Abstract
Compared to conventional neurons that use synaptic mechanisms to communicate with closely apposed targets, peptidergic neuroendocrine neurons release relatively large quantities of peptide into the vasculature to control neuroendocrine function at more distal sites. This means that maintaining adequate amounts of peptide for release through controlled biosynthesis is critical for their function. But the flexible and adaptive responses these neurons generate to many different challenges require synthesis and release must be coordinated in some way. How neuroendocrine—or in fact, any neuropeptide—neurons link appropriate levels of peptide biosynthesis with the patterns of action potentials that drive peptide release is unknown. Here we review possible mechanisms used by CRH neuroendocrine neurons in the paraventricular nucleus of the hypothalamus to achieve this coordination as they are driven by catecholaminergic inputs from the hindbrain. These particular inputs are essential for ACTH responses to glycemic challenges. We then compare this situation to the one seen across the day in the absence of stress when circulating ACTH and corticosterone exhibit predictable variations throughout the day. This activity pattern is driven by a completely different set of inputs. Based on these and other results, we propose that CRH synthesis and release mechanisms are not tightly and invariably linked together as CRH neurons are activated. Instead, complex coupling mechanisms exist both as a property of the pre-motor network that provides direct synaptic inputs to CRH neurons, and in the complex intracellular signal transduction mechanisms engaged by the very diverse set of receptors expressed by CRH neurons. This coupling process provides neuroendocrine CRH neurons with a versatile and dynamic mechanism that links peptide biosynthesis and release in an efficient and highly adaptable manner.
1 Introduction
The principal means of communication for all neurons is the release of chemical signals at spatially defined locations: synapses for neurons in the brain, or the vasculature for neurons with neuroendocrine terminals. Although the vast majority of neurons use fast-acting small molecule neurotransmitters, many neurons also release peptides that modulate the responses of post-synaptic targets, or in the case of most neuroendocrine neurons, as their principal signaling molecules that control the activity of endocrine cells in the pituitary gland and elsewhere in the body.
Like other neurons, neuroendocrine neurons generate action potentials upon appropriate stimulation. These action potentials are responsible for the calcium-dependent release of peptides from neuroendocrine terminals into the vasculature by means of mechanisms that again, are not substantially different from those seen at conventional synapses. The cellular targets of neuroendocrine signals are located some distance from the release sites, and the transmission path is the vasculature rather than the synapse. Peptidergic neuroendocrine neurons must therefore release larger amounts of signal than those that communicate using synaptically released small molecule transmitters. To do this, hypothalamic neuroendocrine neurons synthesize significant amounts of peptide in their cell bodies, which is then packaged into vesicles. These are shipped down axons to terminals that abut fenestrated capillaries at release sites in the neurohypophysis: the median eminence for neurons that control hormones synthesized in the pars distalis of the pituitary gland, or the neural lobe of the pituitary gland for those that release oxytocin and vasopressin.
Peptidergic neuroendocrine neurons are like all other neurons—and indeed secretory cells in general—in that peptide synthesis and peptide release are controlled by two independent intracellular processes. This means that a key feature of neuroendocrine neurons is their ability to transduce information encoded by their diverse neural inputs into the appropriate engagement of synthesis and release programs. To maintain sufficient amounts of releasable peptide for the demands of release there must be coordination between synthesis and release.
2. Aims of This Review
Numerous studies in mammalian systems during the past forty years have revealed much about the physiology and individual cellular components of neuroendocrine synthesis and release mechanisms. But despite the fact that neuroendocrine neurons must coordinate the information they receive from their neural inputs with the appropriate activation of synthesis and release there has been very little consideration of whether these processes are coupled and if they are, how this coupling might be enabled. These coupling mechanisms in neruoendocrine neurons are the subject of this review.
We focus on stimulus-synthesis-release coupling in corticotropin-releasing hormone (CRH) neuroendocrine neurons in the paraventricular nucleus of the hypothalamus (PVH) that control corticotropes in the pituitary and the adrenocortical responses to stress. Although CRH neurons have been intensively investigated for over thirty years because of their importance to stress pathophysiology, it is still not known how they transduce afferent stress-related signals into the appropriate engagement of the programs that coordinate peptide synthesis and release. Furthermore, intracellular coordinating mechanisms have not been identified, and how these processes might be coupled to the specific afferents that encode stressor-related information is also unknown. We have recently investigated these processes by manipulating catecholaminergic inputs to the PVH and adjacent forebrain. These inputs are one of the largest to the PVH and have long been associated with the control of stress responses [Sawchenko & Swanson, 1981; Plotsky et al., 1989; Wittman, 2008].
We begin by briefly describing the neurobiology of CRH neuroendocrine neurons. We then review the basic organization of peptide synthesis and release processes, before presenting our results showing that the coupling of these processes is not fixed but is in fact quite flexible. This coupling process provides neuroendocrine neurons with a versatile and dynamic mechanism that links peptide biosynthesis and release in an efficient and highly adaptable manner. We then present evidence that the phosphoryl-modified forms of the p44/42 MAP kinases (phospho-ERK1/2) are key agents in CRH neuroendocrine neurons that contribute to dynamic stimulus-synthesis-release coupling in response to glycemic challenges that engage catecholaminergic inputs.
3. Stressors, CRH Neurons, and the Hypothalamo-Pituitary-Adrenal (HPA) Axis
3.1 Neuroendocrine CRH neurons
Neuroendocrine CRH neurons are one of the five PVH parvicellular neuroendocrine populations that control hormone release from the pars distalis. Most CRH neurons are located in the medial parvicellular (mp) part of the PVH. They form the principal integrative hub for the adrenocortical stress response [Watts, 2005]. Neuroendocrine CRH neurons release two adrenocorticotropin (ACTH) secretogues, CRH and vasopressin (AVP). CRH is the predominant secretagogue in rodents, but its actions on corticotropes are supplemented by AVP, meaning that these two peptides collectively control ACTH release [see Watts, 1995, 2005 for reviews].
