Abstract
Antigenic diversity shapes immunity in distinct and unexpected ways. This is particularly true of the humoral response generated against influenza A viruses. While it is known that immunological memory developed against previously-encountered influenza A virus strains impacts the outcome of subsequent infections, exactly how sequential exposures to antigenically variant viruses shape the humoral immune response in humans remains poorly understood. To address this important question, a longitudinal analysis of antibody titers against various pandemic and seasonal strains of influenza virus spanning a 20-year period (1987–2008) was performed using samples from 40 individuals (d.o.b. 1917–1952) obtained from the Framingham Heart Study. Longitudinal increases in neutralizing antibody titers were observed against previously-encountered pandemic H2N2, H3N2 and H1N1 influenza A virus strains. Antibody titers against seasonal strains encountered later in life also increased longitudinally at a rate similar to that against their pandemic predecessors. Titers of cross-reactive antibodies specific to the hemagglutinin stalk domain were also investigated, since they are known to be influenced by exposure to antigenically diverse influenza A viruses. These titers rose modestly over time, even in the absence of major antigenic shifts. No sustained increase in neutralizing antibody titers against an antigenically more stable virus (human cytomegalovirus) was observed. The results herein describe a role for antigenic variation in shaping the humoral immune compartment, and provide a rational basis for the hierarchical nature of antibody titers against influenza A viruses in humans.
INTRODUCTION
Antigenic shift and drift are the primary mechanisms through which influenza A viruses (IAVs) evolve to evade adaptive immunity. This antigenic plasticity is the reason that most individuals become infected with IAVs multiple times throughout the course of their lives. It is also the reason that IAV pandemics remain one of the greatest threats to global public health. Immunological memory acquired through exposures to previously encountered IAVs is known to impact the outcome of subsequent infections (1–9). In contrast though, the way in which sequential exposures to antigenically distinct IAVs shapes the humoral immune compartment remains poorly characterized. This is largely due to the combined challenge of recapitulating the complex exposure patterns of humans using animal models, and the inherent difficulties in performing longitudinal studies in humans of sufficient length to gather meaningful results. A previous longitudinal analysis focused on understanding the humoral response against common viral and vaccine antigens (excluding IAV) found striking differences in the half-life of the antibody response specific to each antigen (10). These observations raised major questions regarding how humoral immunity against IAV may evolve and is maintained after multiple exposures to antigenically variable viruses. Understanding these complex immunological interactions is essential for both predicting risk groups upon future IAV epidemics/pandemics, and for the rational design of next-generation vaccines.
One of the most longstanding and poorly understood aspects of the humoral immune response to IAV is the observation that the magnitude of the antibody response against a given subtype of IAV is always greatest against the first strain of that subtype that one encounters. The theories of “original antigenic sin (OAS)” (11–14), or more recently, “antigenic seniority” (15) have been proposed as explanations for this phenomenon. The theory of OAS attempts to explain this phenomenon by the hypothesis that exposure to the “original antigen” may result in the mounting of “suboptimal” responses to future IAVs. In a refinement of this model, Lessler and colleagues recently reported the same basic observations (that individuals tended to have the greatest neutralizing antibody titers to H3N2 IAV strains encountered earliest in life); however, their description of “antigenic seniority” did not necessitate a suppressive role for the original antigen in the apparently lower titers observed against strains encountered later (15). Unfortunately, the cross-sectional nature of the data precluded direct elucidation of a rational basis for these results, highlighting the need to understand how the influenza-specific humoral compartment evolves over time using a longitudinal approach.
The goal of developing a “universal” influenza virus vaccine in which cross-reactive, broadly-neutralizing antibodies specific to the hemagglutinin (HA) stalk domain are elicited has received substantial attention of late. While sequential exposures to antigenically dissimilar IAVs within the same HA group seem to elicit these antibodies most effectively (3, 6, 16–18), plasmablasts producing these antibodies have also been isolated from individuals who recently received a seasonal trivalent vaccine (TIV, 19). These observations have led to uncertainty in assessing how stalk-reactive antibodies are maintained over time, especially during periods of relative antigenic stability. The extent to which this class of antibodies can be boosted upon sequential exposures to distinct HA subtypes are also of major interest. Most studies have focused on antibodies that bind and neutralize IAVs bearing group 1 HAs (H1, H5, etc…). However, little is known about antibodies which exhibit broad neutralization against group 2 HA-carrying IAVs (H3, H7, etc..) (20–22). Interestingly, there has never been a major antigenic shift among group 2 viruses that circulate widely in humans (currently circulating H3N2 viruses are drifted relatives of the “Hong Kong” H3N2 pandemic of 1968). Therefore, the frequency and longevity of group 2 HA stalk-specific antibodies remains a major outstanding question.
Herein, we address the aforementioned gaps in viral immunology by performing serological analyses of longitudinal samples gathered over a 20 year period (Fig. 1). We find that hemagglutination inhibition (HAI) titers specific to pandemic viruses in human circulation between 1957 and 2008 (H2N2, H3N2, H1N1) exhibited sustained increases over the course of the 20 year study period. Increases in total IgG reactivity (endpoint titers) to a given HA were more variable and did not always increase in conjunction with HAI titers. Since the rise in HAI titers against pandemic strains would be consistent with OAS and/or antigenic seniority, we also measured HAI titers against drifted H1N1 and H3N2 strains that emerged over a decade after the respective pandemic strains. Interestingly, we found that the rate of HAI titer increase specific to drifted viruses was similar to that of its pandemic parent. This observation provides a rational basis for the hierarchical nature of the human antibody response to IAVs. Finally, we analyze the longitudinal antibody response against human cytomegalovirus (HCMV), a virus which is antigenically more stable and exhibits lifelong persistence by establishing latency and periodically reactivating to produce infectious virus following primary infection. Titers of reactive and neutralizing antibodies specific to HCMV remained stable for the duration of the study. These data suggest that differential mechanisms of humoral regulation are likely to exist for antigenically more stable and antigenically variable viruses.
