Abstract
Live donation benefits recipients, but the long-term consequences for donors remain uncertain. RELIVE surveyed kidney donors (N=2,455; 61% women; mean age 58, aged 24 – 94; mean time from donation 17 years, range 5 – 48 years) using the SF-36. The 95% CIs for white and African-American donors included or exceeded SF-36 norms. Over 80% of donors reported average or above average health for their age and sex (p<0.0001). Donors’ age-sex adjusted physical component summary [PCS] scores declined by half a point each decade after donation (p=0.0027); there was no decline in mental component summary [MCS] scores. White donors’ PCS scores were 3 points higher (p = 0.0004) than non-whites’; this difference remained constant over time. 9% of donors had impaired health (PCS or MCS score > 1 SD below norm). Obesity, history of psychiatric difficulties, and non-white race were risk factors for impaired physical health; history of psychiatric difficulties was a risk factor for impaired mental health. Education, older donation age, and a first degree relation to the recipient were protective factors. 1% reported that donation affected their health very negatively. Enhanced pre-donation evaluation and counseling may be warranted, along with ongoing monitoring for overweight donors.
Keywords: Kidney donor, Living donor, organ donation, Kidney, quality of life
INTRODUCTION
The benefits of living kidney donation to the recipient are well-established (1–2), but uncertainty remains regarding the long-term impacts on living donors (3). Studies have confirmed that surgical complication rates are low, and serious psychiatric sequelae are rare (4–6). Reports suggest that the majority of living donors experience levels of health-related quality of life (HRQOL) similar to or exceeding that of the general population (7–9). Nevertheless, it has been consistently shown that some donors (< 5%) experience significant psychological distress or retain highly negative attitudes about donation (10–11). Information on the predictors and correlates of poor HRQOL outcomes is extremely limited (5), particularly for minority donors or the growing number of overweight donors (3, 6, 12–14).
The Renal and Lung Living Donors Evaluation Study (RELIVE) provided a unique opportunity to investigate the long-term HRQOL of living donors. RELIVE conducted an extensive chart review to identify all living donor surgeries conducted from 1963 through 2005 at three large US transplant centers (15). Where possible, donor current address and phone contact information were derived from these records, and surviving donors requested to complete HRQOL questionnaires. The SF-36 health survey (16) was included in this questionnaire because it is a standardized instrument with norms for US adults overall and grouped by age and sex. SF-36 results from the African-American Health project (AAHP) (17) provided non-donor comparison data for African-American donors. The study objectives were to provide a comprehensive analysis of the HRQOL of a large and representative sample of living kidney donors, and to identify predictors of poor long-term HRQOL outcomes. We hypothesized that donors’ current HRQOL would be influenced by factors known at the time of donation, and also influenced by perceptions regarding the donation experience such as expected recovery time, time to resumption of usual activities, and comfort with decision to donate, among other factors.
METHODS
The RELIVE study was designed to evaluate the medical and health-related quality of life outcomes of living kidney donors at three large US transplant centers. Details about RELIVE have been published elsewhere (15).
Study Design and Population
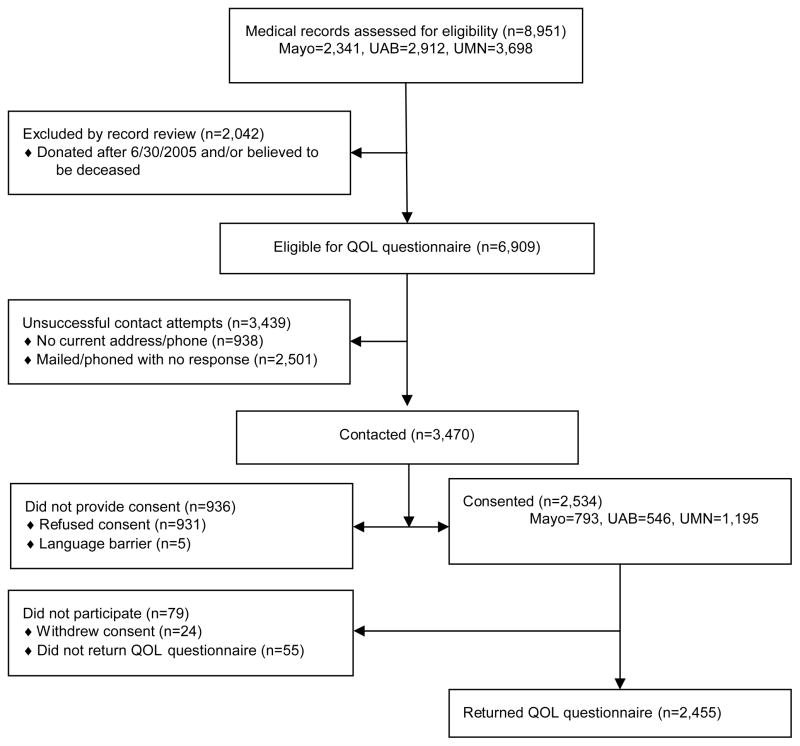
An observational, cross-sectional survey of living kidney donors at least five years from time of donation was conducted. Enrollment is detailed in Figure 1. Medical records from all living kidney donor surgeries conducted at the three study sites (N=8,951) were reviewed. Surgeries performed after July 1, 2005 and known deaths were excluded, leaving 6,909 potentially eligible donors (donation years 1963 – 2005). A study invitation letter was sent to each donor. If no response was received, a follow-up letter and at least two telephone calls were made by study coordinators. Although a fee-based internet service was used to update address and phone numbers, current address and phone information could not be found for 13.6% [n= 938]. Thirty-six percent overall [n= 2,501] did not respond to any study contact attempts. This varied by race: 34% of white donors, 58% of African-American donors, and 27% of donors of other or unknown race did not respond to any study contacts (Supplemental Table A). The true status of these donors is unknown; it is likely that some were passive refusals and others were never contacted. Fifty percent [n= 3,470] of the potentially eligible donors were contacted: 2,455 consented and completed a study questionnaire; 931 refused consent; 79 consented but failed to return the questionnaire, and 5 were unable to participate due to a language barrier. Among those contacted, rates of questionnaire completion were similar across transplant centers (67% to 76%) and for white and African-American donors (71% and 72%, respectively), and somewhat lower (65%) for donors of other or unknown race (Supplemental Table A). Participation rates diminished with increasing time from donor surgery, from 79% for procedures done since January 2000 to 55% for those done in the 1960s.