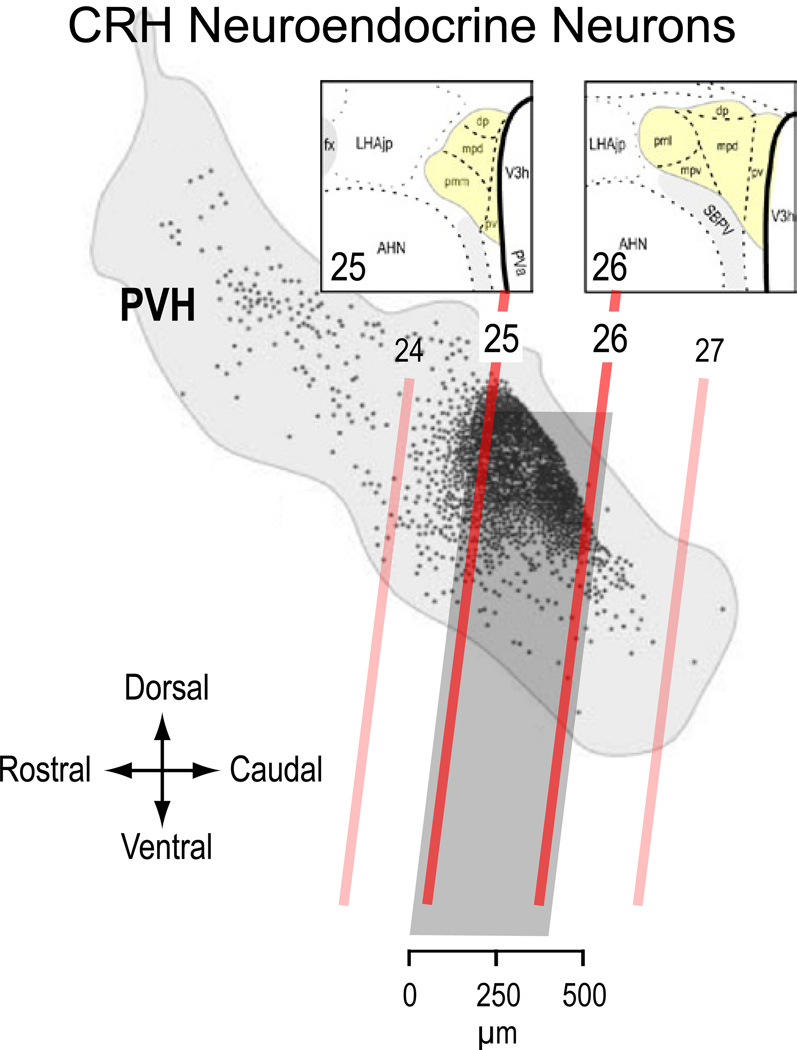
Figure 1 shows that the vast majority of neuroendocrine CRH neurons in the rat are restricted to an approximately 400 µm rostrocaudal PVHmp domain located between levels 25 and 26 [Simmons & Swanson, 2009] of the Swanson rat atlas [Swanson 2004]. Although all these neurons synthesize CRH, they form a heterogenous population of at least 6 different phenotypes, each of which varies in its ability to synthesize CRH, AVP, neurotensin, and pro-enkephalin. This population therefore exhibits considerable plasticity in the degree of peptide co-expression that follows various stressors and a changing glucocorticoid environment [Swanson et al., 1986; Watts & Sanchez-Watts, 1995; Watts, 1996, 2005].
Figure 1.
The location of individual CRH neuroendocrine neurons (black dots) shown on a sagittal view of the rat paraventricular nucleus of the hypothalamus (PVH; light gray outline). The oblique red lines show the corresponding positions of four atlas levels (24–26) from Swanson [2004]. Note that vast majority of CRH neurons are found in the dorsal aspect of the PVH between levels 25 and 26 (darker gray box) Insets show coronal views of these two levels. In the coronal plane most CRH neuroendocrine neurons are found in the dorsal zone of the medial parvicelllular (mpd) part of the PVH. Adapted from Swanson, 2004; Simmons & Swanson, 2009, with permissions.
3.2 Afferent-Dependent CRH Neuronal Activation
To initiate basal and stressor-driven HPA responses, the PVHmp receives numerous regulatory signals that travel along the many distinct afferent pathways [Ulrich-Lai & Herman, 2009], including a major set of catecholaminergic inputs from the hindbrain [Ssawchenko & Swanson, 1981]. Because each stressor has a unique signature in terms of its character, origin (internal or external), priority for action (actual or perceived), intensity, duration etc., it will recruit distinct combinations of humoral and neural signals to modify brain function. A particular stressor will therefore recruit a subset of these PVH afferents, the constituency of which depends on the nature of the stressor or stimulus. This stimulus-defined afferent ‘set’ then mediates a CRH neuronal response to the stressor that is appropriately graded to the nature and strength of the stimulus [Watts, 2005; Watts & Khan, 2011]. It will involve a controlled and coordinated activation of ACTH secretogogue synthesis and release processes, including increased action potential frequency, the accumulation of phospho-(p)CREB, nuclear TORC2, pERK1/2, Fos, CRH hnRNA and mRNA, ACTH secretogogue release, etc [Herman et al., 2003; Khan & Watts, 2004; Liu et al., 2008, 2010, 2012; Wamsteeker & Bains, 2010; Osterlund et al., 2012].
4. The Dynamic Coupling of Neural Inputs, Peptide Synthesis, and Peptide Release
4.1 Parallel cellular programs for synthesis and release
The rate of ACTH secretagogue release from neuroendocrine terminals in the median eminence is determined by those transmitter-activated receptors in the post-synaptic membrane of the CRH neuronal soma and dendrites that drive cell membrane depolarization and the consequent changes in action potential frequency. But transmitter-driven signal transduction mechanisms can also activate biosynthetic processes in CRH neurons, particularly those that converge on cAMP regulatory binding protein (CREB) to control ACTH secretagogue gene expression. Increased biosynthetic rates then maintain adequate levels of CRH and AVP in neuroendocrine terminals to drive continued ACTH release from corticotropes [Watts, 2005].
Figure 2 illustrates that synthesis and release mechanisms in CRH neurons operate in different spatial and temporal domains [Watts, 2005]. They converge in CRH neuroendocrine terminals in the median eminence where action potentials stimulate calcium-dependent release of CRH from vesicles that were originally packaged in the neuronal somata and shipped down axons to the terminals. Despite their clear functional and structural separation, most studies appear to assume that CRH neuronal activation always involves both peptide synthesis and release—ie. that these two processes are always tightly coupled. This is because the system is often examined using acute stressors that activate both processes quickly making them difficult to separate using currently available methodologies. Furthermore, it is not common for studies to measure the behavior of viable markers of CRH synthesis and release in the same animal, again making it difficult to determine functional relationships between them.