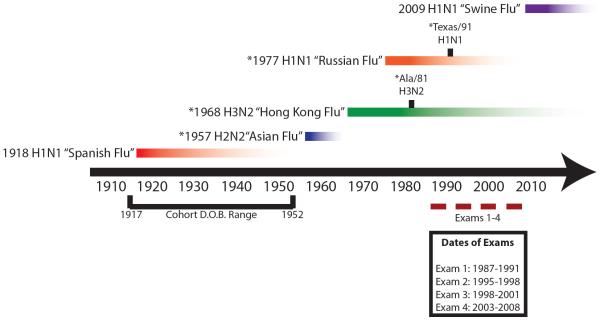
Figure 1. Timeline of IAV circulation, cohort birth dates and sampling period.
All individuals enrolled in this study were born between 1917 and 1952, as indicated on the lower left portion of the timeline. Serum samples were gathered approximately every 5 years (see Materials and Methods) between 1987 and 2008, as depicted in the lower right portion of the timeline. Emergence of pandemic strains, and subsequent circulation of their seasonal descendants are depicted above the timeline: red bar – 1918 H1N1; blue bar – 1957 H2N2; green bar – 1968 H3N2; orange bar – 1977 H1N1; purple bar = 2009 H1N1. * denotes strains specifically tested in this study.
RESULTS
HAI antibody titers against previously encountered pandemic IAVs increase over time
Herein, the antibody responses of 40 individuals were monitored every five years over a 20 year period. The birth dates of these individuals (1917–1952) allowed us to probe their responses to three IAV pandemic strains (1957 H2N2, 1968 H3N2 and 1977 H1N1) and seasonal isolates.
HA is the dominant antibody target upon exposure to IAVs. In order to understand how the humoral response against the HA of pandemic IAVs evolves over time, HAI assays and IgG endpoint titrations were performed. Endpoint titers against purified, recombinant HAs were used to quantify total HA-specific IgG antibodies levels. HAI assays are functionally-based and were performed in order to determine titers of antibodies that bind to conventional neutralizing sites present on the HA head domain. Despite its disappearance from human circulation in 1967, HAI titers against Jap/57 H2 increased significantly over the sampling period, from a geometric mean titer (GMT) of 12.6 at exam 1 (42.5% of individuals were seropositive at this time (HAI ≥ 40)) to a GMT of 60.0 at exam 4 (Fig. 2A). HAI titers against HK/68 H3 and USSR/77 H1 also rose from exam 1 to exam 4, from GMTs of 104.8 and 32.0 to 735.2 and 302.1, respectively (Fig. 2B, C). Notably, the most substantial increases in Jap/57 H2 and HK/68 H3 titers appeared to occur between exams 1 and 2, while for USSR/77 H1 the most pronounced increase in titers were observed between exams 2 and 3. Differences in the magnitude of HAI titer changes between each exam for each HA subtype would be expected based on distinctive patterns of IAV circulation and prevalence/severity of infections during these periods. Although it is difficult to directly compare HAI titers among different subtypes, it is interesting to note that the magnitude of the HAI titers at Exam 4 corresponded well to the relative length of circulation of each subtype, and its degree of drift (Table 1).
Figure 2. HAI and IgG endpoint titers against pandemic H2N2, H3N2 and H1N1 IAV strains.
HAI titers were evaluated using 40 matched serum samples against A) Sing/57 H2 HA VLPs, B) HK/68 H3N2 virus or C) USSR/77 H1N1 virus. Black hatched lines indicate an HAI titer of 40, which is the conventional cutoff used to indicate seroconversion to a given HA. GMTs are depicted by red lines. IgG endpoint titers from the same sera were evaluated by ELISA against recombinant D) Jap/57 HA, E) HK/68 HA or F) USSR/77 HA, with GMTs depicted by orange lines. Data were compared using one-way repeated measures ANOVA. GMTs between exams were then compared using Tukey's multiple comparisons test. Statistically significant differences relative to exam 1 are indicated by asterisks. Ratios of IgG endpoint titers to HAI titers for G) Sing/57 IgG:Jap/57 H2 HAI, H) HK/68 H3 and I) USSR/77 H1 were then determined using the above data and plotted, with means depicted by yellow lines. Data were compared using one-way repeated measures ANOVA. Means between exams were then compared using Tukey's multiple comparisons test to determine statistically significant differences, which are denoted by asterisks.
Table 1.
Comparing drift of H2, H3 and H1 human influenza virus hemagglutinins by amino acid identity
| Subtype | Comparison | Amino Acid Identity(%) |
|---|---|---|
| H3N2 | HK/68 vs. Wisc/05* | 87 |
| H1N1 | USSR/77 vs. SI/2006 HA* | 91 |
| H2N2 | Sing/57 vs. Alb/67 | 94 |
H3N2 and H1N1 strains recommended by the WHO for use in 2007–2008 Northern Hemisphere vaccine were used or sequence comparison
In contrast to the sustained increases in HAI titers observed for Jap/57 H2 and HK/68 H3, endpoint IgG titers for the corresponding proteins (Sing/57 H2 is 99% identical to Jap/57 H2) were more variable and did not always reflect changes observed in HAI titers. HK/68 H3 titers remained relatively stable, despite a surprising and transient decrease between exams 1 and 2. While Sing/57 H2 titers appeared to increase, statistical significance was not reached (Fig. 2D, E). A significant increase in total reactivity was only observed in the context of USSR/77 H1 (Fig. 2F). It is interesting to note that IgG endpoint titers against USSR/77 at exam 1 were much lower than those against H2N2 and H3N2 viruses. This may be a reflection of the fact that USSR/77 emerged most recently, since by Exam 3 mean IgG endpoint titers against USSR/77 were essentially equivalent to those against HK/68 at Exam 1. By this time, both viruses would have circulated for approximately 20 years (Table 1, compare Fig. 2E and F).
