Abstract
Background
Behavioral and psychological symptoms of dementia (BPSD) are often considered to be the greatest challenge in dementia care, leading to increased healthcare costs, caregiver burden, and placement into care facilities. With potential for pharmacological intervention to exacerbate behaviors or even lead to mortality, the development and rigorous testing of non-pharmacological interventions is vital. A pilot of the Tailored Activities Program (TAP) for reducing problem behaviors in people with dementia was conducted in the United States with promising results. This randomized trial will investigate the effectiveness of TAP for reducing the burden of BPSD on persons with dementia and family caregivers within an Australian population. This trial will also examine the cost-effectiveness and willingness to pay for TAP compared with a control group.
Methods
This randomized trial aims to recruit 180 participant dyads of a person with dementia and their caregivers. Participants will have a diagnosis of dementia, exhibit behaviors as scored by the Neuropsychiatric Inventory, and the caregiver must have at least 7 h per week contact. Participants will be randomly allocated to intervention (TAP) or control (phone-based education sessions) groups, both provided by a trained occupational therapist. Primary outcome measure will be the revised Neuropsychiatric Inventory – Clinician rating scale (NPI-C) to measure BPSD exhibited by the person with dementia.
Conclusions
This trial investigates the effectiveness and cost-effectiveness of TAP within an Australian population. Results will address a significant gap in the current Australian community-support base for people living with dementia and their caregivers.
Keywords: dementia, activity, occupational therapy, behavior, non-pharmacological intervention
Introduction
The prevalence of dementia and related progressive neurodegenerative disorders in Australia is on the rise (Australian Institute of Health and Welfare, 2012). These conditions are considered to be the greatest contributor to the burden of disability for older people (Rees, 2009). Neuropsychiatric behaviors, also known as behavioral and psychological symptoms of dementia (BPSD), are common and often considered to be the greatest challenge in dementia care. BPSD often lead to increased healthcare costs, caregiver burden, and placement into permanent care facilities (Cerejeira et al., 2012).
People who exhibit BPSD, which generate higher levels of caregiver burden, such as aggression and agitation, are more likely to be prescribed psychotropic medications (Chiu et al., 2006). Although pharmacological interventions are frequently used to manage these behaviors, they can lead to unwanted or adverse effects, an exacerbation of behaviors, or even add to mortality (Kar, 2009). Secondary to the lack of efficacious pharmacological interventions (Ballard et al., 2006), there has been some focus on the development of non-pharmacological interventions to target different aspects associated with care and management in dementia (Brodaty and Arasaratnam, 2012). The majority of non-pharmacological studies to date have had a focus on caregiver burden and coping (Sorensen et al., 2002; Belle et al., 2006; Brodaty and Donkin, 2009; Brodaty and Arasaratnam, 2012), or on people with dementia already residing in care facilities (Chenoweth et al., 2009; Brodaty and Arasaratnam, 2012). However, a large proportion of people with dementia live in the community, which lends to the need for increased research into community-based interventions (Callahan et al., 2012). In 2011, it was estimated that 70% of people with dementia in Australia were living in the community, with around 200,000 informal caregivers were involved in their care (Australian Institute of Health and Welfare, 2012). A recent meta-analysis investigating non-pharmacological community-based interventions with caregiver involvement found the majority of results across the studies to be positive (Brodaty and Arasaratnam, 2012). Importantly, this study found no adverse side effects resulted from the non-pharmacological interventions reviewed.
The use of purposeful activity as a non-pharmacological intervention for people with dementia has shown promise for enhancing quality of life (QOL) and reducing behaviors such as agitation and depression (Brooker et al., 2007). In particular, activities tailored specifically to a person’s own interests and functional level generate greater levels of engagement (Kolanowski et al., 2005; Kolanowski et al., 2011). The importance of engaging people with dementia based on their interests and roles was highlighted by Kitwood (1997). Kitwood (1997) commented on the importance of maintaining personhood by meeting the unique needs of each individual to be occupied, and that with occupation comes an enhanced sense of self-worth.
One promising study, which implemented the use of activity as an intervention, was a randomized controlled trial (n = 60) of the Tailored Activity Program (TAP) conducted in the United States (Gitlin et al., 2008). The TAP intervention involves an occupational therapist (OT) working with both the caregiver and the person with dementia through tailoring activities (which could include leisure activities or activities of daily living (ADLs) depending on the individual) to the capacity, interests, and roles of the person with dementia. TAP also involves working closely with caregivers for effective implementation (Gitlin et al., 2009). Another study in the Netherlands also demonstrated the potential benefits of a community-based OT intervention in people with dementia and their caregivers. This Dutch intervention involved OT visits to assess the person’s abilities, train family caregivers in skills such as problem-solving and coping strategies, and to implement environmental and compensatory strategies to assist the person with dementia to engage in meaningful activities (Graff et al., 2007). They found that OT improved the daily functioning of the person with dementia (p < 0.0001, as assessed using the process scale of the Assessment of Motor and Process Skills) while also reducing caregiver burden and providing caregivers with a better sense of control over their lives (p < 0.0001; Graff et al., 2006; 2007). These initial positive findings support further research into community-based and OT-based interventions for people living with dementia and their caregivers.
While both of these studies demonstrate the potential benefits for OT in the field of dementia, the outcomes of the Dutch study (Graff et al., 2006) focused on QOL, health status, and mood whereas the TAP program focused on reducing behaviors associated with dementia, which, as aforementioned, are considered to be the most challenging and burdensome aspect of the disease. Therefore, TAP is an OT-based intervention program that shows particular promise within the field of dementia. TAP is novel as caregivers are actively involved in the TAP process from activity development in the beginning to activity simplification for future declines in function of the person with dementia, and generalization of strategies to other care contexts. Through this process, caregivers develop an increased sense of self-efficacy for addressing care issues with their family members. This involvement of both the caregiver and the person with dementia in the community is novel as the majority of non-pharmacological interventions in dementia have been in a residential setting, or involve only one half of the dyad (Zarit and Leitsch, 2001; Schulz et al., 2005; Brodaty and Arasaratnam, 2012).