Figure 2.
Two independent sets of processes control CRH peptide synthesis and its release in CRH neuroendocrine neurons. Each of the processes are found in different temporal and spatial domains, with peptide release being a virtually immediate response to membrane depolarization, action potential generation, and peptide release from vesicles accumulated at terminals in the median eminence. Each process will involve somewhat different intracellular signal transduction mechanism in CRH neurons, but cross-talk between then offers a mechanism to couple the two processes together.
We have generated a large body of evidence over the past 15 years showing that the coupling of synthesis and release of ACTH secretagogues is in fact dynamic, variable, and stimulus-specific, which leads to the notion that each process can be controlled independently [Watts, 2005; Khan et al., 2011]. This flexibility makes the function of CRH neurons much more adaptable to a wide variety of challenges than if synthesis and release were rigidly coupled. The regulated coupling of secretagogue synthetic and release mechanisms to each other and to neural inputs is therefore an essential part of CRH neuronal function. Although much work has shown how afferent signals drive neurotransmitter release, as well as how neuropeptides are synthesized, the nature of afferent-synthesis-release coordination in peptidergic neurons remains an unresolved neurobiological question, not just for CRH neurons, but for all peptidergic neurons.
We propose that the components of these coupling mechanisms exist at two interactive levels: within an afferent control network that is located proximal to the PVH; and inside CRH neurons. Results from our recent experiments using manipulations of the catecholaminergic inputs to the PVH and adjacent forebrain [Ritter et al., 2003; Khan et al., 2011; Kaminski & Watts, 2012] are consistent with cross-talk between intracellular signal transduction pathways being a likely intracellular coupling mechanism (Fig. 2). These studies explore how glycemic challenges engage the catecholaminergic innervation of the PVH to drive HPA responses [Khan et al., 2011]. They provide evidence that the regulation of ERK1/2 phosphorylation is an important contributor to stimulus-synthesis-release coupling processes.
The way that the elements within these two levels are organized and interact remains unclear, not least because a complete and detailed picture of microcircuitry within the PVH is not known. But at least four different models of their interactions are apparent. These are shown in Figure 3. It proposes that separate afferent inputs can each control synthesis and release mechanisms (Fig. 3A). With appropriate stimulation, these might be linked by increasing the activity of one input as opposed to another, perhaps through the recruitment of phospho-ERK1/2 (Fig 3B). The presence of a third set of inputs, for example catecholaminergic neurons that can act on pre-motor inputs as well as CRH neurons themselves, engenders two further mechanisms: one that does not involve phospho-ERK1/2 (Fig. 3C) and one that does (Fig. 3D). A more detailed consideration of how a pre-motor PVH/CRH network that involves catecholaminergic inputs might be organized is presented later (Section 5). Given the diversity of inputs that CRH neurons receive it seems likely that variants of each are present within the PVH and its local microcircuitry.
Figure 3.
Four hypothetical schemes illustrating how differential coupling between peptide synthesis and release could be enabled either at the pre-motor network or intracellular signal transduction levels. A) Synthesis and release are independently controlled by different inputs. B) Synthesis and release are increased by one input through pERK1/2’s ability to activate both processes. C) Synthesis and release are increased by a third indirect input that controls the two direct inputs, each of which operates independent of the other and pERK1/2. D) Synthesis and release are increased by a third direct input that operates independent of the two others. The third input directly controls pERK1/2 and thereby synthesis and release.
4.2 Evidence for dynamic coupling between afferent, synthesis, and release processes
Numerous studies have shown that there are two primary patterns of ACTH and corticosterone release. First, a daily pattern exists where levels of these two hormones peak when activity begins. In rodents this is evident as maximal ACTH release at lights off. Second, stressor-activated release also occurs, the magnitude of which is generally determined by the intensity of the stressor. With the sequencing of Crh in 1985 [Jingami et al., 1985] and the consequent availability of probes to measure changes in Crh expression and CRH mRNA levels, it became clear that to maintain secretogogue levels in the median eminence, stressor-activated ACTH release was accompanied by increase Crh expression [see Watts, 1996 for review]. In these circumstances, CRH synthesis and release quickly increase together. However, a series of studies from our lab has revealed the existence of independent control mechanisms for these processes.
First, because of its slow development, hypovolemia has a temporal profile that allows us to discriminate between the onset times of the various CRH neuronal responses. With this stressor, ACTH release occurs well before Crh expression [Tanimura et al., 1998]. Presumably if one mechanism controlled both processes, they would occur together. Second, when insulin-induced hypoglycemia is relatively mild, ACTH release is activated whereas CRH synthesis is not [Gorton et al., 2007]. Deeper hypoglycemia activates both [Khan et al., 2011]. Third, synthesis and release mechanisms respond asymmetrically to hypovolemia in the presence of a second heterotypic stressor; release responses remain, but synthesis is suppressed [Watts & Sanchez-Watts, 2002]. Finally, during the daily excursions of ACTH and corticosterone secretion, the temporal patterns of ACTH release and Crh transcription are completely out of phase [Watts et al., 2004]. In should be noted that in all these circumstances, CRH rather than AVP, is considered the predominant ACTH secretogogue.
Figure 4 shows the markedly different relationships between synthesis and release across the day in the absence of stressors (Fig. 4A) [Watts et al., 2004], and in response to glycemic challenges that rely on catecholaminergic projections to the PVH (Fig. 4B) [Khan et al., 2011]. These results demonstrate the presence of independently controlled synthesis and release mechanisms within CRH neurons that can be linked together—ie. positively correlated—when required by elevated release. As we have already described, this is not surprising given that the two processes operate in different spatial and temporal domains (Fig. 2). For coupling to occur there must be regulated cross-talk between the two pathways.
Figure 4.
