 - 27; http://dx.doi.org/ 10.1105/tpc.104.023549; PMID: 15258265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. . Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005; 17:616 - 27; http://dx.doi.org/ 10.1105/tpc.104.026690; PMID: 15659623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig-Müller J. . Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 2011; 62:1757 - 73; http://dx.doi.org/ 10.1093/jxb/erq412; PMID: 21307383 [DOI] [PubMed] [Google Scholar]
- 7.Guilfoyle TJ, Hagen G. . Auxin response factors. Curr Opin Plant Biol 2007; 10:453 - 60; http://dx.doi.org/ 10.1016/j.pbi.2007.08.014; PMID: 17900969 [DOI] [PubMed] [Google Scholar]
- 8.Schuller A, Kehr J, Ludwig-Müller J. . Laser microdissection coupled to transcriptional profiling of arabidopsis roots inoculated by Plasmodiophora brassicae indicates a role for brassinosteroids in clubroot formation. Plant Cell Physiol 2014; 55:392 - 411; http://dx.doi.org/ 10.1093/pcp/pct174; PMID: 24285749 [DOI] [PubMed] [Google Scholar]
- 9.Grsic-Rausch S, Kobelt P, Siemens JM, Bischoff M, Ludwig-Müller J. . Expression and localization of nitrilase during symptom development of the clubroot disease in Arabidopsis. Plant Physiol 2000; 122:369 - 78; http://dx.doi.org/ 10.1104/pp.122.2.369; PMID: 10677430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Li Q, Li Z, Staswick PE, Wang M, Zhu Y, He Z. . Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol 2007; 145:450 - 64; http://dx.doi.org/ 10.1104/pp.107.106021; PMID: 17704230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M-Y, Liu X-T, Chen Y, Xu X-J, Yu B, Zhang S-Q, Li Q, He Z-H. . Arabidopsis acetyl-amido synthetase GH3.5 involvement in camalexin biosynthesis through conjugation of indole-3-carboxylic acid and cysteine and upregulation of camalexin biosynthesis genes. J Integr Plant Biol 2012; 54:471 - 85; http://dx.doi.org/ 10.1111/j.1744-7909.2012.01131.x; PMID: 22624950 [DOI] [PubMed] [Google Scholar]
- 12.Lee I, Ambaru B, Thakkar P, Marcotte EM, Rhee SY. . Rational association of genes with traits using a genome-scale gene network for Arabidopsis thaliana.. Nat Biotechnol 2010; 28:149 - 56; http://dx.doi.org/ 10.1038/nbt.1603; PMID: 20118918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siemens J, Keller I, Sarx J, Kunz S, Schuller A, Nagel W, Schmülling T, Parniske M, Ludwig-Müller J. . Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development. Mol Plant Microbe Interact 2006; 19:480 - 94; http://dx.doi.org/ 10.1094/MPMI-19-0480; PMID: 16673935 [DOI] [PubMed] [Google Scholar]
- 14.Dharmasiri N, Dharmasiri S, Estelle M. . The F-box protein TIR1 is an auxin receptor. Nature 2005; 435:441 - 5; http://dx.doi.org/ 10.1038/nature03543; PMID: 15917797 [DOI] [PubMed] [Google Scholar]
- 15.Scherer GFE. . AUXIN-BINDING-PROTEIN1, the second auxin receptor: what is the significance of a two-receptor concept in plant signal transduction?. J Exp Bot 2011; 62:3339 - 57; http://dx.doi.org/ 10.1093/jxb/err033; PMID: 21733909 [DOI] [PubMed] [Google Scholar]
- 16.Siemens J, González MC, Wolf S, Hofmann C, Greiner S, Du Y, Rausch T, Roitsch T, Ludwig-Müller J. . Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol Plant Pathol 2011; 12:247 - 62; http://dx.doi.org/ 10.1111/j.1364-3703.2010.00667.x; PMID: 21355997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Päsold S, Ludwig-Müller J. . Clubroot (Plasmodiophora brassicae) formation in Arabidopsis is reduced after treatment with prohexadione-calcium, an inhibitor for oxoglutaric acid-dependent dioxygenases. Plant Pathol 2013; 62:1357 - 65; http://dx.doi.org/ 10.1111/ppa.12049 [DOI] [Google Scholar]