The US TAP pilot demonstrated the increase in caregiver skills through the following: improved self-efficacy using activities, F(1,43) = 7.1, p = 0.011, Cohen’s d = 0.74; increased mastery, F(1,43) = 6.7, p = 0.013, Cohen’s d = 0.55; and greater use of simplification techniques, F(1,43) = 5.5, p = 0.023, Cohen’s d = 0.71. Caregivers reported benefits of greater ability for the person to be engaged in activity and kept busy, thus leading to fewer hours in which the caregiver was doing things for the person, F(1,42) = 8.8, p = 0.005, Cohen’s d = 1.14, or was “on duty,” F(1,42) = 15.8, p = 0.001, Cohen’s d = 1.01 (Gitlin et al., 2008; 2010). For the person with dementia, this successful TAP pilot trial showed reductions in incidences of BPSD overall, F(1,41) = 7.58, p = 0.009, Cohen’s d = 0.72, with significant reductions in specific behaviors of shadowing, F(1,4) = 58.9, p = 0.003, Cohen’s d = 3.10, agitation, Wald X2(1) = 6.0, p = 0.014, Cohen’s d = 0.75, repetitive questioning, F(1,22) = 5.94, p = 0.023, Cohen’s d = 1.22, and argumentation, Wald X2(1) = 6.6, p = 0.010, Cohen’s d = 0.77 (Gitlin et al., 2008). Although a small-sampled pilot study, these initial results were very promising and thus support further study into TAP.
This trial will investigate the effectiveness and cost-effectiveness for TAP compared with the control group within an Australian population. To date, no such community-based intervention has been investigated in Australia, with most non-pharmacological interventions being tested within the residential care setting. The primary aim of this study is to determine the clinical and cost-effectiveness of TAP in reducing the burden of BPSD in persons with dementia and family caregivers. The primary hypothesis is that TAP will reduce the frequency of behavioral and psychological symptoms of dementia, including agitation, apathy, dysphoria/depression, sleep, and irritability/lability as measured on the revised Neuropsychiatric Inventory – Clinician rating scale (NPI-C) over four months in persons with dementia compared with the control group, who receive education on dementia. Secondary hypotheses are that (i) persons with dementia who receive TAP will have a reduced severity and frequency of all 14 domains of BPSD (NPI-C); (ii) caregivers who receive TAP will report reduced burden, decreased caring time, higher mastery in engaging the person with dementia in activities, and enhanced communication skills; (iii) TAP will be cost-effective; and (iv) persons with dementia who receive TAP will receive less psychotropic medication.
Methods
Study design
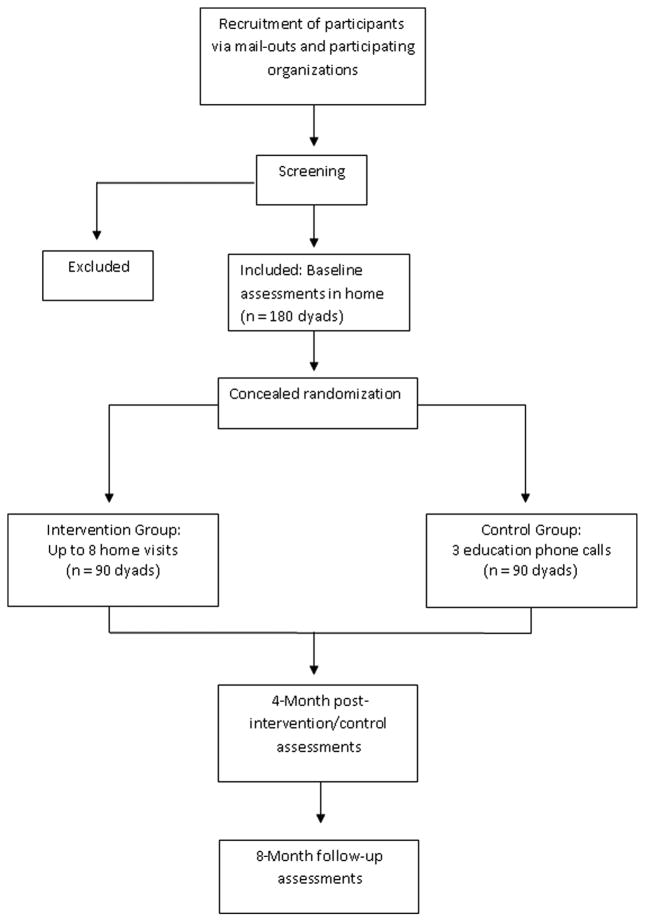
A two-group randomized parallel design clinical trial of 180 dyads is planned in Australia to evaluate the effectiveness of TAP compared with the control group. Once screened, eligible participants will undergo a baseline assessment. Following this, both participants will be either randomized to the TAP program or a control group receiving three educational phone sessions. A blinded research assistant will then conduct post-intervention assessments at four months, and follow-up assessments at eight months. No interaction with participants will occur between the post-intervention and follow-up assessment periods.
Recruitment and participants
Participants will be recruited from community organizations around Sydney via a mail-out strategy. Inclusion criteria for the person with dementia are that they must:
have a diagnosis of dementia;
have a score >3.31 on the Informant Questionnaire on Cognitive Decline for the Elderly (IQCode; Jorm and Jacomb, 1989);
be rated by the caregiver as having a frequency score of at least 2 or above on any item of the Neuropsychiatric Inventory (Cummings et al., 1994) over the past month;
have conversational English;
be able to participate (independently or with some assistance) in at least two ADLs (e.g. bathing, dressing);
if on psychotropic medication, be on a stable dose for the past 60 days; and
if on dementia medication (Ebixa, Reminyl, Exelon, Aricept), be on a stable dose for the past 3 months.