Correlations between CRH synthesis rates (as measured by the accumulation of CRH hnRNA) and CRH releases (as measured by plasma ACTH concentrations) in (A), unstressed rats sampled across a 24h period (inset shows mean values for each variable across the day); and (B) 30 minutes after rats were exposed to either intravenous (iv) insulin-induced hypoglycemia or iv 2-deoxy-D-glucose (see text for details). Note that the correlations were negative (A; F1,77 = 34.43, p<0.0001 R2 = 0.3084) in the absence of stress or positive (B; R2 = 0.6604 F1,29 = 54.45, p<0.0001) in the presence of stress, indicating completely different relationships between synthesis and release in these two states. Data adapted from Watts et al. 2004 and Khan et al., 2011.
Different relationships therefore exist between synthesis and release in CRH neurons depending on whether a stressor is absent or present. If a stressor is present, the strength of the coupling depends on the nature and intensity of the stressor, which in turn is determined by the nature of its afferent coding mechanisms. The situation is further complicated with an additional stressor, or in the presence or absence of corticosterone [Watts & Sanchez-Watts, 2002; Watts et al., 2004] meaning that the multiple mechanisms likely interact at different levels to enable such a complex yet flexible set of responses.
4.3 What is Required of a Coupling Mechanism?
Although a complete picture of the components that coordinate afferent-synthesis-release coupling in CRH neuroendocrine—or indeed any—neurons is unknown, key elements should ideally possess at least three properties. First, they should be able to regulate the appropriate mechanisms for both synthesis and release; second, they must be engaged by diverse stimuli; and third, they should respond in proportion to stimulus intensity. These properties will enable an appropriate coupling strength to match the nature of the stimulus and its demands on the HPA system.
Coupling of this nature could be achieved using at least two different control mechanisms: by way of a pre-motor network comprised of those neurons located proximal to the PVH that synapse directly on CRH neurons; and/or from cellular signaling pathways within the CRH neuron itself that can control the activity of both processes. We now describe candidate mechanisms at each of these two control levels.
5. A Pre-Motor Network That Controls the Neuroendocrine PVH
CRH neurons receive a wide variety of neural inputs that constitute their pre-motor (ie. pre-synaptic) control elements [Watts, 2005]. These inputs include neurons that use glutamate and small amino acid-derived transmitters (GABA and monoamines), and inputs from peptidergic neurons (including neuropeptide Y, Agouti-relate peptide, α–melanocyte stimulating hormone, and opioids). The minimal model for how they control CRH neurons is that each provides varying degrees of excitatory or inhibitory drive directly and with little interaction with other inputs—basically a parallel network. Appropriately graded responses are then generated in a simple integrative manner. Numerous studies show that this model is untenable
[see Herman et al., 2002, 2003; Levy & Tasker, 2012; Wamsteeker & Bains, 2010; Watts, 2005, for reviews]. So a fundamental part of understanding how CRH neuroendocrine control systems function is resolving how stressors impact CRH neurons using a pre-motor network that is comprised of the neurons that form direct synaptic connections with CRH neurons.
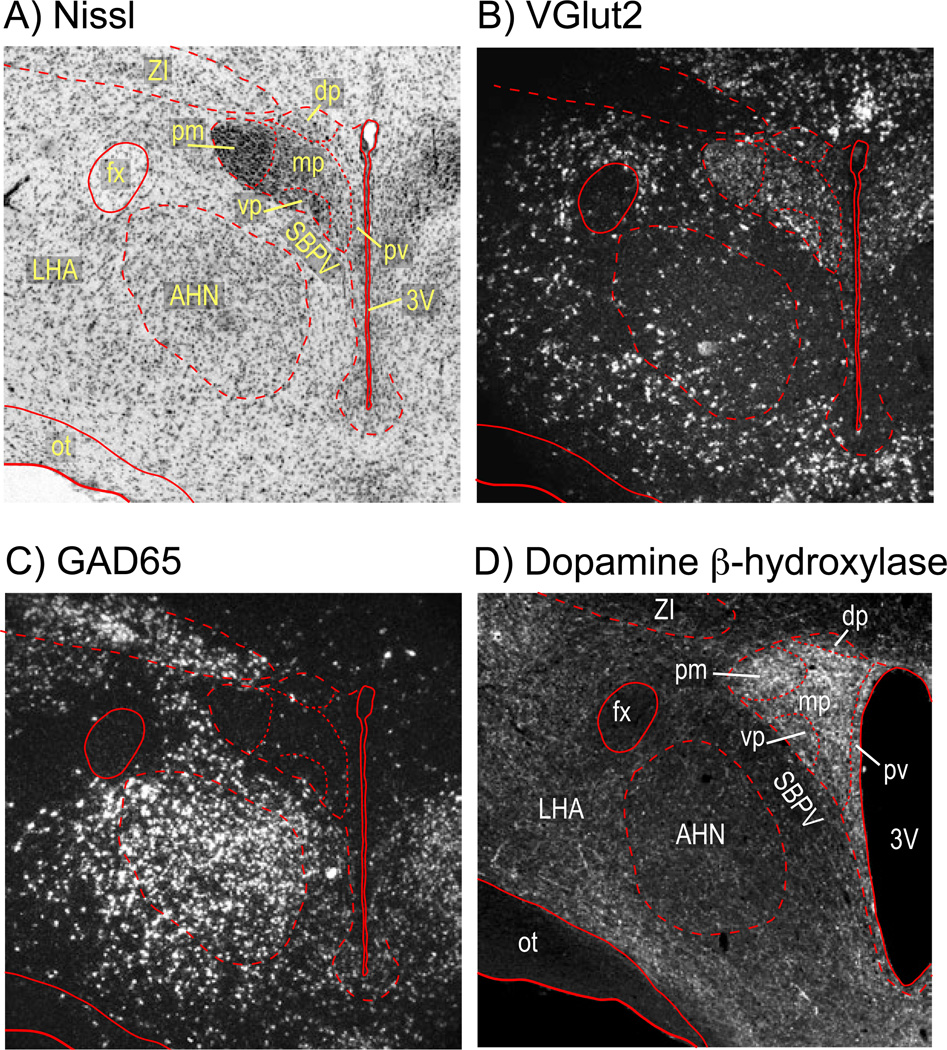
The pre-motor network acts as an ‘integrator’ or ‘gate’ through which all encoded stress and other signals must pass before they can engage CRH neurons [Cullinan et al., 2008; Herman et al., 2002; Levy & Tasker, 2012; Watts, 2005]. We propose that this integrator includes catecholaminergic inputs. Although this network is electrophysiologically well defined [Bains & Ferguson, 1999; Boudaba et al., 1996; Chong et al., 2004; Daftrey et al., 2000; Han et al., 2002; Hewitt et al., 2009; Iremonger et al., 2010; Kuzmiski et al., 2010; Levy & Tasker, 2012; Marty et al., 2011; Verkuyl et al., 2005; Yang et al., 2008], anatomically it remains an enigma, particularly regarding the location of the GABA and glutamate pre-motor neurons. Some of these are located distally [Herman et al., 2003; Ulrich-Lai et al., 2011; Zeigler et al., 2012], but electrophysiological studies using PVH slices—where only elements close to the PVH remain functional—strongly support a more proximal location for others [Boudaba et al., 1996; Cullinan et al., 2008; Daftrey et al., 2000; Herman et al., 2002] including GABA or glutamate interneurons within or close to the PVH [Csáki et al., 2000; Daftrey et al., 2000; Roland et al., 1993]. Although this notion is supported by the many neurons in the PVH that express vGlut2 as well as neurons in peri-PVH regions that express GAD65/67 (Fig. 5), precise relationships between these neurons and the CRH population are very difficult to address anatomically because conventional neuroanatomical tracers are virtually impossible to use effectively in such a confined region.
