Abstract
Recent conceptualizations of alcohol expectancies relate cognitive schemas to their neurobiological underpinnings; cue reactivity paradigms lend themselves well to testing this broadened conceptual framework. In the present study, we examined the relationship between self-reported alcohol expectancies and responses to alcohol-related and affective picture cues among fifty-five young adults. In addition to traditional subjective and psychophysiological indices of cue reactivity, the startle eyeblink reflex was obtained during picture cue presentations to address both attention-arousal (early probes) and affective-motivational (late probes) aspects of cue processing. Analyses indicated that participants reporting greater positive, arousing, and social alcohol expectancies rated alcohol cues as more pleasant, arousing, and craving-inducing. In addition, participants displayed inhibited startle reactivity to late alcohol cue probes, indicative of an appetitive reaction. Finally, startle responding to early probes indicated that participants with greater alcohol expectancies displayed blunted attention to negative affect cues. Findings are discussed in terms of the utility of the startle reflex and cue reactivity paradigms for clarifying the relationship between alcohol expectancies and motivated attention to salient cues.
Keywords: alcohol, cue reactivity, expectancies, startle, affect
Language-based, psychometric indices of alcohol expectancies have been shown to explain up to 50% of the variance in reported alcohol use, to predict and track drinking increases and decreases through adolescence and adulthood, and to mediate the influence of other known alcohol risk factors on future drinking behavior (for a review see Goldman, 2002; Goldman, Darkes, & Del Boca, 1999). It has been theorized that these language-based measures represent but one way of probing a variety of fundamental neurobehavioral processes that have evolved to direct behavioral outputs by using prior experiences to anticipate future rewards and punishments (Goldman, 2002; Goldman et al., 2006). That is, expectancies represent functional mechanisms of adaptation and survival that are manifest across multiple biological systems. In this conceptualization, alcohol expectancies are understood to reflect both cognitive and affective/motivational pathways of risk.
Despite this theoretical link between language-based indices of alcohol expectancies and affective/motivational processes that influence alcohol use, researchers have made few attempts to bridge these two domains thus far. Research methods that have evolved to address alcohol craving in controlled laboratory settings (operationalized as reactivity to alcohol cues) may provide one kind of bridge. In contrast to the predominantly verbal self-report methods in alcohol expectancy research, investigation of cue reactivity has incorporated physiological probes of affective response to alcohol cues. Both alcohol expectancies and craving are thought to reflect prior experience with alcohol consumption, to reflect motivational processes that influence subsequent alcohol intake, and presumably are maximized under environmental conditions that match those associated with prior alcohol consumption.
Because studies of cue reactivity have found traditional non-specific psychophysiological measures of affective response (e.g., heart rate, skin conductance) generally to show smaller effect sizes than self-report measures (e.g., Carter & Tiffany, 1999), the present study also will assess the startle eyeblink reflex, presumably a more specific index of attentional and affective processing. In humans, the startle reflex is typically measured as the magnitude of the eyeblink in response to a sudden, intense stimulus (e.g., noise burst). Evidence that the startle reflex is enhanced in the context of unpleasant affect and avoidance-oriented motivation, and is inhibited when pleasant affect and/or appetitive drive states are engaged, is substantial (see Bradley, Cuthbert, & Lang, 1999). In addition, neurophysiologic pathways involved in affective startle modulation are well delineated in animal models. These same neural systems-- the central nucleus of the amygdala (e.g., Davis et al., 1982) and the nucleus accumbens (e.g., Koch et al., 1996) -- have been shown to reflect anticipatory/expectancy processes in animals and have been theoretically linked to language-based expectancy responses (Holland & Gallagher, 2004).
Although research is at an early stage and findings have been somewhat mixed, the startle reflex has shown initial promise as an objective index of affective/motivational state during substance cue processing (e.g., Drobes et al., 2001; Elash, Tiffany, & Vrana, 1995; Geier et al., 2000; Mucha et al., 2000; Saladin, Drobes, Coffey, & Libet, 2002). Additional research will hopefully determine individual and contextual factors that account for distinct patterns of startle reactivity (e.g., elevation vs. reduction) across drug cue studies. In the present study with non-drug dependent individuals, we expect that individuals who report the greatest alcohol expectancies will show an appetitive pattern of startle responding in the presence of alcohol-related cues.
In addition to its properties as an affect/motivation probe, the startle reflex has been widely used as an index of attention (e.g., Ashare, Hawk, & Mazzullo, 2007; Dawson et al., 1993; Hawk et al., 2002), and expectancies have been extensively linked to attentional processes (Grossberg, 1995). Most notably, there is considerable evidence that the startle reflex is inhibited when a startle-eliciting stimulus is preceded closely by a non-startling stimulus. This pre-pulse inhibition (PPI) effect is thought to reflect largely pre-attentive sensory gating mechanisms. Interestingly, studies have shown that PPI is increased when participants are instructed to pay greater attention to the prepulse stimulus (e.g., Dawson et al., 1993; Hawk, 2002). Within an affective picture viewing paradigm, Bradley and colleagues (Bradley, Cuthbert, & Lang, 1993) presented startle stimuli on some trials in close proximity following picture onset (approximately 300 msec), which presumably would render the onset of the picture as a prepulse stimulus for the early startle probe. Consistent with this interpretation, significant startle inhibition was observed for early probes trials, relative to late-probed trials. Furthermore, the degree of inhibition was greatest for highly arousing pictures, regardless of their hedonic content. This suggested that startle inhibition at this early stage of picture processing could serve as an index of attentional cue processing. By presenting startle probes early and late during picture processing in the current study, the intent is to gauge how alcohol expectancies relate to both attentional (early probes) and affective (late probes) reactions to alcohol-related and affective cues.
