Abstract
We examined the effects of TSP2 deficiency on assembly of collagenous extracellular matrix (ECM) by primary marrow-derived mesenchymal stromal cells (MSC) undergoing osteoblast differentiation in culture. After 30 days, wild-type cells had accumulated and mineralized a collagen-rich insoluble matrix, whereas the TSP2-null cultures contained markedly lower amounts of matrix collagen and displayed reduced mineral. Differences in matrix collagen were seen as early as day 9, at which time wild-type cultures contained more total collagen per cell than did TSP2-null cells. Collagen was unevenly distributed amongst different extracellular compartments in the two cell-types. Collagen levels in conditioned medium of wild-type cells were higher than those of TSP2-null cells, but were roughly equivalent in the acid-soluble, newly cross-linked matrixes. Conversely, the mature, cross-linked acid-insoluble matrix layer of wild-type cells contained about twice as much collagen as TSP2-null cell-derived matrix. Western blot analysis of type I collagen in detergent-soluble and insoluble matrix fractions supported the premise that matrix collagen levels were reduced in TSP2-null MSC undergoing osteoblastic differentiation in vitro. Western blot and immunofluorescent analysis suggested that assembly of fibronectin into matrix was not affected by TSP2 deficiency. Instead, western blots of conditioned medium demonstrated a marked reduction in mature, fully processed type I collagen in the absence of TSP2. Our data suggest that, in the context of osteoblast differentiation, TSP2 promotes the assembly of a type I collagen-rich matrix by facilitating pro-collagen processing.
Keywords: matricellular, collagen pro-peptides, mineralization, TSP2-null mouse
Introduction
Bone formation during development and skeletal regeneration following trauma depend critically on the proper formation of a type I collagen-rich extracellular matrix (ECM) by bone forming osteoblasts. Matrix-bound type I collagen binds integrins on the surface of osteoblast progenitors to activate an ERK-mediated signal transduction cascade that leads to the activation of runx2 and the initiation of the osteoblast differentiation cascade (1–4). These cellular interactions with type I collagen are required for osteoblast differentiation. A variety of intracellular and extracellular proteins facilitate the proper translation and secretion of procollagen, proteolytic processing of pro-collagen to collagen, and assembly of collagen into fibers with tissue-specific structural attributes. Functional deficits in individual accessory proteins are known to impair matrix collagen content, organization and tissue strength (5–8).
Thrombospondin-2 (TSP2) is one matrix protein that facilitates the proper organization of collagen fibers into ECM. Skin and tendons of TSP2-null mice have irregularly sized and loosely packed type I collagen fibers and markedly lax connective tissue (9). In the context of the skeleton, TSP2-null mice display increased endocortical bone thickness associated with an augmented osteoblast progenitor cell pool (10). In addition to affecting marrow stromal cell proliferation, TSP2 promotes osteoblast lineage progression at the expense of adipogenesis (11, 12). Our published data suggest that TSP2 promotes mineralization in an osteoblast cell line, while also facilitating accumulation or retention of collagen and osteocalcin in the ECM (13). Since MSC-collagen interactions are required for osteoblast lineage progression, TSP2 could promote osteoblast differentiation indirectly by facilitating formation of a collagenous matrix. In the current work, we utilized MSC derived from TSP2-null and wild-type mice to address this hypothesis, and our data suggest that TSP2 facilitates matrix collagen assembly by promoting pro-peptide processing. Levels of collagen in the mature insoluble matrix were also substantially reduced in TSP2-null osteoblasts, suggesting that TSP2 promotes incorporation of collagen into matrix or that it affects collagen protein stability.
Materials and Methods
Isolation and culture of primary marrow-derived mesenchymal stromal cells
All mice were housed under specific pathogen free conditions at the University of Michigan. All procedures were approved by the University of Michigan Committee on the Care and Use of Animals and comply with NIH guidelines outlined in the Care and Use of Laboratory Animals. Primary MSC culture was conducted as described previously (11, 14). Whole marrow was flushed from tibiae and femora of 6–8 week old male TSP2 +/+ (wild-type) and TSP2−/− (null) mice. A single cell suspension was obtained using a 21guage needle and the cells were plated in MSC growth medium (α-MEM containing 10% fetal bovine serum (Fisher Scientific, Pittsburgh, PA), 100 IU/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine (Glutamax; Invitrogen, Carlsbad, CA) and 50 µg/ml ascorbate (Sigma-Aldrich, St. Louis, MO) at a ratio of one mouse per 10 cm dish. This heterogenous cell population was cultured for 7–9 days. To maintain the influence of non-adherent cells, 1/3 of the culture medium was replaced every 4 days. Cells were passaged onto 6 well plates at 200,000 cells per well in MSC growth medium. At day 4, the medium was replaced with osteogenic differentiation medium (MSC growth medium supplemented with 10 mM β-glycerolphosphate (Sigma-Aldrich). Medium was replaced completely every 3 to 4 days. Fresh ascorbate was added at each medium change.