Inclusion criteria for the caregivers are that they must:
report occurrence of one or more behaviors (e.g. apathy, passivity, disruptive behaviors) in person with dementia over the past month;
have conversational English;
be a family member aged at least 18 years and living with person with dementia, or have at least four days or 7 h per week contact;
be accessible by phone; and
indicate a willingness to learn use of activities.
Formal approval has been received from the local institutional Human Research Ethics Committee, and informed consent is to be obtained from the person with dementia and the caregiver involved in the study.
Randomization
Randomization into TAP or control group will occur after baseline assessment for each participant (see Figure 1). The randomization will be generated by an investigator external to the recruitment and the assessment process. Group allocation, where people will be assigned to either intervention or control groups, will involve the use of opaque envelopes for concealment, and be conducted by the OT interventionist who will not be involved in any of the post-intervention or follow-up assessment processes.
Figure 1.
Process of randomization and flow of participants through the Australian TAP study.
Intervention
Participants in the intervention group will receive the TAP protocol involving up to eight contacts from a TAP-trained OT over a four-month period (Gitlin et al., 2009). The OT interventionist has been trained in the TAP protocol, and fidelity checks to ensure appropriate adherence to the protocol will be conducted at regular two-month intervals. This approach to fidelity will be achieved via tele-conference with the interventionist and Professor Gitlin and her team, in which a few randomly selected de-identified cases will be discussed from beginning to end of their TAP participation. The TAP intervention process occurs over three phases, which are outlined in Table 1. The majority of these contacts will involve both the person with dementia and their caregiver. For a more detailed overview of TAP, see Gitlin et al. (2009).
Table 1.
Overview of the TAP intervention protocol phases from visits/contacts one to eight
| PHASE | SESSIONS | CONTENT |
|---|---|---|
| 1 | 1 and 2 |
|
| 2 | 3–6 |
|
| 3 | 7 and 8 |
|
Notes: LACLS = Large Allen’s Cognitive Level Screen; ADM = Allen’s Diagnostic Module; TUG = Timed Up and Go.
Activities will be specifically tailored to the person with dementia to match their abilities, interests, and roles, and may therefore vary greatly depending on each individual participant (e.g. gardening, folding laundry, painting, watching a special DVD). In TAP, the OT will write three specific activity prescriptions, including goals and implementation techniques, from which the caregiver (and sometimes person with dementia) will then choose the activity to be introduced first. The prescription will then be reviewed, and the activity is introduced with the OT and caregiver working together. In each session previous prescriptions will be reviewed, with any issues highlighted by the caregiver addressed through problem-solving with the OT. The process will be repeated for each activity, followed by sessions on activity simplification and generalization of strategies to end the intervention period. Activity simplification refers to modifying an activity, such as simplifying the activity itself or providing more prompting throughout activity engagement, to account for the declines that occur throughout the progression of dementia (Gitlin et al., 2009). Caregivers are supplied with a “Caregiver manual” which contains practical information on activity, communication, behavioral management, and health and safety suggestions for both caregiver and person with dementia. The Australian TAP study will include a sub-cohort of persons who have frontotemporal dementia (FTD). For this group an amended version of the “Caregiver manual” will be supplied, which accommodates the unique symptomatology known for FTD (Piguet et al., 2011; Raskovsky et al., 2011).
Control group
Participants in the control group will receive three telephone education sessions of around 20 min, each based on a general book dealing with dementia. These sessions, conducted by the same interventionist OT that conducts the TAP intervention sessions, will be with the caregiver, and will not involve the person with dementia. During these sessions, the OT and caregiver will have some discussion around specific chapters in the book about general dementia-related issues. These sessions are designed to control for the empathetic listening and dementia-related information received by participants in the intervention group, and are not intended to be an equivalent time in social contact to the TAP intervention sessions. Caregivers in the intervention group will receive a copy of the same book to control the TAP intervention as the only difference between the groups.
Data collection
Prior to baseline visits, caregiver participants will self-complete a range of baseline questionnaires covering information such as demographics, QOL, burden, stress, task management, caregiver confidence, and vigilance. Baseline assessments will then be completed by the research assistant in participant’s home, taking approximately 1.5 h to complete. In-home baseline assessment will involve cognitive assessment of the person with dementia and the caregiver. Remaining assessments are about the person with dementia’s behavior, function, resource utilization, and health, completed via proxy with the caregiver. The importance of evaluating anticipated intervention outcomes for both members of the dyad was discussed by Zarit and Leitsch (2001), who highlighted that if an intervention was targeted at only one member of the dyad, still both would be impacted.
These same assessments will be completed post-intervention at four months, and again at eight-month follow-up in the participant’s home by the same blinded research assistant. Additional assessment will include willingness to pay at eight months. Willingness to pay is a cost-effectiveness measure, and is further discussed under the “economic analysis” section of this paper. An appropriate number of baseline assessments will be conducted in tandem to allow for inter-rater reliability analysis of the NPI-C.
Outcome measures
The primary outcome measure of the study is the revised NPI-C to measure BPSD exhibited by the person with dementia. The NPI-C, developed from the original NPI (Cummings et al., 1994) is based on caregiver report, and assesses the frequency (0 = never – 4 = very frequently) and severity (0 = none – 3 = marked: a major source of behavioral abnormality) of 14 behavioral domains, including delusions, hallucinations, agitation, aggression, dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor disturbance, sleep disorders, appetite and eating disorders, and aberrant vocalizations (de Medeiros et al., 2010). Five of these domains have been highlighted as major outcomes of this study: apathy, agitation, dysphoria/depression, sleep, and irritability/lability. Based on previous experience and trials (Brodaty and Burns, 2012), these five domains were highlighted as major outcomes as they were deemed most likely to respond to the intervention rather than the domains such as hallucinations and delusions. The NPI is a widely used tool; the NPI-C version used in this study has been shown to have sound validity and reliability, and allows for clinician judgment to support the caregiver’s validity of rating (de Medeiros et al., 2010).