Finally, as with alcohol expectancies, risk factors for substance use disorders are related to affective startle modulation. For instance, adult children of alcoholics (not diagnosed with an alcohol use disorder; Zimmerman, Spring, Wittchen & Holsboer, 2004) and alcoholics diagnosed with Antisocial Personality Disorder (ASPD) displayed a blunted startle response in the presence of unpleasant stimuli (Miranda, Meyerson, Buchanon & Lovallo, 2002b). Extending this area of research, the present study will examine the relationship between a known risk factor for alcohol problems (alcohol expectancies) and startle reactivity to both alcohol and affective cues, using methods designed to address both attentional and motivational aspects of cue processing.
The primary objective of this study is to investigate how alcohol expectancy measures relate to indices of cue reactivity among young adult drinkers. We will relate language-based alcohol expectancy measures to subjective and psychophysiological assays of motivation, affect, and attention (e.g., craving, startle reflex, autonomic response) in response to alcohol-related and emotionally salient cues. To the extent that alcohol expectancies prove to be related to startle reflex modulation, this would suggest a specific linkage between alcohol expectancies and neurobiological substrates of emotional-motivational and/or attentional processing.
Method
Participants
Fifty-five individuals (21 men, 34 women) between the ages of 18 and 28 participated in the study. Participants were recruited via flyers distributed throughout the University of South Florida campus and surrounding area, as well as screening among college students participating for course credit. Given the established relationship between alcohol consumption and expectancies, approximately half of the sample was recruited as heavy drinkers and the other half as light drinkers in order to ensure a broad range of alcohol expectancies across the sample. Thus, the present sample was meant to represent a wide range of drinking patterns among the college-age population, but not those at the extremes (either completely abstinent, or exhibiting an alcohol use disorder). Heavy drinkers were those who met criteria for binge drinking on four or more occasions per month. As defined by the National Institute on Alcoholism and Alcohol Abuse (NIAAA), binge drinking entails the consumption of 5 or more standard alcohol drinks (12 oz. beer, 5 oz. wine, 1.5 oz. spirits) for men, or 4 or more standard alcohol drinks for women over a 2-hour time period (NIH, 2004). In addition, heavy drinkers were excluded if they reported drinking an average of 4 or more drinks per day (in order to avoid drinking patterns consistent with an alcohol use disorder). Light drinkers consumed less than 12 drinks per month and no more than 3 drinks per occasion, but they were excluded if they reported complete abstinence over the past month.
Materials and Apparatus
Picture cues
Twelve pictures from each of four categories (i.e., alcohol-related, pleasant, neutral, and unpleasant) were shown to each participant in a randomized order. Affective pictures (pleasant, neutral, and unpleasant) were selected from the International Affective Picture System (IAPS; CSEA, 2002), and included those that have elicited reliable subjective and physiological responses in previous studies1. Neutral cues consisted of a range of affectively neutral pictures (e.g. hairdryer, book), rather than non-alcohol beverage items, in order to avoid potential confusion between alcohol and non-alcohol beverage cues. The alcohol-related pictures included those taken from standard picture sets (e.g., Normative Affective Picture System [NAPS]; Strizke, Breiner, Curtin, & Lang, 2004), as well as pictures from various internet and stock photography sources. All alcohol-related pictures included beer, either as a figure-ground representation or in a social context, as beer is the most commonly consumed alcoholic beverage among the college-aged population (Wechsler, Kuo, Lee, & Dowdell, 2000).
Startle stimulus and assessment
The acoustic startle stimulus was delivered, and resulting startle eyeblink responses were measured, via a Pentium-II computer running VPM software (version 12.3; Cook, 1997). The startle probe consisted of a 50 msec, 100 dB white noise burst with near instantaneous rise time, produced digitally by SoundEdit software (Macromedia; San Francisco, CA). The stimulus was delivered via a SoundBlaster (Creative Labs, Milpitas, CA) AWE 64 Gold sound card, externally amplified by an Optimus Integrated Stereo Amplifier (Radio Shack, model SA-155), and presented binaurally via a matched pair of Telephonics (Huntington, NY) TDH-49 earphones. Sound levels were calibrated using a Bruel & Kjaer sound level meter.
The eyeblink response to each startle probe was measured with two small (4 mm) Ag-AgCl electrodes (In Vivo Metrics, Inc.) filled with saline gel and placed along the left orbicularis oculi muscle region, following skin preparation with a mildly abrasive cream (NuPrep). One electrode was placed directly below the pupil and the second was placed immediately lateral to the first. Impedance values were kept below 5 Kohms prior to recording, and were measured following recording to ensure that impedance was maintained at this level. The raw EMG signal was amplified, filtered (bandpass settings of 90 and 150 Hz), full wave rectified, and integrated (250 msec time constant) using Coulbourn Instruments V-series modules. The data were sampled at a rate of 1000 Hz by a LabMaster DMA A/D converter (Scientific Solutions; Solon, OH) from 50 msec prior until 250 msec after probe onset. Data were stored for manual offline scoring and analysis.
