Abstract
Apicomplexan parasites express various Calcium-Dependent Protein Kinases (CDPKs), and some of them play essential roles in invasion and egress. Five of the six CDPKs conserved in most Apicomplexa have been studied at the molecular and cellular levels in Plasmodium species and/or in Toxoplasma gondii parasites, but the function of CDPK7 was so far uncharacterized. In T. gondii, during intracellular replication, two parasites are formed within a mother cell through a unique process called endodyogeny. Here we demonstrate that the knock-down of CDPK7 protein in T. gondii results in pronounced defects in parasite division and a major growth deficiency, while it is dispensable for motility, egress and microneme exocytosis. In cdpk7-depleted parasites, the overall DNA content was not impaired, but the polarity of daughter cells budding and the fate of several sub-cellular structures or proteins involved in cell division were affected, such as the centrosomes and the kinetochore. Overall, our data suggest that CDPK7 is crucial for proper maintenance of centrosome integrity required for the initiation of endodyogeny. Our findings provide a first insight into the probable role of calcium-dependent signalling in parasite multiplication, in addition to its more widely explored role in invasion and egress.
Keywords: Apicomplexa, Toxoplasma gondii, Tet-inducible system, Calcium Dependent Protein Kinase, Replication, Calcium, centrosome, kinetochore, apicoplast, Inner Membrane Complex
Introduction
Apicomplexan parasites are responsible for a wide array of pathologies in humans and animals. They include Plasmodium falciparum, the causative agent of malaria, and Toxoplasma gondii, a life-threatening parasite in immuno-compromised individuals. Members of this phylum generally possess an apical complex composed of secretory organelles (micronemes and rhoptries) that contribute to active host cell invasion and, for most Apicomplexa, to the establishment of the parasitophorous vacuole membrane within which the parasite securely replicates (Dubremetz et al., 2012). The Apicomplexa also have an inner membrane complex (IMC) composed of flattened vesicular sacs laying underneath the plasma membrane (Vivier et al., 1969). During parasite replication, the apical complex and the IMC are formed de novo within the mother cell in a process described as internal budding and the general cell division process, characterized by the formation of two daughter parasites within the mother is called endodyogeny (Gubbels et al., 2008b). This process implies the budding of two nascent parasites in a synchronous and symmetric fashion and according to a highly polarized organization within the mother cell (Gubbels et al., 2006, Nishi et al., 2008, Sheffield et al., 1968). Toxoplasma division is initiated upon elongation of the Golgi apparatus, followed by the duplication of the centrosomes and then by the fission of the Golgi (Gubbels et al., 2008b, Sheffield et al., 1968). Simultaneously, the apicoplast elongates and associates with the centrosomes and enters the growing daughter cells during nuclear division (Hartmann et al., 2006, Pelletier et al., 2002, Striepen et al., 2000). The scaffold of the daughter cells (comprising the conoid, the nascent IMC and the subpellicular MTs), begins to appear slightly before the completion of DNA replication (Hu et al., 2002, Nishi et al., 2008). Mitosis and cytokinesis progress in concert with daughter cell growth and each newly formed daughter cell encapsulates a Golgi stack and an apicoplast (Morrissette et al., 2002, Nishi et al., 2008). Next, the nucleus, the endoplasmic reticulum and finally the mitochondrion are packed into the developing daughter cells (Nishi et al., 2008). Rhoptries and micronemes organelles are made de novo by vesicular budding from the Golgi apparatus (Breinich et al., 2009, Hoppe et al., 2000, Ngo et al., 2003, Nishi et al., 2008, Sloves et al., 2012). Ultimately, the plasma membrane of the mother cell wraps the daughters, and its apical organelles degenerate and are eliminated in a residual body (Hu et al., 2002, Morrissette et al., 2002, Nishi et al., 2008).
In Apicomplexan parasites, little is known about the kinases required for the parasite to progress through the cell division cycle. Calcium dependent protein kinases (CDPKs) are major actors in calcium signalling in plants, ciliates and apicomplexan parasites (Harper et al., 2005). The Apicomplexa CDPKs consist of a serine/threonine protein kinase domain and regulatory motifs containing 2–4 calcium-binding EF hands (Billker et al., 2009). A junction domain has been also identified and described in CDPK1, CDPK3 and CDPK4 proteins that regulates the activity of the CDPKs and is connected to the carboxy-terminal calmodulin-like domain (Ahmed et al., 2012, Chandran et al., 2006, Ranjan et al., 2009, Wernimont et al., 2011, Wernimont et al., 2010). Plasmodium falciparum possesses 7 annotated CDPKs while Toxoplasma gondii possesses 12 CDPKs (Billker et al., 2009). Only 6 CDPKs (CDPK1, CDPK3, CDPK4, CDPK5, CDPK6 and CDPK7) are expressed and conserved in almost all apicomplexan parasites. Among the 6 well-conserved CDPKs, only 5 (CDPK1, CDPK3, CDPK4, CDPK5 and CDPK6) have been studied and their functions revealed. These CDPKs have been involved as a link between calcium signalling and differentiation, motility, invasion, and egress. Down regulation of TgCDPK1 protein resulted in loss of parasite motility, host cell invasion, and egress abilities (Lourido et al., 2010). In Plasmodium, CDPK1 regulates microneme discharge, erythrocyte invasion by merozoites, activates repressed mRNA to warrant appropriate and stage-specific protein expression, and is also playing a role in early schizont development (Azevedo et al., 2013, Bansal et al., 2012, Sebastian et al., 2012). In P. berghei, the knockout of PbCDPK3 drastically inhibits the ability of ookinetes to traverse the peritrophic membrane in the mosquito gut and stops oocysts production (Ishino et al., 2006, Siden-Kiamos et al., 2006). A pronounced defect was observed on egress upon disruption of TgCDPK3 and PfCDPK5 (Dvorin et al., 2010, Garrison et al., 2012, McCoy et al., 2012). In P. berghei gametocytes, genetic disruption of CDPK4 interrupted microgamete differentiation (Billker et al., 2004). Finally, PbCDPK6 controls the sporozoites switch from a migratory to an invasive mode (Coppi et al., 2007).
In the present study, we report the crucial contribution of Toxoplasma CDPK7 (a protein broadly conserved across the Apicomplexa phylum) in the early steps of parasite division, as depletion of TgCDPK7 led to a reduced number of vacuoles undergoing division. More precisely, it appears that TgCDPK7 participates in the correct positioning and partitioning of the centrosomes during parasite division. Centrosome defects most likely generated directly or indirectly several abnormalities in the cdpk7 mutant, such as the incorrect distribution of kinetochore and spindle pole proteins, the inaccurate positioning of the daughter cells during endodyogeny, the reduced numbers of vacuoles undergoing division, the asynchronisation of budding during parasite division and the atypical presence of dense material (that corresponds probably to chromatin) in the nucleus.
Results
TgCDPK7 is produced in the tachyzoites and its PH domain binds to monophosphate phosphoinositides
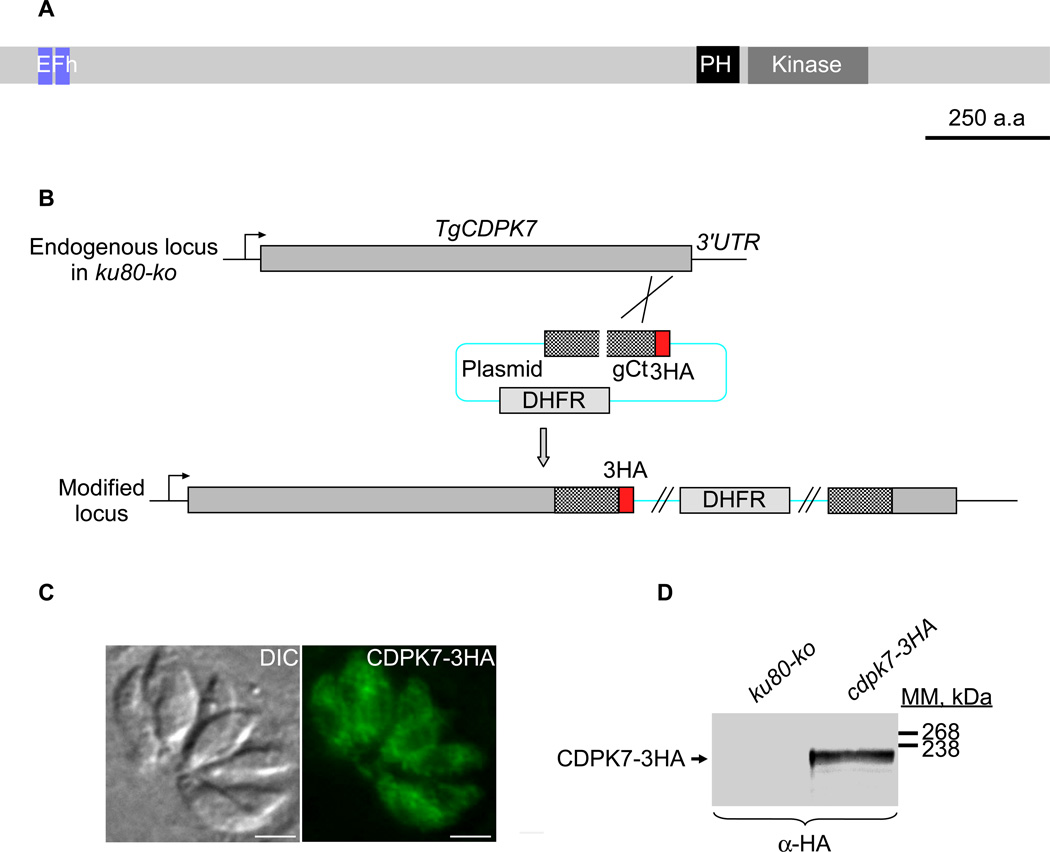
Among the 12 CDPKs present in T. gondii, TgCDPK1, TgCDPK3, TgCDPK4, TgCDPK5, TgCDPK6 and TgCDPK7 are the only 6 conserved across the phylum (Billker et al., 2009). TgCDPK7 protein has orthologs in all Apicomplexa (except Cryptosporidia), and also in alveolates (Miranda-Saavedra et al., 2012). T. gondii CDPK7 protein, in addition to the two calcium-binding EF-hand domains present at the beginning of the protein sequence, is harbouring a pleckstrin-homology domain (PH) just upstream its serine/threonine kinase domain found at the end of the protein sequence (Figure 1A). To localize TgCDPK7, a C-terminal triple epitope-tag was inserted in the open reading frame by single homologous recombination at the endogenous TgCDPK7 locus in the RH-ku80ko strain (Huynh et al., 2009) (Figure 1B). The resulting transgenic parasites expressed CDPK7-3HA of the expected mass (around 220 kDa) as assessed by western blot (Figure 1D) and localized as a diffuse cytoplasmic signal in intracellular or extracellular parasites as seen by immunofluorescence assay (IFA) (Figure 1C and data not shown). This cytoplasmic signal also locally appeared as punctuate, hinting that CDPK7 could be associated to vesicles, possibly via its PH domain (known to bind to phosphoinositides). To determine the potential interactions of TgCDPK7 PH domain with various phosphoinositides, we performed lipid dot-blot assays using recombinant wild type TgCDPK7 PH domain fused to a glutathione s-transferase (GST) protein and purified from bacteria (Supplementary figure 1A). The PH domain of TgCDPK7 protein was found to bind in vitro to three different phosphoinositides: PI(3)P, PI(4)P and PI(5)P (Supplementary figure 1B). A stronger intensity was observed for the binding of the PH-GST recombinant protein to the PI(4)P phosphoinositide (Supplementary figure 1B). No binding was detected for the recombinant GST protein used as negative control, for any of the phosphoinositides tested (Supplementary figure 1B).
Figure 1. Expression and localization of TgCDPK7 in tachyzoites.
(A) Schematic representation of Toxoplasma gondii CDPK7 (TgCDPK7) showing the domains of homology to EF hands, the pleckstrin homology (PH) and the kinase domains. All domains were predicted using SMART EMBL. Scale bar represents 250 aa. (B) Insertion of three HA epitope tags at the C-terminus of TgCDPK7, by single homologous recombination at the 3’ of the corresponding gene (knock-in in RH-ku80ko strain). (C) IFA performed on intracellular transgenic parasites using anti-HA antibodies. Scale bars represent 2 µm. (D) Western blot analysis performed on transgenic or RH-ku80ko parasite lysates probed with anti-HA antibodies. TgCDPK7-3HA is found at the expected molecular mass (220 kDa).