Secondary measures will assess all other behavioral domains on the NPI-C, as well as QOL, carer objective and subjective burden, disease state and cognitive status, and cost-effectiveness. All measures, including information on medication use, will be collected at baseline at four and eight months. The only exception being the “Willingness to Pay” cost-effectiveness measure, which is only collected at eight-month follow-up to ensure rater blinding at four months is maintained due to the use of separate intervention and control versions of this study. Table 2 provides an overview of the Australian TAP study outcome measures.
Table 2.
Overview of outcome measures used in the Australian TAP study
| OUTCOME MEASURE | DETAILS OF MEASURE AND PROPERTIES | GENERAL CATEGORY OF USE IN AUS TAP STUDY |
|---|---|---|
| Caregiver Assessment of Function and Upset (CAFU) (Gitlin et al., 2005) | A 15-item measure of caregiver upset with level of dependence of person with dementia in both IADLs and BADLs. Items covered: “telephone,” “shopping,” “food preparation,” “housekeeping,” “laundry,” “travel,” “medications,” “finances,” “mobility,” “eating,” “bathing,” “dressing,” “toileting,” and “grooming,” Internal consistency measured using Cronbach’s α ranged from 0.80–0.91 across all ADL dependence and upset scores. Discriminant validity was demonstrated using SRCCs to find dependence not significantly associated with factors, such as caregiver’s age or number of BPSD. Convergent validity was established using SRCCs to highlight associations between factors such as caregiver’s level of upset and caregiver’s reaction to behaviors for ADLs (rs = 0.34, p < 0.001). | ADL function of person with dementia; caregiver burden |
| Clinical Dementia Rating scale-FTLD (CDR-FTLD) (Morris, 1993; Knopman et al., 2008) | The CDR is a six-domain tool which provides a global assessment of function and cognition. Domains included: “memory,” “orientation,” “judgment and problem-solving,” “community affairs,” “home and hobbies,” and “personal care.” The FTLD version has two extra domains developed to cover the “language” and “behavior, comportment, and personality” aspects of FTD. Domains are scored on a scale from 0 = no impairments, to 3 = severe dementia. Inter-rater reliability measured using κ s ranges from 0.66–0.94 (Oremus et al., 2000). Internal reliability as measured using Cronbach’s α ranges from 0.85–0.92, and convergent validity measured using SRCCs for correlation between CDR and other cognitive and functional tools ranges from 0.53–0.66 (Cedarbaum et al., 2013). | Disease state and cognitive status of person with dementia |
| Disability Assessment for Dementia (DAD) (Gelinas et al., 1999) | A 40-item, informant-based scale with 17 BADL items and 23 IADL items. BADL items include: “hygiene,” “dressing,” “continence,” and “eating,” while IADL items include: “meal preparation,” “telephoning,” “going on an outing,” “finance and correspondence,” “medications,” and “leisure and housework,” DAD total score is corrected to 100, so non-applicable questions are excluded to avoid bias toward activities (e.g. finances; cooking). Lower scores represent greater impairment. Inter-rater and test-retest reliability measured using ICCs were found to be good (0.95 and 0.96 respectively), and found to have a high degree of internal consistency (Cronbach’s α > 0.80). Criterion validity was assessed through significant correlations between total DAD score and scores on the MMSE (Pearson’s r = 0.54) (Folstein et al., 1975) and the GDS (Pearson’s r = −0.70) (Reisberg et al., 1982). | ADL function of person with dementia |
| Depression, Anxiety, and Stress Scale-21 (DASS-21) (Lovibond and Lovibond, 1995) | Self-complete measure consisting of 21 items in total; seven items each for the depression, anxiety, and stress scales. Each item ranges from 0 = “did not apply to me at all,” to 4 = “applied to me very much or most of the time.” Internal consistencies of the individual scales as measured using Cronbach’s α have been reported as 0.947 for depression, 0.897 for anxiety, and 0.933 for stress (Crawford and Henry, 2003). Convergent validity has been demonstrated using PCCs, which found significant correlations between DASS depression and the BDI-II (r = 0.76) (Beck et al., 1996), DASS anxiety and the BAI (r = 0.74) (Beck and Steer, 1990), and DASS stress with the PANAS-N (r = 0.74) (Watson et al., 1998; Gloster et al., 2008). | Caregiver burden |
| EuroQol 5-D (proxy) (Nord, 1991; Brooks and EuroQol Group, 1996) | Proxy measure asking the caregiver to rate how they think the person with dementia would rate their own health if they were able to appropriately communicate it. Consists of a descriptive system of general health status questions, and a visual analogue scale to measure overall health. PCCs have been used to demonstrate construct validity through significant correlations between the EQ-5D and measures such as the Barthel Index (0.67) (Mahoney and Barthel, 1965; Kunz, 2010). | Quality of life of person with dementia |
| Frontotemporal Dementia Rating Scale (FRS) (Mioshi et al., 2010) | A 30-item tool measuring changes in ADL functioning, and behavior, which then indicates a stage in disease progression (from very mild to profound) in people with FTD. Domains include: “behavior,” “outing and shopping,” “household chores and telephone,” “finances,” “medications,” “meal preparation and eating,” and “self-care and mobility.” Unidimensionality of the scale was confirmed using a principal component analysis of the scale items, which found a good level of raw variance (46.4%). Cronbach’s α was used to find a good test consistency of 0.93, and intra-class reliability coefficients were used to demonstrate an inter-rater reliability of 0.994. | Disease state and cognitive status of person with dementia |
| Health Utilities Index (HUI) (Feeny et al., 1995) | A 40-item tool which provides a measure of health status, health-related quality of life, and produces a utility score. Domains include: “vision,” “hearing,” “speech,” “getting around,” “hands and fingers,” “self-care,” “feelings,” “memory,” “thinking,” and “pain and discomfort.” The HUI can be used to describe outcomes, such as efficacy and efficiency, of an intervention and is responsive to changes in health status over time. Demonstrated to have acceptable test-retest reliability with ICCs ranging from 0.62–0.87 across a range of studies (Feeny et al., 2004; Fisk et al., 2005). SRCCs have been used to demonstrate construct validity between the HUI with tools such as the SF-6D (0.69) (Brazier et al., 1998) and the EQ-5D (0.80) (Fisk et al., 2005). | Cost-effectiveness |