Autonomic measures
Electrodes were attached according to standard guidelines for measuring heart rate and skin conductance. For heart rate, a large (8 mm) Ag-AgCl electrode filled with saline gel was placed on the underside of each forearm, after skin preparation with a mildly abrasive alcohol pad. The EKG signal was amplified and filtered (Coulbourn V75-01 with bandpass settings of 8 and 40 Hz), prior to detection of inter-beat (R-R) intervals (to the nearest msec) using a Schmitt trigger. The stored inter-beat intervals (IBIs) were edited off-line to correct for missed or extra triggers due to movement or other sources of artifact, based on evaluation of each stored IBI according to a range of absolute (600 to 1200 msec) and relative (65% to 135% of rolling average) minimum and maximum IBI values. The corrected IBIs were then converted to heart rate (beats per minute, or bpm) for analysis. For skin conductance, two large electrodes were filled with an isotonic gel, then placed on the hypothenar eminence on the non-dominant palm. An isolated skin conductance coupler (Coulbourn V71-23) was used to create a constant voltage (.5 V) circuit using these electrodes, and the signal was sampled at a rate of 20 Hz, and calibrated to detect a range of 0–40 μS.
Subjective ratings
Four subjective ratings in response to each picture cue were obtained. The first three (valence, arousal, dominance) were obtained using a computerized version of the self-assessment manikin (SAM; Bradley & Lang, 1994). SAM is a graphical cartoon figure that can be manipulated with a computer trackball to represent varying degrees of each affective dimension. For the valence dimension, SAM can range from a happy, smiling figure to one that is unhappy and frowning. For the arousal dimension, SAM can range from an excited, jittery figure to one that appears sleepy and relaxed. For the dominance dimension, SAM ranges from extremely large to extremely small. Standard adjectives were provided to the participant as anchors for each end of the continuum. Finally, craving to drink alcohol was obtained using a visual analog scale, with anchors of Not at all and Extremely provided at either end of the scale. All subjective ratings were coded on a 0 to 20 scale.
Procedure
Individuals who were interested in participating were initially screened over the telephone. If they met study criteria, they were scheduled to attend a two-hour afternoon laboratory session. Upon arrival at the laboratory, participants read and signed an approved Informed Consent document. The participant was then seated in a comfortable chair and provided a breath sample to confirm that they were not intoxicated (BAC = 0) at the time of the assessment (AlcoholSensor IV, Intoximeters, Inc.). Next, electrodes were attached according to established guidelines. Following a five-minute acclimation period, participants were presented with two sample cue trials, followed by a randomized sequence of 48 picture cues. Four random picture orders were utilized such that each cue category followed each other category an equal number of times within and across orders. Thus, there was no systematic bias for alcohol cues preceding or following any other cue category. Indeed, this may be considered an advantage of this cue reactivity procedure, relative to a more traditional in vivo paradigm in which only one trial of each type is presented and order effects are often observed (e.g., Monti et al., 1987). Furthermore, the brevity of each picture presentation (6 secs) relative to inter-trial intervals (15 secs plus rating time) renders carry-over effects less of an issue within the present paradigm. Pictures were presented on a 20-inch computer monitor placed directly in front of the participant, using the following sequence for each picture trial: (1) variable (15-sec average) inter-trial interval (2) 2-sec baseline, (3) 6-sec picture presentation, (4) approximately 20-secs for subjective ratings. The participant entered subjective ratings directly into the computer. The startle eyeblink reflex was assessed during ten out of the twelve pictures in each category, with half of the probes presented during the early part of the viewing interval (250–350 msec following picture onset) and half later in the viewing interval (4 – 5.5 secs following picture onset). Early probes provide information regarding attention to cues; late probes are relevant to emotion/motivational processing (see Bradley, Cuthbert & Lang, 1993). Startle probes were also presented during ten of the inter-trial intervals. Heart rate and skin conductance were measured continuously throughout each trial. The cue reactivity assessment lasted for approximately 40 minutes. Following the last set of ratings, all electrodes were removed.
Following electrode removal, the participant completed several questionnaire and interview assessments. A Demographic Form recorded data on age, gender, race/ethnicity, education, marital status, occupation, and household income. A short (24-item) version of the Alcohol Expectancy Multi-Axial Assessment (A.E.Max; Goldman & Darkes, 2004) was administered, in which a 7-point Likert scale (0 = never, 6 = always) is rated for various expected effects of alcohol: horny; social; egotistical; attractive; sick; sleepy; woozy; and dangerous. Higher order factor scales of positive-negative and arousal-sedation derived from this measure were of particular interest for the present analysis. Furthermore, since social alcohol expectancies best predict drinking behavior in college-aged drinkers compared to any other expectancy category, the 9-item Alcohol Expectancy Questionnaire – Physical and Social Pleasure Scale (AEQ; Brown et al., 1987) was also administered. In addition, a 30-Day Timeline Follow-Back (TLFB; Sobell & Sobell, 1992) interview assessment was used to quantify drinking amounts and patterns during the preceding 30 days, such as total number of drinks and number of drinks per occasion. After all assessments were completed, participants were debriefed, given cash or course credit, and dismissed from the session. The entire session lasted an average of 75–90 minutes.