Figure 5.
Photomicrographs of coronal sections through the hypothalamus at the level of the paraventricular nucleus of the hypothalamus (PVH) showing a), a Nissl stained section and the cytoarchitecture of the region; b), in situ hybridization (ISH) signal for the vesicular glutamate transporter 2 mRNA; c), ISH signal for the glutamic acid decarboxylase 65 mRNA, which identifies GABAergic neurons; and d) dopamine β-hydroxylase immunoreactivity. a) – c) are from the same animal; d) is from a different animal.
Abbreviations: 3V, third ventricle; AHN, anterior hypothalamic nucleus; fx, fornix; LHA, lateral hypothalamic area; mp, medial parvicellular part of the PVH; ot, optic tract; pm, posterior magnocellular part of the PVH; pv, periventricular part of the PVH; SBPV, subparaventricular zone; vp, ventral parvicellular part of the PVH; ZI, zona incerta;
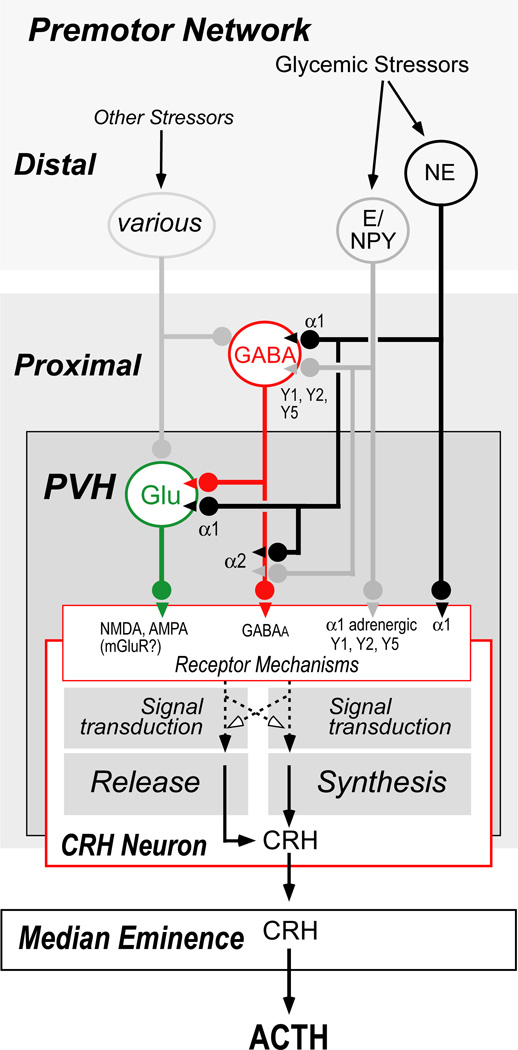
Figure 6 shows a model of a pre-motor control network for CRH neurons that derives largely from the electrophysiological experiments in PVH slices, we have just discussed. It illustrates that catecholaminergic inputs from the ventrolateral medulla (A1/C1) and to a lesser extent the medial part of the nucleus of the solitary tract (A2/C2) [Sawchenko & Swanson, 1981]—some of which express NPY—interact with CRH neurons directly using α1 adrenergic receptors expressed by CRH neurons. Some of these catecholaminergic neurons appear to engage CRH neurons indirectly by way of glutamatergic and GABAergic neurons that have direct connections with CRH neurons. These interactions occur both at the GABAergic and glutamatergic cell bodies, as well as presynaptically at GABAergic and glutamatergic terminals. Even without considering other monoaminergic and peptidergic inputs to CRH neurons, this model already engenders considerable complexity to afferent interactions with CRH neurons. Given the diversity of the transmitter/receptor mechanisms within this model, the possibility arises that these different neuronal elements can differentially control synthesis and release; one need not be controlled by the same neural input mechanisms as the other.
Figure 6.
A model of the pre-motor network that regulates CRH neuroendocrine activity based primarily on electrophysiological studies (see text for details). In brief, a number of studies support the existence of a set of GABAergic and glutamatergic neurons located in close proximity to the paraventricular nucleus of the hypothalamus (PVH). In this model, catecholaminergic inputs to the PVH can regulate synthesis and release mechanisms in CRH neurons either by direct synaptic interactions or by way of the pre-motor GABA/glutamatergic network. Because some catecholaminergic neurons also express neuropeptide Y, this peptide may also modulate synthesis and release processes in CRH neurons. Although electrophysiological studies provide strong evidence for interactions of this type, their anatomical bases remain elusive.
Abbreviations: α1, α2, adrenoreceptor subtypes; E, epinephrine; Glu, glutamate; mGluR, metabotropic glutamate receptors; NE, nor-epinephrine; NPY, neuropeptide Y; PVH, paraventricular nucleus of the hypothalamus; Y1, Y2, Y5, NPY receptor subtypes.