| Life Satisfaction Scale (LSS) (Diener et al., 1985). | Self-complete scale measuring an individual’s global judgment of their own life satisfaction. Consists of five items reflecting life satisfaction and well-being, each on a seven-point Likert scale (1 = strongly disagree; 7 = strongly agree). Test-retest reliability has been demonstrated with a coefficient α of 0.85 (Pavot et al., 1991), and inter-rater reliability of 0.73 (Diener et al., 1985). Internal consistency of the scale has been presented as high with coefficient α ranging from 0.79–0.89 (Pavot and Diener, 1993). | Quality of life of caregiver |
| Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) | Brief 30-item measure of general cognitive function covering memory, visuospatial abilities, executive functioning, attention, concentration, language and orientation. Originally designed to detect mild cognitive impairment, is now used globally as a screening tool for cognitive impairment. Test-retest reliability has been demonstrated with a high correlation coefficient of 0.92 (p < 0.001), and good internal consistency found with Cronbach’s α of 0.83. Construct validity of the MoCA has been demonstrated through correlation with the MMSE (r = 0.87, p < 0.001). The MoCA has been shown to exhibit high – excellent sensitivity for identifying mild cognitive impairment (83%–90%) and dementia (94%–100%) when using a cut-off score of 26 (Nasreddine et al., 2005; Smith et al., 2007). Specificity of the MoCA ranges depending on the cut-off score used. One study demonstrated that when using a cut-off of 26, specificity was only 52%; however, if the cut-off was lowered to 24, specificity was raised to 75%, and sensitivity remained high at 87% (Damian et al., 2011). | Cognitive status of both person with dementia and their caregiver |
| Neuropsychiatric Inventory – Clinician rating scale (NPI-C)* (de Medeiros et al. 2010) | A 14-domain tool using caregiver report to assess frequency and severity of 14 behaviors: “delusions,” “hallucinations,” “agitation,” “aggression,” “dysphoria,” “anxiety,” “elation/euphoria,” “apathy/indifference,” “disinhibition,” “irritability/lability,” “aberrant motor disturbance,” “sleep disorders,” “appetite and eating disorders,” and “aberrant vocalizations.” The tool also allows for clinician rating of items based on clinical interviews and any additional information from patient files, and assesses level of caregiver distress related to each behavior reported. Administration time varies due to ability to omit entire domains based on initial screening questions. Inter-rater reliability for each NPI-C item was determined by estimating ICCs, which ranged from 0.50–0.97. PCCs were used to determine convergent validity between NPI-C items and already established measures of BPSD, such as the CSDD with NPI-C depression (r = 0.61); the CMAI with NPI-C agitation and aberrant vocalizations (r = 0.60); and the BPRS with NPI-C delusions and hallucinations (r = 0.60). | BPSD; Major outcomes: apathy, agitation, dysphoria/depression, sleep, and irritability/lability |
| Resource Utilization in Dementia (RUD) (Wimo et al., 1998) | Standardized tool used to collect information about informal and formal resource used in dementia. Administered as an interview with caregiver. Intra-rater reliability has been demonstrated as high, with Cronbach’s α ranging from 0.80–1.0 across all items on the RUD. Validity of responses was supported through comparison of interview-based data with institutionally recorded data (Cronbach’s α ranging from 0.52–0.99), and with actual observations of caregiving time (ICC = 0.74–0.81) (Wimo and Nordberg, 2007; Wimo et al., 2010). | Cost-effectiveness; medication use |
| Vigilance items (Feeney Mahoney et al., 2003) | A brief four-item scale to measure both tangible (“doing things”) and non-tangible (“being there”) aspects of caring. Items require an estimate of time spent in different care situations; the greater the time spent caring, the greater the vigilance score. Internal consistency for ranked item responses was found to have a reliability coefficient Cronbach’s α of 0.66. Scaled vigilance and MMSE scores were shown to be negatively correlated (−0.34, p < 0.001) indicating that as MMSE score declines, scaled vigilance increases. Divergent validity, indicating that the vigilance items measure a new concept, was supported through weak correlation between vigilance and the total RMBPC (Teri et al., 1992) score (r = 0.15, p < 0.001). | Caregiver burden; cost-effectiveness |
| Willingness to Pay (WTP)** (O’Brien and Viramontes, 1994) | A contingent valuation method using surveys of elicitly stated monetary values to provide a direct estimate of a person’s willingness to pay for an intervention or other service. Test-retest reliability has been demonstrated with an acceptable ICC of 0.66, and PCCs found WTP to be correlated with the standard gamble method of measuring health state and utility (von Neumann and Morgenstern, 1953) (r = −0.046), indicating that as a person’s current health state declines, WTP increases. | Cost-effectiveness |
| Zarit Burden Inventory – short (ZBI) (Zarit et al. 1980) | A 22-item self-complete measure assessing disease impact on caregiver in terms of quality of life, psychological impact, and impact on family relationships. Higher scores reflect a greater amount of burden. Test-retest reliability has been demonstrated with an ICC of 0.89, and internal consistency measured using Cronbach’s α was found to have a coefficient of 0.85 (Hébert et al. 1993). | Caregiver burden. |
Notes: BADL = Basic Activities of Daily Living; IADL = Instrumental Activities of Daily Living; FTLD = Frontotemporal Lobar Degeneration; BPSD = Behavioral and Psychological Symptoms of Dementia; BDI-II = Beck Depression Inventory II; BDA = Beck Anxiety Inventory; BPRS = Brief Psychiatric Rating Scale; CMAI = Cohen–Mansfield Agitation Inventory; CSDD = Cornell Scale for Depression in Dementia; GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; PANAS-N = Positive Affect Negative Affect Scale-Negative; RMBPC = Revised Memory and Behavior Problems Checklist; SF-6D = Short Form preference based measure of health; ICC = Intra-class Correlation Coefficient; PCC = Pearson Correlation Coefficient; SRCC = Spearman Rank Correlation Coefficients.