TgCDPK7 protein is essential for parasite survival and replication
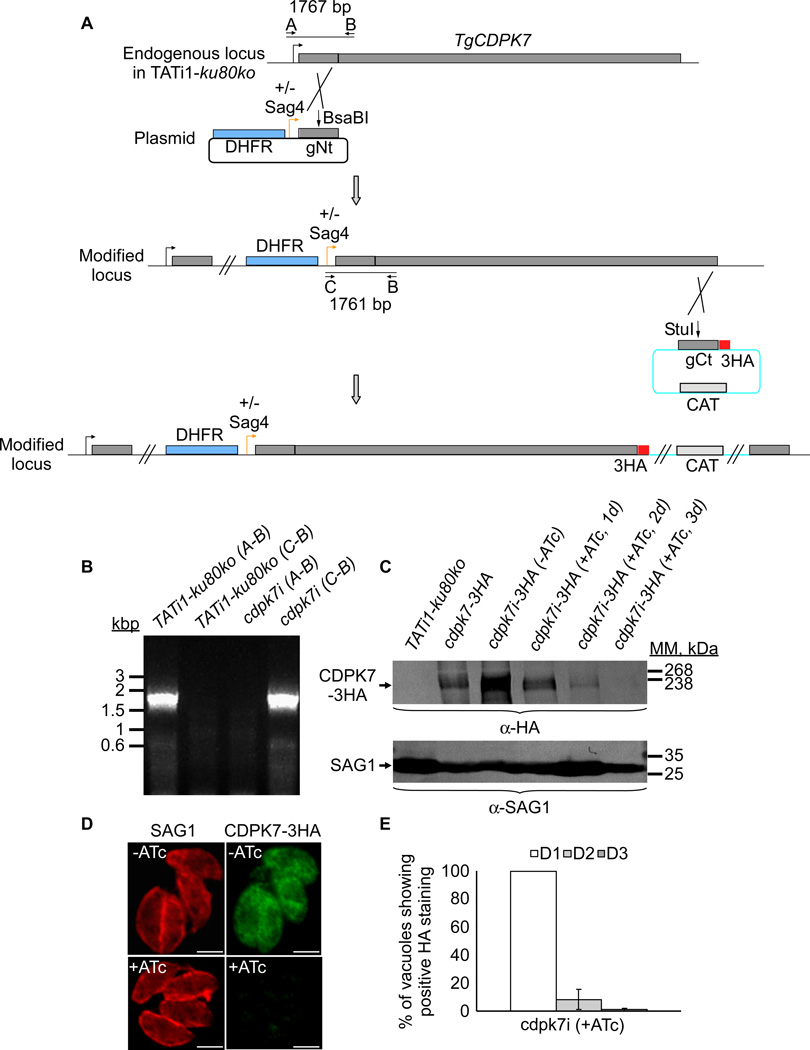
To investigate the function of the TgCDPK7 protein, conditional knockdown was attempted in the TATi1-ku80ko strain, using the tetracycline-based transactivator system previously developed for T. gondii (Meissner et al., 2001). The TATi1-ku80ko cell line that we use is a T. gondii type I strain impaired in non-homologous end joining to favour homologous recombination at a specific locus with high efficiency (Fox et al., 2009, Huynh et al., 2009). We opted for a promoter exchange approach (Figure 2A) instead of the two-step knockout strategy that requires the integration of a second inducible copy (Daher et al., 2010). Our strategy is based on a single homologous recombination in the TgCDPK7 locus, leading to the replacement of the TgCDPK7 promoter with the 7-tet-OpSag4-inducible promoter (Figure 2A). We generated a vector consisting of DHFR resistance cassette for selection, the tetO7-Sag4 inducible cassette, and finally the genomic NtCDPK7 (Figure 2A). Briefly, a region of genomic DNA corresponding to the NtCDPK7 sequence, starting from the annotated ATG start codon was cloned in the conditional vector (Figure 2A). The primers used to generate the vector are listed in the table 1. Parasites that successfully integrated the plasmid were isolated by applying pyrimethamine selection, and then cloned by limiting dilution. To confirm homologous integration of the plasmid and thus modification of the TgCDPK7 locus, a series of PCRs were performed on the genomic DNA isolated from selected clones (Figure 2B and supplementary table 1). Primers were chosen to assess the integration of the plasmid in the TgCDPK7 locus, and to check for the presence of wild-type locus (Figures 2A and 2B). The results showed that resulting Tgcdpk7i parasites do possess the correctly integrated plasmid (Figure 2B). One clone was thereafter selected for phenotypic analysis. To study the regulation of TgCDPK7 protein in presence or in absence of Anhydrotetracycline (ATc), a C-terminal triple epitope-tag (HA-tag) was inserted by single homologous recombination at the inducible TgCDPK7 locus in the Tgcdpk7i strain (Figure 2A). The chloramphenicol acetyltransferase resistance cassette was used to select transgenic parasites expressing the inducible TgCDPK7-3HA protein (Figure 2A). Total protein extracts of the corresponding transgenic parasites were analysed by western blot using anti-HA antibodies and the CDPK7-3HA protein was detected at its expected molecular mass (Figure 2C). As expected, CDPK7i-3HA localized to the cytoplasm of replicating (intracellular) parasites, as observed with the endogenous CDPK7-3HA protein (Figure 2D). To check for the regulation of CDPK7i-3HA expression in the presence or absence of ATc, total protein extracts of the transgenic parasites were analysed by western blot and IFAs using anti-HA antibodies and compared to the endogenous CDPK7 expression level (Figure 2C). Putting the CDPK7 gene under the control of the minimal Sag4 promoter causes an overexpression of the CDPK7i protein compared with endogenous CDPK7 levels, as revealed by western blot (Figure 2C). Assessing the expression of CDPK7i-3HA by western blotting and immunofluorescence assay in the presence of ATc revealed that the protein was no longer detectable after 72 hours of treatment (Figures 2C, 2D, and 2E), with already a marked decrease in CDPK7i-3HA expression at days 1 and 2. To compare the amount of CDPK7-3HA protein in RH-ku80ko or TATi1-ku80ko strains in presence or absence of ATc, a western blot analysis was performed by loading an equal amount of total protein extracts and the SAG1 protein was used as loading control (Figure 2C).
Figure 2. TgCDPK7 conditional knock-in by promoter exchange strategy.
(A) Schematic representation of the strategy used to replace the endogenous promoter of TgCDPK7 with the tetracycline inducible promoter. The DHFR-tetO7-SAG4NtgCDPK7 plasmid contains the dihydrofolate reductase (DHFR) gene (in blue) and the N-terminal genomic coding sequence of TgCDPK7 (in grey, 1455 bp) under the control of the inducible tetO7SAG4 promoter (Orange arrow). Black arrows represent the primers used for PCR analysis and the length of the PCR fragments generated is indicated. To study the regulation of TgCDPK7i gene, we inserted by single homologous recombination at the 3’ of the gene, a sequence coding for three HA epitope tags at the C-terminus of the corresponding TgCDPK7i protein. (B) PCR analysis performed on Tgcdpk7i, showing that single homologous recombination occurred. Genomic DNA from TATi1-ku80ko parasites was used as negative control. (C) Western blot analysis of TATi1-ku80ko and cdpk7i-3HA strains in presence or in absence of ATc. Parasites were treated for 1 or 2 or 3 days (d) with ATc. SAG1 was used as loading control. (D) Down regulation of TgCDPK7-3HA in the cdpk7i strain as shown by IFA with anti-HA antibodies 3 days after ATc treatment. Scale bars represent 2 µm. (E) Quantification of the percentage of vacuoles showing positive HA staining upon treatment with ATc at days 1 or 2 or 3 respectively using anti-HA antibodies.
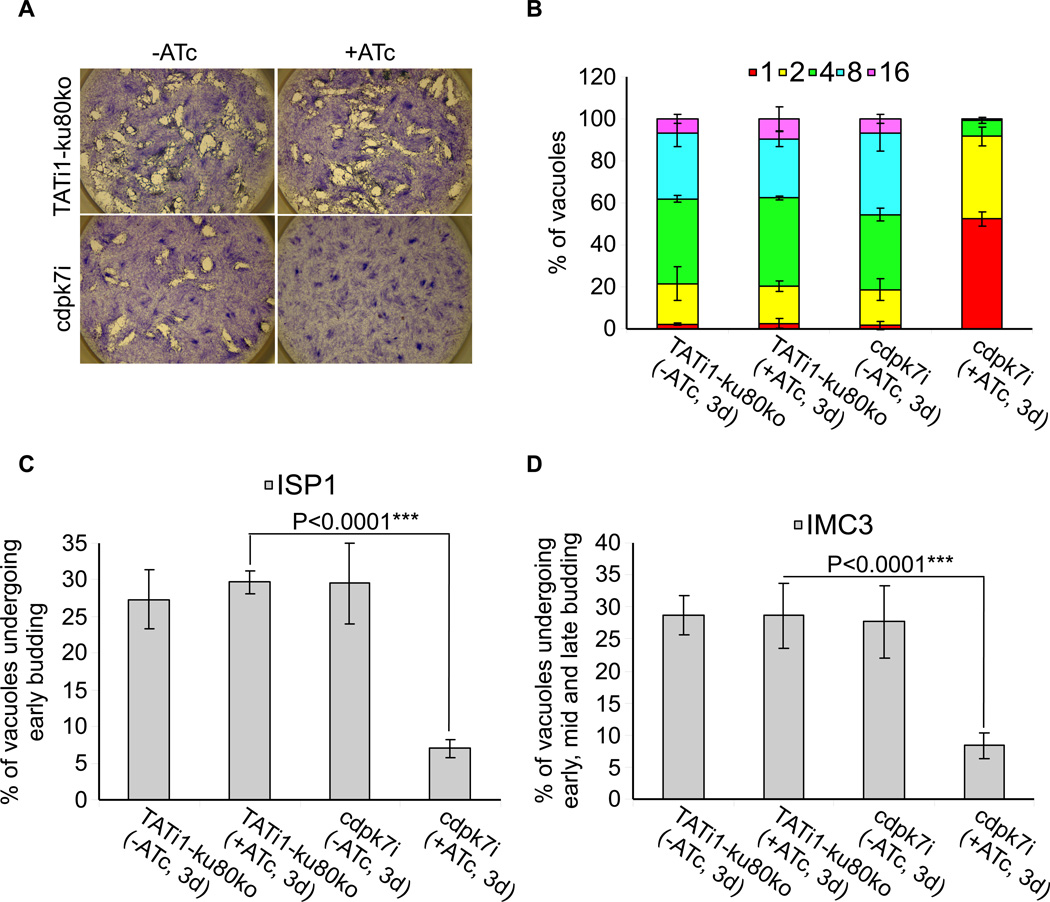
The phenotypic consequences of CDPK7 knock-down were first investigated by plaque assays. Fresh monolayers of HFF cells were infected with parasites in the presence or absence of 1.5 Hg/ml ATc for 7 days. The lytic cycle of the parasite is a multi-step process that involves invasion, several rounds of replication and egress. The plaque assay corresponds to plaques of lysis formed in a monolayer of HFF that recapitulates multiple lytic cycles over several days. When pre-treated (during 48 hours) cdpk7i parasites were depleted of CDPK7i-3HA by 7 days of ATc treatment, no plaques were formed, while non-treated cdpk7i parasites formed plaques similar in size to those of the TATi1-ku80ko recipient strain (Figure 3A). Thus, the plaque assay revealed that CDPK7 is critical for T. gondii parasite survival. We then investigated the role of CDPK7 during the different steps of the lytic cycle: invasion, egress, gliding motility and replication. We established that depletion in CDPK7 caused a modest but significant defect in invasion of 32% when compared to the control grown in the presence of ATc (Supplementary figure 2A). As microneme secretion is involved in invasion process, we then examined the ability of cdpk7i parasites to secrete the microneme protein 2 (MIC2) involved in adhesion (Carruthers et al., 1999b). After secretion onto the parasite surface, MIC2 is translocated to the cell posterior and shed from the parasite plasma membrane by proteolysis, allowing the detection of secreted MIC2 in the supernatant (Carruthers et al., 2000). MIC2 was detected in the supernatant of wild-type or cdpk7i parasites stimulated with ethanol (a potent secretagogue that may act through phospholipase C) demonstrating that in absence of CDPK7 protein, the parasites are still able to secrete the MIC2 protein (Carruthers et al., 1999a) (Supplementary figure 2B). The GRA1 protein was used as a loading control for the secreted proteins (Supplementary figure 2B). The importance of CDPK7 protein for parasite egress was also investigated upon addition of the calcium ionophore A23187. The cdpk7i parasites grown in the presence of ATc behaved like the wild type and rapidly egressed from host cells in response to treatment with calcium ionophore (Supplementary figure 2C). To monitor if parasites depleted in CDPK7 could still accomplish the three forms of gliding movement (helical gliding, circular gliding and twirling), trails deposited by moving parasites on coated Poly-Lysine cover slips were visualized by IFA. The cdpk7i strain showed a significant reduction in trail formation when compared to the control grown in the presence of ATc (Supplementary figure 2D).
Figure 3. Phenotypic consequences of TgCDPK7 depletion in cdpk7i strain.
(A) Plaque assay performed on HFF monolayer infected with TATi1-ku80ko or cdpk7i parasites pretreated first during 48 hours with ATc. After 7 days ± ATc, the HFF were stained with Giemsa. (B) Intracellular growth of TATi1-ku80ko and cdpk7i cultivated in presence or absence of ATc for 48 hours and allowed to invade new HFF cells. Numbers of parasites per vacuole (X axis) were counted 24 hours after inoculation. The percentages of vacuoles containing varying numbers of parasites are represented on the Y-axis. Values are means ± SD for three independent experiments. (C and D) Endodyogeny assay performed on TATi-ku80ko or cdpk7i strains cultivated in presence or absence of ATc for 48 hours and allowed to invade new HFF cells. Numbers of vacuoles showing the formation of newly formed buds (Y axis) were counted 24 hours after inoculation using anti-ISP1 or anti-IMC3 antibodies. Values are means ± SD for three independent experiments. Statistical significance was evaluated using the student’s t test. ***P<0.0001 (C, ISP1), ***P<0.0001 (D, IMC3).