To better understand the relationship between total HA-reactive antibodies and neutralizing antibodies, ratios of IgG endpoint titers to HAI titers were determined for each individual and plotted at each exam (Fig. 2G, H and I). Stable IgG:HAI ratios would suggest that a linear relationship between total IgG antibody levels and HAI antibodies, whereas increases would be indicative of enhanced reactivity in the absence of HAI and vice-versa with regard to decreases. Between exams 1 and 3, Sing/57 IgG: Jap/57 HAI titers remained relatively stable. However, a significant increase in these ratios was observed between exams 3 and 4 suggesting a rise in H2 reactive, but not neutralizing antibodies (Fig. 2G). In contrast, IgG:HAI ratios specific for HK/68 H3 dropped substantially between exams 1 and 2, before increasing and leveling off again between exams 3 and 4 (Fig. 2H). This decline in ratios between exams 1 and 2 is likely indicative of a boost from a virus(es) containing substantial conservation in the conventional antigenic sites present in the H3 HA head domain. USSR/77 IgG:HAI ratios remained much more stable during the sampling period, with a significant increase in reactivity occurring only between exams 1 and 3 (Fig. 2I).
Taken together, these results suggest that HAI titers against previously-encountered strains of IAV are periodically boosted over time; probably upon exposure to newly-emerged IAV strains bearing conserved, neutralizing B cell epitopes.
Humoral response against drifted H1N1 and H3N2 viruses is consistent with `antigenic seniority'
In order to understand how periodic boosting of antibody titers against previously-encountered pandemic IAV strains impacts the humoral response to subsequent IAV exposures, HAI titers and IgG endpoint titers were determined for drifted H1N1 (Texas/91 H1N1) and H3N2 (Ala/81 H3N2) IAV strains (Fig. 3). HAI titers against both TX/91 and Ala/81 rose significantly between exams 1 and 4 (Fig 3A and B). In the case of TX/91, the most pronounced increase between exams 1 (GMT = 2.2) and 2 (GMT = 94.6) corresponded with the appearance of this strain in human circulation (Fig. 1). As was the case against most pandemic strains, IgG endpoint titers tended to increase with time, although these increases were not statistically significant (Fig. 3C, D). While the absolute magnitude of HAI titers against pandemic strains exceeded those of their drifted counterparts, the rate of increase over time was comparable against both pandemic and seasonal strains (Table 2). This data is consistent with the model of “antigenic seniority,” whereby HAI titers against all previously-encountered strains of a given subtype are periodically boosted, resulting in the highest overall HAI titers for strains encountered earliest in life.
Figure 3. HAI and IgG endpoint titers against seasonal H1N1 and H3N2 IAV strains over a 20 year period.
HAI titers were evaluated using 40 matched serum samples against A) Texas/91 H1N1 virus or B) Ala/81 H3N2 virus. Black hatched lines indicate an HAI titer of 40, which is the conventional cutoff used to indicate seroconversion to a given HA. GMTs are depicted by red lines IgG endpoint titers from the same sera were evaluated by ELISA against recombinant C) Texas/91 HA or D) Ala/81 HA, with GMTs depicted by orange lines. Data were compared using one-way repeated measures ANOVA. GMTs between exams were then compared using Tukey's multiple comparisons test. Statistically significant differences relative to exam 1 are indicated by asterisks. n.s. = not significant. Ratios of IgG endpoint titers to HAI titers for E) TX/91 HA and F) Ala/81 H3 were then determined using the above data and plotted, with means depicted by yellow lines. Data were compared using one-way repeated measures ANOVA. Means between exams were then compared using Tukey's multiple comparisons test to determine statistically significant differences, which are denoted by asterisks. n.s. = not significant.
Table 2.
Comparison of HAI titer increases for seasonal and pandemic IAVs
| Exams | H1 | H3 | ||
|---|---|---|---|---|
|
|
||||
| USSR/77 (Pandemic) | Texas/91 (Seasonal) | HK/68 (Pandemic) | Alabama/81 (Seasonal) | |
| 1 – 2 | 2.4 | 43# | 3.9 | 1.5 |
| 2 – 3 | 2.9 | 1.6 | 1.2 | 2.5 |
| 3 – 4 | 1.4 | 1.4 | 1.5 | 1.0 |
Texas/91 began circulating between exams 1–2
As would be expected, a substantial drop in TX/91 H1 IgG:HAI ratios occurred between exams 1 and 2, in concordance with the appearance of this strain in human circulation and the subsequent seroconversion (Fig. 3E). In contrast, IgG:HAI ratios against Ala/81 H3 remained stable during the entire period of sampling (Fig. 3F).
Magnitude of HAI titer increases was age-independent
Since age has previously been shown to influence both the magnitude and the clonality of antibodies against particular IAV strains (15, 23), it was important to establish the impact of each individual's age on the overall magnitude of HAI titer change they experienced for each HA subtype between exams 1 and 4. This was of particular interest in the context of H1 titers, since some of the older individuals in our sampling population may have been exposed to H1 viruses prior to their re-emergence in 1977. Therefore, overall differences in HAI titers between exams 1 and 4 were plotted against age for each individual and for each HA subtype tested in figures 2 and 3 (Fig. 4). As expected, the magnitude of HAI titer changes for Sing/57 H2 (Fig. 4A), HK/68 H3 (Fig. 4B) and Ala/81 H3 (Fig. 4E) were independent of age, since all individuals would have had equal probability of exposure to these viruses. Similarly, age did not seem impact rises in the magnitude of HAI titer changes against either USSR/77 H1 (Fig. 4C) or TX/91 H1 (Fig. 4D). This suggests a minimal impact for any potential H1 exposure prior to 1977 in influencing HAI titer changes, and is also likely to reflect the relatively close clustering of our sample population between the ages of 40 and 60 at the time of exam 1. Finally, it is clear from this analysis that for all HAs tested, the vast majority of individuals experienced boosts in HAI titers against all strains tested.
Figure 4. Magnitude of HAI titer changes against IAV strains was age-independent.
The age of each individual at exam 1 was plotted against their total change in HAI titer between exams 1 and 4 for A) Sing/57 H2, B) HK/68 H3, C) USSR/77 H1, D) TX/91 H1 or E) Ala/81 H3. Pearson correlations were performed to derive r values which were used for determination of statistical significance using two-tailed p-values. n.s. = not significant.