6. Phospho-ERK1/2 as a stimulus-synthesis-release coupler in CRH neurons
Intracellular signaling molecules in neuroendocrine neurons are fundamental for linking the many afferent inputs that encode stressor-related information to peptide synthesis and release. Those molecules that respond to diverse afferent signals and can regulate both biosynthesis and neuronal firing rates will be well placed to enable the coupling of afferent signals to downstream synthetic and release responses. These signaling molecules include the phosphorylated forms of p44/42 mitogen-activated protein kinases (ERK1/2) that belong to the mitogen-activated protein (MAP) kinase family. They can influence both neuronal excitability and gene expression [Thomas & Huganair, 2004]. ERK1/2 are phosphorylated by mitogen-activated protein kinase kinase (MEK), which in turn is activated by way of components in calcium- and cAMP-signaling pathways [Impey et al., 1998; Pierce et al., 2001; Stork & Schmiitt, 2002}
6.1 The Behavior of Phospho-ERK1/2 Following Different Stressors
Phospho-ERK1/2 levels rapidly increase in PVHmp neurons—including CRH neurons—following various systemic challenges, as well as after central delivery of neurotransmitters, growth factors, and receptor agonists [Daniels et al., 2003; Khan et al., 2007; Blume et al., 2009; Manfredsson et al., 2009]. Four sets of observations from our laboratory support the idea that MEK and ERK1/2 are strategically positioned between membrane receptors and the downstream synthetic and release processes in a way that can coordinate these two critical processes during various stress responses.
6.1.1 Phospho-ERK1/2 closely tracks phospho-CREB and Crh transcription in the PVHmpd
CREB phosphorylation is required for increased Crh transcription [Liu et al., 2008], and so it is striking that levels of phospho-ERK1/2 and CRH hnRNA (Fig. 7A), phospho-ERK1/2 and phospho-CREB, and phospho-CREB and CRH hnRNA, are all strongly correlated in the PVHmpd following three different stressors: intravenous injections of 2-deoxyglucose, insulin, or isoflurane anesthesia combined with hypertonic saline injections (A+HS) [Khan et al., 2011]. There is also widespread co-localization of cytoplasmic phospho-ERK1/2 immunoreactivity (-ir) and nuclear phospho-CREB-ir in stimulated PVHmp neurons, showing how phospho-ERK1/2 could act in the same spatial and temporal domains as phospho-CREB to mediate Crh transcription [Khan et al., 2011].
Figure 7.
The relationships between A) CRH hnRNA levels (an index of Crh transcription rates) and phosopho-ERK1/2 levels in the medial parvicellular part (mp) of the paraventricular nucleus of the hypothalamus (PVH); and B) plasma ACTH concentrations and phosopho-ERK1/2 levels in the PVHmp, 30 minutes after rats were exposed to either intravenous (iv) saline (open symbols) or insulin-induced hypoglycemia or iv 2-deoxy-D-glucose (solid symbols). Note the positive correlation (closed symbols, red lines) between CRH hnRNA and phospho-ERK1/2 (A), and ACTH and phospho-ERK1/2 in the stressed animals (B), but not in saline-injected control animals (open symbols, red lines). The dashed lines represents the positive correlations between both sets of variables across the full datasets. See Khan et al., 2011 for more detail. Data adapted from Khan et al., 2011, with permissions.
6.1.2 Phospho-ERK1/2 in the PVHmpd closely tracks increases in plasma ACTH
Concomitant with the increases in CRH transcripts, the graded increases in plasma ACTH that follow insulin and 2-DG injections are significantly correlated to increases of both the signal intensity and the area occupied by phospho-ERK1/2-ir in the PVHmpd [Khan et al., 2011]. This shows that the magnitude of ACTH secretagogue release from CRH neurons closely tracks the amount of phospho-ERK1/2 in PVHmp neurons (Fig. 7B), and that the recruitment of more neurons with elevated phospho-ERK1/2-ir is associated with increased ACTH responses.
6.1.3 ERK1/2 phosphorylation relies on appropriate stimulus-afferent coupling
Phospho-ERK1/2 accumulation is observed in the PVHmpd after anesthesia [Khan & Watts, 2004], immune [Nadjar et al., 2005; Singru et al., 2008], and nociceptive challenges [Choi et al., 2006], as well as drug administration and withdrawal [Valjent et al., 2004; Selcher et al., 2003]. Furthermore, PVH phospho-ERK1/2-ir is elevated after centrally delivered neurotransmitters, growth factors, and pharmacological agents [Daniels et al., 2003; Khan et al., 2007; Blume et al., 2009; Manfredsson et al., 2009]. Together these results show that ERK1/2 phosphorylation is regulated by a broad array of neural inputs to CRH neurons. However, if MAP kinase pathways do function as multi-responsive intermediates that couple afferent signals to downstream synthesis and release programs, then disrupting a neural input that leads to a loss of CRH neuroendocrine function must also be accompanied by a loss of phospho-ERK1/2. We showed that this occurs for glycemic challenges that use catecholaminergic pathways to drive CRH synthesis and release [Khan et al., 2011]. On the other hand, stimuli encoded by broader afferent sets should retain the ability to recruit phospho-ERK1/2 in the absence of catecholaminergic pathways. This prediction, too, was borne out by our results, which showed that full phospho-ERK1/2 recruitment in the PVHmpd after insulin and 2-DG challenges requires intact catecholaminergic afferents, whereas the ERK1/2 phosphorylation evoked by A+HS does not. Collectively, our results demonstrate that although existing pools of unphosphorylated ERK1/2 are ostensibly ‘primed’ to respond to multiple types of afferent stimulation, their activation by phosphorylation is selectively coupled to specific afferent sets engaged by a particular stimulus.
6.1.4. MEK controls intracellular mechanisms critical for CRH synthesis and release
Although our in vivo results provide strong support for a central coordinating role for MAP kinase pathways in CRH synthesis and release following glycemic challenges, we have also obtained direct mechanistic evidence for this function in two sets of experiments utilizing ex vivo tissue slices containing the paraventricular hypothalamus [Khan et al., 2011]. First, we showed that the ability of norepinephrine to increase CREB phosphorylation, an obligatory step for initiating Crh transcription, requires intervening MEK-dependent processes. Second, a MEK-dependent mechanism is required for norepinephrine to increase PVHmp neuroendocrine neuronal firing rates, which is consistent with a reported role for phospho-ERK1/2 in modulating neuronal excitability [Selcher et al., 2003].