Figure 1. Enrollment Flow Diagram.
This diagram describes how the sample of living organ donors who completed quality of life (QOL) questionnaires for this study (N=2,455) was derived from a comprehensive review of the medical records of all live donor kidney transplants conducted at three large clinical sites: University of Minnesota (UMN), The Mayo Clinic - Rochester, MN, and University of Alabama, Birmingham (UAB).
This study was approved by the institutional review boards of the transplant (UAB: IRB approval number X070604010; UMN: IRB approval number 0905M66501; Mayo: IRB approval number 09-001345) and data coordinating centers (IRB approval number CR00032674 and protocol number HUM00004345). Informed consent was given by each participant.
Data Collection
HRQOL questionnaires were mailed, self-administered, and returned in postage-paid envelopes. Questionnaires included the SF-36, version 2 (16,18), a self-assessment of day-to-day function and well-being over the previous four weeks in eight domains of HRQOL. Domain scores are standardized to the age and sex distribution of the US adult population and combined to form physical and mental component summary (PCS and MCS) scores. Higher scores indicate better health. SF-36 results were compared to the National Health Measurement Survey (NHMS) (19). Supplemental Table B provides additional details about scoring and interpreting the SF-36.
Statistical Analyses
Differences between respondents and non-respondents by demographic characteristics, and differences among donors grouped by race, were examined using chi-square or Fisher’s exact tests and one-way analyses of variance or rank tests for categorical and continuous predictors, respectively. Donors’ SF-36 scores were compared to norms and other samples using t-tests and rank tests. Quantile regression estimated the impact of race, procedure (laparoscopic or open), and time from donation on sex-by-age adjusted PCS and MCS scores. Multivariable logistic regression analyses identified factors that predicted a poor HRQOL outcome. Factors known prior to the time of donation surgery were used to fit an initial prediction model using a best subsets approach. Additional models tested whether donation experience variables were associated with poor HRQOL, after adjustment for the initial model. Missing data were imputed using IVEWare (20) and analyses were conducted using SAS 9.2. See the online supplement for statistical methodology details. A meaningful difference was defined as 3 points on the SF-36 MCS or PCS. Statistical significance was determined using a threshold of p < 0.01 to control Type I error and to focus on results where differences were most likely to be both statistically significant and clinically meaningful.
RESULTS
RELIVE donors (n=2,455) were 15 to 74 years old at time of donation and 24 to 94 years old at time of study. The mean age at donation was 41 years, and women outnumbered men (61%). On average, 17 ±10 years had elapsed since donation surgery. The majority of donors were white (93%), married (75%), educated beyond high school (77%), and working full (52%) or part-time (12%) (Table 1). Most were biologically related to their recipient as a sibling (41%), parent (18%), or child (13%). Nine percent were spouses. Seven percent were a friend of the recipient and 6% were not a relative or friend of the recipient. Five percent of donors identified themselves as black or African-American (n=113), and 1% as Hispanic or Latino (n=31). White donors were older than African-American donors, and were more likely to be currently married. Weight at time of donation, measured by body mass index (BMI), did not differ by race.
Table 1.
Characteristics of Donors, Overall and Grouped by Self-Reported Race
| Race | ||||
|---|---|---|---|---|
| White or European American | Black or African- American | Other or Unknown | ||
| All Donors | (n = 2455) | (n = 2282) | (n = 113) | (n = 60) |
|
| ||||
| M(SD) | M(SD) | M(SD) | M(SD) | |
|
| ||||
| Age at donation* | 41(11) | 41(11) | 36(11) | 38(11) |
| Age at survey* | 58(11) | 58(11) | 52(10) | 53(12) |
| Years since donation | 17(10) | 17(10) | 15(8) | 15(8) |
| BMI at donation | 26(5) | 26(5) | 27(5) | 26(4) |
|
| ||||
| N(%) | N(%) | N(%) | N(%) | |
|
| ||||
| Female | 1505(61) | 1403(61) | 71(63) | 31(52) |
| Hispanic/Latino | 31(1) | 18(1) | 0( 0) | 13(22) |
| Relationship of donor to recipient | ||||
| Parent | 450(18) | 422(18) | 17(15) | 11(18) |
| Child | 316(13) | 283(12) | 21(19) | 12(20) |
| Sibling | 1011(41) | 942(41) | 50(44) | 19(32) |
| Other relative | 130(5) | 116(5) | 9(8) | 5(8) |
| Spouse | 219(9) | 208(9) | 8(7) | 3(5) |
| Friend | 173(7) | 165(7) | 4(4) | 4(7) |
| Other unrelated | 149(6) | 140(6) | 3(3) | 6(10) |
| Missing | 7( 0) | 6( 0) | 1(1) | 0( 0) |
| BMI at donation | ||||
| Less than 25 | 1092(44) | 1021(45) | 42(37) | 29(48) |
| 25 to 29.9 | 883(36) | 823(36) | 42(37) | 18(30) |
| 30 to 34.9 | 329(13) | 296(13) | 21(19) | 12(20) |
| 35 or higher | 102(4) | 95(4) | 6(5) | 1(2) |
| Missing | 49(2) | 47(2) | 2(2) | 0( 0) |
| Type of surgical procedure | ||||
| Open | 1630(66) | 1514(66) | 81(72) | 35(58) |
| Laparoscopic | 822(33) | 765(34) | 32(28) | 25(42) |
| Unknown | 3( 0) | 3( 0) | 0( 0) | 0( 0) |
| Educational attainment at survey | ||||
| Less than high school | 66(3) | 59(3) | 5(4) | 2(3) |
| High school | 497(20) | 468(21) | 16(14) | 13(22) |
| Some college or tech school | 920(37) | 846(37) | 50(44) | 24(40) |
| College degree | 510(21) | 477(21) | 22(19) | 11(18) |
| Graduate degree | 449(18) | 421(18) | 20(18) | 8(13) |
| Missing | 13(1) | 11( 0) | 0( 0) | 2(3) |
| Marital status at survey* | ||||
| Married or living together | 1852(75) | 1750(77) | 63(56) | 39(65) |
| Separated, divorced, widowed | 449(18) | 408(18) | 30(27) | 11(18) |
| Never married | 141(6) | 113(5) | 20(18) | 8(13) |
| Missing | 13(1) | 11( 0) | 0( 0) | 2(3) |
| Employment at survey* | ||||
| Working full-time | 1272(52) | 1181(52) | 67(59) | 24(40) |
| Working part-time | 299(12) | 280(12) | 7(6) | 12(20) |
| Not working for pay | 770(31) | 729(32) | 27(24) | 14(23) |
| Unemployed | 80(3) | 63(3) | 10(9) | 7(12) |
| Missing | 34(1) | 29(1) | 2(2) | 3(5) |
| Transplant center* | ||||
| Mayo Clinic | 773(31) | 748(33) | 6(5) | 19(32) |
| University of Alabama | 544(22) | 442(19) | 92(81) | 10(17) |
| University of Minnesota | 1138(46) | 1092(48) | 15(13) | 31(52) |
| Recipient vital status (according to donor) | ||||
| Alive | 1467(60) | 1368(60) | 67(59) | 32(53) |
| Deceased | 952(39) | 882(39) | 46(41) | 24(40) |
| Unknown or missing | 36(2) | 32(2) | 0( 0) | 4(7) |
| Recipient graft status (according to donor) | ||||
| Functioning | 1060(43) | 997(44) | 41(36) | 22(37) |
| Functioning, but with problems | 98(4) | 87(4) | 7(6) | 4(7) |
| Not functioning | 307(13) | 283(12) | 19(17) | 5(8) |
| Unknown or missing | 990(40) | 915(40) | 46(41) | 29(48) |
Significant differences between white and African-American donors, p<0.01. Eight donors were missing information about ethnicity. Individuals not working for pay include homemakers, seasonal workers, retirees, and students.