Group 1 and group 2 HA stalk antibodies expand over time in the absence of antigenic shift
Broadly-neutralizing HA stalk-specific antibodies are thought to be induced most efficiently upon sequential exposures to viruses exhibiting substantial antigenic diversity (5, 24). Recent studies have shown that broadly neutralizing antibodies specific to the group 1 HA stalk were widely boosted in at least two instances: in individuals who received the A/New Jersey/1976 H1N1 vaccine, and in those vaccinated or infected with the pandemic 2009 H1N1 (p2009 H1N1) virus (3, 6, 16, 25). However, the status of these antibodies during extended periods in which no major antigenic changes occur to the HA of circulating IAVs remains unclear. During the period over which samples were collected for this study (1987–2008), only seasonal strains originating from the USSR/77 H1N1 pandemic (group 1) and from the HK/68 H3N2 pandemic (group 2) circulated (Fig. 1). To determine how titers of HA stalk antibodies specific to group 1 HAs evolved over this period, ELISA plates were coated with recombinant cH6/1 protein and endpoint IgG titrations were performed. Recombinant cH6/1 protein has proven to be a reliable reagent for detection of group 1 HA stalk specific antibodies in several previous studies (3, 24). Between exams 1 and 4, the IgG GMT against cH6/1 rose significantly, from 1369 to 4371 (Fig.5A). The most pronounced rise in titers occurred between exams 1 and 3, while the apparent decline in titers between exams 3 and 4 was not statistically significant. This suggests that even during extended periods of relatively minor antigenic changes to the HA head domain, group 1 HA stalk titers expanded modestly.
Figure 5. Group 1 and group 2 HA stalk-reactive antibody titers.

IgG endpoint titers against group 1 and group 2 HA stalks were evaluated by ELISA against A) cH6/1 HA to determine group 1 HA stalk antibody titers and B) cH5/3 HA to determine group 2 HA stalk antibody titers, from 40 matched samples collected at exams 1, 2, 3 and 4. Red lines denote GMTs. Data were compared using one-way repeated measures ANOVA. GMTs between exams were then compared using Tukey's multiple comparisons test to determine statistically significant differences relative to exam 1, denoted by asterisks. C) Sera collected at Exam 1 were evaluated for HAI activity against NJ/76 virus and Sing/57 VLPs. Individuals were then split into two groups according to whether they had seroconverted (HAI ≥ 40) to NJ/76 only (NJ/76+/Sing/57−) or to both NJ/76 and Sing/57 (NJ/76+/Sing/57+). IgG endpoint titers to group 1 HA stalk were then evaluated by ELISA against cH6/1 recombinant HA. Titers (GMTs) of both groups were compared by Student T test, from which a two-tailed p-value was calculated to evaluate significance.
Unlike group 1 HAs, of which several distinct antigenic lineages have circulated in humans, only group 2 viruses of the HK/68 H3N2 lineage have circulated widely (Fig. 1). The paucity of a group 2 IAV antigenic shift in humans has led to uncertainty regarding the presence and the overall magnitudes of anti-HA stalk antibodies specific to the group 2 HA stalk. To address this, IgG endpoint titers against the group 2 HA stalk were determined using cH5/3 recombinant HA. At the time of exams 1 and 2, these titers fell below the assay's limit of detection. By exams 3 and 4 though, titers against the HA stalk of group 2 viruses rose to levels that could be detected in the majority of individuals (Fig. 5B). Consistent with the model that robust titers of anti-HA stalk antibodies require exposure to HAs with substantial differences in globular head antigenicity, the overall magnitude of group 2 HA stalk IgG titers was substantially lower than those observed against group 1 HA stalk (compare Fig. 5A and 4B).
Given that at least three antigenically distinct lineages of viruses harboring group 1 HAs have circulated in humans (classical swine H1N1, H2N2 and seasonal H1N1), it was of interest to determine whether individuals exposed to more diverse group 1 IAVs also had elevated titers of group 1 stalk antibodies. This question is especially important in the translational context of developing universal influenza virus vaccination strategies that seek to achieve broad and long-lasting immunity by eliciting protective levels of HA stalk antibodies. To address this, our cohort was separated into two groups: one that was seropositive for NJ/76 H1 but seronegative for Sing/57 H2 HA at exam 1 (NJ/76+/Sing/57−) and a group that was seropositive for both NJ/76 H1 and Sing/57 H2 at exam 1(NJ/76+/Sing/57+). Endpoint IgG titers against the group 1 HA stalk domain were determined for each individual within the two groups and were significantly higher (3.8-fold) in those who were seropositive for both NJ/76 H1 and Sing/57 H2 (776 vs. 2949) (Fig. 5C). There was no significant difference in the age of the individuals belonging to each group. These data support the notion that multiple substantial boosts in HA stalk antibodies can be achieved in humans with complex and varied pre-exposure histories. This could be achieved by exposure to HAs bearing head domains with sufficiently different antigenicity from those encountered previously.
No sustained increase in neutralizing titers against an antigenically stable virus
In order to assess how the humoral response is impacted by sequential exposures to antigenically variant viruses, it was necessary to compare the response observed against IAVs to that generated against an antigenically more stable virus. In a natural setting, herpesviruses provide an excellent model for this phenomenon. Following primary infection, herpesviruses establish lifelong latency and periodically reactivate to produce infectious virus facilitating transmission. To enable this life-long persistence, these viruses encode numerous immunomodulatory factors. However, unlike IAVs the virus remains antigenically identical. Therefore, we evaluated antibody titers specific to HCMV, a ubiquitous betaherpesvirus. Levels of neutralizing anti-HCMV antibodies at each exam were assessed from individuals who were seropositive for HCMV at Exam 1 (based on commercial ELISA, 15/40 individuals (37.5%) were determined to be seropositive at exam 1). AD169IE2-YFP was preincubated with serum before inoculation of MRC-5 fibroblasts. YFP signal was measured and was normalized against a HCMV-negative control sample to determine infectivity. In contrast to IAV, titers of HCMV neutralizing antibodies remained stable for the duration of the study (Fig. 6A). Titers of HCMV-reactive IgG antibody levels were also measured for those individuals who tested seropositive at Exam 1 (Fig. 6B). Over the course of the four exams, no statistically significant increase in IgG titers was observed. These results indicate that HCMV neutralizing antibody titers and HCMV-reactive antibody titers remain relatively stable over extended periods of time. Therefore, the humoral response appears to be differentially affected by sequential exposures to antigenically identical or antigenically variable viruses.