Extraction of extracellular matrix
At the end of the indicated culture times, conditioned medium was collected into tubes containing a cocktail of protease inhibitors (1mM PMSF, 5 mM sodium fluoride, 1 mM sodium orthovanadate and the complete EDTA-free protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)), snap-frozen and stored at −80°C. Culture wells were then washed twice with PBS, and matrix fractions were obtained by two methods. To obtain acid soluble and insoluble fractions, cells were scraped into 0.5M acetic acid and incubated overnight at 4°C with rocking. Acid-insoluble components were pelleted for 1 hour at 14,000g in a 15°C centrifuge, and denatured 1 hour at 55°C in PBS containing proteinaseK (60 mAU/ml). Amounts of total fibrillar collagen in the resulting acid soluble and insoluble matrix fractions were determined using the Sircoll assay as described below.
Alternatively, cells and matrix proteins not strongly incorporated into the ECM were extracted using deoxycholate (15). Here, cells were serum-starved in αMEM containing 50 µg/ml ascorbate and 2 mM L-glutamine. After four hours, conditioned medium was collected as outlined above for acid extraction, cells were washed twice with PBS, cell layers were scraped into 4% deoxycholate in 20mM Tris-HCL, pH 8.8, passed 8 times through a 27-1/2gauge needle and then incubated overnight at 4°C with rocking. Deoxycholate-insoluble ECM was pelleted by centrifugation at 17000× g for 30 minutes at 4°C, and solubulized in 1% SDS in 25mM Tris-HCL, pH 8.0. The protease inhibitor cocktail was added to all matrix samples before snap freezing and storing at −80°C. Type I collagen levels in deoxycholate soluble and insoluble matrix fractions were determined by Western blot as outlined below.
Sircol Assay
As described previously (13), fibrillar collagen levels in acid-soluble and insoluble matrix fractions were determined using a sirius red dye binding method (Sircol soluble collagen assay; Biocolor Ltd, Carrickfergus, Northern Ireland). After 9 or 30 days in osteoblastic culture, conditioned medium plus acid-soluble and insoluble matrix fractions were collected as outlined above under extraction of extracellular matrix. Concentrations of collagen in conditioned medium and the acid-soluble cell layer were determined by comparing optical density values to those derived from collagen standards provided with the Sircol kit. Standards were prepared in MSC growth medium containing serum. Relative amounts of gelatin in the heat-denatured, acid-insoluble matrix fraction were also determined by spectrophotometry
Western blot analysis of type I collagen
Cell-conditioned medium was subjected to TCA precipitation and equal volumes were diluted in 6× gel sample buffer (100 mM Tris-HCL (pH 6.8), 30% glycerol, 10% SDS and 0.012% bromphenol blue) containing freshly added 2-mercaptoethanol (5%) and protease inhibitors. Equal volumes of deoxycholate-soluble or insoluble matrix extracts were diluted in gel sample buffer. After boiling, proteins were separated on 7.5% SDS-PAGE gels and transferred to nitrocellulose membranes in transfer buffer (192 mM glycine and 20% methanol in 25 mM Tris, pH 8.3) at 4°C. Non-specific sites were blocked with Tris-buffered saline containing 0.05% Tween 20 and 3% BSA. Next, blots were incubated with a monoclonal anti-α1(I) collagen antibody that binds unprocessed pro-collagen α1(I), partially processed pro-α1(I) collagen with the N pro-peptide removed (pC-collagen α1(I)), and fully processed α1(I) collagen (MD Bioproducts, St. Paul, MN). The membranes were washed and incubated with horseradish peroxidase conjugated goat-anti-rabbit immunoglobin-G (Thermo Scientific, Rockford, IL). Membranes were washed again in TBS-T and immunoreactive proteins were detected using enhanced chemiluminescence (ECL; Thermo Scientific, Rockford, IL). The deoxycholate soluble fractions were also subjected to fibronectin (Sigma antibody F6140) and actin (Novus Biologicals, Littleton, CO) immunoblot analysis.
For each of the three collagen-immunoreactive species detected, band intensities were determined from histograms generated using ImageJ gel analysis software (National Institutes of Health, Bethesda, MD) and compared to values obtained from wild-type cultures at each time-point. Since the antibody displayed preferential binding to the intermediately processed forms, films with different exposure times were utilized to quantify each species.
Gene expression analysis
After 4 days in osteogenic culture, wells were washed twice with PBS and total RNA was harvested using the RNeasy kit (Qiagen, Valencia, CA), DNase treated using the on-column RNAse-free DNase system (Qiagen), and one microgram RNA was reverse transcribed using qScript-cDNA supermix (Quanta Biosciences, Gaithersburg, MD). Type I Collagen (Col1A1) mRNA levels were determined by real time PCR using SYBR green (Invitrogen, Carlsbad, CA) and published primers (13). Thermal cycling was performed using a 7500 Fast Real-Time System (Applied Biosystems, Foster City, CA). Proper amplicon formation was confirmed by agarose gel electrophoresis or by melt-curve analysis, and relative mRNA expression levels were calculated using the double delta-Ct method.