Data processing and analyses
Startle reflex data was scored manually offline for peak amplitude (the maximum EMG voltage deflection in response to the acoustic startle probe) and onset latency (in msec, the length of time from probe onset to response initiation), using VPM software (Cook, 1997). Based on visual inspection of the integrated EMG waveform for each startle trial, those trials with excessive baseline activity, post-stimulus activity incompatible with a startle eyeblink response, or multiple post-stimulus eyeblinks were rejected and not included in analyses. Startle amplitude and latency measures showed a strong negative correlation. To avoid redundancy, the present analyses focus only on startle amplitude. To minimize the impact of individual variability in startle responding, each participant’s responses were converted to T scores. Continuous physiological responses (heart rate, skin conductance) were expressed as the deviation of each half-sec value during picture viewing from the 2-sec trial baseline. Based on a clear triphasic waveform (e.g., Bohlin & Kjellberg, 1979), we scored the initial deceleration (D1) as the peak downward deflection during the first 3.5 seconds after picture onset and the initial acceleration (A1) as the peak increase during seconds 2 and 5 following picture onset. For skin conductance, the maximum change between seconds 2 and 6 following picture onset was scored. All cue reactivity variables were summed over trials within each picture category (pleasant, neutral, unpleasant, alcohol-related).
Initial analyses focused on the overall pattern of cue reactivity via a series of one-way repeated measures analysis of variance (ANOVA). For affective pictures, cue type (pleasant, neutral, unpleasant) was the within-subjects variable, with ratings (valence, arousal, dominance, and craving) and psychophysiological reactions (early [250–350 msec] startle, late [4–5.5 secs] startle, skin conductance, heart rate) as the dependent variables. Separate t-tests compared responses between alcohol and neutral cues. We considered the alternate strategy of examining all four cue categories as a single repeated measures factor within an omnibus model. However, we conceptualized affective and alcohol-related cue reactivity as separate issues, with no hypotheses involving direct comparisons between reactions across these cue types (cf., Drobes et al., 2001). Finally, correlational analyses examined the relationship between alcohol expectancies and each cue reactivity measure. Given the number of potential comparisons between several expectancy subscales and the range of dependent cue reactivity indices, we employed a modified Bonferroni procedure (Simes, 1986) to reduce the experiment-wise Type I error rate.
Results
Sample Description
The sample consisted of 55 individuals (20 males and 35 females) with an average age of 21.1 years (SD = 2.7). The racial distribution was consistent with that of the Tampa Bay area (76.3% Caucasian, 16.4% African-American, 7.3% Asian). Twenty-five “heavy drinkers” (17 female) and thirty “light drinkers” (18 female) were recruited. As intended, the recruitment strategy provided a sample endorsing a wide range of drinking behaviors and alcohol expectancies. On average, participants consumed 31.15 (SD = 37.23; range = 0–190) total alcoholic beverages during the preceding month, and 3.74 (SD = 1.91; range = 1–9) drinks per drinking occasion. Consistent with other young adult (mostly college-aged) samples, there was a normal distribution of positive and arousing alcohol expectancies, and a negatively skewed distribution for social alcohol expectancies.
Alcohol Cue Reactivity and its Relation to Expectancy Measures
Subjective Ratings
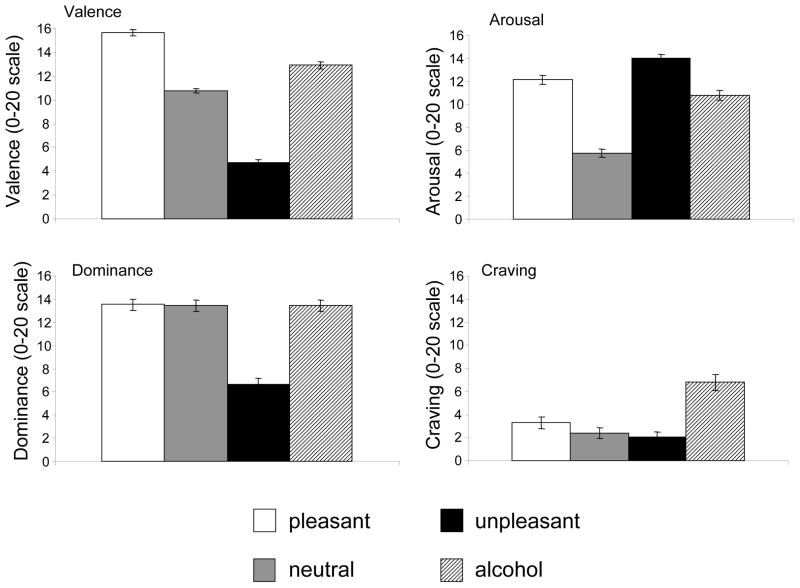
Ratings for affective cues were consistent with previous research, such that there were significant cue category main effects for valence, arousal, dominance, and craving ratings, F’s (2,108) > 9.0, p’s < .001 (see Figure 1). In addition, alcohol-related cues elicited greater subjective craving, valence, and arousal ratings than neutral cues (p’s < .001; see Figure 1).
Figure 1.
Mean subjective ratings for affective and alcohol-related cue categories. Each rating is based on a 0–20 Likert-type scale. Double-sided error-bars represent standard errors of the mean.
Significant positive relationships between subjective ratings to alcohol cues and expectancy subscales were found. In particular, a pattern emerged in which individuals with the greatest social expectations from alcohol consumption rated alcohol cues as more pleasing, arousing, and craving-inducing (see Table 1). Interestingly, those with relatively greater positive expectancies from alcohol rated unpleasant picture cues as less aversive than their peers. This finding suggests that individuals with more positive alcohol expectancies have blunted responding to unpleasant stimuli.