Figure 6. HCMV neutralization titers and IgG indexes.
A) Titers of HCMV-neutralizing antibodies from subjects seropositive for HCMV at exam 1 were determined at exams 1-4 by neutralization assay. HCMV strain AD169IE2-YFP was incubated with a 1:100 dilution of serum prior to infection of MRC5 fibroblasts for 16h. YFP signal was quantified and all samples were normalized to a HCMV seronegative control. Mean titers are denoted by the purple line. B) HCMV IgG antibody indexes of individuals who were seropositive at exam 1 were also determined at exams 1-4 by commercial ELISA, using a 1:21 dilution of sera, as per the manufacturer's recommendations. Means are denoted by the blue line. Data were analyzed by one-way repeated measures ANOVA. Statistical differences between means were evaluated using Tukey's multiple comparison's test, where n.s. = not significant.
DISCUSSION
Despite decades of intensive study, a great deal of mystery continues to surround the development of humoral immunity against viral agents. One of the most elusive problems regarding the immune response to IAV is the question of how sequential exposures to drifted/antigenically variable viruses shape the humoral compartment. While cross-sectional studies have provided useful observations regarding differential responses among age-groups to particular strains, a lack of longitudinal analyses has precluded more direct elucidation of the overall impact of sequential exposures on shaping the immune response. The insights gained from these types of studies are essential to understanding the development of immunological memory, and particularly how the humoral compartment evolves over time.
Herein, the antibody responses against several pandemic and seasonal IAV strains have been assessed. In order to differentiate between response quality and overall magnitude, both neutralizing antibody titers (as measured by HAI) and total HA-specific antibody reactivity was measured. Interestingly, neutralizing antibody titers specific to all pandemic strains tested rose significantly over the 20 year course of study. Previous work has shown that antigenic drift of IAVs occurs in a punctuated fashion, often with few amino acid changes differentiating one strain from the next (26). This means that many epitopes remain conserved even as the virus drifts. These epitopes are capable of stimulating memory cells generated during previous exposures. Sustained circulation of H3N2 and H1N1 viruses during the sampling period would thus account for the periodic boosting of antibody titers to previously-encountered strains in a manner consistent with that observed in Fig. 2. The rise in H2N2 neutralizing antibody titers was more surprising, since this strain disappeared from widespread human circulation in approximately 1968. However, antibodies with HAI activity that cross-react with both H2 and H3 HAs have been described previously (27). Given the relatively modest boost in H2N2 HAI titers compared to those observed for H1N1 and H3N2 pandemic strains, it is likely that cross-reactive antibodies elicited by exposures to H3N2 viruses could account for the observed increases. The ability to maintain, and even boost antibody titers against virus subtypes encountered in the distant past without periodic re-exposure to viruses of the same subtype is an important finding and should be carefully considered when evaluating the future pandemic potential of distinct IAV subtypes.
As expected, HA reactivity as measured by IgG endpoint titers was much more variable than neutralizing antibody titers. These titers are likely to be influenced more substantially by non-specific, polyclonal stimulation that occurs upon exposure to foreign agents (28). While H2N2 and H1N1 IgG endpoint titers tended to mirror changes in HAI antibody titers, H3N2 endpoint titers remained relatively stable despite substantial increases in neutralizing antibody titers. Indeed, HAI titers would be expected to increase upon further affinity maturation of cells already present in the memory compartment with certain HA head domain specificities, which would not necessarily increase overall reactivity as measured by ELISA. A recent study profiling the lineage structure of the human antibody repertoire to influenza virus vaccination brings some clarity to this finding (23). The authors found that the number of antibody lineages found in elderly subjects was substantially reduced relative to their younger counterparts. However, their pre-vaccination mutational loads were much higher, suggesting that clones selected by more frequent re-stimulation are maintained while the overall pool of clonal lineages contracts.
In a manner consistent with “OAS,” the magnitude of neutralizing antibody titers specific to pandemic IAV strains exceeded those of their seasonal counterparts. The results described herein lend support to the notion that the hierarchical nature of the human antibody response to IAVs is likely a result of “antigenic seniority.” While titers were greatest against pandemic strains of each subtype, protective responses were also observed against seasonal viruses that circulated over a decade later. Thus, the phenomenon of OAS appears to apply equally to both pandemic and seasonal IAV strains. Indeed, the magnitudes of HAI titer increases between exams were similar for both seasonal and pandemic strains, and were independent of age in this sample population. These data provide a rational basis for one of the most well-known and peculiar phenomena relating to humoral immunity against IAV. Specifically, these results would support a model whereby antibodies specific to previously-encountered strains are periodically boosted by later exposures to viruses of the same subtype. This ultimately results in highest antibody titers against strains encountered earliest in life; since antibodies generated against strains encountered progressively earlier have the opportunity to be boosted the greatest number of times during future exposures.
In addition to driving the expansion of antibody titers to epitopes present on the HA head, sequential exposures to IAV are also known to influence titers of antibodies specific to the HA stalk domain. These antibodies are of particular interest, since they are known to possess broad neutralization activity (29). Specific scenarios wherein broadly-neutralizing antibodies have been elicited in humans has been the focus of several recent studies (3, 6, 16–18, 21). These studies suggest that broadly-neutralizing antibodies are boosted more efficiently in humans upon sequential exposure to HAs bearing head domains with substantial antigenic differences. Plasmablasts with HA stalk specificity have also been detected following vaccination with seasonal trivalent inactivated vaccine (TIV) (19). This has led to uncertainty regarding the longevity of these antibodies, especially during prolonged periods without substantial antigenic shift. During the period of sample collection described herein, only seasonal H1 and H3 IAV viruses circulated in humans (Fig. 1). Interestingly, titers of group 1 HA and group 2 HA stalk-reactive antibodies rose modestly, but significantly throughout this period of minimal antigenic variation. The apparent absence of antibodies specific to group 2 HA stalk (cH5/3) during exams 1 and 2 may reflect the detection limit of the assay, rather than their complete absence. Indeed, infection with H3 viruses have been shown to effectively elicit these antibodies (21). However, it is not entirely surprising that their titers appear substantially lower than those observed against group 1 HAs, since no major antigenic shift has occurred in group 2 viruses that circulate in humans.