Compared to donors who declined study participation, RELIVE donors were slightly younger at donation (mean ages 40.5 vs. 41.5), donated more recently (16.3 vs. 19.5 years), had higher educational attainment at time of donation (56% vs. 39% educated beyond high school), and were more likely to be unrelated to their recipient (13% vs. 6%, all p < 0.01) (See Supplemental Table A).
To investigate the representativeness of our sample, we compared the demographics of RELIVE donors to 2009 US kidney donors, using data published in the OPTN/SRTR 2010 Annual Report (See Supplemental Table C). Although the mean age at donation was very close (about 41 years) and the percent of women (61%) was the same, substantially fewer RELIVE donors were African-American (5% versus 15%) or Hispanic/Latino (1% versus 14%) compared to the 2009 US donor data. Moreover, most of the African-American RELIVE donors (81%) had donated at a single site: the University of Alabama at Birmingham. Thus, the RELIVE donors are less ethnically diverse than current donors.
Donor HRQOL Profile
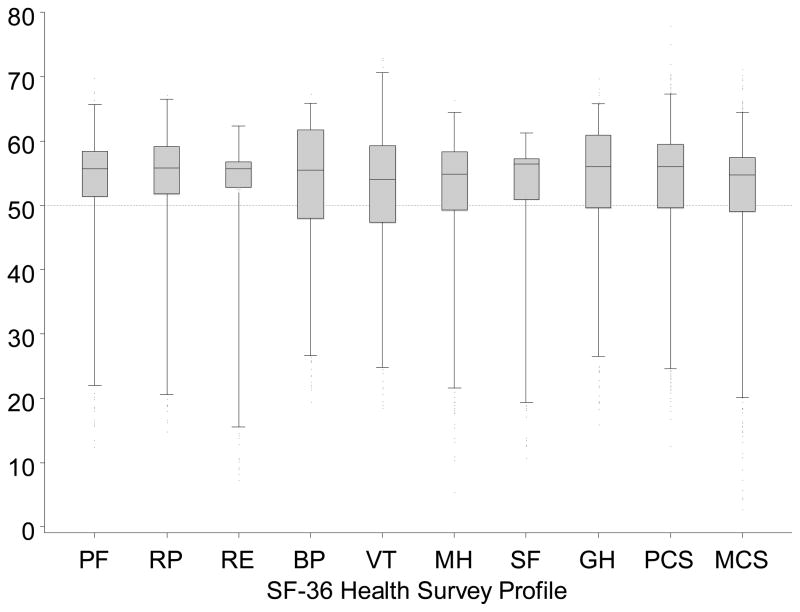
Donors’ SF-36 profile scores are depicted in Figure 2. Donors reported significantly better function and well-being relative to the US adult population in all domains (Table 2, p<0.0001 all). Overall physical and mental health were also higher (PCS scores, 51±9, p<0.0001; MCS scores, 53 ± 9, p<0.0001). Compared to expected SF-36 profiles for people of their own sex and age, donors’ scores were significantly higher (p<0.0001, for all). Between 80% and 87% of donors’ domain scores and 84% of their MCS and PCS values were in the average or above average range for their sex and age (Table 2).
Figure 2. SF-36 profile of RELIVE donors.
Boxplots display donor scores for all the scales in the SF-36 profile. Higher scores indicate better health states. All SF-36 scales are standardized to have a mean of 50 and standard deviation of 10 in the US general population. A dotted line indicates the population mean on the y-axis. Boxes extend from the 25th to 75th percentiles, whiskers (vertical lines) extend from the 1st to the 99th percentiles. Abbreviations: physical component summary score (PCS), mental component summary score (MCS), physical health (PH), impact of physical health on role functioning at home and at work (RP), bodily pain (BP), vitality (VT), general health perceptions (GH), social functioning (SF), impact of mental health on role functioning (RE), and mental health (MH).
Table 2.
RELIVE donors’ SF-36 Profile and Distribution of Scores relative to US Norms
| SF-36 scores | Percent of donors with above or below average scores based on their sex and age (in 10 year categories) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Scale1 | Mean2, 3 | SD | Below Average (>5 points below) % | Average (within 5 points) % | Above Average (>5 point above) % | |
| PCS (N=2415) | 51 | 9 | 16 | 28 | 56 | |
| MCS (N=2415) | 53 | 9 | 16 | 37 | 47 | |
| PF (N=2450) | 51 | 9 | 14 | 29 | 57 | |
| RP (N=2433) | 52 | 9 | 14 | 23 | 63 | |
| BP (N=2435) | 52 | 10 | 20 | 30 | 50 | |
| GH (N=2437) | 53 | 9 | 15 | 32 | 54 | |
| VT (N=2439) | 53 | 9 | 19 | 31 | 49 | |
| SF (N=2441) | 52 | 9 | 14 | 19 | 68 | |
| RE (N=2434) | 52 | 8 | 13 | 26 | 61 | |
| MH (N=2439) | 53 | 9 | 17 | 33 | 50 | |
Abbreviations: physical component summary (PCS) score and mental component summary (MCS) score, physical health (PH), impact of physical health on role functioning at home and at work (RP), bodily pain (BP), vitality (VT), general health perceptions (GH), social functioning (SF), impact of mental health on role functioning (RE) and mental health (MH).