Interestingly, however, TgCDPK7-depleted parasites showed a severe growth defect as compared to controls, and they did not progress through cell division as shown by the accumulation of vacuoles with only one or two parasites at day 3 (57% and 35% of the vacuoles contain 1 or 2 parasite(s) respectively) (Figure 3B). At day 1 the development of cdpk7i parasites was comparable to the wild type strain treated or not with ATc (Supplementary figure 3A). In contrast, we observed a strong defect in growth at day 2 that corresponded to the partial depletion of the CDPK7 protein (Supplementary figure 3B). At day 4 we observed no additional defect in parasite growth (Supplementary figure 3C). Quantification of the percentage of vacuoles undergoing endodyogeny after 3 days of treatment with ATc indicated that the cdpk7i strain was significantly impaired in initiation of daughter cells formation within the mother parasite (Figures 3C and 3D). The IMC Sub-compartment Protein 1 (ISP1) marking exclusively the apical cap of the IMC of both daughter and mother cells and the IMC Protein 3 (IMC3) staining mainly the newly formed daughter parasites, were used as markers to monitor the percentage of parasites undergoing endodyogeny (Anderson-White et al., 2011, Beck et al., 2010). After 2 days of ATc treatment, the percentage of vacuoles displaying endodyogeny markers is divided roughly by 2 (data not shown) and after 3 days around 8% of the vacuoles showed signs of division (Figures 3C and 3D). Taken together these results indicated a major role for TgCDPK7 in early steps of parasite division and growth.
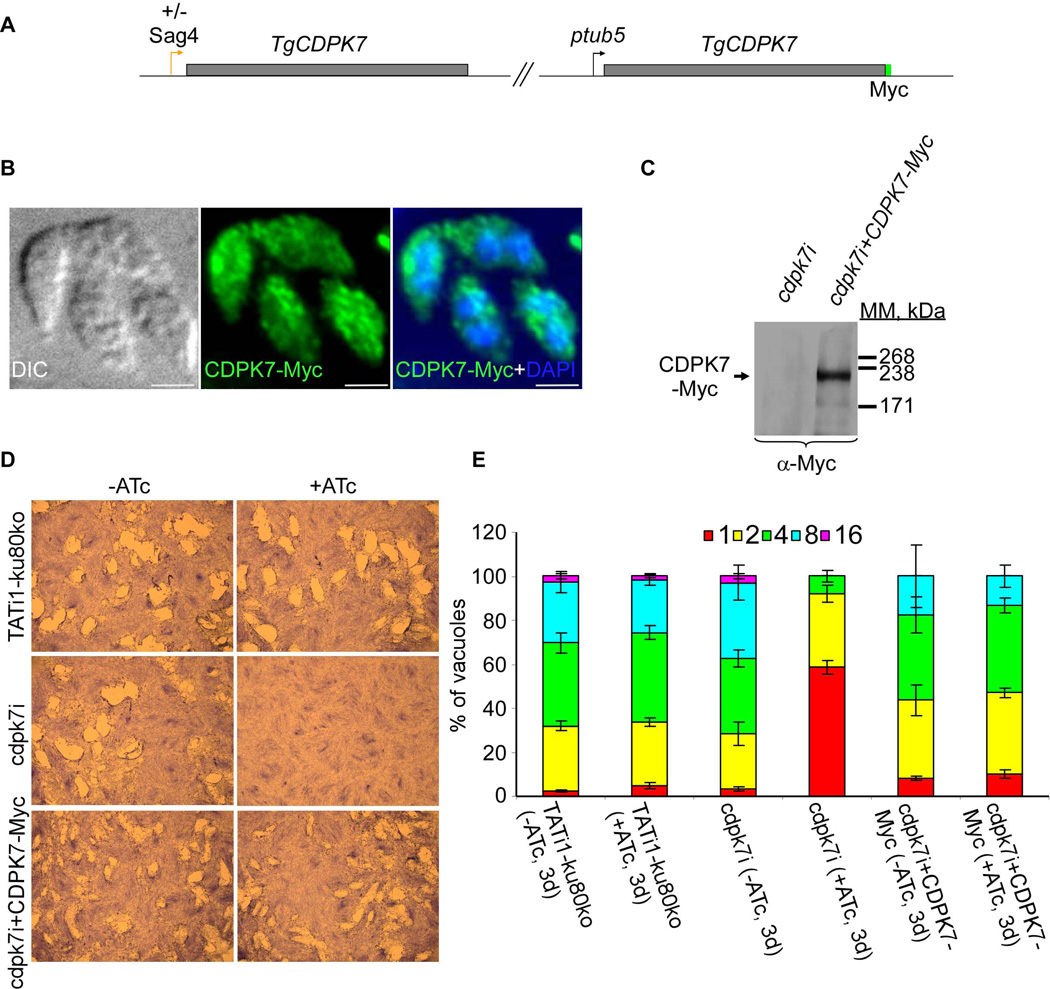
To confirm that TgCDPK7 alone is responsible for the phenotypic defect observed in cdpk7i, we complemented this strain by stably integrating a C-terminally Myc-tagged TgCDPK7 copy controlled by the tubulin promoter (Figure 4A). IFA analysis showed that CDPK7-Myc localization is comparable to the native protein cytosolic/vesicular dual localization (Figure 4B). The CDPK7-Myc protein was detectable in the complemented cdpk7i strain by western blot at the expected molecular mass (Figure 4C). Expression of CDPK7-Myc in complemented ATc-treated cdpk7i parasites restored the ability of parasites to form lysis plaques and to grow at a rate comparable to the wild type strain (Figures 4D and 4E). A slight defect on parasite growth was observed in the cdpk7i+CDPK7-Myc strain overexpressing the CDPK7 protein controlled by the tubulin promoter (Figure 4E) suggesting that overexpression of CDPK7 protein is toxic for parasites over a certain threshold.
Figure 4. Functional complementation of the cdpk7i strain.
(A) The scheme depicts the strategy used for complementation of cdpk7i parasites with CDPK7-Myc controlled by Tgtub5 promoter. (B) cdpk7i parasites stably expressing CDPK7-Myc were grown in presence of ATc during 3 days and visualized by IFA using anti-Myc antibodies. (C) Constitutive expression of integrated CDPK7-Myc in cdpk7i strain was confirmed by western blotting using anti-Myc antibodies. (D) Plaque assays were carried out by infecting HFF monolayers with TATi1-ku80ko or cdpk7i or cdpk7i stably expressing CDPK7-Myc for 7 days ± ATc. (E) Intracellular growth of TATi1-ku80ko, cdpk7i and cdpk7i stably expressing CDPK7-Myc cultivated in presence or absence of ATc for 48 hours and allowed to invade new HFF cells. Numbers of parasites per vacuole (X axis) were counted 24 hours after inoculation. The percentages of vacuoles containing varying numbers of parasites are represented on the Y-axis. Values are means ± SD for three independent experiments.
TgCDPK7 knockdown affects the orchestrated division of parasites and the polarity of the budding
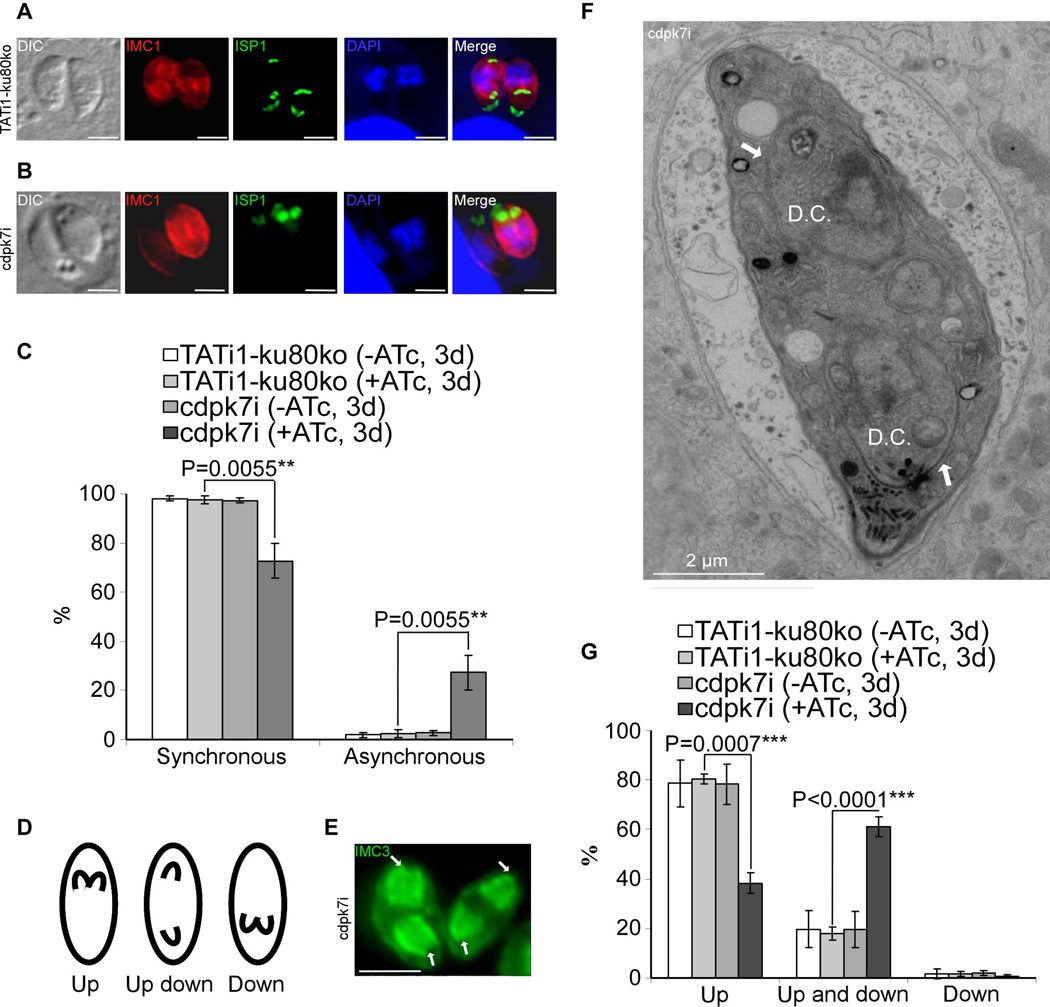
As a T. gondii parasite begins to divide, two daughter cells begin to develop synchronously in all the parasites present within a given vacuole, which are detectable by anti-IMC antibodies (Figures 5A and 5C). Intriguingly, in cdpk7-depleted parasites, only 72.6%±7 of the cells undergoing endodyogeny in a same vacuole were synchronized in the creation of new IMC buds (versus 97.6%±1.1 in the WT strain) (Figure 5C). In cdpk7i parasites treated with ATc, a significant percentage of vacuoles (27.3%±7) contained a pair of parasites with one parasite exhibiting newly formed IMCs, while the second one was devoid of these sub-cellular structures (Figures 5B, 5C and 6G).
Figure 5. cdpk7i mutant parasites display asynchronous division and reveal that TgCDPK7 contributes to the close positioning of daughter cells during division.
(A) and (B): IFA analysis of vacuoles containing 2 parasites undergoing division after 3 days of ATc treatment. (A): IFA of representative TATi1-ku80ko vacuole which was compared to IFA of representative cdpk7i vacuole, (B). (A) and (B): anti-IMC1 (in red) and anti-ISP1 (in green) antibodies, respectively, were used to detect the nascent buds. In (A) TATi1-ku80ko vacuole exhibits synchronous IMC buds, while in the cdpk7i vacuole shown in (B), the formation of IMC buds appears only in one parasite. (C) Scoring of synchronous and asynchronous dividing parasites containing vacuoles by IFA, using anti-IMC3 antibodies. Statistical significance was evaluated using the student’s t test. **P=0.0055 (synchronous), **P=0.0055 (asynchronous). (D) A scheme showing the three directions adopted by the IMC buds during parasite division. (E) IFA of representative cdpk7i parasites showing an up/down division (with anti-IMC3 antibodies staining), one nascent IMC grows towards the apical end, while the second buds towards the posterior pole of the parasite (white arrows). (F) Electron micrograph of a dividing cdpk7i parasite (treated with ATc during 3 days). Two daughters are assembled within a mother cell in up/down orientation (white arrows). D.C.: daughter cells. (G) Scoring of daughter cell orientation during parasite division by IFA using anti-ISP1 antibodies. Interference with TgCDPK7 function favoured the up/down topology whereas wild-type or untreated parasites adopted mainly the up topology. Scale bars represent 2 µm. Statistical significance was evaluated using the student’s t test. ***P=0.0007 (up), ***P<0.0001 (up and down).