Individuals who received the NJ/76 H1N1 vaccine, Cal/09 vaccine or were infected with Cal/09 virus are known to have elevated titers against group 1 HA stalk (3, 6). However, those exposed to both NJ/76 and Cal/09 did not have higher titers than those exposed to NJ/76 virus only (3). This is likely due to the high degree of antigenic conservation shared by these two strains. Since multiple boosts of HA stalk antibodies has never been observed in humans, it was of interest to determine whether exposure to HAs with sufficiently large antigenic variation could produce such a phenomenon. Consistent with the notion that antigenic shift drives robust expansion of stalk antibodies, individuals who were seropositive for both NJ/76 H1N1 and Sing/57 H2N2 at exam 1 had significantly greater titers of group 1 HA stalk antibodies than those who were seropositive for NJ/76 alone. These results provide compelling evidence that antigenic variation is essential to drive the expansion of HA stalk antibodies in humans. As proposed previously, such results are consistent with a model whereby substantial antigenic changes to the immunodominant HA head domain are required to permit expansion of broadly-neutralizing antibodies to subdominant stalk epitopes (5). This in turn raises the possibility that vaccination strategies that mimic these natural scenarios could be utilized as an effective means by which to provide broad and long-lasting protection against IAV.
To explore how sequential exposures to antigenically variable viruses shape humoral immunity in comparison to antigenically stable viruses, reactivity and neutralizing antibody titers specific to HCMV were also measured. HCMV is a ubiquitous betaherpesvirus that establishes life-long latency in the hematopoietic compartment following primary infection. As has been observed for other herpesviruses, HCMV reactivation occurs periodically in healthy individuals. Though the signals that trigger reactivation are not fully understood, they include immunosuppression, elevated levels of tumor necrosis factor α, inflammatory prostaglandins and stress-induced catecholamines (30). In healthy individuals, effective adaptive immunity prevents the development of symptoms. However, serious pathology can occur in those who are immunocompromised (31). Though one cannot specifically control for frequency and magnitude of exposures to different viral agents in a human population, previous work has shown that HCMV antibody titers are greater in elderly individuals than in the young (32). Nonetheless, reactivation becomes more frequent with age. In fact, HCMV DNA could be found in the urine of 91% of elderly seropositive subjects monitored over a 6 month period (33). The mean age of the cohort included in this study was 49 at the beginning of exam 1, and 70 by the end of exam 4. In order to avoid the possible confounding effect of measuring increases that could be due to primary CMV infection during the examination period, all subjects who tested seronegative for HCMV at exam 1 were excluded from further analysis. Interestingly, in contrast to the sustained increases in neutralizing antibody titers observed against IAVs, HCMV reactive and neutralizing antibody titers remained stable for the duration of the study. These results are consistent with the stable antibody titers observed against other herpesviruses, including varicella-zoster virus and Epstein-Barr virus (10). Taken together, these data suggest that by adulthood, antibody titers against viruses that establish persistent/chronic infections without changing antigenicity tend to plateau. This is likely because at a certain level, high circulating titers of neutralizing antibodies become sufficient to neutralize any reactivated virus.
By undertaking a longitudinal approach to understanding the effects of sequential virus exposures on shaping the humoral immune compartment we have been able to investigate several important questions not easily addressed by studies in animal models or cross-sectional analyses. However, due to the inability to control for exposure histories in a study of this nature, potential effects dependent upon type and frequency of exposure (ie. infection vs. vaccination) cannot be easily resolved. The cohort examined in this study consisted of adults 35 years and older at the time of initial sample collection. Whether these same changes occur in younger, less exposed individuals remains to be seen. Still, the use of longitudinal human samples is the most direct and relevant way to investigate the global effects of complex immunological interactions.
Specifically, we have demonstrated that sequential exposures to antigenically distinct strains of IAV drive an expansion of neutralizing antibody titers against previously-encountered strains. This observation provides a rational basis for the long-term persistence of antibodies against strains encountered in the distant past despite a lack of boosting by identical strains in the future. In addition, we have helped to clarify the elusive immunological basis for what has been historically called “OAS.” Our data suggest that the hierarchical nature of neutralizing antibody titers to strains encountered progressively earlier in life stem from periodic boosting of these titers upon subsequent exposures to related strains. However, we did not find any evidence of suboptimal responses to seasonal strains encountered long after each pandemic. This phenomenon is consistent with the model of “antigenic seniority,” that has recently been described (15). Our work also addresses several outstanding questions regarding the immunobiology of HA stalk-reactive antibodies, which appear critical for conferring broad protection against IAV. Antibody titers specific to group 1 and 2 HA stalks also rose modestly during the study period despite a lack of any major antigenic shifts. However, group 1 HA stalk antibody titers were greatest in individuals who were exposed to the most antigenically diverse group 1 viruses, namely Sing/57 H2N2 and NJ/76 H1N1. These observations support the notion that antigenic diversity strongly influences HA stalk titers in humans, and provides support for vaccination strategies seeking to replicate this phenomenon. Finally, we show that antibody titers against a viral agent causing persistent infection (like HCMV) that are antigenically fixed seem to remain stable at a particular plateau. These results are consistent with those obtained for similar viral agents (10). It is likely that other infectious agents to which individuals are repeatedly exposed will have similar effects on shaping humoral memory, depending on whether or not they remain antigenically identical (ie. herpesviruses) or variant (i.e. noroviruses, coronaviruses, etc…). Together, these results have provided insights regarding the regulation of adaptive immunity and the role played by antigenic variation in shaping the humoral immune compartment. Understanding the complex patterns of immunological memory development in human populations should be a focus for those attempting to design more long-lasting and efficacious vaccines.