Scores were transformed to have a mean of 50 and standard deviation of 10 in the general U.S. population. Higher scores indicate better health states for all scales. All donor means are significantly different from SF-36 version 2 norms for the U.S. general population: one-sample t-tests, p-values <.0001, all.
N= between 2,415 for PCS and MCS and 2,450 for PF. Missing values ranged from 5 (for PF) to 40 (for the MCS and PCS)
The HRQOL profiles of white and African-American donors are shown in Figure 3. The 95% confidence intervals for both white and African-American donors either included or exceeded US norms. White donors reported higher levels of social functioning than African-American donors (p = 0.0007); there were no other differences. Norms for people aged 55 to 64 are 5 points lower (worse health) on the PCS (unadjusted) than norms for those aged 35 to 44, yet these donors (current mean age 58) reported higher PCS values than the younger US population (1998 US census population: 51% women, mean age 35 years).
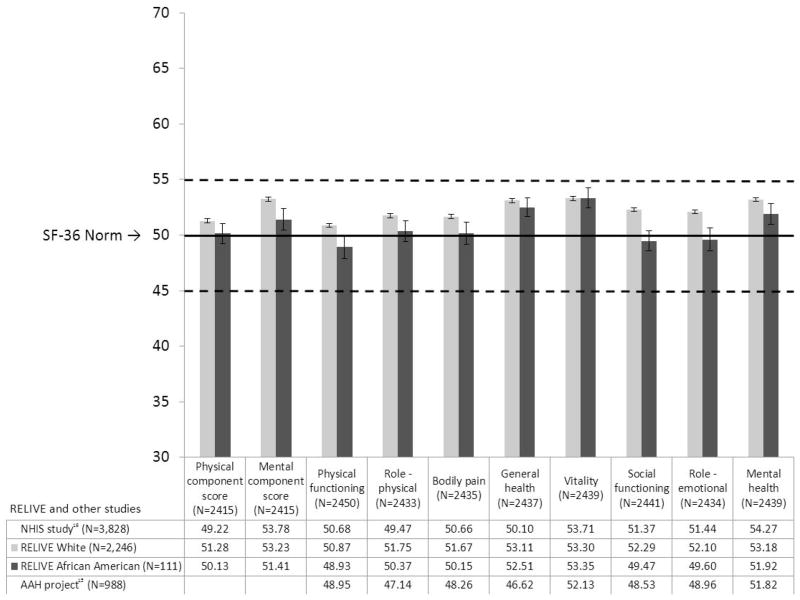
Figure 3. SF-36 Profiles of RELIVE white and African-American donors.
Mean unadjusted SF-36 scores of white and African-American donors are shown as bars. Race data were obtained by self-report. The horizontal solid line marks the population norm and dotted lines mark ± 0.5 SD (the range for average scores). White donors had higher scores for social functioning than African-American donors (p = 0.0007); other SF-36 scores were not significantly different at p=0.01. Mean (± 1 SE) scores for RELIVE donors and two comparison groups are presented below the chart. Results from the National Health Measurement Survey (NHMS), a representative telephone survey of US adults 35–89 years old conducted in 2005–2006 (17) are shown in the first line. RELIVE donors’ (all races combined) SF-36 scores were significantly higher than NHMS results for the PCS, role physical, bodily pain, general health perceptions, and social functioning (ps from 0.006 to <0.0001); scores for physical functioning, vitality, role emotional, and the MCS did not differ; and NHMS mental health results were higher than RELIVE donors’ scores (p <0.0001). Results from the African-American Health project (AAHP), a population-based, in-home survey of 998 African-American adults aged 49 to 65 living in Missouri conducted 2000–2001 (18), are shown in the bottom line beneath the chart. SF-36 overall and domain scores of African-American donors and AAHP participants were not significantly different, except for general health perceptions, where African-American donors reported higher scores (p < 0.0001).
Comparisons to Other US Populations
Donor SF-36 scores are shown at the bottom of Figure 3, along with results from the NHMS and the AAHP (17, 19). Most NHMS profile scores are higher than the SF-36 norms. Despite this, donors’ profiles compared favorably with NHMS results. Donors’ PCS, MCS, and scores on 7 of 8 domains were significantly higher or not different than NHMS results, and when the NHMS mental health score was higher, the difference was less than 1.2 points, below the SF-36 threshold for a clinically meaningful difference. HRQOL reports from African-American donors compared favorably to AAHP results, with donors reporting better general health perceptions (p<0.0001), and no other differences.
Time from Donation and HRQOL
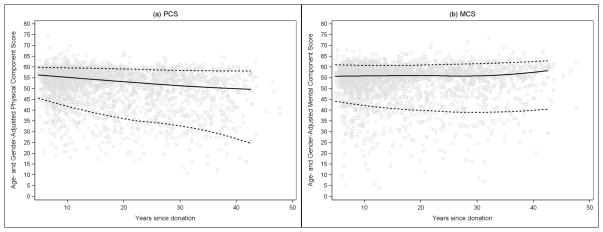
Sex and age-adjusted PCS and MCS scores are depicted against time from donation in Figure 4. In general, the most recent donors reported the highest PCS, while donors furthest away from donation reported the lowest scores. PCS declined by half a point with each decade after donation (slope (SE) =−0.54 (0.18) per decade, p=0.0027). For comparison, a change in the PCS score from 51 (mean of this sample) to 50.5 (drop of half a point), represents moving from the 47th to the 45th percentile score in the US general population. There was not a statistically significant decline in MCS over time at the 0.01 level (slope (SE) = −0.32 (0.15) per decade, p = 0.03). White donors’ PCS scores were 3 points higher than non-white donors (beta (SE) = 3.02 (0.85), p = 0.0004), the threshold for a minimally important difference (16). This difference did not change over time (race by time interaction, p = 0.11). Race did not influence the trajectory of MCS scores over time (beta (SE) = 1.31 (0.81), p = 0.11). Procedure (laparoscopic vs. open) did not influence the trajectory of either PCS (p = 0.31) or MCS (p = 0.73) scores over time.