Determination of DNA content
Wells were washed twice with PBS and DNA was extracted using the DNeasy extraction kit (Qiagen, Valencia, CA). DNA was quantified by UV spectrophotometry using a SpectraMax5 plate reader (Molecular Devices, Eugene, Oregon).
Alizarin Red-S staining
Mineral was visualized by Alizarin Red-S staining. Plates were washed with PBS, air dried, fixed in 50% ethanol, stained for 5 minutes with a 1.0% solution of Alizarin Red S, and rinsed thoroughly in ddH20. Photographic images of the plates were obtained using a flatbed scanner.
Fibronectin Immunofluorescent Analysis
Primary MSC were passaged onto 4 well glass chamber slides at 10,000 cells per well in MSC growth medium. After 3 days, cells were washed with PBS and fixed for 15 minutes at room temperature in 3.7% formaldehyde. After washing three times with PBS, cells were permeabilized in 0.2% triton-x-100 in PBS for 30 minutes. The cells were again washed three times with PBS, incubated overnight at 4°C with 10% goat serum, and then with a polyclonal antibody raised against cellular fibronectin (F6140; Sigma-Aldrich, St. Louis, MO). Cells were then incubated with DyLight 594-conjugated IgM (Bethyl Laboratories, Montgomery, TX). Both primary and secondary antibodies were prepared in 10% goat serum at a 1:250 dilution. The slides were cover-slipped in Prolong Gold anti-fade with dapi (Molecular Probes, Eugene Oregon). Microscopic analysis was conducted using an Axiovert 200M inverted microscope equipped with epifluorescence and the Axiovert image analysis software suite (Carl Zeiss, Gottingen, Germany). Using a consistent pattern of locations, 14 images per chamber well were captured with a black and white camera using 400 (dapi) and 500 (fibronectin) ms exposure times. A uniform window-level adjustment was made to all images in Image J.
Statistics
Data are mean and standard deviation from 2 to 4 independent cell harvests, totaling 6–12 separate wells. TSP2-null samples were compared to wild-type using a t-test corrected for unequal variance using Welch’s test (Prism, GraphPad Software, La Jolla, CA). Significance was set at p<0.05.
Results
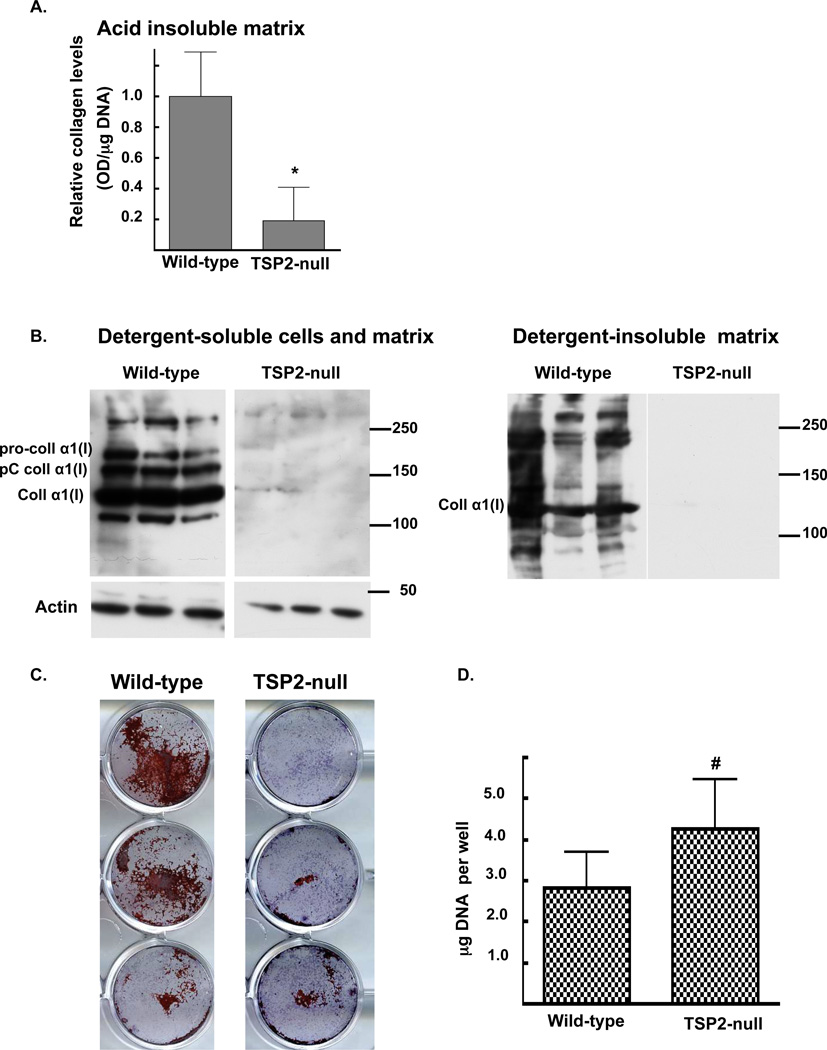
To further define the relationship between TSP2 expression, matrix collagen levels and mineralization in osteoblasts (13), osteoblastic differentiation was induced in primary MSC harvested from TSP2-null and wild-type mice. After 30 days, Sircoll analysis showed that wild-type cells had accumulated a collagen rich acid-insoluble matrix, whereas the TSP2-null cells displayed markedly lower levels (1.0 ± 0.41 and 0.04± 0.02 for wild-type and TSP2-null cultures respectively; p<0.001, figure 1A). Western blot analysis of type I collagen in detergent-soluble and insoluble cell-matrix protein extracts showed similar results (figure 1B). Alizarin Red-S staining demonstrated noticeably higher mineralization in wild-type compared to TSP2-null cells (Figure 1C). Lower matrix collagen levels and mineralization in the TSP2-null cells occurred despite slightly higher DNA levels (4.2 ± 1.2 and 2.8 ± 0.9 µg DNA per well in TSP2-null and wild-type cells, respectively; p<0.05; figure 1D).