Table 1.
Correlations between alcohol expectancy subscales and subjective ratings
| Valence | Arousal | Dominance | Craving | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| P | N | U | A | P | N | U | A | P | N | U | A | P | N | U | A | |
| AEM Arousing | .12 | .09 | .23 | .19 | −.05 | .17 | −.06 | .06 | −.01 | .13 | .28 | .13 | −.14 | −.12 | −.03 | −.05 |
| AEM Sedating | −.11 | .17 | .15 | −.05 | −.07 | −.03 | .11 | −.06 | .10 | −.05 | .11 | −.01 | −.11 | −.06 | .12 | .00 |
| AEM Positive | −.07 | .29 | .36** | .27 | −.11 | −.02 | .11 | .22 | .05 | .00 | .23 | .06 | .03 | .00 | .09 | .19 |
| AEM Negative | .10 | .00 | .09 | −.04 | −.02 | −.18 | −.06 | −.15 | .03 | .10 | .21 | .09 | −.25 | −.17 | −.01 | −.19 |
| AEM Social | .03 | .17 | .19 | .45** | −.19 | .00 | .18 | .32* | −.28 | −.09 | .00 | −.13 | .13 | .02 | −.12 | .32* |
| AEQ Social | −.16 | .01 | .14 | .46** | −.15 | −.11 | −.02 | .27 | −.10 | .05 | .13 | .17 | .28 | .25 | .06 | .34 |
Note. AEM Arousing = Alcohol Expectancy Multi-Axial Assessment (A.E.Max) Arousal Subscale; AEM Sedating = A.E.Max Sedating Subscale; AEM Positive = A.E.Max Positive Subscale; AEM Negative = A.E.Max Negative Subscale; AEM Social = A.E.Max Social Subscale; AEQ Social = Alcohol Expectancy Questionnaire Social and Physical Pleasure Subscale; P = pleasant cues; N = neutral cues; U = unpleasant; A= alcohol-related cues. Significant correlations are in bold.
p < .05
p < .01. These zero-order correlations were repeated while controlling for neutral cues, and the same correlations maintained significance.
Autonomic reactivity: heart rate
Consistent with prior research, the D1 component was significantly greater to unpleasant than to neutral cues, F(1,54) = 4.08, p < .05, suggesting an elevated orienting response to the unpleasant cues. This establishes that unpleasant cues in the present experimental context were perceived as particularly attention-grabbing and potentially threatening. However, heart rate responses to pleasant and alcohol cues were not significantly different from neutral cues.
Consistent with the idea that overlap would be found between cue reactivity and expectancy measures, a significant positive relationship was found between arousing alcohol expectancies and heart rate acceleration during unpleasant cue processing (r = .37, p < .001). Several weaker relationships between expectancy subscales and the D1 component were not statistically significant following the modified Bonferroni adjustment.
Autonomic reactivity: skin conductance
As in prior research, a significant main effect for cue category was found for skin conductance level following picture onset (F [3,162] = 6.42, p < .001). Post hoc analyses showed that unpleasant pictures were associated with greater skin conductance activity than each of the other categories (i.e., unpleasant cues were processed as arousing; p’s < .05). However, no significant correlations between skin conductance responses and alcohol expectancies were found.
Startle reactivity
Based on previous research, one would expect to find an early startle pattern such that reactivity to both pleasant and unpleasant cues would be attenuated compared to neutral, and a late startle pattern such that reactivity to pleasant cues would be attenuated and reactivity to unpleasant cues would be potentiated compared to neutral. In this sample, however, no main effects for early or late startle as a function of affective picture category appeared (F’s [2,98] = 1.46 and 0.58, respectively, p’s > .20; see Figure 2). Although the pattern of means for early startle reactivity to affective pictures (as well as startle inhibition for late pleasant pictures) was generally in the expected direction, polynomial trend analyses did not reveal any significant linear or quadratic patterns for early or late startle reactivity across the affective categories. This failure to replicate prior findings within a sample of college-aged drinkers may be due to the inclusion of a fourth, alcohol-related, category interspersed with the three typical affective categories. Despite this constraint, concordances between alcohol expectancy measures and cue-elicited startle reactivity to both alcohol and affective cues were observed.
Figure 2.
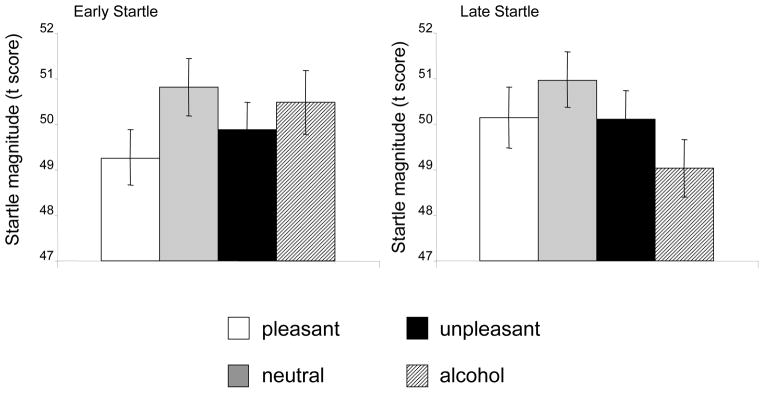
Mean early and late startle response magnitude for affective and alcohol-related cue categories. Startle magnitudes are expressed as standardized t-scores based on individual means and standard deviations across all four cue types.