We have already demonstrated that norepinephrine-driven increases in ERK1/2 phosphorylation in CRH neurons are both α1-adrenoceptor- and MEK-dependent [Khan et al., 2007]. Together, these in vivo and ex vivo experiments show that the ability of norepinephrine to increase CREB phosphorylation and firing rates involves α1-adrenoceptors and MEK activity. Importantly, this demonstrates that α1-adrenoceptor- and MEK-dependent mechanisms can regulate the firing rate and thereby the release of ACTH secretagogues from PVHmp neurons. Although the nature of this mechanism is unknown, it may involve MEK and/or ERK1/2 interactions with chloride homeostasis, which is an α1-adrenoceptor-dependent in PVHmp neurons [Hewitt et al., 2009]. Together these results strongly support a key role for this MAPK pathway in regulating Crh transcription and ACTH secretagogue release both after exogenous norepinephrine application in vivo [Khan et al., 2007], and as part of the catecholaminergic afferent-dependent increases after insulin and 2-DG [also see Ritter et al., 2003].
6.2 The Behavior of Phospho-ERK1/2 In the Absence of Stress
If levels of phospho-ERK1/2 are significantly correlated with the increased synthesis and release of CRH we see during stress (Fig. 7), then how does phospho-ERK1/2 behave in the absence of stress? For the most part, phospho-ERK1/2 levels remain low and uncorrelated to CRH hnRNA or ACTH levels in unstressed control animals (Fig. 7). We have also found that under conditions where ACTH secretagogue release and Crh transcription are negatively correlated across the day (Fig. 4A) phospho-ERK1/2 levels remain consistently low [Gorton et al., 2007]. Considered together, these results show that in the absence of stress Crh transcription and ACTH release each proceeds without significant amounts of phospho-ERK1/2 in CRH neurons. Presumably different afferent sets are recruited to maintain activity in the basal state compared to those responsible for responses seen following stress. This conclusion is supported by the finding that a complete loss of catecholaminergic inputs to the PVH has no effect on the daily rhythm of corticosterone release [Ritter et al., 2003], yet this lesion completely abolishes responses to glycemic challenges [Ritter et al., 2003; Khan et al., 2011].
7. Conclusion
Afferent-encoded information must first engage the appropriate receptors expressed by CRH neurons to increase Crh transcription and ACTH secretagogue release. The downstream effects of these receptor arrays are channeled through multiple intracellular signaling pathways that converge on CREB phosphorylation on the one hand [Aguilera & Liu, 2012], and on membrane depolarization and increased firing rate on the other [Watts, 2005]. Our results strongly support the idea that phospho-ERK1/2 are obligatory constituents in the pathways used by catecholaminergic afferents to increase both Crh transcription and ACTH secretagogue release. They are also consistent with the idea that the amount of ERK1/2 phosphorylation present in CRH neurons regulates the degree of afferent-synthesis-release coupling across a range of stimulus intensities.
Since phospho-ERK1/2 can act as a ‘gain-controller’ for a variety of intracellular processes [Selcher et al., 2003, Hazzalin & Mahadevan, 2002], it is tempting to speculate that the loose coupling we have previously observed between ACTH secretagogue release and Crh transcription [Watts, 2005; Gorton et al., 2007; Tanimura et al., 1998; Watts & Sanchez-Watts, 2002; Watts et al., 2004] is related to the degree of ERK1/2 phosphorylation that is driven by altered afferent activity. Thus, under conditions where the coupling of ACTH secretagogue release and Crh transcription is negatively correlated (Fig. 3A) phospho-ERK1/2 levels remain consistently low [Gorton et al., 2007]. In contrast, the increasingly tight coupling of afferent signaling, synthesis, and secretagogue release in CRH neurons is achieved with the significantly higher levels of phospho-ERK1/2 generated by glycemic challenges and other stressors.
Afferent-synthesis-release coupling is a crucial function in all neurons, allowing them to adapt their activities in both an ongoing and anticipatory manner. By examining the role of ERK1/2 phosphorylation in CRH neuroendocrine neurons following stimuli that require catecholaminergic projections from the hindbrain to the PVH and adjacent regions, we have shown that this particular MAPK pathway is strategically located to contribute to this coupling process, and raises the possibility that it plays a similar key role in other peptidergic neurons.
Acknowledgements
AGW would like to acknowledge all the members of the Watts lab who have contributed to the development of these concepts over the years. The work was supported by NS029728 and JDRF 2-710-2008 from the Juvenile Diabetes Research Foundation (AGW). Work in the UTEP Systems Neuroscience Laboratory is supported by DK081937 (AMK) and pilot project funds from the Border Biomedical Research Center (5G12RR008124).