Figure 1. After 30 days in culture, matrix collagen levels and mineralization are reduced in TSP2-null MSC undergoing osteoblastic differentiation in vitro.
A: Relative collagen levels in acid-insoluble matrix were determined using a Siruis red dye-binding assay. B: Western blot analysis of type I collagen was conducted on detergent soluble and insoluble cell-matrix protein preparations. C: Cytochemical determination of calcified matrix was conducted by Alizarin Red-S staining. D: DNA levels were quantified by UV spectrophotometry. Bars are mean and SD of data obtained from 3 independent cell harvests totaling 12 wells per genotype. * p<0.0001, # p<0.05.
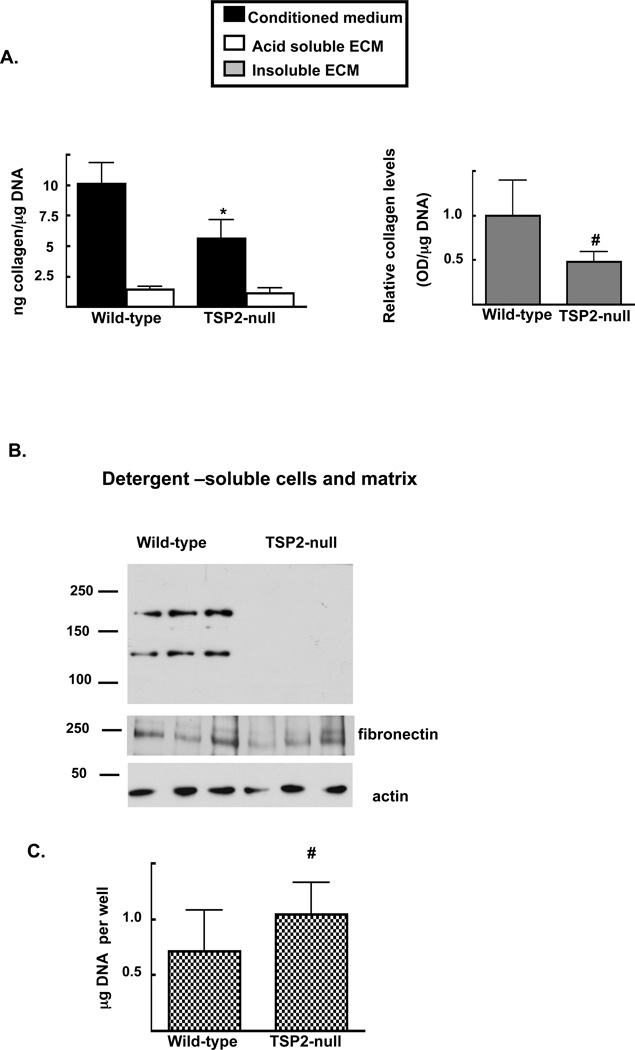
We also examined collagen at earlier time points. After 9 days, wild-type cells had accumulated more total acid-soluble fibrillar collagen (medium plus acetic acid-soluble cell-ECM layer) per cell than did TSP2-null cells (11.5 ± 1.9 vs 7.1 ± 2.1 ng collagen per µg DNA in wild-type vs. TSP2-null cultures respectively; p <0.001). Our data also suggest that the relative distribution of collagen between the medium, acid-soluble and acid-insoluble matrix compartments was different in TSP2-null and wild-type cells. While the conditioned medium of wild-type MSC contained about twice as much collagen as that of TSP2-null cell-conditioned medium (10.1±1.7 vs. 5.6 ±1.5 ng collagen per µg DNA in wild-type vs. TSP2-null cultures, respectively; p<0.0001, Figure 2A left panel, black bars), levels of collagen in the acid-soluble matrix were roughly equivalent (1.4 ± 0.2 vs. 1.1 ± 0.4 ng collagen per µg DNA in wild-type vs. TSP2-null cultures, respectively, Figure 2A left panel, open bars). Similar to the day 30 results presented in figure 1, the acid-insoluble matrix of wild-type cells contained about twice as much collagen relative that of TSP2-null cells (1.0 ± 0.4 and 0.5 ± 0.1 for wild-type and TSP2-null cells respectively; p<0.05, Figure 2A, right panel).
