Abstract
Purpose
Triptolide induces cancer cell apoptosis by inhibiting RNA synthesis and signaling pathways like NF-κB. We compared triptolide prodrug MRx102 to triptolide to determine if it displayed comparable efficacy and improved toxicology and toxicokinetic profiles.
Methods
MV4–11 AML cells and cells from AML patients were analyzed for MRx102− and triptolide-induced cytotoxicity/apoptosis. MRx102 and triptolide were compared in toxicology/toxicokinetics studies in rat and dog using a new emulsion formulation.
Results
MRx102 induced cytotoxicity in MV4–11 cells (IC50 = 15.2 nM, 7.29 nM for triptolide) and apoptosis in cells from AML patients (EC50 = 40.6 nM and 2.13 nM for triptolide). MRx102 and triptolide induced apoptosis in CD34+CD38− AML stem/progenitor cells with a similar difference in activity (EC50, MRx102 = 40.8 nM, triptolide = 2.14 nM). In a rat toxicology comparison using a new intravenous emulsion formulation, the MRx102 MTD was 4.5 mg/kg for males, 3 mg/kg for females; the triptolide MTD was 0.63 mg/kg for males, 0.317 mg/kg for females. The MRx102 NOAEL was 1.5–3.0 mg/kg, and the triptolide NOAEL was 0.05–0.15 mg/kg. Mean plasma concentrations for both MRx102 and triptolide decreased rapidly from a high Cmax following i.v. injection. Plasma triptolide levels stabilized at a consistent level through 2 hours after MRx102 injection. Triptolide T1/2,e values for MRx102-injected rats (~0.85 to ~3.7 hours) were markedly greater than triptolide injected rats (~0.15 to ~0.39 hours), indicating more extended triptolide exposure with MRx102. MRx102 dog toxicology and toxicokinetics results are presented.
Conclusions
MRx102 was 20− to 60-fold safer than triptolide comparing rat NOAELs. This may be due to the improved toxicokinetic profile of MRx102 compared to triptolide using the emulsion formulation, with no high Cmax and more consistent early exposure to triptolide.
Keywords: AML, prodrug, leukemia, triptolide, MRx102, toxicology, toxicokinetics
Introduction
Triptolide is a natural product extracted from the medicinal plant, Tripterygium wilfordii, [1] used traditionally to treat inflammatory and autoimmune diseases. Triptolide has been used clinically to treat rheumatoid arthritis [2], nephritis [3] and certain cancers. Despite complete remissions for some patients with triptolide in acute leukemia [4] and the F60008 triptolide prodrug in acute myeloid leukemia (AML) [5], significant safety challenges have been encountered [6]. F60008 underwent incomplete conversion in human plasma. High interindividual variability of pharmacokinetic parameters between patients reflected widely divergent circulating triptolide levels, and the most severe adverse events were due to overexposure (evident as a high Cmax) [6]. Thus, the major problem for further development of triptolide and triptolide-related compounds is the toxicity profile that appears to result from poor pharmacokinetics.
Our development of triptolide prodrug MRx102 is intended to optimize the activity for clinical application and produce, essentially, an improved and safer triptolide derivative. Our requirements for an optimized compound include complete prodrug triptolide conversion, and avoiding very high initial exposure to triptolide in preclinical animal studies by maintenance of relatively constant triptolide blood levels early after i.v. injection. A new formulation was developed to help achieve the goal of improved pharmacokinetics for the lipophilic MRx102 prodrug and to avoid excipient-related side effects.
The activity of MRx102 was compared to triptolide for in vitro cytotoxicity with the MV4–11 AML cell line. We evaluated MRx102 and triptolide activity with AML patient cells, using flow cytometric characterization of CD34+CD38− stem/progenitor cells that are thought to be responsible for AML disease progression, resistance to therapy and relapse. The new formulation was developed for intravenous studies, and was utilized in toxicology and toxicokinetic comparisons. The combination of the MRx102 triptolide prodrug and the emulsion formulation produced an altered and improved triptolide exposure profile, resulting in an apparently much safer triptolide.
Materials and Methods
Animals
Male and female Sprague-Dawley rats from Harlan (Frederick, MD) were 7–8 weeks old at the start of dosing in the toxicology and toxicokinetics studies. Rats were individually housed upon assignment to the studies in compliance with National Research Council “Guide for the Care and Use of Laboratory Animals.” Male and female beagle dogs from Marshall BioResources USA (North Rose, NY) were 6.6–8.8 kilograms in the 7-day repeat dose toxicology and toxicokinetics studies. Dogs were individually housed in compliance with USDA guidelines.
Cells, cell culture and treatment of cells
The MV4–11 human AML cell line was obtained from the American Type Culture Collection (Manassas VA, CRL-9591) for studies at Murigenics Inc. (Vallejo, CA) and cultured in Iscove’s Modified Dulbecco’s Medium (Invitrogen, Inc, Carlsbad CA) containing glutamine, penicillin/streptomycin and 10% heat inactivated fetal calf serum (FCS).