Figure 4. Sex- and age-adjusted PCS and MCS scores of RELIVE donors by time from donor surgery.
Each donor’s sex- and age-adjusted physical and mental component summary score is plotted by time from donor surgery in years in this scatterplot. Data points above the norm mean (50) on the y-axis indicate donors with better health and functioning than peers of the same age and sex. The solid line is a loess curve fitted to the observed median score and the dotted lines follow the 5th and 95th percentiles at each point in time. The overall adjusted physical and mental component mean scores are 54±9 (range 13 to 78) and 52±9 (range 3 to 71), respectively.
Donor Self-Rated Health Status
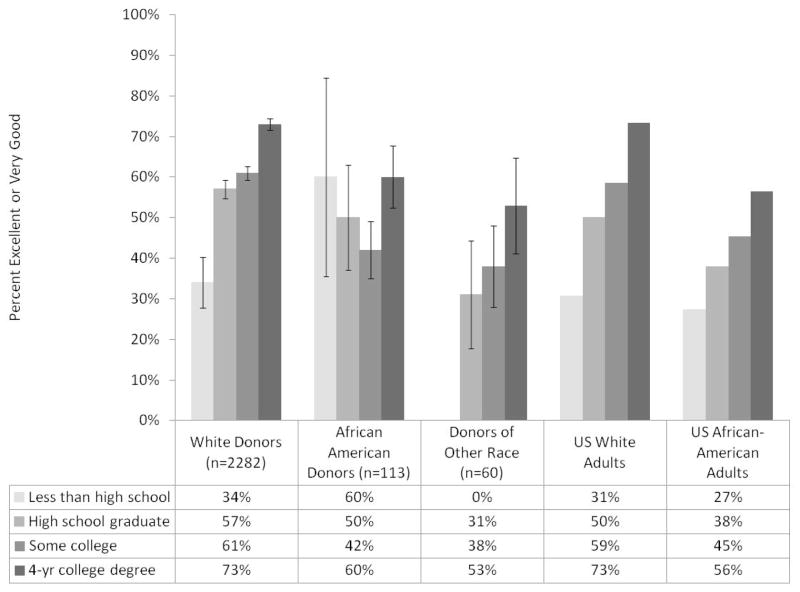
When donors were asked to rate their current health, the common responses were good (31.3%), very good (44.2%), or excellent (18.5%). Few donors selected fair (5.2%) or poor (1%). Reports of fair or poor health are more common in the US population, 12.1% and 3.4%, respectively (19). Figure 5 shows the proportions of RELIVE donors with excellent or very good self-rated health by race and education (used here as a surrogate for socioeconomic status), and comparable rates for US white and African-American adults. After adjustment for education, white donors remained more likely to report excellent or very good health than either African-American donors (p = 0.0034) or donors of other races (p =0.0004). Education was strongly correlated with self-rated health among US adults, white donors, and donors of other or unknown race, but not among African-American donors.
Figure 5. Proportions of donors reporting “excellent” or “very good” health, according to race and education and comparable data for US white and African-American adults.
Bar heights indicate the proportion of donors reporting excellent or good health within four categories of educational attainment: less than high school, high school graduate, some college, or 4-year college graduate. Vertical lines atop the bars indicate ± 1 standard error. The last 8 bars are comparable data on self-reported health by educational attainment for US white and African-American adults aged 25–74 from the 2005–2007 Behavioral Risk Factor Surveillance Systems as reported by Braveman et al (24). Standard errors are not provided for the US population estimates because they have been weighted to reflect the entire population. Sixteen donors with missing values for education or self-rated health status were omitted. After adjustment for education, white donors were more likely to report excellent or very good health than either African-American donors or donors of other races (p = 0.0034 and p = 0.0004, respectively). Overall, patterns of self-rated health status are similar for donors and US adults.
Most donors reported that donation had no impact on their general health (73%). The remaining donors reported very positive (10%), somewhat positive (6%), somewhat negative (10%), or very negative (1%) impacts. Perceived impact of donation on health was related to race and to self-rated health (p<0.0001, both). Donors of other or unknown race perceived a very negative health impact more often than white or African-American donors (6.8% vs. 0.8% and 2.7%, respectively). Despite perceiving a very negative impact of donation on their health, these donors rated their current health as reasonably good: excellent (4%), very good (8%), good (52%), fair (20%), or poor (12%), with missing data for one donor. In response to questions about their donation experience, about half (n=11 of 25) reported medical complications and/or emotional, psychological, or substance abuse difficulties as a result of donation, and five reported that they never recovered from donor surgery. The others (14 of 25) did not report these problems; they resumed usual daily activities (e.g., driving, shopping for groceries) less than 3 months after donor surgery, and reported no medical complications or emotional, psychological, or substance abuse difficulties as a result of donation.
Predictors of Impaired HRQOL
Nine percent of donors (n=211) had significantly impaired physical HRQOL, scoring more than 10 points below their sex-by-age adjusted PCS norm (See Supplemental Table B). These individuals would typically be unable to do vigorous activity, have difficulty working, and/or have pain or other chronic conditions. Nine percent of donors (n=233) had significantly impaired mental HRQOL based on the MCS. These individuals would typically have depressed or anxious moods some or most of the time, impacting their ability to socialize and function at work or home.
Pre-donation obesity, history of psychiatric difficulties, and race were independent risk factors for impaired physical HRQOL, after adjustment for time since donation (Table 3). The influence of excess body weight is supported by evidence of a dose-response relationship. Donors with a BMI of 35 or higher at time of donation had more than four times the risk (OR=4.32), donors with a BMI of 30 to 34.9 had nearly triple the risk (OR = 2.85), and donors with a BMI of 25 to 29.9 had almost double the risk (OR = 1.84) of impaired physical HRQOL compared to other donors. History of psychiatric difficulties pre-donation more than doubled (OR=2.46) the risk of being in the impaired group. Non-white donors were also about twice as likely (OR=2.05) to be in the impaired group. Although the likelihood of impaired physical HRQOL was associated with race, this risk was stable over time from donation. On the other hand, higher educational attainment at time of donation and first-degree relationship to the recipient were independent protective factors. For each additional level of education, the odds of having significant physical HRQOL impairment decreased by about 25% (OR=0.77). First-degree relatives were half as likely as more distant relations or unrelated donors to have significant physical HRQOL impairment.