Figure 2. Deficits in matrix collagen associated with TSP2-deficiency are observed as early as day 9.
A: At day 9, collagen levels in conditioned medium (A; left panel, black bars), acid soluble matrix (A; left panel, open bars) and relative levels in acid insoluble matrix (A; right panel) were determined using a Sirius red dye-binding assay. B: Western blot analysis of type I collagen, fibronectin and actin was conducted on detergent soluble cell-matrix protein preparations. Blot is representative of data obtained from material harvested at culture days 7–10 from 3 independent cell harvests. C: DNA levels at day 9 were quantified by UV spectrophotometry. Bars are mean and SD of data obtained from 3 independent cell harvests totaling 12 wells per genotype, # p<0.05.
Western blot analysis of type I collagen in detergent soluble cell-ECM fractions suggested that its accumulation into the matrix is reduced in TSP2-null cells. Here, type I collagen was detectable in only a few detergent-insoluble ECM fractions of wild-type wells, so this fraction was not analyzed at this time point. To address the possibility that the DOC-soluble fractions harvested from TSP2-null cultures contained very little ECM, we also conducted fibronectin Western blots of the same samples. Levels of fibronectin in the detergent soluble ECM were not significantly affected by TSP2-deficiency (Figure 2B middle panel; 8098.03 ± 3197.3 and 8318.56 ± 6490.0 pixels per area in wild-type and TSP2-null cells, respectively.) As with the day 30 data presented in figure 1, the TSP2-null cultures displayed slightly higher DNA levels compared to wild-type cells (1.0 ± 0.30 and 0.71 ± 0.58 µg DNA per well in TSP2-null and wild-type cells, respectively; p<0.05, figure 2C).
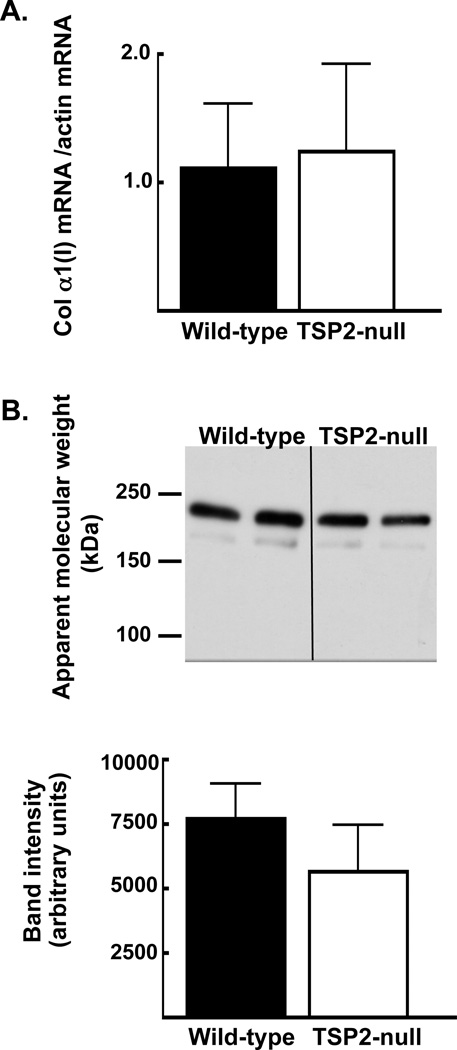
Next, we addressed the possibility that TSP2 affects the amount of type I collagen available to be incorporated into ECM. After 4 days of osteoblast differentiation, levels of pro-collagen in cell-conditioned medium were not significantly affected by TSP2 deficiency (7,694.7 ± 1,355.1 and 5,649.0 ± 1,814.8 pixels per area for wild-type and TSP2-null cells, respectively; figure 3B and C). Accordingly, gene expression analysis at day 4 suggested that TSP2 does not affect α-1(I) collagen mRNA levels during the first few days of osteoblast differentiation (figure 3A).
Figure 3. In MSC undergoing osteoblast differentiation, TSP2 deficiency does not affect the amount of type I collagen present in conditioned medium.
A: After 4 days in osteoblastic culture, collagen gene expression levels, normalized to actin, were determined by rtPCR. B: Collagen protein levels in cell-conditioned medium were determined by Western blot (top panel). Band intensities were determined using histograms generated in ImageJ (bottom panel). Bars are mean and SD of data obtained from 2 independent cell harvests totaling 6 wells per genotype.