Primary Outcome measure;
Collected at 8-month follow-up only to maintain rater blinding at 4 months due to use of separate intervention and control versions in the current study.
Economic analysis
The primary economic outcome of cost-effectiveness analysis will be used incrementally to evaluate differences in costs and health effects between TAP and control conditions (World Health Organization, 2003). Secondary economic outcomes will include cost per caregiver hour saved as measured by time spent “on duty” and time spent “doing things,” and caregiver’s willingness to pay measured via a contingent valuation method (Oremus and Tarride, 2008).
Statistical analysis
Between-group differences will be analyzed across outcome variables using general linear models of analysis of covariance (ANCOVA), using baseline measures as a covariate. Baseline measures, such as disease stage, psychotropic medication, and gender, will be analyzed univariantly with the difference score. Variables with significant associations (p < 0.2) will be included as covariates in the general linear model. Repeated measures of general linear models will be used to include all three time points across the study. Data will be analyzed using Statistical Package for Social Sciences (SPSS).
Sample size calculation
A total of 180 participant dyads (90 dyads per group) are required to be recruited into this randomized trial. The sample size calculation is based on a small effect size of f = 0.20 in the primary behavioral outcomes at four months, and adjusting for a 20% dropout/missing data rate. This sample size calculation is based on previous trials, which have used the NPI as an outcome measure (Feldman et al., 2001; Campbell et al., 2008). The estimated effect size for this study is calculated to give a power of 80%. The study currently has Department of Health and Ageing (DoHA) seeding funding (trial registration ACTRN12612001161819) for 60 participant dyads, and we will seek further funding to complete the full trial.
Discussion
The results of this study have the potential to inform the development of clinical guidelines for non-pharmacological management of BPSD exhibited by community-living people with dementia. Development of evidence-based non-pharmacological interventions in community dementia care settings is a need supported by a growing body of evidence (Brodaty and Arasaratnam, 2012; Gitlin, 2012; Górska et al., 2013).
From a governmental economic perspective, it is favorable for people with dementia to remain at home with their family caregivers for as long as possible rather than being institutionalized (Brodaty and Donkin, 2009). A prospective study found that caregiver distress related to behaviors exhibited by the person with dementia was a significant predictor of placement into a care facility (de Vugt et al., 2005). The TAP pilot found that 86% of caregivers reported less upset with BPSD (Gitlin et al., 2009), suggesting the potential for TAP to delay institutionalization. The cost-effectiveness of TAP was further suggested through a reduction in time spent caring, and through caregiver’s willingness to pay for the intervention (Gitlin et al., 2009), lending support to a trial of TAP as a potential community-based intervention for dementia management in an Australian setting.
Potential limitations for this Australian TAP study include difficulties with recruiting required number of participants to demonstrate effect and the confounding impact of any dementia interventions participants may be concomitantly involved in such as community care services. To address these issues, a research assistant will be employed throughout the study to ensure ongoing recruitment, participants will be excluded at the screening phase if they are involved in any other clinical trials, and any other involvement with community care services will be noted for analyses.
It is predicted that participation in the TAP intervention will improve outcomes for the person with dementia and their caregiver, such as improved QOL and delayed placement into care facilities. The basis of this is the benefit of purposeful activity, which has been shown to reduce certain behaviors in dementia within the context of care facility (Cohen-Mansfield, 2001; Marshall and Hutchinson, 2001). The US TAP pilot has shown that these initial findings for the benefit of activity are translatable to the community context, and has also suggested the benefit for specifically tailored activities to the individual (Gitlin et al., 2008). The Australian TAP project is a unique opportunity to replicate the original TAP pilot in a definitive and confirmatory trial and to extend its translation globally, while also addressing a significant gap in the current Australian community-support base for people living with dementia and their caregivers. The Australian TAP project will also provide new information about TAP, including an assessment of cost-effectiveness prospectively.
Acknowledgments
This study is funded by an Australian Department of Health and Ageing (DoHA) Seeding Grant (trial registration: ACTRN126120011611819). Laura N Gitlin developed the TAP program with a team of occupational therapists at Thomas Jefferson University, Philadelphia, USA.
Footnotes
Conflict of interest
None.
Description of authors’ roles
C.M. O’Connor drafted the manuscript, and is the research project coordinator on the trial. L. Clemson led the development of the Australian TAP research protocol, contributed to conceptualization and study design, and contributed to the drafting of the manuscript. H. Brodaty jointly developed the Australian TAP research protocol, contributed to conceptualization and study design, and contributed to the drafting of the manuscript. Y.H. Jeon jointly developed the Australian TAP research protocol, contributed to conceptualization and study design, and contributed to the drafting of the manuscript. E. Mioshi jointly developed the Australian TAP research protocol, contributed to conceptualization and study design, and contributed to the drafting of the manuscript. L. Gitlin developed the TAP protocol, contributed to the Australian TAP research protocol, and to the drafting of the manuscript.