As shown in Table 2, early startle reactivity during unpleasant cues was positively correlated with arousing (r = .38, p < .01), positive (r = .31, p < .05), and social alcohol expectancies (r = .34, p < .05). Given that a smaller startle response in this picture period is associated with greater attentional cue processing, these observed relationships suggest that those with greater alcohol expectancies exhibit blunted attentional processing of unpleasant cues. To further illustrate this pattern, Figure 3 shows startle magnitudes during early unpleasant cue processing for participants split into low and high groups for each of these expectancy subscales. This pattern is consistent with a growing literature suggesting reduced affective reactivity as a marker of drinking risk.
Table 2.
Correlations between alcohol expectancy scales and startle reactivity
| Early Startle | Late Startle | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| P | N | U | A | P | N | U | A | |
| AEM Arousing | −.02 | .02 | .38** | −.09 | .14 | −.16 | −.15 | .11 |
| AEM Sedating | .12 | −.02 | .13 | −.02 | −.08 | −.20 | .00 | −.01 |
| AEM Positive | .13 | .05 | .31* | −.04 | −.06 | −.23 | −.05 | −.03 |
| AEM Negative | −.02 | −.03 | .26 | −.08 | .12 | −.16 | −.12 | .13 |
| AEM Social | .15 | .00 | .21 | −.06 | .04 | −.12 | .18 | −.20 |
| AEQ Social | .21 | −.03 | .34* | −.08 | −.17 | −.27 | .00 | .18 |
Note. AEM Arousing = Alcohol Expectancy Multi-Axial Assessment (A.E.Max) Arousal Subscale; AEM Sedating = A.E.Max Sedating Subscale; AEM Positive = A.E.Max Positive Subscale; AEM Negative = A.E.Max Negative Subscale; AEM Social = A.E.Max Social Subscale; AEQ Social = Alcohol Expectancy Questionnaire Social and Physical Pleasure Subscale; P = pleasant cues; N = neutral cues; U = unpleasant; A= alcohol-related cues. Significant correlations are in bold.
p < .05
p < .01.
Figure 3.
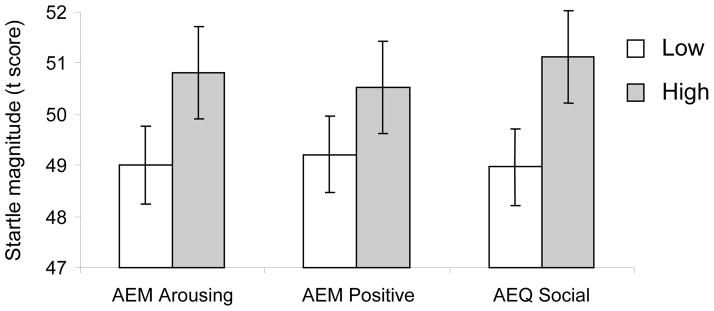
Mean early startle response to unpleasant cues as a function of several expectancy subscales. Low and high expectancy groups are based separate median splits for each expectancy subscale. AEM Arousing = A.E.Max Arousal; AEM Positive = A.E.Max Positive; AEQ Social = Alcohol Expectancy Questionnaire Social and Physical Pleasure.
In the late trial portion, startle reactivity to alcohol-related cues was significantly attenuated relative to neutral cues (t [49] = 2.1, p < .05), indicating that alcohol-related cues were processed as highly appetitive. However, late startle reactivity to alcohol cues was not significantly related to self-rated alcohol expectancies.
Discussion
In this first study of the relationship between alcohol expectancies and reactivity to alcohol and affective stimuli, a number of concordances were found. After subjective ratings of affective cues were shown to be consistent with those reported in prior studies, ratings of alcohol cues were found to be positively related to alcohol expectancies. Specifically, those who reported having greater positive and social alcohol expectancies rated alcohol pictures as more arousing, pleasant, and craving-inducing. These findings begin to establish a bridge between constructs that have been separately embodied within the study of alcohol expectancies, craving and cue reactivity.
Although we did not observe early startle reactivity to differ significantly as a function of affective or alcohol picture categories, important relationships were found between alcohol expectancies and early startle reactivity. In particular, individuals with greater positive, arousing and social alcohol expectancies showed relatively greater early startle response magnitudes in response to unpleasant pictures. If we interpret this to reflect a blunted startle inhibition to this picture category, an implication is that individuals with greater expectancies for these key alcohol effects are less likely to be engaged by negative affect stimuli at a fundamental level. Prior work has shown that those at elevated risk for alcohol problems (e.g., positive family history, antisocial personality disorder) have reduced processing of aversive stimuli (Miranda et al., 2002a; Miranda et al., 2002b). As alcohol expectancies have been shown to predict alcohol problems, this adds to an emerging literature regarding alcohol risk and dulled aversive emotional processing.
Startle reflex activity during the later picture period has been reliably modulated by cue valence, with startle activity elevated while viewing aversive pictures and diminished during appetitive picture processing (e.g., Lang, 1995). In the present study, we did not find this expected pattern. However, prior studies almost always examine affective modulation of startle within a balanced set of pleasant, neutral, and unpleasant cues. Inclusion of a fourth category (alcohol) in the present study may have disrupted the typical affective modulation of startle, via a change in the local context whereby each pictures is viewed.