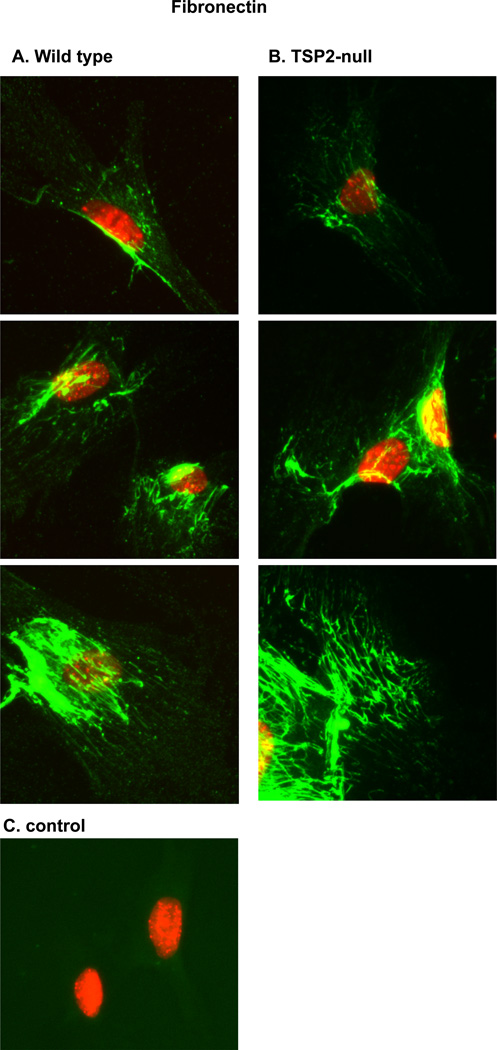
Collagen fibrillogenesis occurs in conjunction with assembly of a pericellular fibronectin matrix (18). To address the possibility that fibronectin matrix assembly is affected by TSP2-deficiency, we visualized cell-associated fibronectin in day 4 cultures using an anti-cellular fibronectin antibody. Representative images are shown in figure 4. A similar range of patterns and relative intensities were observed for both wild-type and TSP2-null MSC. Together, our Western blot analysis at day 10 (figure 2B) and our immunofluorescent data at day 4 (figure 4) suggest that fibronectin matrix assembly is not impaired in TSP2-null osteoblasts.
Figure 4. TSP2-deficiency does not significantly impair pericellular accumulation of fibronectin.
MSC were cultured from three days. A: Three representative images from wild-type cultures. B: Three representative images from TSP2-null cultures. C: Control wells in which the primary anti-fibronectin antibody was omitted.
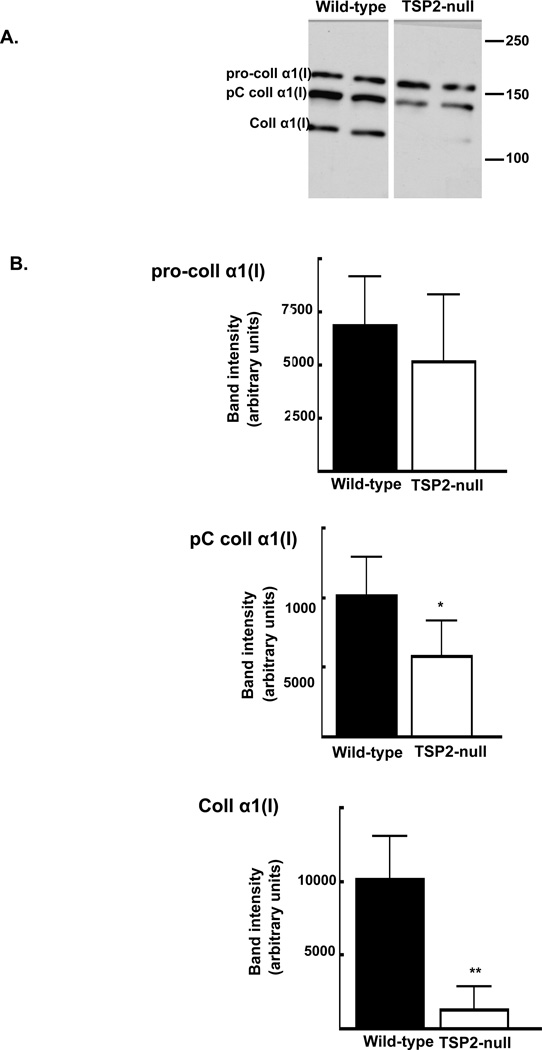
Pro-collagen is proteolytically processed upon secretion and prior to being incorporated into mature fibrillar matrix (19). This natural history of collagen maturation was affected by TSP2-deficiency. Figure 5A shows a representative blot of conditioned medium collected after 10 days of osteoblast differentiation. Consistent with observations at day 4 (figure 3), levels of procollagen were roughly equivalent at day 10 (6,870.0 ± 2,260.0 and 5,166.4 ± 3135.8 pixels per area for wild-type and TSP2-null cells, respectively, figure 5B, top panel). Values of intermediately processed type I collagen (pC collagen α1(I) band) were approximately double in the presence of TSP2 (10,132.6 ± 2,743.8 and 5,815.5 ± 2,510.6 in wild-type and TSP2-null cells, respectively; p < 0.001; figure 4B middle panel). At this time, levels of fully-processed mature type I collagen in cell-conditioned medium were markedly reduced in the absence of TSP2 (9,669.7 ± 3,069.7 and 1,082 ± 1,325.2 pixels per area in wild-type and TSP2-null cells, respectively; p<0.0001, figure 5B, bottom panel).