Table 3.
Multivariable Logistic Regressions Predicting Significantly Impaired Physical HRQOL1 and Mental HRQOL2
| Physical HRQOL Impairment (n=211 out of 2,455) | Mental HRQOL Impairment (n=233 out of 2,455) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| (1) Model based on pre-donation characteristics | ||||||
| Age at donation, per 10 yearsa | 0.74 | (0.65, 0.86) | <0.0001 | |||
| 10 years after donationa,b | 4.38 | * | 2.86 | ** | ||
| 20 years after donationa,b | 11.30 | 5.47 | ||||
| 30 years after donationa,b | 17.14 | 6.97 | ||||
| 40 years after donationa,b | 15.29 | 5.92 | ||||
| Non-white (ref: white) | 2.05 | (1.27, 3.30) | 0.0034 | |||
| 1st degree relative of recipient (ref: extended relation or not related) | 0.54 | (0.36, 0.80) | 0.0025 | |||
| BMI 25–29.9 (ref: < 25)b | 1.84 | (1.31, 2.65) | 0.0005 | |||
| BMI 30–34.9 (ref: < 25)b | 2.85 | (1.84, 4.42) | <0.0001 | |||
| BMI ≥35 (ref: < 25)b | 4.32 | (2.37, 7.87) | <0.0001 | |||
| Education at donation (continuous, 5 levels)b | 0.77 | (0.64, 0.91) | 0.0027 | 0.81 | (0.70, 0.95) | 0.0092 |
| History of psychiatric difficulties prior to donation (ref: no history or unknown)a,b,c | 2.46 | (1.57, 3.84) | <0.0001 | 3.82 | (2.61, 5.60) | <0.0001 |
| Model (1) plus the following, entered one at a time: | ||||||
| Overall donation experience (poor – excellent) | 0.79 | (0.68, 0.92) | 0.0024 | 0.67 | (0.59, 0.77) | <0.0001 |
| Recovery time compared to expected (shorter – longer) | 1.26 | (1.10, 1.44) | 0.0008 | |||
| Overall recovery time (<3 months – never) | 1.63 | (1.31, 2.02) | <0.0001 | 1.87 | (1.53, 2.28) | <0.0001 |
| Recovery time for daily activities (<3 months – never)a,b | 3.05 | (2.26, 4.12) | <0.0001 | 2.81 | (2.11, 3.75) | <0.0001 |
| I felt depressed for a while after the surgery | 1.38 | (1.22, 1.56) | <0.0001 | 1.49 | (1.33, 1.67) | <0.0001 |
| Once surgery was over, no one paid attention | 1.31 | (1.15, 1.49) | <0.0001 | 1.51 | (1.34, 1.69) | <0.0001 |
| There was support available to me from the health care providers | 0.80 | (0.71, 0.89) | 0.0001 | 0.72 | (0.65, 0.80) | <0.0001 |
| My family or friends supported me throughout the donor surgery | 0.78 | (0.67, 0.90) | 0.0007 | 0.69 | (0.61, 0.79) | <0.0001 |
| Comfort now with the decision to donate | 0.64 | (0.52, 0.78) | <0.0001 | |||
| Self-reported complication (ref: no, unknown) | 1.92 | (1.39, 2.64) | <0.0001 | 2.26 | (1.68, 3.03) | <0.0001 |
| How did your donation affect your general health? (very negatively – very positively)a | 0.68 | (0.55, 0.84) | 0.0002 | 0.52 | (0.42, 0.64) | <0.0001 |
| Married or partnered at questionnaire completion | 0.64 | (0.47, 0.86) | 0.0032 | |||
| Educational attainment at questionnaire completiona | 0.60 | (0.48, 0.76) | <0.0001 | |||
Notes: Data were missing for less than 5% of donors for all variables except educational attainment at donation (missing for 19%); missing values were multiply imputed using the sequential regression imputation method.
Significantly impaired physical HRQOL was defined as a PCS >−1 SD below sex-by-age norms (n=211 out of 2455) 9% of the sample.
Significantly impaired mental HRQOL was defined as a MCS >−1 SD below sex-by-age norms (n = 233 of 2455) 9% of the sample.
These variables remained significant (p < 0.01) when all variables in this table were simultaneously included in the mental HRQOL model (c-statistic = 0.760). Mental HRQOL base model c-statistic = 0.660. Additional model c-statistics for Mental HRQOL with covariates were = 0.670 to 0.705.
These variables remained significant (p < 0.01) when all variables in this table were simultaneously included in the physical HRQOL model (c-statistic = 0.762). Base Model Physical HRQOL c-statistic = 0.704. Additional model c-statistics for Physical HRQOL with covariates were 0.715 to 0.741.
History of psychiatric difficulties was ascertained from medical records from the time of donation and included mentions of depression, anxiety, bi-polar disorder, post-traumatic stress disorder, and other psychiatric diagnoses.
Physical HRQOL: time since donation p < 0.0001, time since donation squared p = 0.0010.
Mental HRQOL: time since donation p = 0.0003, time since donation squared p = 0.0095.
After adjustment for the factors listed above, the following aspects of the donation experience were associated with increased risk of impaired physical HRQOL: estimated time to recover from surgery, longer than expected recovery time, longer time to resumption of usual activities, feeling depressed or ignored, and medical or psychological difficulty after donation. With each increment of time to resumption of usual activities, the adjusted risk of impaired physical HRQOL increased 3-fold (OR=3.05). Perceptions of strong support from health providers, family, and friends throughout the donation process were protective. Donors who reported positive impacts of donation on their health were also less likely to have impaired physical HRQOL.
History of psychiatric difficulties prior to donation more than tripled the risk of impaired mental HRQOL (OR=3.82), after adjustment for time (Table 3). Being older at time of donation and having more education were protective. After adjustment for these predictors, increased risk of impaired mental HRQOL was associated with: time to recovery from surgery, time to resumption of usual activities, feeling depressed or ignored, and medical or psychological difficulty after donation. Factors associated with reduced risk were positive perceptions of the donation experience, support from health providers, family, and friends, comfort with decision to donate, positive impacts of donation on health, and current education and marital status.
Factors not associated with impaired HRQOL are listed in Supplemental Table D.