References
- Australian Institute of Health and Welfare. Dementia in Australia. Vol. 70. Canberra, Australia: AIHW; 2012. Cat. No. AGE. [Google Scholar]
- Ballard CG, Waite J, Birks J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database of Systematic Reviews. 2006;1:CD003476. doi: 10.1002/14651858.CD003476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Belle SH, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Annals of Internal Medicine. 2006;145:727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. Journal of Clinical Epidemiology. 1998;51:1115–1128. doi: 10.1016/s0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. American Journal of Psychiatry. 2012;169:946–953. doi: 10.1176/appi.ajp.2012.11101529. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Burns K. Nonpharmacologcial management of apathy in dementia: a systematic review. American Journal of Geriatric Psychiatry. 2012;20:549–564. doi: 10.1097/JGP.0b013e31822be242. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues in Clinical Neuroscience. 2009;11:217–228. doi: 10.31887/DCNS.2009.11.2/hbrodaty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker DJ, Woolley RJ, Lee D. Enriching opportunities for people living with dementia in nursing homes: an evaluation of a multi-level activity-based model of care. Aging & Mental Health. 2007;11:361–370. doi: 10.1080/13607860600963679. [DOI] [PubMed] [Google Scholar]
- Brooks R, EuroQol Group. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Callahan CM, et al. Transitions in care for older adults with and without dementia. Journal of the American Geriatrics Society. 2012;60:813–820. doi: 10.1111/j.1532-5415.2012.03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N, et al. Impact of cholinesterase inhibitors on behavioral and psychological symptoms of Alzheimer’s disease: a meta-analysis. Clinical Interventions in Aging. 2008;3:719–728. doi: 10.2147/cia.s4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, et al. Rationale for use of the clinical dementia rating sum of boxes as a primary outcome measure for Alzheimer’s disease clinical trials. Alzheimer’s & Dementia. 2013;9:S45–S55. doi: 10.1016/j.jalz.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Frontiers in Neurology. 2012;3:1–21. doi: 10.3389/fneur.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth L, et al. Caring for Aged Dementia Care REsident Study (CADRES), a cluster-randomised trial of person-centred-care, dementia care mapping and usual care in dementia. Lancet Neurology. 2009;8:317–325. doi: 10.1016/S1474-4422(09)70045-6. [DOI] [PubMed] [Google Scholar]
- Chiu MJ, Chen TF, Yip PK, Hua MS, Tang LY. Behavioral and psychologic symptoms in different types of dementia. Journal of the Formosan Medical Association. 2006;105:556–562. doi: 10.1016/S0929-6646(09)60150-9. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. American Journal of Geriatric Psychiatry. 2001;9:361–381. [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. British Journal of Clinical Psychology. 2003;42:111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/WNL.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Damian AM, et al. The Montreal Cognitive Assessment and the Mini-Mental State Examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dementia and Geriatric Cognitive Disorders. 2011;31:126–131. doi: 10.1159/000323867. [DOI] [PubMed] [Google Scholar]
- de Medeiros K, et al. The neuropsychiatric inventory-clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. International Psychogeriatrics. 2010;22:984–994. doi: 10.1017/S1041610210000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Verhey FRJ. A prospective study of the effects of behavioural symptoms on the institutionalization of patients with dementia. International Psychogeriatrics. 2005;17:577–589. doi: 10.1017/S1041610205002292. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larson RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Earhart CA. Allen Diagnostic Modules: Manual. Colchester, CT: S&S Worldwide; 2006. [Google Scholar]
- Feeney Mahoney D, Jones RN, Coon DW, Mendelsohn AB, Gitlin LN, Ory M. The caregiver vigilance scale: application and validation in the Resources for Anhancing Alzheimer’s Caregiver Health (REACH) project. American Journal of Alzheimer’s Disease and Other Dementias. 2003;18:39–48. doi: 10.1177/153331750301800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny CM, et al. The stability of utility scores: test-retest reliability and the interpretation of utility scores in elective total hip arthroplasty. Quality of Life Research. 2004;13:15–22. doi: 10.1023/B:QURE.0000015307.33811.2d. [DOI] [PubMed] [Google Scholar]
- Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems: health utilities index. Pharmacoeconomics. 1995;7:490–502. doi: 10.2165/00019053-199507060-00004. [DOI] [PubMed] [Google Scholar]
- Feldman H, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57:613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Brown MG, Sketris IS, Metz LM, Murray TJ, Stadnyk KJ. A comparison of health utility measures for the evaluation of multiple sclerosis treatments. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:58–63. doi: 10.1136/jnnp.2003.017897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gelinas I, Gauthier L, Mcintyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. American Journal of Occupational Therapy. 1999;53:471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- Gitlin LN. Good news for dementia care: caregiver interventions reduce behavioral symptoms in people with dementia and family distress. American Journal of Psychiatry. 2012;169:894–897. doi: 10.1176/appi.ajp.2012.12060774. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, et al. Caregiver appraisals of functional dependence in individuals with dementia and associated caregiver upset: psychometric properties of a new scale and response patterns by caregiver and care recipient characteristics. Journal of Aging and Health. 2005;17:148–171. doi: 10.1177/0898264304274184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Huack WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. American Journal of Geriatric Psychiatry. 2008;16:229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, et al. The tailored activity program to reduce behavioral symptoms in individuals with dementia: feasibility, acceptability, and replication potential. Gerontologist. 2009;49:428–439. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Hodgson N, Jutkowitz E, Pizzi L. The cost-effectiveness of a nonpharmacologic intervention for individuals with dementia and family caregivers: the tailored activity program. American Journal of Geriatric Psychiatry. 2010;18:510–519. doi: 10.1097/JGP.0b013e3181c37d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloster AT, et al. Psychometric properties of the Depression Anxiety and Stress Scale-21 in older primary care patients. Journal of Affective Disorders. 2008;110:248–259. doi: 10.1016/j.jad.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górska S, et al. Service-related needs of older people with dementia: perspectives of service users and their unpaid caregivers. International Psychogeriatrics. 2013;25:1107–1114. doi: 10.1017/S1041610213000343. [DOI] [PubMed] [Google Scholar]