Despite this disruption, an appetitive pattern of inhibited late startle reactivity to alcohol cues was found. However, no significant relationships between late startle activity and alcohol expectancies were observed. In contrast, there were significant relationships between alcohol expectancies and early startle (to affect cues), as well as with several dimensions of subjective report. In terms of startle, it appears that a more immediate, attention-based reactivity probe provides a better linkage with explicit expectancies than a later valence-based probe.
Given that alcohol expectancies can serve as a proxy for increased risk, the blunted startle responding to unpleasant cues among individuals with greater alcohol expectancies may provide a biologically based risk marker for future alcohol use disorders. At higher levels of risk, relationships between reactivity to salient stimuli and alcohol expectancies may not exist as predicted. The current study suggests that increased alcohol expectancies predict decreased reactivity to salient stimuli, particularly those of an aversive nature. It is reasonable to predict that higher risk individuals may respond to other salient stimuli, including pictures of alcohol, in an altered way. Future research comparing reactivity to both affective and alcohol-related cues among high and low risk individuals can hopefully determine variability in the relationships between alcohol expectancies and cue reactivity across level of risk. Furthermore, since the literature indicates that variables such as antisocial personality disorder and family history of alcoholism are related to blunted affective responding (Miranda et al, 2002a; Miranda et al, 2002b), and since this current study finds the same effect among individuals with greater alcohol expectancies, future cue reactivity research would benefit from analyzing the interactions between these three risk variables and their impact on affective processing.
In contrast to the startle response, we observed limited relationships between alcohol expectancies and autonomic cue reactions. Nonetheless, and consistent with the literature, initial cardiac deceleration and skin conductance responding was greatest in the presence of unpleasant cues (e.g., Lang, Bradley, & Cuthbert, 1998), indicating that these cues were processed as particularly arousing and aversive. Indeed, autonomic reactions to unpleasant cues were significantly greater than for pleasant cues, which in turn were not differentiated from neutral cues. It may be that cues selected for the pleasant category in this study were not as salient or emotionally arousing as the unpleasant cues. Alternatively, the inclusion of alcohol cues in the present cue context may have diminished the saliency of generic pleasant cues.
The study has several limitations that should be noted. First, the sample size was relatively small for examining the moderating effect of alcohol expectancies. Nonetheless, several significant relationships were observed, generally in the predicted direction. Second, although the affective pictures were based on normative data, the alcohol cue set was developed specifically for this study. One issue is that, although beer is the most commonly consumed alcoholic beverage in the college-age population (Wechsler et al., 2000), we did not specifically recruit participants for whom beer was the preferred alcohol beverage. Furthermore, the alcohol pictures contained a range of content, including simple figure-ground pictures (e.g., glass of beer), as well as those with a more complex social context (e.g., bar scene). An interesting avenue for further research will be to examine whether these and other characteristics of alcohol pictures (e.g., beverage type) influence reactivity, and the extent to which variability in these reactions are related to alcohol expectancies. Third, as mentioned above, the inclusion of alcohol pictures may have played a role in reducing attention to the other cue types presented. Prior research has not shown that other appetitive pictures (e.g., food) disrupted affective picture processing (e.g., Drobes et al., 2001), yet it is possible that alcohol pictures shown to a young adult sample of drinkers changes the overall picture context sufficiently as to change these patterns.
Despite these limitations, there were several findings of note. To summarize, we observed relationships between self-reported alcohol expectancies and a range of alcohol and affective cue reactions. Those who reported relatively greater positive, arousing, and social alcohol expectancies experienced more cravings, pleasure, and arousal upon exposure to salient alcohol cues, as well as discernible patterns of physiological responding. In particular, those with greater alcohol expectancies exhibited blunted attentional orienting toward negative affect cues, as demonstrated by reduced inhibition of the startle reflex immediately after picture onset. Furthermore, this sample demonstrated an appetitive pattern of responding to alcohol cues, although startle reactions in the presence of these cues were not moderated by alcohol expectancies. Overall, startle reactions during a phase of picture processing that was more immediate, and possibly more unconscious, showed closer associations with traditional alcohol expectancy indices. This suggests that individual alcohol expectancies may be related at a fundamental biological level with more implicit cognitive processes (e.g., attention, orienting) that can be indexed by objective indices of immediate cue engagement. Further research should seek to replicate these findings, determine whether this response pattern can predict future drinking behavior, and explore whether these patterns are modifiable.
Acknowledgments
This study was supported in part by National Institute on Alcohol and Alcoholism grants R01 AA11157 (DD), R37 AA08333 (MG), R01 AA11925 (MG), R01 AA016091 (MG), and National Institute on Drug Abuse grant R21 DA 017906 (DD).
The authors wish to thank the staff of the Alcohol and Substance Use Research Institute at the University of South Florida, and the Tobacco Research and Intervention Program at the Moffitt Cancer Center, for their help in conceptualizing and implementing this research.
Footnotes
The IAPS slide numbers for each affective category were as follows: pleasant – 1710, 2150, 4610, 5600, 7230, 7260, 7330, 8030, 8180, 8200, 8940, 8500; neutral - 1670, 2200, 5520, 7010, 7050, 7080, 7090, 7130, 7150, 7190, 7224, 7490, 7500, 7710; unpleasant - 1090, 1300, 2690, 3000, 3010, 3130, 3150, 3170, 3250, 6200, 8480, 9410. Alcohol images from the Normative Alcohol Picture System were as follows: beer1, beer2, beer 5, beer6. Additional alcohol pictures were selected from internet and stock photography sources, and are available from the authors upon request.