DISCUSSION
The kidney donors in the present study reported better physical and mental functioning and well-being than their counterparts in the general US population. With a larger sample and longer-term follow-up (average 17 years), these results confirm and extend earlier reports from single center series (5, 8, 21), and recent international studies (7, 9). Worldwide, about 27,000 living kidney donations are performed each year (22). In Norway, Mjoen et al. (9) surveyed 1,414 donors who were on average 12.6 years from donation. These donors reported better HRQOL on all SF-36 domains compared to a population-based sample of adult Norwegians. An international collaborative (7) compared the HRQOL of non-donor controls to donors (N=203) who were on average 5.5 years from donation and found no differences in SF-36 scores.
This study presents new information on the outcomes of African-American kidney donors. African-American donors’ SF-36 scores compared favorably to norms and results from a population-based sample of African-American adults, (17) and were generally similar to white donors’ scores. After adjustment for age and sex, white donors had better overall physical functioning than non-white donors, but trends over time from donation did not differ by race, indicating that non-white donors did not have an accelerated decline in function or well-being in the decades following donation compared to white donors. These findings are reassuring, given evidence from administrative databases that African-American and Hispanic donors have higher risks of developing hypertension, diabetes, and chronic kidney disease after donation than white donors, although not higher rates than minority non-donors (23).
It has been shown that differences in self-rated health are closely linked to socioeconomic status (24), and therefore we expected donors with greater educational attainments to rate their current health higher than those with less education. This was true for white donors, but not for African-American donors. Reasons for this are unknown, but we are mindful that the majority of the RELIVE African-American donors hail from a single center and may differ from African-American donors elsewhere in ways we were unable to assess.
A novel finding from our work is the long-term HRQOL impact of excess body weight in otherwise healthy adults. We did not have current BMI information, and could not determine if donor BMIs increased or decreased in the years since donation, but obesity at time of donation increased risk of significant physical impairment (PCS) between 5 and 48 years later. The credibility of this finding is supported by a graded relationship: the higher the BMI category, the greater the risk. When BMI and the SF-36 are measured concurrently, worse physical HRQOL (PCS) is consistently reported by the obese and overweight, compared to those in the normal weight range (25–26). Bodily pain and mobility are most often negatively affected by obesity. The relationship between weight and mental HRQOL is more nuanced. Longitudinal cohort studies and weight loss trials generally find that gaining weight diminishes physical HRQOL, and losing weight improves both physical and mental HRQOL. Our findings for overweight and obese donors may have important implications for the future HRQOL of all adults who are currently overweight, but otherwise healthy (e.g., have no major comorbidities).
The association of pre-donation obesity (BMIs>30) with adverse long-term HRQOL is also notable in light of recent trends to accept heavier donors in the United States. Although the proportion of very obese donors (BMIs >35) changed little between 1999 and 2009 (from 2% to 3.1%), the proportion of obese donors (BMIs>30–35) more than doubled (from 8% to 21.8%) during this interval (3). Increased follow-up and study of these donors is warranted. Our findings also add support to current practices of excluding very obese donors, given higher surgical complication rates, the natural history of metabolic syndrome, and recent evidence of poor long-term medical outcomes (14). Nogueira and others found 42% of obese kidney donors (N=36, average 7 years post-donation) were hypertensive and 47% had compromised renal function.
Donor experience of long recovery time, complications, and low donor support and attention were associated with impaired function and well-being many years after donation. These associations suggest that initiatives to improve the donor experience should be seriously considered.
In the past, it has been difficult to identify robust predictors of poor HRQOL among donors (5), and center differences, small sample sizes, and limited follow-up have been identified as contributing to this problem. Varying definitions of HRQOL may also contribute, as we found differences in the pre-donation factors that predicted physical versus mental HRQOL impairment. Notably, obesity and non-white race were risk factors specifically for impaired physical HRQOL and older age at donation was specifically associated with reduced risk of impaired mental HRQOL. Donor history of psychiatric difficulties (donor chart documentation of history or treatment for depression, anxiety, post-traumatic stress disorder, or other disorders), which has been regularly identified as a risk factor for poor outcomes (5, 27–28) was a risk factor for both poor physical and mental HRQOL in our sample.
The main strength of RELIVE is its size, multi-center collaboration, and length of follow-up. Limitations include the absence of pre-donation HRQOL data, and substantial under-representation of minority donors. Although 9.6% of donors in our study eligibility window were African-American, proportionately fewer African-Americans than white donors responded to study contacts, and as a result, African-Americans comprised only 5% of the RELIVE donor sample. Fewer than 2% of the donors in our eligibility window were Hispanic or Latino, and these donors were also less likely than white donors to respond to study contacts. In retrospect, greater efforts should have been made to devise more effective approaches for contacting minority donors. Overall, only 50% of the donors whose surgeries were identified from medical records responded to study contact attempts. No current contact information was available for over 900 donors, despite use of a fee-based internet search service to locate current addresses and phone numbers. These limitations reflect the ambitious nature of securing follow-up as long as 50 years after donation. Finally, it should be kept in mind that RELIVE donors are from three centers, and are not a representative national sample.
A number of factors, in addition to race, differed between donors who did and did not participate in RELIVE, including age, education, time from donation, and relationship to the recipient. Because our primary HRQOL results were age-adjusted and effects of all these factors were estimated in multivariate analyses, we do not believe that these factors served to bias our findings.
In conclusion, the majority of living kidney donors maintain average or above average HRQOL over the long-term. Findings suggest potential donors who are overweight or obese, less educated, have prior psychiatric difficulties, are not white, or not first-degree relatives of the recipient represent groups at risk for poor HRQOL. New or enhanced efforts of pre-donation counseling and education, particularly weight loss counseling, and post-donation monitoring efforts could improve outcomes of these donors.
Supplementary Material
Acknowledgments
This research was performed as a project of the Renal and Lung Living Donors Evaluation Study (RELIVE), a collaborative clinical research project sponsored by the National Institute of Allergy and Infectious Diseases, Health Resources and Services Administration and National Heart, Lung, and Blood Institute. We thank the donors who generously shared their experiences with RELIVE.
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance.