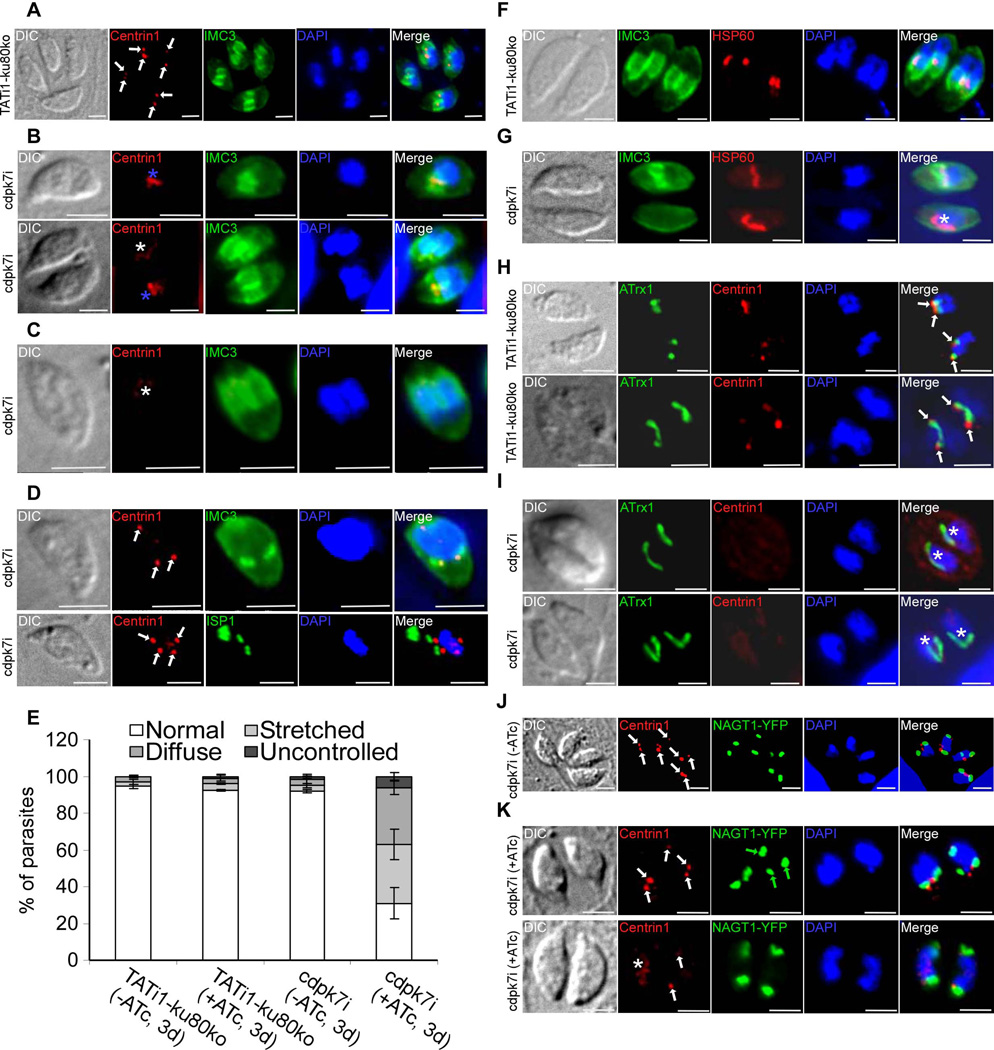
Figure 6. cdpk7i mutant parasites display centrosomal defects, reveal a plastid elongation independent of centrin 1 protein and IMC buds and show the dependency of the Golgi positioning and number with that of centrosome duplication.
A) to (D): IFA analysis of parasites undergoing division after 3 days of ATc treatment. (A) to (D): anti-centrin1 and anti-IMC3 or anti-ISP1 antibodies were used to stain the centrosome and daughter buds, respectively. (A) A representative TATi1-ku80ko vacuole containing 4 parasites is shown. The duplicated centrosomes (in red) in each parasite are marked with a white arrow. Each newly formed IMC (in green) encapsulates one centrosome. (B) to (D): Representative examples of cdpk7i parasites showing centrosomes or centrin 1 staining defects. (B) A stretched centrosome (blue asterisks) which is located in between two newly formed daughter buds (upper image) or partitioned without being split between the nascent IMCs (lower image) is shown. (C) A diffused centrin 1 staining (white asterisk) is shown. (D) Two parasites in division showing an abnormal number of centrosomes per nucleus (white arrows) (3 in the upper image and 4 in the lower picture). (E) Scoring of centrosome defects during parasite division by IFA using anti-centrin1 antibodies. Interference with TgCDPK7 function led to stretched centrosomes, affected the distribution of centrin 1 protein and impaired the regular number of centrosome duplication. Data are mean values ± SD for three independent experiments. (F) to (I): IFA analysis of vacuoles containing 2 parasites undergoing division after 3 days of ATc treatment. (F) and (H): IFAs of representative TATi1-ku80ko vacuoles which were compared to IFAs of representative cdpk7i vacuoles, (G) and (I). (F) and (G): anti-IMC3 and anti-HSP60 antibodies were used to stain the daughter buds and the apicoplast, respectively. (F): a TATi1-ku80ko vacuole with newly formed IMC (in green) encapsulating each apicoplast. (G) A cdpk7i vacuole exhibiting the formation of IMC buds in only one parasite out of two. In the parasite lacking the 2 IMC buds, the apicoplast adopts its elongated shape and lies on the nucleus (white asterisk). (H) and (I): anti-centrin 1 and anti-ATrx1 antibodies were used to stain the centrosome and the apicoplast, respectively. (H) The ends of the plastid in dividing apicoplasts of the TATi1-ku80ko parasites are consistently associated with the parasite’s centrosomes. This association is maintained even after apicoplast division (white arrows). (I) Two representative cdpk7i vacuoles showing the diffused staining of centrin 1 protein. In the parasite lacking a concentrated centrin 1 dotted-like staining, the apicoplast adopts its elongated shape and lies on the nucleus (white asterisk). (J) and (K): anti-centrin 1 antibodies and NAGT1-YFP were used to detect the centrosome and the Golgi, respectively. (J) The two centrosomes (white arrows) are found at the inner ends of the newly-divided Golgi (in green). (K) Two representative cdpk7i vacuoles treated with ATc showing an abnormal numbers of centrosomes and Golgi per nucleus (white and green arrows, upper image), and a diffuse staining of centrin 1 protein (white asterisk) or two centrosomes in up and down position (white arrows) (lower image). Scale bars represent 2 µm.
To gain more insights into the polarity of budding in the mutant parasites, we analysed by IFA the T. gondii IMC subcompartment protein 1 (TgISP1) and also the TgIMC3 proteins (Anderson-White et al., 2011, Beck et al., 2010). In wild-type parasites and under our culture conditions, most of the daughter cells (80.1%±6.4) grow towards the apical end of the mother cell (up orientation) (Figures 5D and 5G and supplementary figures 4B and 4C), 18.1%±5.4 were observed with opposite dividing daughter cells (up and down orientation) (Figures 5D, 5E, 5F and 5G) and finally 1.7%±1.3 of the dividing parasites show newly formed IMCs directed towards the basal end of the mother cell (down orientation) (Figures 5D and 5G). Interestingly, in cdpk7-depleted parasites, the proportion between up and up/down was affected, with 61%±4 of the parasites dividing in opposite directions and only 38.3%±4 in the same way (Figures 5D, 5E, 5F and 5G).
TgCDPK7 knockdown affects the partitioning and the number of centrosomes during parasite division
To further dissect the origin of the parasite cell cycle arrest, we performed a detailed phenotypic analysis using a battery of developmental markers in immunofluorescence. First, we stained the centrosomes and the budding IMC with anti-centrin1 and anti-IMC3 antibodies respectively. Combining these two markers allows the differentiation between dividing and non-dividing parasites. In T. gondii, duplication of the centrosome occurs in G1/S phase transition and is followed by the initiation of the budding late in S phase (Anderson-White et al., 2012). Once the components of the cytoskeleton begin to assemble apically to the duplicated centrosomes, each newly formed IMC encapsulates one centrosome (marked by white arrows in Figure 6A) (Anderson-White et al., 2012, Chen et al., 2013, Hu et al., 2002, Nishi et al., 2008). However, only around 30% of the cdpk7i parasites treated with ATc and undergoing endodyogeny are presenting a normal situation in the duplication of the centrosomes (versus 92% in the wild-type (WT) strain) (Figure 6E). In the cdpk7-depleted parasites and during division, the state of the centrosomes or the centrin1 staining are presenting abnormalities: around 32% of the centrosomes are stretched and found in between the two IMC buds (versus 4% in the WT strain), around 31% of the dividing parasites are showing a diffuse centrin1 labelling and not a dotted-like typical staining (versus 3% in the WT strain), and finally around 6% of the centrosomes are illustrating uncontrolled duplication (more than 2 centrosomes per nuclei are detected using anti-centrin1 antibodies) (versus 1% in the WT strain) (Figures 6B, 6C, 6D and 6E, respectively).
The diffuse centrin 1 staining does not affect apicoplast elongation
In T. gondii, the apicoplast division is closely linked to centrosome duplication, nucleus differentiation and IMC budding (Striepen et al., 2000). Daughter cell growth resulted in the formation of a U-shaped apicoplast, which at last underwent fission and partitioned between the two daughter cells in the apical zone of the nucleus (Figure 6F) (van Dooren et al., 2009). Interestingly, in cdpk7-depleted parasites where the vacuoles harboured a pair of asynchronous parasites, the apicoplast located in the parasite devoid of nascent daughter cells was able to elongate and lounge on one side along the nucleus (marked by white asterisk in Figure 6G). In addition, it has been shown that during parasite division, each apicoplast end is associated with one centrosome (marked by white arrows in Figure 6H) (Striepen et al., 2000). In cdpk7-depleted cells and more specifically in the parasites devoid of centrin1 dotted-like staining, the apicoplast again showed an elongated shape and lied along one side of the nucleus or looked like V-shape which is a characteristic of dividing apicoplasts (white asterisks) (Figure 6I). These observations suggest that the elongated shape of the apicoplast does not depend on the centrin 1 protein, or on the formation of nascent IMC buds.
Centrosomal defects affect the number and the positioning of the Golgi apparatus
The Golgi grows by a process of lateral extension followed by medial fission; one copy is then encapsulated into each daughter cell. When the duplicated centrosomes separated, they then migrated back to the apical end of the nucleus, associating most commonly with opposite ends of the dividing Golgi (Figures 6J and 6K, white arrows) (Hartmann et al., 2006, Pelletier et al., 2002). The association of the centrosomes with the inner ends of the newly-divided Golgi suggested a role in organellar segregation (Hartmann et al., 2006). We observed irregular number of Golgi apparatus in the parasites containing more than two centrosomes per nucleus. Indeed, three Golgi organelles were observed in association with the three centrosomes detected using anti-centrin1 antibodies (Figure 6K, upper image, green arrows). Interestingly, the up and down position of the centrosomes and the diffuse staining of centrin 1 (white asterisk) did not affect the duplication of the Golgi (Figure 6K, lower image). These observations suggest that CDPK7-depletion disrupts proper centrosome duplication which might result in abnormal Golgi positioning and number.