References
- Ashare RL, Hawk LW, Mazzullo RJ. Motivated attention: Incentive effects on attentional modification of prepulse inhibition. Psychophysiology. 2007;44:839–845. doi: 10.1111/j.1469-8986.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin G, Kjellberg A. Orienting activity in two-stimulus paradigms as reflected in heart rate. In: Kimmel HD, Van Olst EH, Orlebeke JF, editors. The orienting reflex in humans. Hillsdale, NJ: Erlbaum; 1979. [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: attention and emotion in startle modification. Psychophysiology. 1993;30:541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. Cambridge: Cambridge University Press; 1999. pp. 157–182. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brown SA, Christiansen BA, Goldman MS. The alcohol expectancy questionnaire: An instrument for the assessment of adolescent and adult alcohol expectancies. Journal of Studies on Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention [CSEA-NIMH] The International Affective Picture System [photographic slides] The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 2002. [Google Scholar]
- Cook EW., III . VPM reference manual. Birmingham, AL: Author; 1997. [Google Scholar]
- Dawson ME, Hazlett EA, Filion DL, Nuetcherlein KH, Schell AM. Attention and schizophrenia: Impaired modulation of the startle reflex. Journal of Abnormal Psychology. 1993;102:633–641. doi: 10.1037//0021-843x.102.4.633. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. Journal of Neuroscience. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Miller EJ, Hillman CH, Bradley MM, Cuthbert BN, Lang PJ. Food deprivation and emotional reactions to food cues: Implications for eating disorders. Biological Psychology. 2001;57:153–177. doi: 10.1016/s0301-0511(01)00093-x. [DOI] [PubMed] [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: Self-report, psychophysiological, and startle probe responses. Experimental & Clinical Psychopharmacology. 1995;3:156–162. [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Expectancy and risk for alcoholism: The unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcoholism: Clinical and Experimental Research. 2002;26:737–746. [PubMed] [Google Scholar]
- Goldman MS, Darkes J. Alcohol expectancy multi-axial assessment: A memory network-based approach. Psychological Assessment. 2004;16:4–15. doi: 10.1037/1040-3590.16.1.4. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Darkes J, Reich RR, Brandon KO. From DNA to cconscious thought: The influence of anticipatory processes on human alcohol consumption. In: Munafo MR, Albery IP, editors. Cognition and addiction. London: Oxford University Press; 2006. pp. 149–187. [Google Scholar]
- Goldman MS, Del Boca FK, Darkes J. Alcohol expectancy theory: the application of cognitive neuroscience. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. New York: Guilford Publications; 1999. [Google Scholar]
- Grossberg S. The attentive brain. American Scientist. 1995;83:438–449. [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Pelham WE, Yartz AR. Attentional modification of short-lead prepulse inhibition and long-lead prepulse facilitation of acoustic startle among preadolescent boys. Psychophysiology. 2002;39:333–339. doi: 10.1017/s0048577201393071. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Pleasure-attenuation of startle is disrupted by lesions of the nucleus accumbens. Neuroreport. 1996;7:1442–1446. doi: 10.1097/00001756-199605310-00024. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion and motivation: Measuring affective perception. Journal of Clinical Neurophysiology. 1998;15:387–408. doi: 10.1097/00004691-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Miranda R, Meyerson LA, Buchanan TW, Lovallo WR. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2002a;26:441–448. [PubMed] [Google Scholar]
- Miranda R, Meyerson LA, Buchanan TW, Lovallo WR. Altered affective modulation of the startle reflex in alcoholics with antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 2002b;27:1901–1911. doi: 10.1097/01.ALC.0000099263.71214.F9. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Stuhlinger M, Mundle G. Appetitive effects of drug cues modeled by pictures of the intake ritual: Generality of cue-modulated startle examined with inpatient alcoholics. Psychopharmacology. 2000;151:428–432. doi: 10.1007/s002130000508. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health: Task Force of the National Advisory Council on Alcohol Abuse and Alcoholism. A Call to Action: Changing the Culture of Drinking at U.S. Colleges. 2004 Retrieved September 24, 2007, Available on line: www.collegedrinkingprevention.gov.
- Saladin ME, Drobes DJ, Coffey SF, Libet JM. The human startle reflex and alcohol cue reactivity: Effects of early versus late abstinence. Psychology of Addictive Behaviors. 2002;16:98–105. doi: 10.1037//0893-164x.16.2.98. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Clifton, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Strizke WGK, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychology of Addictive Behaviors. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Kuo M, Lee H, Dowdell GW. Environmental correlates of underage alcohol use and related problems of college students. American Journal of Preventative Medicine. 2000;19:24–29. doi: 10.1016/s0749-3797(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman U, Spring K, Wittchen H, Holsboer F. Effects of ethanol administration and induction of anxiety-related affective states on the acoustic startle reflex in sons of alcohol-dependent fathers. Alcoholism: Clinical & Experimental Research. 2004;28:424–432. doi: 10.1097/01.alc.0000117835.49673.cf. [DOI] [PubMed] [Google Scholar]