Figure 5. Levels of mature α1(I) collagen are reduced in the absence of TSP2.
After 10 days in osteoblastic culture, type I collagen levels determined in cell-conditioned medium by Western. A: Representative blot. B: Intensities of the pro-collagen α1(I) (top panel), pC collagen α1(I) (middle panel), and fully processed collagen α1(I) (bottom panel) were determined using histograms generated in ImageJ. Bars are mean and SD of data obtained from 3 independent experiments totaling of 12 wells per genotype. * p<0.001, ** p<0.0001.
Discussion
TSP2 modulates MSC proliferation and promotes osteoblast lineage progression. Here, we extended previous data by demonstrating reduced collagen levels in the cell-derived matrix assembled by osteoblastic cells isolated from TSP2-null animals. Although the amount of type I collagen detected in conditioned medium was not different at an early time point, ECM collagen levels were substantially reduced in mature TSP2-null osteoblast cultures.
Individual pro-collagen α chains are translated on endoplasmic reticulum-bound ribosomes where select prolines and lysines are post-translationally modified by hydroxylation and glycosylation. While still inside the ER-golgi complex, three pro-collagen molecules associate via their pro-peptides and form a pro-collagen triple helix, which is then secreted into the extracellular space. Outside the cell, the pro-peptides are removed by N- and C- pro-collagen proteinases allowing lateral association of individual triple helixes into insoluble fibrils and fibers. Lysyl oxidase catalyzes cross-links between lysine and hydroxylysine. Overtime, these cross-links are modified and become highly insoluble as a collagenous matrix ages (20).
We analyzed collagen in cell culture using complimentary biochemical methods. Dilute acetic acid solubilizes newly secreted pro-collagen molecules, as well as nascent multimers with young lysyl oxidase-mediated cross-links. In order to assess collagen levels in the more mature cross-linked matrix, we measured relative levels of gelatin in the acid-insoluble fractions by denaturing fibrillar collagens using heat and proteinaseK. These acetic acid soluble and insoluble fractions were analyized using a Sirius red dye-binding assay that will reveal information about all fibrillar collagens.
In order to obtain information about type I collagen specifically, we conducted western analysis on deoxycholate cell-ECM extracts (15). Deoxycholate is a detergent that solubilizes cellular and membrane components and, like acetic acid, is expected to recover newly secreted nascent multimers with limited cross-linking. The deoxycholate insoluble matrix was dissolved in SDS; a method that recovers insoluble cross-linked collagen, but may not dissolve very highly reduced, aged cross-links. Thus, of the two methods utilized, heat denaturation of the acid-insoluble fraction likely leads to more complete recovery of insoluble matrix collagen.
Over all, our data obtained using both methods indicate that matrix collagen levels are reduced in the absence of TSP2. One notable difference was observed in the acetic acid and detergent soluble protein extracts of day 9 cultures. Sircoll analysis, which is reflective of total fibrillar collagen, showed that levels of acetic-acid soluble collagen were similar in TSP2-null and wild-type cultures. Conversely, Western blot showed that levels of type I collagen were reduced in TSP2-null detergent soluble extracts. Since Sirius red binds to all fibrillar collagens, collagens type III and V, which are also secreted by osteoblasts, likely contribute to the Sircoll data. Whether TSP2 affects other fibrillar collagens is not currently known.
The virtual absence of the fully processed collagen-immunoreactive band in Western blots of TSP2-null cell-conditioned medium, as well as reduced levels of the partially processed pC α-1 (I) collagen, suggest that TSP2 facilitates removal of one or both pro-peptides from the ends of newly secreted triple helixes. Members of the tolloid family of zinc-dependent metalloproteinases, cleave the C terminal pro-peptide from collagen (21). ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family members process the N terminal pro-peptide (22). Removal of the C terminal pro-peptide promotes self-assembly of fibrils (23). Collagen molecules with retained N-pro-peptides can be incorporated along with properly processed triple helixes into fibrils (24), leading to improper packing and fragility, as documented in the rare human skin disease dermatosparaxis (25.)
One factor that could influence the amount of collagen detected in mature TSP2-null osteoblast cultures is increased degradation. In the current work, levels of collagen in acid- and detergent-insoluble ECM fractions were markedly reduced in TSP2-null cells, suggesting that the partially processed collagen might be degraded rather than being incorrectly incorporated into the ECM. The mechanisms behind our observations have not been defined, but increased bioavailability of matrix metalloproteinase 2 and decreased tissue transglutaminase activity contribute to collagen degradation in TSP2-null dermal fibroblasts (16, 17, 26). Similarly, the possibility that procollagen peptide processing is impaired during osteoblast differentiation in TSP2-null MSC remains to be explored.