TgCDPK7 knockdown affects the distribution of MORN1 and Nuf2 proteins, two components of the centrocone and the kinetochore, respectively
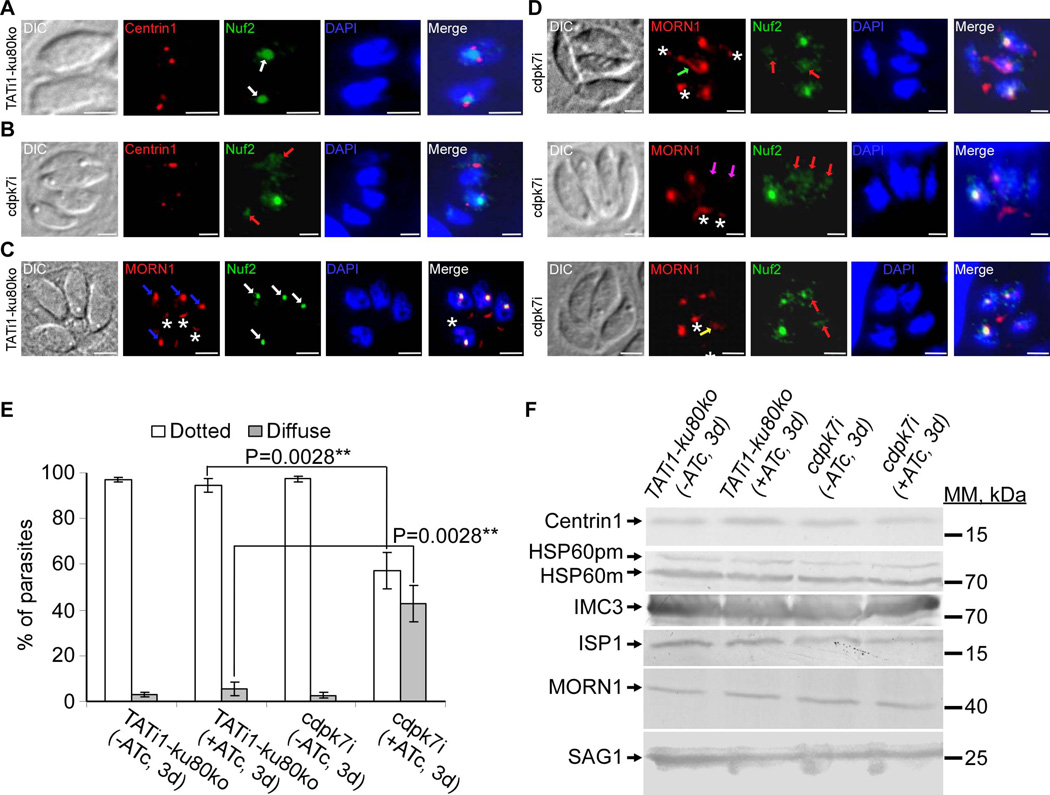
Having shown that the partitioning and/or the positioning of the centrosome are affected in the cdpk7-depleted parasites, we next wanted to check the status of the centrocone and kinetochore in the mutant. It has been shown that centromeres of T. gondii chromosomes are maintained in close proximity to the centrocone and centrosomes throughout the cell cycle of the parasite (Brooks et al., 2011). In T. gondii, DNA duplication occurs next to centrosome duplication and the condensed chromosomes are linked to the spindle microtubules via the kinetochore (Chan et al., 2005, Cheeseman et al., 2008). At least, two types of microtubules are found in the nucleus: those that extend from one centrosome to the other, and those that extend from the centrosomes to contact the kinetochores which are linked to centromeres. To study the kinetochore and the centrocone in the mutant, we decided to detect by IFA the native TgNuf2 protein (a conserved component of the kinetochore protein complex mediating contact with the microtubules, M. Farrell, and M. J. Gubbels, unpublished) and the double Myc-tagged TgMORN1 protein (MORN1 is associated with the spindle pole in the centrocone sub-cellular structure in T. gondii; (Gubbels et al., 2006)) expressed in the mutant under the control of its endogenous promoter. We next double-labelled parasites to compare Nuf2 localization with that of centrin1, a component of the centrosome, with MORN1, which is found in the spindle pole (blue arrows) as well as at the basal complex (white asterisks) (Figure 7C) (Anderson-White et al., 2011, Hu et al., 2006). Figures 7A and 7C show images representing wild-type parasites during cell division. During division we observed one Nuf2 spot (white arrows) that appeared in between the two duplicated centrosomes and close to markers of the spindle (Figures 7A and 7C). Interestingly, in a given vacuole of cdpk7-depleted parasites undergoing division, the characteristic Nuf2 spot was not detectable in all parasites, but a diffused or a fragmented Nuf2 staining was observed (red arrows) (Figures 7B and 7D). The diffused Nuf2 staining was seen in the parasites showing a stretched centrosome or a dispersed centrin 1 staining (Figure 7B). The MORN1 staining at the spindle pole was also impaired in cdpk7-depleted parasites, it appeared as an atypical scattered labelling (yellow arrow), or straight out undetectable (pink arrows), or emerged as a pipe shape linking the basal MORN1 staining to the spindle pole MORN1 localization (green arrow) (Figure 7D). In the vacuoles undergoing endodyogeny, quantification of the percentage of parasites with a diffuse Nuf2 staining represents 42.6%±8 of the parasites (versus 3.6%±2.1 in the WT strain) (Figure 7E). These observations suggested that CDPK7-depletion causes centrosome alterations and disruption sub-cellular structures of centrocone and kinetochore.
Figure 7. TgCDPK7 knockdown affects the distribution of MORN1 and Nuf2 proteins.
(A) to (D): IFA analysis of parasites undergoing division after 3 days of ATc treatment. (A) and (C): IFAs of representative TATi1-ku80ko parasites which were compared to IFAs of representative cdpk7i mutant parasites, (B) and (D). (A) and (B): anti-centrin1 (in red) and anti-Nuf2 (in green) antibodies were used to stain the centrosome and kinetochore respectively. (A) Two TATi1-ku80ko vacuoles were shown with each containing 1 parasite undergoing division. The kinetochore (in green) in each parasite is marked with a white arrow. TgNuf2 protein which is a component of the kinetochore complex is concentrated in the parasite nucleus and is flanked by two centrosomes during division (white arrows). (B) A cdpk7i vacuole showing the typical dotted-like staining of Nuf2 protein surrounded by a duplicated centrosome in only 1 parasite out of 4. In the parasites displaying a stretched centrosome or an undetectable centrin 1 labelling, the Nuf2 protein was found diffused throughout the nucleus (pink arrows). (C) and (D): anti-Myc (TATi1-ku80ko and cdpk7i parasites express a Myc tagged MORN1 protein) and anti-Nuf2 antibodies were used to label the spindle pole and kinetochore sub-cellular structures respectively. (C) A TATi1-ku80ko vacuole containing 4 parasites is shown. The kinetochore (in green) in each parasite is marked with a white arrow. TgNuf2 protein (white arrows) shows a partial colocalisation with MORN1 protein which is a component of the spindle pole structure located at the nuclear envelope of the parasite (blue arrows). The MORN1 protein marked also the basal end of the parasites (white asterisks). (D) IFA showed abnormal MORN1 protein distribution at the spindle pole in cdpk7i parasites displaying a diffused Nuf2 protein (pink arrows). The MORN1 protein was distributed in the form of a pipe (green arrow), was found undetectable (the two white arrows) or diffused (yellow arrow). Scale bars represent 2 µm. (E) Scoring of Nuf2 protein distribution defect by IFA using anti-Nuf2 antibodies. Data are mean values ± SD for three independent experiments. Statistical significance was evaluated using the student’s t test. **P=0.0028 (dotted), **P=0.0028 (diffuse). (F) Western blot analyses performed on mutant or TATi1-ku80ko parasite lysates probed with anti-centrin1 or anti-HSP60 or anti-IMC3 or anti-ISP1 or anti-Myc or anti-SAG1 antibodies. pm: pre-mature; m: mature.
To know if the level of expression of all division markers used in this study is affected in the mutant parasites, we performed western blot analyses using different antibodies allowing us to detect the following proteins: Centrin1, HSP60, IMC3, ISP1 and MORN1. The data we obtained showed that there are no obvious effects on the level of expression of those different proteins (Figure 7F). The SAG1 protein was used as loading control (Figure 7F).
TgCDPK7 knockdown leads to accumulation of intact secretory apical organelles in residual bodies and to the formation of abortive parasite bodies
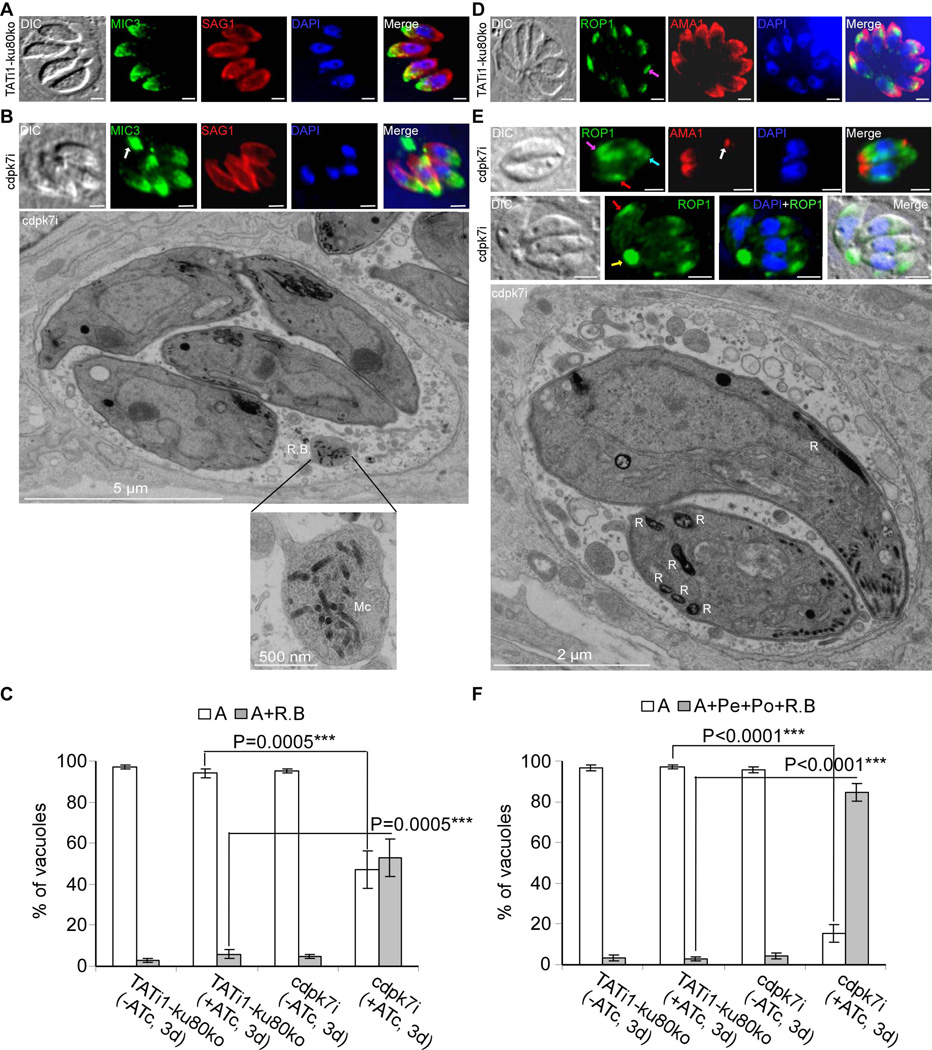
As CDPK7 protein depletion affected host cell entry, we decided to look at micronemes and rhoptries in the mutant, two organelles that are required for invasion. Micronemes and rhoptries, which are normally localized in the apical region of the wild type parasites (Figures 8A and 8D, respectively) were partially mislocalized in absence of CDPK7 (Figures 8B and 8E, respectively). Indeed, micronemes were found at the apex as well as accumulating in large residual bodies (white arrow) (Figure 8B) and rhoptries were found at the apex (pink arrows), accumulating in the residual bodies (yellow arrow) or dispersed at the periphery (red arrows) and at the basal end of the mother cells (blue arrow) (Figures 8D and 8E). Electron microscopy (EM) was used to confirm IFA observations and revealed the presence of intact micronemes in residual bodies and rhoptries at the periphery or at the basal pole of parasites (Figures 8B and 8E). Despite this, some microneme and rhoptry organelles are properly positioned to the apical pole of the parasite (Figures 8B, 8E and supplementary figures 4A and 4B). Quantification of the percentage of vacuoles containing specific micronemal protein 3 (MIC3) staining in residual bodies represents 53%±9.1 of the vacuoles in cdpk7-depleted parasites (versus 4.6%±1.8 in the WT strain) (Figure 8C). A more drastic defect was observed on the positioning of rhoptries in cdpk7-depleted parasites, 84.6%±4.5 of the vacuoles show a mis-positioning of rhoptry organelles, that were found dispersed at the periphery, or at the basal pole or in residual bodies (versus 3.5%±1.3 in the WT strain) (Figure 8F). Examining additional organelles by IFA in the cdpk7-depleted parasites revealed that dense granules and the mitochondrion were not affected, but we could not exclude a putative role of CDPK7 in maintaining these two organelles in case the ATc treatment of mutant parasites would not completely deplete the protein (Supplementary figure 5). In addition to the accumulation of organelles in residual bodies we also noticed the presence of unknown parasite bodies in some vacuoles. These unknown parasite body structures probably correspond to abortive immature parasites. As shown in Supplementary figures 6A and 6B, these unknown parasite bodies which are stained with IMC1 but not with ISP1 antibodies, were found within some vacuoles in the vacuolar space, either at the apical zone of the ISP1 staining of complete parasites (Supplementary figure 6A) or at the basal end of developing parasites (Supplementary figures 6B and 6C). It seems that these bodies are attached to the mature parasite as shown by EM in supplementary figure 6C. Quantification of the percentage of vacuoles harbouring these unknown parasite bodies represents 39.3%±4.5 of the vacuoles in cdpk7-depleted parasites (versus 4.2%±1.5 in the WT strain) (Supplementary figure 6D).
Figure 8. TgCDPK7 knockdown accumulates micronemes in residual bodies and impairs rhoptries positioning to the apical end of the parasite.
(A), (B), (D) and (E): IFA analysis of parasites after 3 days of ATc treatment. Scale bars represent 2 µm. (A) TATi1-ku80ko parasites showed a normal apical staining of MIC3 protein (in green). The SAG1 protein localizes at the parasite plasma membrane (in red). (B) cdpk7i parasites showed accumulation of micronemes in residual bodies (white arrow). Thin section electron micrographs were taken from cdpk7i that had grown for a total of 3 days in presence of ATc. A vacuole with 4 parasites is presented showing a residual body containing intact micronemes (Mc). Some micronemes and rhoptries were also found correctly localized to the parasite apical pole. Scale bar, 5 µm. (C) Scoring of vacuoles showing accumulation of MIC3 protein in residual bodies by IFA using anti-MIC3 antibodies. Data are mean values ± SD for three independent experiments. Statistical significance was evaluated using the student’s t test. ***P=0.0005 (A), ***P=0.0005 (A+ R.B). A: apical, R.B: residual body. (D) TATi1-ku80ko parasites showed a normal apical staining of ROP1 (a rhoptry marker, in green) (pink arrows) and AMA1 proteins (a microneme marker, in red). (E) In the cdpk7i parasites, rhoptries were dispersed at the periphery (red arrows), at the posterior ends (blue arrow) and in residual bodies (yellow arrow). Thin section electron micrographs depict rhoptry organelles (R) at the periphery or at the posterior ends of cdpk7i parasites that had grown for a total of 3 days with ATc. Scale bar, 2 µm. (F) Scoring of vacuoles showing abnormal distribution of ROP1 protein at the periphery, at the posterior ends and in residual bodies by IFA using anti-ROP1 antibodies. Data are mean values ± SD for three independent experiments. Statistical significance was evaluated using the student’s t test. ***P<0.0001 (A), ***P<0.0001 (A+ Pe + Po + R.B). A: apical, Pe: periphery, Po: Posterior ends, R.B: residual body.