- Graff MJL, Vernooij-Dassen MJM, Thijssen M, Dekker J, Hoefnagels WHL, Olde-Rikkert MGM. Community-based occupational therapy for patients with dementia and their care givers: randomised controlled trial. British Medical Journal. 2006;333:1196. doi: 10.1136/bmj.39001.688843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff MJL, Vernooij-Dassen MJM, Thijssen M, Dekker J, Hoefnagels WHL, Olde-Rikkert MGM. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. Journal of Gerontology: Medical Sciences. 2007;62A:1002–1009. doi: 10.1093/gerona/62.9.1002. [DOI] [PubMed] [Google Scholar]
- Hébert R, Bravo G, Girouard D. Fidélité de la traduction française de trois instruments d’évaluation des aidants naturels de malades déments. Canadian Journal on Aging. 1993;12:324–337. [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): sociodemographic correlates, reliability, validity and some norms. Psychological Medicine. 1989;19:1015–1022. doi: 10.1017/S0033291700005742. [DOI] [PubMed] [Google Scholar]
- Kar N. Behavioral and psychological symptoms of dementia and their management. Indian Journal of Psychiatry. 2009;51:S77–S86. [PMC free article] [PubMed] [Google Scholar]
- Kitwood T. The experience of dementia. Aging & Mental Health. 1997;1:13–22. doi: 10.1080/13607869757344. [DOI] [Google Scholar]
- Knopman DS, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski A, Litaker M, Buettner L. Efficacy of theory-based activites for behavioral symptoms of dementia. Nursing Research. 2005;54:219–228. doi: 10.1097/00006199-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT. A randomized clinical trial of theory-based activities for the behavioural symptoms of dementia in nursing home residents. Journal of the American Geriatrics Society. 2011;59:1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S. Psychometric properties of the EQ-5D in a study of people with mild to moderate dementia. Quality of Life Research. 2010;19:425–434. doi: 10.1007/s11136-010-9600-1. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal. 1965;14:61–65. [PubMed] [Google Scholar]
- Marshall MJ, Hutchinson SA. A critique of research on the use of activities with persons with Alzheimer’s disease: a systematic literature review. Journal of Advanced Nursing. 2001;35:488–496. doi: 10.1046/j.1365-2648.2001.01887.x. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, et al. The Montreal Cocnitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nord E. EuroQol: health-related quality of life measurement. Valuations of health states by the general public in Norway. Health Policy. 1991;18:25–36. doi: 10.1016/0168-8510(91)90141-J. [DOI] [PubMed] [Google Scholar]
- O’Brien B, Viramontes JL. Willingness to pay: a valid and reliable measure of health state preference? Medical Decision Making. 1994;14:289–297. doi: 10.1177/0272989X9401400311. [DOI] [PubMed] [Google Scholar]
- Oremus M, Perrault A, Demers L, Wolfson C. Review of outcome measurement instruments in Alzheimer’s disease drug trials: psychometric properties of global scales. Journal of Geriatric Psychiatry and Neurology. 2000;13:197–205. doi: 10.1177/089198870001300404. [DOI] [PubMed] [Google Scholar]
- Oremus M, Tarride JE. A systematic review of the use of contingent valuation in Alzheimer’s disesase research. Dementia. 2008;7:461–480. doi: 10.1177/1471301208096630. [DOI] [Google Scholar]
- Pavot W, Diener E. Review of the satisfaction with life scale. Psychological Assessment. 1993;5:164–172. [Google Scholar]
- Pavot W, Diener E, Colvin CR, Sandvik E. Further validation of the satisfaction with life scale: evidence for the cross-method convergence of well-being measures. Journal of Personality Assessment. 1991;57:149–161. doi: 10.1207/s15327752jpa5701_17. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurology. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Raskovsky K, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G. Dementia: Facing the Epidemic. A Vision for a World Class Dementia Care System. Sydney, Australia: Alzheimer’s Australia; 2009. [Google Scholar]
- Reisberg B, Ferris SH, De Leon MJ, Crook T. The global deterioration scale (GDS) for assessment of primary degenerative dementia. American Journal of Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM, Klinger JN. Evidence-based caregiver interventions in geriatric psychiatry. Psychiatric Clinics of North America. 2005;28:1007–1038. doi: 10.1016/j.psc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Canadian Journal of Psychiatry. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: The revised memory and behavior problems checklist. Psychology and Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- von Neumann J, Morgenstern O. Theory of Games and Economic Behavior. Princeton, NJ: Princeton University Press; 1953. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1998;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wimo A, Nordberg G. Validity and reliability of assessments of time comparisons of direct observations amd estimates of time by the use of the resource utilization in dementia (RUD) instrument. Archives of Gerontology and Geriatrics. 2007;44:71–81. doi: 10.1016/j.archger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Wimo A, Wetterholm AL, Mastey V, Winblad B. Evaluation of the resource utilization and caregiver time in anti-dementia drug trials – a quantitative battery. In: Wimo A, Jonsson B, Karlsson G, Winblad B, editors. The Health Economics of Dementia. London: John Wiley and sons; 1998. pp. 465–499. [Google Scholar]
- Wimo A, Jonsson L, Zbrozek A. The Resource Utilization in Dementia (RUD) instrument is valid for assessing informal care time in community-living patients with dementia. Journal of Nutrition, Health & Aging. 2010;14:685–690. doi: 10.1007/s12603-010-0316-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Methods for generalized cost-effectiveness analysis. In: Edejer TTT, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL, editors. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organisation; 2003. pp. 3–97. [Google Scholar]
- Zarit SH, Leitsch SA. Developing and evaluating community-based intervention programs for Alzheimer’s patients and their caregivers. Aging & Mental Health. 2001;5:84–98. doi: 10.1080/13607860120044864. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, Back-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]