A variety of non-collagenous matrix proteins are known to promote proper tissue-specific packing, fiber size and assembly of collagen matrixes (5). As modulators of cellular interactions with their extracellular environment, matricellular proteins have been implicated in collagen matrix assembly. Data supporting a role for TSP2 in facilitating proper collagen matrix formation have been published in the context of dermal tissues (9, 25). Another matricellular protein, SPARC (secreted protein acidic and rich in cysteine) facilitates proper pro-collagen processing and assembly of a mature cross-linked collagenous matrix by attenuating procollagen processing (27,28). In the context of bone, formation of a collagenous ECM precedes mineralization, and small leucine-rich proteoglycans are required for optimal mineralization (29). Thus, the absence of a collagen-rich ECM in TSP2-null osteoblasts could contribute to reductions in matrix mineralization that we have observed in the current work and previously (13).
Conclusions
Interaction with a type I collagen-rich ECM is required for progenitor cells to undergo terminal osteoblast differentiation. Our results suggest that TSP2 may promote osteoblast differentiation indirectly by facilitating the formation and/or stability of this osteogenic matrix.
Acknowledgements
The authors are indebted to Bonnie Nolan for expert animal care and technical assistance and to Dr. Renny T. Franceschi for thoughtful discussions and review of the manuscript.
This work was supported by NIAMS/NIH K01 AR057427 (AIA).
Footnotes
Declaration of Interests
The authors report no conflicts of interest.
Bibliography
- 1.Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, Franceschi RT. Interactions between extracellular signal-regulated kinase 1/2 and P38 map kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. J Bone Miner Res. 2012;27:538–551. doi: 10.1002/jbmr.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 3.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 4.Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103–1113. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- 5.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M, Septier D, Rapoport O, Iozzo RV, Young MF, Ameye LG. Targeted disruption of two small leucine-rich proteoglycans, biglycan and decorin, excerpts divergent effects on enamel and dentin formation. Calcif Tissue Int. 2005;77:297–310. doi: 10.1007/s00223-005-0026-7. [DOI] [PubMed] [Google Scholar]
- 7.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J Investig Dermatol Symp Proc. 2000;5:61–66. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 10.Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. J Bone Miner Res. 2000;15:851–862. doi: 10.1359/jbmr.2000.15.5.851. [DOI] [PubMed] [Google Scholar]
- 11.Hankenson KD, Bornstein P. The secreted protein thrombospondin 2 is an autocrine inhibitor of marrow stromal cell proliferation. J Bone Miner Res. 2002;17:415–425. doi: 10.1359/jbmr.2002.17.3.415. [DOI] [PubMed] [Google Scholar]
- 12.Shitaye HS, Terkhorn SP, Combs JA, Hankenson KD. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biol. 2010;29:549–556. doi: 10.1016/j.matbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alford AI, Terkhorn SP, Reddy AB, Hankenson KD. Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone. 2010;46:464–471. doi: 10.1016/j.bone.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alford AI, Reddy AB, Goldstein SA, Murthy P, Tayim R, Sharma G. Two molecular weight species of thrombospondin-2 are present in bone and differentially modulated in fractured and nonfractured tibiae in a murine model of bone healing. Calcif Tissue Int. 2012;90:420–428. doi: 10.1007/s00223-012-9580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Duyn Graham L, Sweetwyne MT, Pallero MA, Murphy-Ullrich JE. Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix. J Biol Chem. 2010;285:7067–7078. doi: 10.1074/jbc.M109.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 17.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 18.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 19.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 20.Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, Li SW, Prockop DJ, Lapiere CM, Nusgens BV. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277:5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- 21.Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]
- 22.Hulmes DJ, Kadler KE, Mould AP, Hojima Y, Holmes DF, Cummings C, Chapman JA, Prockop DJ. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J Mol Biol. 1989;210:337–345. doi: 10.1016/0022-2836(89)90335-5. [DOI] [PubMed] [Google Scholar]
- 23.Watson RB, Holmes DF, Graham HK, Nusgens BV, Kadler KE. Surface located procollagen N-propeptides on dermatosparactic collagen fibrils are not cleaved by procollagen N-proteinase and do not inhibit binding of decorin to the fibril surface. J Mol Biol. 1998;278:195–204. doi: 10.1006/jmbi.1998.1680. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agah A, Kyriakides TR, Bornstein P. Proteolysis of cell-surface tissue transglutaminase by matrix metalloproteinase-2 contributes to the adhesive defect and matrix abnormalities in thrombospondin-2-null fibroblasts and mice. Am J Pathol. 2005;167:81–88. doi: 10.1016/S0002-9440(10)62955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 27.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–22671. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- 28.Harris BS, Zhang Y, Card L, Rivera LB, Brekken RA, Bradshaw AD. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol. 2011;301:H841–H847. doi: 10.1152/ajpheart.01247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haruyama N, Sreenath TL, Suzuki S, Yao X, Wang Z, Wang Y, Honeycutt C, Iozzo RV, Young MF, Kulkarni AB. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix Biol. 2009;28:129–136. doi: 10.1016/j.matbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]