Finally, a closer examination of the nucleus by EM in cdpk7-depleted parasites revealed the accumulation of a large mass of dense material in the nuclei (Supplementary figure 6E). To examine whether these morphological changes are associated with changes in the amount of DNA, we performed DNA content analysis on cdpk7-depleted parasites. As shown in supplementary figure 7, after 96 hours incubation with ATc, cdpk7-depleted cells showed a minor defect in DNA replication: the two peaks representing 1N and 1.8N containing population are broader compared to wild type controls, and a slight increase of parasites in S phase was observed. This suggests that the vast majority of the cdpk7-depleted cells do not arrest by a natural checkpoint. We also performed FACS analysis using parasites incubated with ATc in a shorter period of time (three days) to capture any phenotype developed within three-day incubation of ATc, and observed not much difference of DNA content compared to four-day incubation samples. We also collected the parasites with larger pore polycarbonate filter to avoid separation of big abnormal parasites containing multi-N DNA, and again, no obvious change in DNA content comparing to parental line and cdpk7-depleted parasites. These results suggest CDPK7 is not involved in a cell cycle checkpoint.
Discussion
This study establishes that TgCDPK7 plays a crucial role in parasite division. TgCDPK7 knockdown alters the partitioning and the positioning of the centrosomes, two early steps required not only for a successful coordination between mitosis and cytokinesis but also for the polarity of nascent IMC budding.
TgCDPK7 is a high molecular mass protein and possesses a PH domain which is absent in the other Apicomplexa CDPKs. Unlike the canonical domains architecture previously described (Billker et al., 2009), the CDPK7 contains 2 N-terminally EF hands (instead of being downstream of the kinase domain) and a PH domain followed by a putative catalytic kinase domain located at the end of the protein sequence. In between the kinase domain and the EF hand motifs, an autoinhibitory (or a junction) domain was reported for other CDPKs (Azevedo et al., 2013, Ranjan et al., 2009). In the absence of calcium, a part of the junction domain occupies the catalytic site, keeping the kinase in an inactive state. The binding of calcium to the calmodulin like domain (CLD) promotes the release of the pseudo-substrate region from the catalytic cleft and favours the interaction between the kinase and its substrate(s) (Ranjan et al., 2009). Putative regulatory CDPKs elements corresponding to the pseudo-substrate region (NΦR/KxΦ) and CLD consensus sequence (KLxxΦAΦxxΦAxxΦ) motifs are absent from TgCDPK7 protein sequence (data not shown). Unlike other described CDPKs, the TgCDPK7 protein sequence that separates the EF hands and the kinase domain and includes the PH domain (1387 amino acids) is extremely long (representing more than the half of the TgCDPK7 protein). To establish a relationship between TgCDPK7 uncommon structure and it(s) function(s) as well as its regulation, further investigations are required.
Since CDPK7 has a PH domain (known to interact with phosphoinositides, (Kutateladze, 2010), it implies that this putative kinase might have additional functions in the intracellular trafficking. Protein lipid overlay assays showed that the TgCDPK7-PH domain interacts with three phosphoinositides: PI(3)P, PI(4)P and PI(5)P. Our unpublished work has shown that disruption of lipid kinases for producing PI(3)P and PI(5)P leads to phenotypes (W. Daher et al, in preparation) which were different from the one described upon depletion of the CDPK7 protein. Thus, to which extent the lipid interaction domain of CDPK7 is involved in its function in cell division is currently unknown.
Among all already studied calcium dependent protein kinases, CDPK7 is the only one showing a direct role in parasite division. Our results demonstrated that CDPK7 knockdown causes a significant reduction in the percentage of the parasites which can initiate the formation of IMC buds (the percentage drops at day 3 from 29% to 8%), and that the centrosome was impaired during division. Indeed the centrosome was stretched between or in between two nascent IMCs, or present in several copies by nucleus, or finally seems to disappear (centrin1 staining was diffuse). In addition, IFA using different division markers revealed that the daughter cells adopt an atypical orientation during endodyogeny. Thus, in absence of TgCDPK7, the majority of daughter cells grow on opposite sides of the nucleus instead of developing side by side towards the apical tip of the mother cell, as observed in wild-type parasites. In T. gondii, at the beginning of cell division, centrosomes were reported to migrate to the basal end of the nucleus, divide and move back to the apical end and re-associate with the Golgi (Hartmann et al., 2006). Recently, it was formally established that the centrosome provides the spatial platform for daughter assembly, which is consistent with our results (Chen et al., 2013). In absence of CDPK7, normal paired centrosomes were observed in thirty percent of dividing parasites, meaning that this putative kinase would play a role in centrosome duplication. Many kinases have also been reported in the literature as being key players involved in centrosome disengagement (Aki1 and Plk1 kinases) or separation (Nek2A, Aurora A and Plk1 kinases) or maturation (Aurora A and Plk1 kinases) or duplication (CDK2, MPS1 and Plk4) or inhibiting centrosome overduplication (CDK2, MPS1 and Chk1 kinases) (Brownlee et al., 2012, Mardin et al., 2012). Recently it was shown that the Toxoplasma ortholog of NsNek2 is TgNek1 and is required for centrosome splitting (Chen et al., 2013). We might consider the CDPK7 as a new putative kinase class implied in the control of the centrosome cycle in T. gondii parasite. Further investigations are now needed to understand the role of CDPK7 in centrosome duplication and its biology.
Regarding the asynchronisation observed in the mutant parasites, it is still premature to say if it is due to centrosome abnormalities and/or simply to suboptimal conditions upon depletion of CDPK7 protein. Indeed, our data revealed that among the 8% of the vacuoles showing signs of division, 27% are asynchronous. In the light of all division defects discussed previously, CDPK7 could be a key kinase controlling one or more step(s) during centrosome duplication and also involved in the checkpoint required for the initiation of nascent IMC buds (at the end of the S phase).
In T. gondii tachyzoites, the apicoplast is encapsulated within the two newly formed IMC buds and elongates with both extremities apparently associated to the closely positioned centrosomes (Striepen et al., 2000). As it was shown in figures 6G and 6I, in absence of newly formed IMC buds or of a dotted-like centrin1 staining, the apicoplast is still able to lengthen. Thus, it seems that this elongation does not depend on IMC budding or on centrin 1 protein. Various hypothesis can explain our observations: (i) the apicoplast does not require the centrin 1, but another component of the centrosome and, although the centrosome integrity is probably affected, its architecture would be maintained and sufficient to allow the inheritance of the apicoplast, (ii) in the mutant parasites, a fraction of the diffused centrin 1 staining could be associated to and thus maintain centrosome sub-cellular structure necessary to the division of the apicoplast, (iii) the centrin 2 and 3 are also present at the centrosome and other centrins are encoded by Toxoplasma genome (Hu, 2008) (M.J. Gubbels, unpublished), hitherto we cannot exclude their role either in centrosome duplication and/or in apicoplast elongation and fission. The diffuse centrin 1 staining may suggest that this protein is a direct or indirect putative substrate of the CDPK7 and that a phosphorylation defect may hamper its localisation at the centrosome. Indeed, it has been shown that a phosphorylation defect of centrin by the PKA in Hela cells affects centrosome duplication (Lutz et al., 2001). Further experiments are required to identify the component(s) of the centrosome involved in the anchoring of the apicoplast for guiding its elongation and inheritance.
Knockdown of CDPK7 also affects the distribution of both Nuf2 and MORN1 proteins, two components of the kinetochore and the spindle pole respectively (Gubbels et al., 2006) (M. Farrell, and M. J. Gubbels, unpublished). The centrocone is composed of the spindle pole, the nuclear envelope and the nuclear microtubules (passing through the nuclear envelope) (Brooks et al., 2011). This sub-cellular structure is closely associated to both the centrosomes and kinetochores via microtubules (Dubremetz, 1973, Ferguson et al., 2008, Gerald et al., 2011, Hu et al., 2006, Nabetani et al., 2001, Schrevel et al., 1977, Vaishnava et al., 2005). Thus, in cdpk7-depleted parasites, centrosome defects may compromise the mitotic structures, as illustrated by the abnormal distribution of MORN1 and Nuf2 proteins, while, interestingly, DNA synthesis is not affected, suggesting that there is a checkpoint activated to prevent re-entry in S-phase and preventing the accumulation of polyploid DNA.
In accordance with this hypothesis and as shown in supplementary figure 6, a significant percentage of vacuoles accumulated unknown parasite bodies, thus highlighting abortive or unsuccessful rounds of division. In addition we observed the accumulation of nuclear dense material at one side of the nucleus (Supplementary figure 6E). Further experiments are required to determine if this dense material may correspond to condensed and duplicated DNA. However, the DNA content analysis shows a nearly intact cell cycle pattern in cdpk7 depleted parasites (Supplementary figure 7).
In mammalian cells, the centrosome duplication cycle is tightly linked to the cell division cycle. T. gondii deviates from this rule as many temperature sensitive (ts) cell cycle mutants show an increase in ploidity in the absence of daughter budding (Gubbels et al., 2008a, Gubbels et al., 2008b). However, the increase in ploidity is accompanied by a corresponding increase in centrosome number, indicating a tight connection between centrosome number and the DNA replication cycle (Chen et al., 2013, Gubbels et al., 2008b). As shown here, cdpk7 depleted parasites show a defect in centrosome position and partition, and the DNA replication cycle remains intact. This suggests that the communication with the centrosome can fail at any point in the cell cycle. This is consistent with observations in a TgNek1 ts-mutant with defective centrosome splitting but intact centrosome duplication, wherein a > 2N population accumulates along with progression of the cell cycle (Chen et al., 2013). Even though cdpk7 depleted cells adhere to the “centrosome number keeps pace with ploidity rule” it is unusual that they do not arrest in a particular part of the cell cycle, which is likely caused by disintegration of the entire centrosome and losing it as a signalling hub. Moreover, the Golgi defects observed in cdpk7-depleted cells was not observed in the ts-TgNek1 parasites, indicating the Golgi divides independently of centrosome segregation (C.T. Chen, and M.J. Gubbels, unpublished). In short, these results suggest that the centrosome serves as a signalling hub and coordinates, directly or indirectly, the replication of the Golgi apparatus, DNA replication, and cell cycle progression.
TgCDPK7 knockdown also alters proper positioning of rhoptries, and to a lesser extent the micronemes that accumulate in residual bodies. The mis-positioning of rhoptries organelles to the apex or their accumulation with micronemes in the residual bodies suggests an involvement of TgCDPK7 in either anchoring or trafficking of these two secretory organelles. It is also possible that, whereas the rhoptries and micronemes are nevertheless formed, the disorganization of the buds lead to secretory orgenelles anarchistic dispersion. Regardless of the accumulation of micronemes and rhoptries in residual bodies, conditional perturbation of TgCDPK7 function altered modestly but significantly on host cell invasion (a modest defect on invasion was observed, supplementary figure 2A), suggesting that enough organelles reach the apical pole to fulfil their function.
TgCDPK7 appears to carry out multiple roles such as the successful duplication and the correct positioning of the centrosomes at the onset of parasite division and also the apical transport and/or anchoring of secretory organelles. Identification of TgCDPK7 substrates will be helpful to scrutinize mechanistically the mode of action of this putative kinase which is crucial for probably most of the apicomplexan parasites. An interesting strategy based on the exploitation of the kinase gatekeeper residue in presence of ATPγS was recently developed to identify parasite CDPKs substrates (Lourido et al., 2013). This strategy could be used and would potentially help us explaining the complex broad range phenotype we observed upon depletion of the putative CDPK7 kinase. Furthermore, we cannot exclude possible crosstalk between CDPK7 and other non-identified kinases and phosphatases involved in the control of parasite division.
CDPK7 represents an interesting model to study the link between calcium signalling and parasite division in apicomplexans. Moreover, because this putative kinase only exists in Apicomplexa, this makes it an attractive target to develop drugs against T. gondii and related apicomplexan parasites.
Experimental procedures
Parasites culture
T. gondii RH strains RH-ku80ko (Huynh et al., 2009) and TATi1-ku80ko (Sheiner et al., 2011) were grown in human foreskin fibroblasts (HFF) maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; GIBCO, Invitrogen) supplemented with 5% fetal calf serum and 2 mM glutamine. Selections of transgenic parasites were performed with chloramphenicol for CAT selection (Kim et al., 1993); pyrimethamine for DHFR-TS selection (Donald et al., 1993) and anhydrotetracycline (ATc) at 1.5 µg/ml for the inducible system (Meissner et al., 2001).