Abbreviations
- AAHP
African-American Health project
- HRQOL
health-related quality of life
- MCS
mental component summary
- NHMS
National Health Measurement Survey
- PCS
physical component summary
- RELIVE
Renal and Lung Living Donors Evaluation Study
- SF-36
Short Form -36 Health Survey
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
Detailed Statistical Methods
- Supplemental Table A: Characteristics of donors eligible for RELIVE and demographic comparisons between participating and non-participating donors, among those with confirmed study contact
- Supplemental Table B: Scoring and Interpretation the SF-36 version 2 Health Profile based on the User’s Guide by Ware et al. (2007)
- Supplemental Table C: Comparisons between RELIVE Donors and US 2009 Kidney Donors
- Supplemental Table D: Variables tested but not included in the HRQOL models because they were not significant
References
- 1.Matas AJ, Payne WD, Sutherland DE, Humar A, Gruessner RW, Kandaswamy R, et al. 2,500 living donor kidney transplants: a single-center experience. Ann Surg. 2001 Aug;234(2):149–164. doi: 10.1097/00000658-200108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.OPTN. [Accessed June 18, 2012];Survival of kidney recipients by donor type - living or cadaveric. 2012 Available at: http://optn.transplant.hrsa.gov/latestData/rptStrat.asp.
- 3.Leichtman A, Abecassis M, Barr M, Charlton M, Cohen D, Confer D, et al. Living kidney donor follow-up: state-of-the-art and future directions, conference summary and recommendations. Am J Transplant. 2011 Dec;11(12):2561–2568. doi: 10.1111/j.1600-6143.2011.03816.x. [DOI] [PubMed] [Google Scholar]
- 4.Abecassis M, Adams M, Adams P, Arnold RM, Atkins CR, Barr ML, et al. Consensus statement on the live organ donor. JAMA. 2000 Dec 13;284(22):2919–2926. doi: 10.1001/jama.284.22.2919. [DOI] [PubMed] [Google Scholar]
- 5.Dew MA, Switzer GE, DiMartini AF, Myaskovsky L, Crowley-Matoka M. Psychosocial aspects of living organ donation. In: Tan HP, Marcos A, Shapiro R, editors. Living Donor Transplantation. NYC: Informa Healthcare USA, Inc; 2007. [Google Scholar]
- 6.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010 Mar 10;303(10):959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 7.Clemens K, Boudville N, Dew MA, Geddes C, Gill JS, Jassal V, et al. The long-term quality of life of living kidney donors: a multicenter cohort study. Am J Transplant. 2011 Mar;11(3):463–469. doi: 10.1111/j.1600-6143.2010.03424.x. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. N Engl J Med. 2009 Jan 29;360(5):459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mjoen G, Stavem K, Westlie L, Midtvedt K, Fauchald P, Norby G, et al. Quality of life in kidney donors. Am J Transplant. 2011 Jun;11(6):1315–1319. doi: 10.1111/j.1600-6143.2011.03517.x. [DOI] [PubMed] [Google Scholar]
- 10.Simmons RG, Marine SK, Simmons RL, editors. Gift of Life: The Effect of Organ Transplantation on Individual, Family and Societal Dynamics. 2. New Brunswick, New Jersey: John Wiley & Sons; 1987. [Google Scholar]
- 11.Feltrin A, Pegoraro R, Rago C, Benciolini P, Pasquato S, Frasson P, et al. Experience of donation and quality of life in living kidney and liver donors. Transpl Int. 2008 May;21(5):466–472. doi: 10.1111/j.1432-2277.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 12.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR. Living kidney donors requiring transplantation: focus on African Americans. Transplantation. 2007 Sep 15;84(5):647–649. doi: 10.1097/01.tp.0000277288.78771.c2. [DOI] [PubMed] [Google Scholar]
- 13.Nogueira JM, Weir MR, Jacobs S, Haririan A, Breault D, Klassen D, et al. A study of renal outcomes in African American living kidney donors. Transplantation. 2009 Dec 27;88(12):1371–1376. doi: 10.1097/TP.0b013e3181c1e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueira JM, Weir MR, Jacobs S, Breault D, Klassen D, Evans DA, et al. A study of renal outcomes in obese living kidney donors. Transplantation. 2010 Nov 15;90(9):993–999. doi: 10.1097/TP.0b013e3181f6a058. [DOI] [PubMed] [Google Scholar]
- 15.Taler SJ, Messersmith EE, Leichtman AB, Gillespie BW, Kew CE, Stegall MD, et al. Demographic, Metabolic, and Blood Pressure Characteristics of Living Kidney Donors Spanning Five Decades. Am J Transplant. 2013;13(2):390–398. doi: 10.1111/j.1600-6143.2012.04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User’s manual for the SF-36v2™ Health Survey. 2. Lincoln, R.I: QualityMetric Incorporated; 2007. 3d. [Google Scholar]
- 17.Wolinsky FD, Miller DK, Andresen EM, Malmstrom TK, Miller JP. Health-related quality of life in middle-aged African Americans. J Gerontol B Psychol Sci Soc Sci. 2004 Mar;59(2):S118–123. doi: 10.1093/geronb/59.2.s118. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 19.Maglinte GA, Hays RD, Kaplan RM. US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol. 2012 May;65(5):497–502. doi: 10.1016/j.jclinepi.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghunathan TE, Solenberger P, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software Users Guide. Ann Arbor: Institute for Social Research, University of Michigan; 2002. [Google Scholar]
- 21.Clemens KK, Thiessen-Philborrk H, Parikh CR, Yang RC, Karley ML, Boudville N, et al. Psychosocial health of living kidney donors: A systematic review. Am J Transplant. 2006;6(12):2965–2977. doi: 10.1111/j.1600-6143.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- 22.Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int. 2009 May;75(10):1088–1098. doi: 10.1038/ki.2009.20. [DOI] [PubMed] [Google Scholar]
- 23.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010 Aug 19;363(8):724–732. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010 Apr 1;(Suppl 1):S186–196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2(3):173–82. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 26.Ul-Haq Z, Mackay DF, Fenwick E, Pell JP. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity (Silver Spring) 2013;21(3):E322–7. doi: 10.1002/oby.20107. [DOI] [PubMed] [Google Scholar]
- 27.Reimer J, Rensing A, Haasen C, Philipp T, Pietruck F, Franke GH. The impact of living-related kidney transplantation on the donor’s life. Transplantation. 2006;81(9):1268–1273. doi: 10.1097/01.tp.0000210009.96816.db. [DOI] [PubMed] [Google Scholar]
- 28.Rowley AA, Hong BA, Martin S, Jones L, Vijayan A, Shenoy S, et al. Psychiatric disorders: are they an absolute contraindication to living donation? Prog Transplant. 2009 Jun;19(2):128–131. doi: 10.1177/152692480901900206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.