References
- Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Activation of N-methyl-D-aspartate receptors evokes calcium spikes in the dendrites of rat hypothalamic paraventricular nucleus neurons. Neuroscience. 1999;90:885–891. doi: 10.1016/s0306-4522(98)00525-9. [DOI] [PubMed] [Google Scholar]
- Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neumann ID. Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinol. 2009;150:1841–1849. doi: 10.1210/en.2008-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-S, Seo Y-J, Shim E-J, Kwon M-S, Lee J-Y, Ham Y-O, Suh H-W. Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res. 2006;1108:28–38. doi: 10.1016/j.brainres.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Chong W, Li LH, Lee K, Lee MH, Park JB, Ryu PD. Subtypes of α1- and α2-adrenoceptors mediating noradrenergic modulation of spontaneous inhibitory postsynaptic currents in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2004;16:450–457. doi: 10.1111/j.1365-2826.2004.01180.x. [DOI] [PubMed] [Google Scholar]
- Csáki A, Kocsis K, Halász B, Kiss J. Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience. 2000;101:637–655. doi: 10.1016/s0306-4522(00)00411-5. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Boudaba C, Tasker JG. Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience. 2000;96:743–751. doi: 10.1016/s0306-4522(00)00003-8. [DOI] [PubMed] [Google Scholar]
- Daniels D, Patten CS, Roth JD, Yee DK, Fluharty SJ. Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res. 2003;986:1–11. doi: 10.1016/s0006-8993(03)03162-7. [DOI] [PubMed] [Google Scholar]
- Gorton LM, Khan AM, Bohland M, Sanchez-Watts G, Donovan CM, Watts AG. A role for the forebrain in mediating time-of-day differences in glucocorticoid counterregulatory responses to hypoglycemia in rats. Endocrinol. 2007;148:6026–6039. doi: 10.1210/en.2007-0194. [DOI] [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J. Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme C, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent nuclear transcription and ERK nuclear translocation. Neuron. 1998;21(4):869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Benediktsson AM, Bains JS. Glutamatergic synaptic transmission in neuroendocrine cells: Basic principles and mechanisms of plasticity. Front Neuroendocrinol. 2010;31:296–306. doi: 10.1016/j.yfrne.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Jingami H, Mizuno N, Takahashi H, Shihahara S, Furutani Y, Imura H, Numa S. Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett. 1985;191:63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- Kaminski KL, Watts AG. Intact catecholamine inputs to the forebrain are required for appropriate regulation of corticotrophin-releasing hormone and vasopressin gene expression by corticosterone in the rat paraventricular nucleus. J Neuroendocrinol. 2012;24:1517–1526. doi: 10.1111/j.1365-2826.2012.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Kaminski KL, Sanchez-Watts G, Ponzio TA, Kuzmiski JB, Bains JS, Watts AG. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci. 2011;31:18479–18491. doi: 10.1523/JNEUROSCI.4785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. J Neurosci. 2007;27:7344–7360. doi: 10.1523/JNEUROSCI.0873-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Watts AG. Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinol. 2004;145:351–359. doi: 10.1210/en.2003-0539. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, Marty V, Baimoukhametova DV, Bains JS. Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat Neurosci. 2010 Oct;13(10):1257–1264. doi: 10.1038/nn.2629. 2010. [DOI] [PubMed] [Google Scholar]
- Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci. 2012;6:24. doi: 10.3389/fncel.2012.00024. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Coello AG, Grinevich V, Aguilera G. Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription. Endocrinology. 2010;151:1109–1118. doi: 10.1210/en.2009-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3',5'-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149:3512–3520. doi: 10.1210/en.2008-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Poon V, Sanchez-Watts G, Watts AG, Takemori H, Aguilera G. Salt-inducible kinase is involved in the regulation of corticotropin-releasing hormone transcription in hypothalamic neurons in rats. Endocrinology. 2012;153:223–233. doi: 10.1210/en.2011-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Coello AG, Grinevich V, Aguilera G. Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription. Endocrinol. 2010;151:1109–1118. doi: 10.1210/en.2009-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, et al. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol. Ther. 2009;17:980–991. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty V, Kuzmiski JB, Baimoukhametova DV, Bains JS. Short-term plasticity impacts information transfer at glutamate synapses onto parvocellular neuroendocrine cells in the paraventricular nucleus of the hypothalamus. J Physiol. 2011;589:4259–4270. doi: 10.1113/jphysiol.2011.208082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, Combe C, Busquet P, Dantzer R, Parnet P. Signaling pathways of interleukin-1 actions in the brain: anatomical distribution of phospho-ERK1/2 in the brain of rat treated systemically with interleukin-1b. Neurosci. 2005;134:921–932. doi: 10.1016/j.neuroscience.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Osterlund C, Jarvis E, Chadayammuri A, Unnithan R, Weiser MJ, Spencer RL. Tonic, but not phasic corticosterone, constrains stress activated extracellular-regulated-kinase 1/2 immunoreactivity within the hypothalamic paraventricular nucleus. J. Neuroendocrinol. 2011;23:1241–1251. doi: 10.1111/j.1365-2826.2011.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20(13):1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinol. 2003;144:1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn. Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516:423–441. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- Singru PS, Sánchez E, Acharya R, Fekete C, Lechan RM. Mitogen-activated protein kinase contributes to lipopolysaccharide-induced activation of corticotropin-releasing hormone synthesizing neurons in the hypothalamic paraventricular nucleus. Endocrinol. 2008;149:2283–2292. doi: 10.1210/en.2007-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork PJS, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12(6):258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Lind RW. Regulation of multiple peptides in CRF parvocellular neurosecretory: Implications for the stress response. In: Hökfelt T, Fuxe K, Pernow B, editors. Prog Brain Res. Amsterdam: Elsevier; 1986. pp. 169–190. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Third Edition. San Diego, California, USA: Academic Press; 2004. [Google Scholar]
- Tanimura SM, Sanchez-Watts G, Watts AG. Peptide gene activation, secretion, and steroid feedback during stimulation of rat neuroendocrine corticotropin-releasing hormone neurons. Endocrinol. 1998;139:3822–3829. doi: 10.1210/endo.139.9.6191. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, et al. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. EurJNeurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–121. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- Wamsteeker JI, Bains JS. A synaptocentric view of the neuroendocrine response to stress. Eur J Neurosci. 2010;32:2011–2021. doi: 10.1111/j.1460-9568.2010.07513.x. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Physiological regulation of peptide messenger RNA colocalization in rat hypothalamic paraventricular medial parvicellular neurons. J Comp Neurol. 1995;352:501–514. doi: 10.1002/cne.903520403. [DOI] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J. Neurosci. 2002;22:6282–6289. doi: 10.1523/JNEUROSCI.22-14-06282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinol. 2004;145:529–540. doi: 10.1210/en.2003-0394. [DOI] [PubMed] [Google Scholar]
- Wittmann G. Regulation of hypophysiotrophic corticotrophin-releasing hormone- and thyrotrophin-releasing hormone-synthesising neurones by brainstem catecholaminergic neurones. J Neuroendocrinol. 2008;20(7):952–960. doi: 10.1111/j.1365-2826.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- Yang JH, Li LH, Shin SY, Lee S, Lee SY, Han SK, Ryu PD. Adrenalectomy potentiates noradrenergic suppression of GABAergic transmission in parvocellular neurosecretory neurons of hypothalamic paraventricular nucleus. J Neurophysiol. 2008;99:514–523. doi: 10.1152/jn.00568.2007. 2008. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Edwards MR, Ulrich-Lai YM, Herman JP, Cullinan WE. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 2012;520:2369–2394. doi: 10.1002/cne.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]