Toxoplasma and E. coli vectors
Primers used in this study are listed in table S1. A 1584 bp fragment corresponding to the 3’ of TgCDPK7 was amplified from genomic DNA and cloned into LIC-DHFR-3HA or LIC-CAT-3HA vectors (Huynh et al., 2009). 40 µg of these plasmids were digested by NcoI and StuI respectively, prior to transfection. A 1455 bp fragment corresponding to the 5’ of the coding region (downstream of the codon corresponding to the first predicted in-frame methionine residue) was amplified by PCR from T. gondii genomic DNA and then cloned in the DHFR-TetO7SAG4 plasmid between BglII and NotI restriction sites (Sheiner et al., 2011) downstream the DHFR selection marker, tetO7 tet operator and pSAG4 promoter. This construct was linearised by BsaBI prior to transfection. Transfected parasites were selected with pyrimethamine and cloned by limit dilution. Positive clones were verified by PCR to detect the native locus or the single homologous recombination of the inducible vector in CDPK7 locus. To obtain a complemented cell line, we transfected the cdpk7i strain with 100 µg of the ptub5-CDPK7Myc-SAGCAT vector. The CDPK7 open reading frame (6402 bp) was amplified by PCR from cDNA and then cloned in fusion with a Myc tag in the pMORN1-Myc(2)MORN1-SAGCAT vector between BglII and AscI restriction sites. The pMORN1 promoter was removed from the pMORN1-CDPK7Myc-SAGCAT vector using PmeI and BglII restriction sites and then exchanged by the ptub5 promoter to generate the ptub5-CDPK7Myc-SAGCAT construct.
To study the localization of MORN1 protein, the cdpk7i strain was transfected with 100 µg of pMORN1-Myc(2)MORN1-SAGCAT vector. To study the morphology of the Golgi apparatus, the cdpk7i strain was transfected with 100 µg of NAGT1-YFP-SAGCAT vector (Pelletier et al., 2002).
The bacterial expression was achieved by insertion of CDPK7-PH domain (324 bp) between EcoRI and NotI in pGEX4T3 vector. The GST alone or the GST-TgCDPK7PH recombinant proteins were purified on Glutathione Sepharose beads according to manufacturer’s instructions and then assessed by SDS-PAGE and Coomassie Blue staining.
Generation of transgenic T. gondii
LIC-DHFR-CDPK7Ctg-3HA construct was transfected in the RH-ku80ko strain using 40 µg of vector linearized by NcoI and subjected to pyrimethamine selection (Fox et al., 2009, Huynh et al., 2009). LIC-CAT-CDPK7Ctg-3HA construct was transfected in the cdpk7i strain using 40 µg of vector linearized by StuI and subjected to chloramphenicol selection. pMORN1-Myc(2)MORN1-SAGCAT, NAGT1-YFP-SAGCAT and ptub5-CDPK7Myc-SAGCAT constructs were transfected in the cdpk7i strain using 100 µg of circular vectors and subjected to chloramphenicol selection. DHFR-tetO7-SAG4-NtgCDPK7 inducible construct was transfected in the TATi1-ku80ko strain using 40 µg of vector linearized by BsaBI and subjected to pyrimethamine selection (Sheiner et al., 2011).
Proteins detection by western blot
To detect TgCDPK7-3HA or TgCDPK7-Myc, SAG1 or GRA1, MIC2, HSP60 or IMC3 or Myc-MORN1, Centrin1 or ISP1 proteins, parasite lysates were fractionated on 6%, 12%, 8%, 10% and 15% acrylamide gels respectively prior to detection. Separated proteins were transferred to nitrocellulose membranes and probed with appropriate antibodies in 5% non-fat milk powder in TNT buffer (50 mM Tris pH 8.0; 150 mM NaCl; 0.05% Tween20). The primary antibodies used for detection and their respective dilutions were: rat anti-HA antibodies (Roche) at 1/300, mouse anti-Myc antibodies (SANTA CRUZ BIOTECHNOLOGY) at 1/100, rabbit polyclonal anti-TgSAG1 at 1/1000 (Daher et al., 2010), mouse monoclonal anti-GRA1 antibodies at 1/500 (Charif et al., 1990), mouse monoclonal anti-MIC2 antibodies at 1/100 (Achbarou et al., 1991). Bound secondary conjugated antibodies were visualized using either the ECL system (Amersham Corp) or using alkaline phosphatase kit according to manufacturer’s instructions (Promega).
Fluorescent staining of cells
Briefly, for IFAs of intracellular or extracellular parasites, infected confluent HFF monolayers were fixed for 20 min in 4% paraformaldehyde in PBS, permeabilized with 0.2% triton X-100, blocked with 10% FCS in PBS, and then incubated with primary antibodies (anti-HA (Roche) 1:100, anti-SAG1 1:1000 (Daher et al., 2010), anti-IMC3 1:500 (Anderson-White et al., 2011), anti-centrin 1:500 (kindly provided by Dr Iain Cheeseman), anti-IMC1 1:1000 (Mann et al., 2001), anti-ISP1 1:1000 (Beck et al., 2010), anti-HSP60 1:2000 (Agrawal et al., 2009), anti-ATrx1 1:1000 (kindly provided by Dr Peter Bradley), anti-Nuf2 1:2000 (TGME49_309380) (M. Farrell, and M. J. Gubbels, unpublished), anti-Myc 1:100 (SANTA CRUZ BIOTECHNOLOGY), anti-MIC3 1:500 (El Hajj et al., 2008), anti-ROP1 1:1000 (Soldati et al., 1998), anti-AMA1 1:1000 (Lamarque et al., 2011), anti-GRA1 1:500 (Charif et al., 1990), anti-GRA2 1:500 (Achbarou et al., 1991), anti-mitochondrial F1 beta ATPase (P. Bradley, unpublished)) followed by goat-anti-rabbit or goat-anti-mouse or goat-anti-Guinea pig immunoglobulin G (IgG) conjugated to Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, Invitrogen). Coverslips were mounted onto microscope slides using Immu-mount (Calbiochem). Samples were observed with a Zeiss Axioimager epifluorescence microscope equipped with an apotome and a Zeiss Axiocam MRmCCD camera driven by the Axiovision software (Zeiss), at the Montpellier RIO imaging facility.
Fat blot
The protocol to produce lipid–arrays was developed from (Dowler et al., 2000). Briefly, lipids were dissolved in adequate solvent mixture (chlorophorm/methanol/water 1:1:0.8, by volume) and 1 Hl of lipid solution containing 200 pmoles of each phosphoinositide was spotted on to nitrocellulose membrane (PROTRAN BA 85, GE Healthcare) and allowed to dry at room temperature. All the samples were spotted in quadruplicate and the arrays were stored at 4°C. The membrane was blocked overnight in blocking buffer (3% fatty-acid-free BSA in TNT buffer) and then incubated for 4 h with the bound GST-fusion proteins (1 Hg/ml) at room temperature. The arrays were then washed 5 times with TNT and then incubated for 1h with 1/100 dilution of anti-GST monoclonal antibody (Sigma). The membranes were washed 5 times with TNT and then incubated for 1h with 1/7000 dilution of goat anti-rat IgG alkaline phosphatase conjugate (Promega).
Electron Microscopy
Parasites were pretreated for 48 hours with or without 1.5 Hg/ml ATc, collected promptly after egress and inoculated onto new HFF monolayers in presence of ATc during 24 hours. Infected HFF monolayers on coverslips were fixed for 4 hours at room temperature with 2.5% glutaraldehyde (EMS) in 0.1M phosphate buffer pH7.2, washed in buffer and post-fixed for 1 hour in 1% OsO4, washed in water and stained overnight in 2% uranylacetate. Coverslips were then dehydrated in ethanol series and embedded in Epon (Embed 812, EMS). Ultrathin sections were prepared with a Leica ultracut E microtome, contrasted with 2% uranylacetate in ethanol and lead citrate and observed with a JEOL 1200E electron microscope.
DNA content analysis
The protocol was modified from (Jammallo et al., 2011). Low numbers of TATi1-ku80ko and cdpk7i tachyzoites were seeded and grown in the presence and absence of ATc for four days. The extracellular parasites were removed by 1X PBS wash, and only the intracellular parasites were collected. Parasites were filtered through a 12 µm pore polycarbonate filter (Millipore) after needle passage to release them from the host cell. After 24 hour fixation with methanol at −20°C, DNA was stained with Sytox green and RNA removed by RNase treatment followed by analysis on a FACSCanto flow cytometer (Becton Dickinson) with FITC filter set. Data were analysed using FloJo software (Treestar).
MIC2 secretion assay
Parasites were pretreated for 24 hours with or without 1.5 Hg/ml ATc, collected promptly after egress and inoculated onto new HFF monolayers in presence of ATc during 48 hours. T. gondii tachyzoites freshly lysed from their host cells were harvested by centrifugation and washed twice in IM (Dulbecco’s modified Eagle’s medium, 3% fetal bovine serum, 10 mM HEPES) prewarmed to 37 °C. A pellet of 5.108 parasites was resuspended in 1 ml of IM, and an aliquot of 50 µl was taken as reference standard to allow estimation of the degree of secretion, and microneme secretion was stimulated by adding 10 µl of 100% EtOH to the remaining 950 µl (Carruthers et al., 1999b). The sample was incubated for 10 min at room temperature, then 40 min at 37 °C, and finally cooled down to 0°C in an ice-water bath for 5 min. Parasites were pelleted at 4 °C and then the supernatant was transferred to a new tube and centrifuged again at 4°C. Pellet and supernatant samples were then analyzed by western blot, probing with indicated antibodies.
Plaque assay
Fresh monolayers of HFF on circular coverslips were infected with parasites in the presence or absence of 1.5 Hg/ml ATc for 7 days. Fixation, staining and visualization were performed as previously described (Daher et al., 2010).
Intracellular growth, endodyogeny and Daughter cell orientation assays
Parasites were pretreated for 48 hours with or without 1.5 Hg/ml ATc, collected promptly after egress and inoculated onto new HFF monolayers in presence of ATc during 24 hours. 24 hours later, culture was fixed with PFA and staining with anti-TgSAG1. The numbers of parasites per vacuole were counted for more than 300 vacuoles for each condition. Replication defect was determined by staining of the nascent apical cones of the mother and daughter parasites (anti-ISP1 antibodies). Asynchronicity was evaluated by labelling of the IMCs of the mother and daughter cells (anti-IMC3 antibodies). Formation of rosette structures and daughter cell orientation was assessed using anti-ISP1 or anti-IMC3 antibodies. Data are mean values±s.d. from three independent biological experiments. For each condition, 300 parasites were observed.
Invasion assay
Parasites were pretreated for 24 hours with or without 1.5 Hg/ml ATc, collected promptly after egress and inoculated onto new HFF monolayers in presence of ATc during 48 hours. For invasion assays, 5.106 freshly released tachyzoites were sedimented on confluent cells at 4°C for 30 minutes on ice and warmed up for invasion during 5 minutes at 38.5°C. Invasion was stopped by fixation in 4% PAF and parasites were further processed for IFA. Prior to triton permeabilization, extracellular parasites were labelled with anti-SAG1 antibodies, while following permeabilization, intracellular parasites were stained with anti-ROP1 antibodies that labelled the nascent PV (Lebrun et al., 2005). Data are mean values±s.d. from three independent biological experiments. For each condition, 300 parasites were observed.
Egress assay
Parasites were pretreated for 39 hours with or without 1.5 Hg/ml ATc, collected promptly after egress and inoculated onto new HFF monolayers in presence of ATc during 33 hours. After 33 hours of intracellular growth, media was changed and incubated for 5 minutes at 37°C with DMEM containing 0.06% of DMSO or 3 HM of the Ca2+ ionophore A23187 (from Streptomyces chartreusensis, Calbiochem 100105) as previously described (Daher et al., 2010). Data are mean values±s.d. from three independent biological experiments. For each condition, 300 parasites were observed.
Gliding motility assay
Parasites were pretreated for 24 hours with or without 1.5 Hg/ml ATc, collected promptly after egress and inoculated onto new HFF monolayers in presence of ATc during 48 hours. Freshly released tachyzoites were collected by centrifugation, resuspended in 100 µl and deposited onto Poly-L-Lysine coated coverslips (1 mg/ml, 2 hrs at RT) in a wet environment for 15 minutes at 37°C previously. Parasites were fixed with PAF/GA and IFA using the anti-SAG1 antibody was performed to visualize the trails.
Statistics
P values were calculated in Excel using the Student’s t-test assuming equal variance, unpaired samples, and using 2-tailed distribution. Means and standard deviations (SD) were also calculated in Excel.
Supplementary Material
Acknowledgments
We thank Dr Iain Cheeseman for providing anti-centrin antibodies. We thank Dr Peter Bradley for providing the anti-ISP1, anti-ATrx1 and anti-α-F1-ATPase β-subunit antibodies, Dr Boris Striepen for the anti-HSP60 antibodies, Dr Boris Striepen and Dr Lilach Sheiner for the DHFR-tetO7-SAG4 and LIC-CAT-3HA vectors, Dr Con Beckers for the anti-IMC1 antibodies, Dr Vern Carruthers for providing the RH-ku80ko strain. We are grateful to Eliane Rubio, Renaud Burrer and Yann Bordat for their technical assistance. We thank Veronique Richard and Frank Godiard for their technical assistance on the Electron Microscopy Service of the University of Montpellier 2. We are also grateful to Montpellier RIO imaging facility at the University of Montpellier 2. We are thankful to Dr Jean-Francois Dubremetz and Dr Sebastien Besteiro for critical reading of the manuscript. Dr Wassim DAHER and Dr Maryse LEBRUN are INSERM researchers. This work was supported by the Laboratoire d'Excellence (LabEx) (ParaFrap ANR-11-LABX-0024) to Drs Maryse Lebrun and DAHER Wassim, and by the National Institutes of Health grants AI081924 and AI107475 to Dr Marc-Jan Gubbels.