MATERIALS & METHODS
Study Design
Sera were obtained from 40 random individuals enrolled in the Framingham Heart Study (FHS) Offspring Cohort (http://www.framinghamheartstudy.org/participants/offspring.html) in order to approach a normal distribution. Sampling was performed with individual consent in accordance with the Institutional Review Board framework of the FHS. All individuals were born between 1917 and 1952 in order to ensure potential exposure to pandemic and seasonal IAV strains circulating between 1957 and 2008. Samples were thawed a maximum of one time prior to arrival at our facility, and then were handled in an identical manner. The geometric mean date of birth was 1938. Four samples were acquired from each individual, collected in approximately five year intervals. These samples will be identified as exams 1, 2, 3 and 4 for the purpose of this study. Sample collection periods were as follows: exam 1 (1987 to 1991), exam 2 (1995 to 1998), exam 3 (1998 to 2001) and exam 4 (2005 to 2008). See Fig. 1 for a timeline displaying dates of birth, sampling period and circulation of IAV strains. No metadata was gathered beyond subject age and dates of sample collection. Total IAV and HCMV antibody titers were assessed by ELISA assays. Neutralizing antibodies against IAV were measured by HAI assays, while HCMV neutralizing antibodies were measured by microneutralization assay. Individual antibody titers were determined at each exam in order to assess longitudinal changes.
Expression and purification of recombinant influenza virus proteins
HA proteins of A/Singapore/1/1957 H2N2 (Sing/57), A/Hong Kong/1/1968 H3N2 (HK/68), A/USSR/90/1977 H1N1 (USSR/77), A/Alabama/1/1981 H3N2 (Ala/81), A/Texas/36/1991 H1N1 (Texas/91), cH6/1 (contains globular head domain from A/mallard/Sweden/81/02 fused to the PR8 stalk domain) and cH5/3 (H5 globular head domain from A/Vietnam/1203/2004 fused to the stalk domain from A/Perth/16/2009) were expressed in BTI-TN5B1-4 (High Five) cells as described previously (3, 21, 34). Briefly, ectodomain coding sequences of each protein were cloned into a modified pFastBac vector (Invitrogen) with a C-terminal hexahistidine tag and a T4 trimerization domain. Recombinant baculovirus (rBV) expressing each HA was generated according to the manufacturer's recommendations. High Five cells were infected with the rBVs at a multiplicity of infection (MOI) of 10. Cells were harvested 72–96 h post-infection (hpi). Proteins were purified using Ni-NTA resin (Qiagen).
Hemagglutinin inhibition (HAI) assays
HAI assays were performed according to standard procedures, as described previously (3). Briefly, sera were inactivated with TPCK trypsin at 56 °C for 30 min. For H2 assays, VLPs generated in 293T cells expressing A/Japan/305/1957 (Jap/57) H2 and N3 from A/Swine/Missouri/4296424/06 (Miss/06) were used (35). For all other assays, whole virus particles were used (HK/68, USSR/77, TX/91, Ala/81 or A/New Jersey/1976 H1N1 (NJ/76)). Viruses and sera were mixed and were incubated at RT for 30 min. Chicken red blood cells (0.5%) were then added to each well and plates were incubated at 4 °C for approximately 30 min prior to reading. In accordance with convention, seropositive samples were considered to be those that exhibited HAI activity greater than or equal to 40 against a given HA. Controls with PBS only in the HAI tests resulted in negative readings.
Immunoglobulin G (IgG) endpoint titer determination
IgG endpoint titers were determined by enzyme-linked immunosorbent assay (ELISA) on 96 well plates as described previously (3). Briefly, plates were coated overnight with purified, recombinant HA or BSA (2 μg/ml). Plates were blocked with 5% non-fat milk. Serum was diluted serially in 5% non-fat milk and was incubated on plates for 1h at RT. Plates were washed with PBS/0.025% Tween-20 (PBS-T). Secondary goat anti-human IgG-horseradish peroxidase (HRP, Meridan Life Science Inc) was incubated on plates at a 1:5000 dilution in 5% non-fat milk for 1h at RT prior to washing of plates and addition of HRP substrate (SigmaFAST OPD, Sigma Aldrich). Reactions were stopped after 10 min by addition of 3M HCl and optical density measurements were taken at 490 nm. PBS-only negative control samples were subtracted from readings given by serum samples prior to calculating endpoint titers.
HCMV ELISA and neutralization assays
HCMV IgG antibody indices were determined using a commercial ELISA kit (Calbiotech) according to the manufacturer's instructions. HCMV neutralization assays were performed as described previously (36). Briefly, HCMV strain AD169IE2-YFP (37) (MOI = 2) was incubated with or without a 1:100 dilution of human serum for 2.5 h at 4 °C. Serum/antibody mixtures were then added to MRC5 fibroblasts (1.5 × 104 cells/well) in 96 well plates, and were incubated for 16 h at 37 °C. At this time, inoculum was removed and was replaced with fresh medium prior to quantification of YFP signal using an Acumen eX3 laser scanning fluorescence microplate cytometer. Signal greater than two standard deviations above background (uninfected control) was considered positive. All samples were normalized against a confirmed HCMV seronegative control sample to determine the percentage of neutralization.
Statistical Analyses
All longitudinal analyses were performed by considering linked titers of individuals' as measured at each exam. For evaluation of IgG endpoint titers and HAI titers, repeated measures one-way ANOVA analyses were performed, followed by Tukey's repeated measures test to determine statistical differences in GMTs between each exam. To determine possible correlations between the age of subjects and their overall change in HAI titers between exams 1 and 4, two-tailed P values were derived from Pearson correlation coefficients (r).
ACKNOWLEDGEMENTS
The authors would like to thank the Framingham Heart Study (http://www.framinghamheartstudy.org/participants/offspring.html) for the generous donation of samples and Chen Wang for providing excellent technical support.
FUNDING M.S.M. was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship. T.J.G. was supported in part by a USPHS Institutional Research Training Award T32-AI07647 and a Helmsley Trust Fellowship. F.K. was partially supported by an Erwin Schrödinger Fellowship (J 3232) from the Austrian Science Fund (FWF). This work was supported by PATH and by National Institutes of Health [HHSN26620070010C to P.P], [AI085306 to C.F.B.] and an American Heart Association grant to D.T.
Footnotes
AUTHOR CONTRIBUTIONS All authors were involved in the design of experiments and analysis of data. M.S.M., T.J.G., F.K. and L.C.A. performed experiments. M.S.M. and P.P. wrote the manuscript.