Abbreviations
- CDPK7
Calcium Dependent Protein Kinase 7
- TATi-1
Trans-activator Trap identified
- IMC
Inner Membrane Complex
- Nuf2
Nuclear Filamentous 2
- MORN1
Membrane Occupation and Recognition Nexus Protein 1
- PH
Pleckstrin Homology
References
- Achbarou A, Mercereau-Puijalon O, Autheman JM, Fortier B, Camus D, Dubremetz JF. Characterization of microneme proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1991;47:223–233. doi: 10.1016/0166-6851(91)90182-6. [DOI] [PubMed] [Google Scholar]
- Agrawal S, van Dooren GG, Beatty WL, Striepen B. Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins. J Biol Chem. 2009;284:33683–33691. doi: 10.1074/jbc.M109.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Gaadhe K, Sharma GP, Kumar N, Neculai M, Hui R, et al. Novel insights into the regulation of malarial calcium-dependent protein kinase 1. FASEB J. 2012;26:3212–3221. doi: 10.1096/fj.12-203877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-White B, Beck JR, Chen CT, Meissner M, Bradley PJ, Gubbels MJ. Cytoskeleton assembly in Toxoplasma gondii cell division. Int Rev Cell Mol Biol. 2012;298:1–31. doi: 10.1016/B978-0-12-394309-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, et al. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol. 2011;13:18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo MF, Sanders PR, Krejany E, Nie CQ, Fu P, Bach LA, et al. Inhibition of Plasmodium falciparum CDPK1 by conditional expression of its J-domain demonstrates a key role in schizont development. Biochem J. 2013;452:433–441. doi: 10.1042/BJ20130124. [DOI] [PubMed] [Google Scholar]
- Bansal A, Singh S, More KR, Hans D, Nangalia K, Yogavel M, et al. Characterization of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1) and its role in microneme secretion during erythrocyte invasion. J Biol Chem. 2012;288:1590–1602. doi: 10.1074/jbc.M112.411934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JR, Rodriguez-Fernandez IA, Cruz de Leon J, Huynh MH, Carruthers VB, Morrissette NS, Bradley PJ. A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 2010;6:e1001094. doi: 10.1371/journal.ppat.1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinich MS, Ferguson DJ, Foth BJ, van Dooren GG, Lebrun M, Quon DV, et al. A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Curr Biol. 2009;19:277–286. doi: 10.1016/j.cub.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CF, Francia ME, Gissot M, Croken MM, Kim K, Striepen B. Toxoplasma gondii sequesters centromeres to a specific nuclear region throughout the cell cycle. Proc Natl Acad Sci U S A. 2011;108:3767–3772. doi: 10.1073/pnas.1006741108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee CW, Rogers GC. Show me your license, please: deregulation of centriole duplication mechanisms that promote amplification. Cell Mol Life Sci. 2012;70:1021–1034. doi: 10.1007/s00018-012-1102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Moreno SN, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999a;342(Pt 2):379–386. [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Sherman GD, Sibley LD. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J Biol Chem. 2000;275:14346–14353. doi: 10.1074/jbc.275.19.14346. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999b;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- Chan GK, Liu ST, Yen TJ. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–598. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Chandran V, Stollar EJ, Lindorff-Larsen K, Harper JF, Chazin WJ, Dobson CM, et al. Structure of the regulatory apparatus of a calcium-dependent protein kinase (CDPK): a novel mode of calmodulin-target recognition. J Mol Biol. 2006;357:400–410. doi: 10.1016/j.jmb.2005.11.093. [DOI] [PubMed] [Google Scholar]
- Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol. 1990;71:114–124. doi: 10.1016/0014-4894(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Chen CT, Gubbels MJ. The Toxoplasma gondii centrosome is the platform for internal daughter budding as revealed by a Nek1 kinase mutant. J Cell Sci. 2013 doi: 10.1242/jcs.123364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi A, Tewari R, Bishop JR, Bennett BL, Lawrence R, Esko JD, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher W, Plattner F, Carlier MF, Soldati-Favre D. Concerted action of two formins in gliding motility and host cell invasion by Toxoplasma gondii. PLoS Pathog. 2010;6: e1001132. doi: 10.1371/journal.ppat.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RG, Roos DS. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci U S A. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubremetz JF. [Ultrastructural study of schizogonic mitosis in the coccidian, Eimeria necatrix (Johnson 1930)] J Ultrastruct Res. 1973;42:354–376. [PubMed] [Google Scholar]
- Dubremetz JF, Lebrun M. Virulence factors of Toxoplasma gondii. Microbes Infect. 2012;14:1403–1410. doi: 10.1016/j.micinf.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hajj H, Papoin J, Cerede O, Garcia-Reguet N, Soete M, Dubremetz JF, Lebrun M. Molecular signals in the trafficking of Toxoplasma gondii protein MIC3 to the micronemes. Eukaryot Cell. 2008;7:1019–1028. doi: 10.1128/EC.00413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ, Sahoo N, Pinches RA, Bumstead JM, Tomley FM, Gubbels MJ. MORN1 has a conserved role in asexual and sexual development across the apicomplexa. Eukaryot Cell. 2008;7:698–711. doi: 10.1128/EC.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell. 2009;8:520–529. doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, Oswald BP, et al. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 2012;8:e1003049. doi: 10.1371/journal.ppat.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald N, Mahajan B, Kumar S. Mitosis in the human malaria parasite Plasmodium falciparum. Eukaryot Cell. 2011;10:474–482. doi: 10.1128/EC.00314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, Szatanek T, et al. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog. 2008a;4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci. 2006;119:2236–2245. doi: 10.1242/jcs.02949. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, White M, Szatanek T. The cell cycle and Toxoplasma gondii cell division: tightly knit or loosely stitched? Int J Parasitol. 2008b;38:1343–1358. doi: 10.1016/j.ijpara.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Hu K, He CY, Pelletier L, Roos DS, Warren G. Golgi and centrosome cycles in Toxoplasma gondii. Mol Biochem Parasitol. 2006;145:125–127. doi: 10.1016/j.molbiopara.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Hoppe HC, Ngo HM, Yang M, Joiner KA. Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nat Cell Biol. 2000;2:449–456. doi: 10.1038/35017090. [DOI] [PubMed] [Google Scholar]
- Hu K. Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii. PLoS Pathog. 2008;4:e10. doi: 10.1371/journal.ppat.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Johnson J, Florens L, Fraunholz M, Suravajjala S, DiLullo C, et al. Cytoskeletal components of an invasion machine--the apical complex of Toxoplasma gondii. PLoS Pathog. 2006;2:e13. doi: 10.1371/journal.ppat.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Mann T, Striepen B, Beckers CJ, Roos DS, Murray JM. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol Biol Cell. 2002;13:593–606. doi: 10.1091/mbc.01-06-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- Jammallo L, Eidell K, Davis PH, Dufort FJ, Cronin C, Thirugnanam S, et al. An insertional trap for conditional gene expression in Toxoplasma gondii: identification of TAF250 as an essential gene. Mol Biochem Parasitol. 2011;175:133–143. doi: 10.1016/j.molbiopara.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol. 2010;6:507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, et al. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 2011;7:e1001276. doi: 10.1371/journal.ppat.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, Vial H, Dubremetz JF. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol. 2005;7:1823–1833. doi: 10.1111/j.1462-5822.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- Lourido S, Jeschke GR, Turk BE, Sibley LD. Exploiting the Unique ATP-Binding Pocket of Toxoplasma Calcium-Dependent Protein Kinase 1 To Identify Its Substrates. ACS Chem Biol. 2013 doi: 10.1021/cb400115y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W, Lingle WL, McCormick D, Greenwood TM, Salisbury JL. Phosphorylation of centrin during the cell cycle and its role in centriole separation preceding centrosome duplication. J Biol Chem. 2001;276:20774–20780. doi: 10.1074/jbc.M101324200. [DOI] [PubMed] [Google Scholar]
- Mann T, Beckers C. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:257–268. doi: 10.1016/s0166-6851(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Mardin BR, Schiebel E. Breaking the ties that bind: new advances in centrosome biology. J Cell Biol. 2012;197:11–18. doi: 10.1083/jcb.201108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 2012;8:e1003066. doi: 10.1371/journal.ppat.1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Brecht S, Bujard H, Soldati D. Modulation of myosin A expression by a newly established tetracycline repressor-based inducible system in Toxoplasma gondii. Nucleic Acids Res. 2001;29:E115. doi: 10.1093/nar/29.22.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Saavedra D, Gabaldon T, Barton GJ, Langsley G, Doerig C. The kinomes of apicomplexan parasites. Microbes Infect. 2012;14:796–810. doi: 10.1016/j.micinf.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Morrissette NS, Sibley LD. Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J Cell Sci. 2002;115:1017–1025. doi: 10.1242/jcs.115.5.1017. [DOI] [PubMed] [Google Scholar]
- Nabetani A, Koujin T, Tsutsumi C, Haraguchi T, Hiraoka Y. A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma. 2001;110:322–334. doi: 10.1007/s004120100153. [DOI] [PubMed] [Google Scholar]
- Ngo HM, Yang M, Paprotka K, Pypaert M, Hoppe H, Joiner KA. AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J Biol Chem. 2003;278:5343–5352. doi: 10.1074/jbc.M208291200. [DOI] [PubMed] [Google Scholar]
- Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci. 2008;121:1559–1568. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L, Stern CA, Pypaert M, Sheff D, Ngo HM, Roper N, et al. Golgi biogenesis in Toxoplasma gondii. Nature. 2002;418:548–552. doi: 10.1038/nature00946. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Ahmed A, Gourinath S, Sharma P. Dissection of mechanisms involved in the regulation of Plasmodium falciparum calcium-dependent protein kinase 4. J Biol Chem. 2009;284:15267–15276. doi: 10.1074/jbc.M900656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrevel J, Asfaux-Foucher G, Bafort JM. [Ultrastructural study of multiple mitoses during sporogony of Plasmodium b. berghei] J Ultrastruct Res. 1977;59:332–350. doi: 10.1016/s0022-5320(77)90043-0. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Brochet M, Collins MO, Schwach F, Jones ML, Goulding D, et al. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe. 2012;12:9–19. doi: 10.1016/j.chom.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield HG, Melton ML. The fine structure and reproduction of Toxoplasma gondii. J Parasitol. 1968;54:209–226. [PubMed] [Google Scholar]
- Sheiner L, Demerly JL, Poulsen N, Beatty WL, Lucas O, Behnke MS, et al. A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathog. 2011;7:e1002392. doi: 10.1371/journal.ppat.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden-Kiamos I, Ecker A, Nyback S, Louis C, Sinden RE, Billker O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol Microbiol. 2006;60:1355–1363. doi: 10.1111/j.1365-2958.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloves PJ, Delhaye S, Mouveaux T, Werkmeister E, Slomianny C, Hovasse A, et al. Toxoplasma sortilin-like receptor regulates protein transport and is essential for apical secretory organelle biogenesis and host infection. Cell Host Microbe. 2012;11:515–527. doi: 10.1016/j.chom.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Soldati D, Lassen A, Dubremetz JF, Boothroyd JC. Processing of Toxoplasma ROP1 protein in nascent rhoptries. Mol Biochem Parasitol. 1998;96:37–48. doi: 10.1016/s0166-6851(98)00090-5. [DOI] [PubMed] [Google Scholar]
- Striepen B, Crawford MJ, Shaw MK, Tilney LG, Seeber F, Roos DS. The plastid of Toxoplasma gondii is divided by association with the centrosomes. J Cell Biol. 2000;151:1423–1434. doi: 10.1083/jcb.151.7.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Morrison DP, Gaji RY, Murray JM, Entzeroth R, Howe DK, Striepen B. Plastid segregation and cell division in the apicomplexan parasite Sarcocystis neurona. J Cell Sci. 2005;118:3397–3407. doi: 10.1242/jcs.02458. [DOI] [PubMed] [Google Scholar]
- van Dooren GG, Reiff SB, Tomova C, Meissner M, Humbel BM, Striepen B. A novel dynamin-related protein has been recruited for apicoplast fission in Toxoplasma gondii. Curr Biol. 2009;19:267–276. doi: 10.1016/j.cub.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Petitprez A. [The outer membrane complex and its development at the time of the formation of daughter cells in Toxoplasma gondii] J Cell Biol. 1969;43:329–342. [PMC free article] [PubMed] [Google Scholar]
- Wernimont AK, Amani M, Qiu W, Pizarro JC, Artz JD, Lin YH, et al. Structures of parasitic CDPK domains point to a common mechanism of activation. Proteins. 2011;79:803–820. doi: 10.1002/prot.22919. [DOI] [PubMed] [Google Scholar]
- Wernimont AK, Artz JD, Finerty P, Jr, Lin YH, Amani M, Allali-Hassani A, et al. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol. 2010;17:596–601. doi: 10.1038/nsmb.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.