COMPETING INTERESTS The authors declare no competing interests.
REFERENCES
- 1.McCullers JA, Van De Velde L-A, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:1487–92. doi: 10.1086/652441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manicassamy B, Medina RA, Hai R, Tsibane T, Stertz S, Nistal-Villán E, Palese P, Basler CF, García-Sastre A, Fouchier RAM. Ed. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS pathogens. 2010;6:e1000745. doi: 10.1371/journal.ppat.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 1976 and 2009 H1N1 Influenza Virus Vaccines Boost Anti-Hemagglutinin Stalk Antibodies in Humans. The Journal of infectious diseases. 2013;207:98–105. doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gras S, Kedzierski L, Valkenburg SA, Laurie K, Liu YC, Denholm JT, Richards MJ, Rimmelzwaan GF, Kelso A, Doherty PC, Turner SJ, Rossjohn J, Kedzierska K. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12599–604. doi: 10.1073/pnas.1007270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palese P, Wang TT. Why do influenza virus subtypes die out? A hypothesis. mBio. 2011;2 doi: 10.1128/mBio.00150-11. doi:10.1128/mBio.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2573–8. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster RG. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. Journal of immunology (Baltimore, Md.: 1950) 1966;97:177–83. [PubMed] [Google Scholar]
- 8.FRANCIS T, DAVENPORT FM, HENNESSY AV. A serological recapitulation of human infection with different strains of influenza virus. Transactions of the Association of American Physicians. 1953;66:231–9. [PubMed] [Google Scholar]
- 9.Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979;1:33–5. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- 10.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. The New England journal of medicine. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 11.ORIGINAL antigenic sin. The New England journal of medicine. 1958;258:1016–7. doi: 10.1056/NEJM195805152582014. [DOI] [PubMed] [Google Scholar]
- 12.de St Groth Fazekas, Webster RG. Disquisitions of Original Antigenic Sin. I. Evidence in man. The Journal of experimental medicine. 1966;124:331–45. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de St Groth Fazekas, Webster RG. Disquisitions on Original Antigenic Sin. II. Proof in lower creatures. The Journal of experimental medicine. 1966;124:347–61. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DAVENPORT FM, HENNESSY AV, FRANCIS T. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. The Journal of experimental medicine. 1953;98:641–56. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJD, Guan Y, Jiang CQ, Cummings DAT. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS pathogens. 2012;8:e1002802. doi: 10.1371/journal.ppat.1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G-M, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng N-Y, Lee J-H, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9047–52. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui J, Sheehan J, Hwang WC, Bankston LA, Burchett SK, Huang C-Y, Liddington RC, Beigel JH, Marasco WA. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:1003–9. doi: 10.1093/cid/cir121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrammert J, Koutsonanos D, Li G-M, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng N-Y, Lee J-H, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. The Journal of experimental medicine. 2011;208:181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corti D, Suguitan AL, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. The Journal of clinical investigation. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JPM, Skehel JJ, Lanzavecchia A. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science (New York, N.Y.) 2011;333:850–6. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 21.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. Journal of virology. 2013 doi: 10.1128/JVI.03509-12. doi:10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekiert DC, Friesen RHE, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJWM, Brandenburg B, Vogels R, Brakenhoff JPJ, Kompier R, Koldijk MH, Cornelissen LAHM, Poon LLM, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science (New York, N.Y.) 2011;333:843–50. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He X-S, Dekker CL, Zheng N-Y, Huang M, Sullivan M, Wilson PC, Greenberg HB, Davis MM, Fisher DS, Quake SR. Lineage structure of the human antibody repertoire in response to influenza vaccination. Science translational medicine. 2013;5:171ra19. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer F, Pica N, Hai R, Tan GS, Palese P. Hemagglutinin Stalk-Reactive Antibodies Are Boosted following Sequential Infection with Seasonal and Pandemic H1N1 Influenza Virus in Mice. Journal of virology. 2012;86:10302–7. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, Gubbay J, Pasick J, Petric M, Jean F, Allen VG, Brown EG, Rini JM, Schrader JW. Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem. Frontiers in immunology. 2012;3:87. doi: 10.3389/fimmu.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus ADME, Fouchier RAM. Mapping the antigenic and genetic evolution of influenza virus. Science (New York, N.Y.) 2004;305:371–6. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 27.Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Albrecht R, Blum DL, Ramos I, Fernandez-Sesma A, Edwards KM, García-Sastre A, Basler CF, Crowe JE. Human monoclonal antibodies to pandemic 1957 H2N2 and pandemic 1968 H3N2 influenza viruses. Journal of virology. 2012;86:6334–40. doi: 10.1128/JVI.07158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science (New York, N.Y.) 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 29.Corti D, Lanzavecchia A. Broadly Neutralizing Antiviral Antibodies. Annual review of immunology. 2013 doi: 10.1146/annurev-immunol-032712-095916. doi:10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 30.Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011;2:6. doi: 10.1186/2042-4280-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Current topics in microbiology and immunology. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- 32.Alonso Arias R, Moro-García MA, Echeverría A, Solano-Jaurrieta JJ, Suárez-García FM, López-Larrea C. Intensity of the humoral response to cytomegalovirus is associated with the phenotypic and functional status of the immune system. Journal of virology. 2013;87:4486–95. doi: 10.1128/JVI.02425-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Experimental gerontology. 2007;42:563–70. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PloS one. 2012;7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. Journal of virology. 2012;86:5774–81. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner TJ, Bolovan-Fritts C, Teng MW, Redmann V, Kraus TA, Sperling R, Moran T, Britt W, Weinberger LS, Tortorella D. Development of a high-throughput assay to measure the neutralization capability of anti-CMV antibodies. Clinical and vaccine immunology : CVI. 2013 doi: 10.1128/CVI.00644-12. doi:10.1128/CVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng MW, Bolovan-Fritts C, Dar RD, Womack A, Simpson ML, Shenk T, Weinberger LS. An endogenous accelerator for viral gene expression confers a fitness advantage. Cell. 2012;151:1569–80. doi: 10.1016/j.cell.2012.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]