Fresh peripheral blood samples from AML patients with high blast counts (95% or higher) were acquired after written informed consent had been obtained according to the Declaration of Helsinki, and the study protocol was approved by the MD Anderson Institutional Review Board. Mononuclear cells were purified by Ficoll-Hypaque (Sigma, St Louis, MO) density-gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2mM glutamine, and penicillin/streptomycin.
The MS-5 murine mesenchymal stromal cell (MSC) line known to support primitive human progenitor and to mimic the bone marrow microenvironment was kindly provided by Dr. K. Itoh (Niigata University, Niigata, Japan) [7–9].
Cell viability assay
Viable cell counts were determined by flow cytometry using CountBright beads (Invitrogen, Carlsbad, CA) on annexin V/7-amino-actinomycin D negative cell events. Apoptosis was assessed by flow cytometry with annexin V Cy5 (BD Biosciences, San Diego, CA) using a FACSArray Bioanalyzer (BD Biosciences). For AML patient samples, apoptosis was determined after cells were stained with anti-CD34 and anti-CD38 antibodies. For AML cells cocultured with MS-5 MSC or bone marrow derived MSC, leukemic cells were distinguished by gating on CD45+ populations. Cells were stained with CD45 APC H7, CD34 PE, CD38 PE Cy7 and annexin V Cy5 (BD Biosciences). Apoptosis was determined by flow cytometry of annexin V Cy5 positivity. Specific apoptosis was calculated as 100%×(% apoptosis in treated cells - % apoptosis in untreated cells)/% viable cells in untreated cells.
Cell proliferation assay
Exponentially growing MV4–11 cells were added at 2.5x104 cells/well in 96 well flat bottom culture plates and incubated at 37°C with 5% CO2. After 24 hours, MRx102 (18-benzoyloxy-19-benzoylfuranotriptolide) and triptolide were added to triplicate cultures at three-fold dilutions ranging 2–1000 nM. The cells were incubated for an additional 24, 48 and 72 hours. Alamar Blue (Invitrogen, Inc) was added and the plates incubated for 4 additional hours. The supernatant was harvested and read on a SpectraMax M5 fluorescent plate reader (Molecular Devices, Sunnyvale, CA) using 560 nm for excitation and 590 nm for emission. The fluorescence is a measure of viable cells. IC50 values were calculated in SoftMax Pro (Molecular Devices).
Preparation of MRx102 and triptolide emulsions
Glyceryl trioctanoate (1 g), soybean oil (1 g), and phospholipids (L-lecithin, 0.1 g) were mixed and fully dispersed using a probe sonicator. MRx102 (5 mg) was added, and the fluid was sonicated with cooling until the compound was completely dissolved. Glycerin (0.125 g) was dissolved in a solution of sodium cholate (0.01 g) in water (2.77 ml), and the mixture was added in increments to the phospholipid/oil/MRx102 in a cold water bath and sonicated until completely dissolved, forming a creamy opaque suspension. The pH was adjusted to 7.5 to 8.5 using 0.1N sodium hydroxide. The emulsion was sonicated continuously for 8 minutes in the cold water bath, the result being opaque white, thick and creamy. The emulsion was filtered through a sterile 0.45 µm polyethersulfone membrane filter, appearing unchanged. The triptolide emulsion was prepared in a similar manner. All emulsion components were from Sigma.
7-Day Repeat Dose Toxicology Comparison Study of MRx102 and Triptolide in Rats
In the 7-day repeat dose rat toxicology comparison study of MRx102 and triptolide conducted at Calvert Labs, the test articles prepared using the emulsion formulation were administered i.v. to naïve rats (5/sex/group) once daily for seven days. The emulsion was prepared at 1.0 mg/ml MRx102 and 0.1 mg/ml triptolide, and the volumes were adjusted according to dose. Blood for hematology, coagulation and clinical chemistry was collected from all surviving animals prior to sacrifice on Day 8. Tissues were harvested at necropsy and were later evaluated microscopically for the histopathology report.
7-Day Repeat Dose Toxicology Comparison Study of MRx102 and Triptolide in Dogs
The 7-day repeat dose toxicology study of MRx102 in dogs was conducted at Calvert Labs similar to the 7-day repeat dose toxicology study in rats described above. MRx102 prepared at 1.0 mg/ml using the emulsion formulation was administered i.v. to naïve beagle dogs (2/sex/group) once daily for seven days.
Analysis of triptolide and MRx102 contents of dosing preparations
The MRx102 and triptolide content of the formulations prepared for dosing was determined at Calvert Labs by HPLC analysis before dosing, so that the intended dose level could be administered. An Agilent 1200 Series Modular System (Agilent Technologies, Inc., Santa Clara, CA) was used, with a Phenomenex Gemini-NX C18 column.
Toxicokinetics study and analysis of triptolide and MRx102 content
For the toxicokinetic component of the 7-day rat toxicology study, eighteen animals (9 males, 9 females) per group were dosed i.v. by tail vein with MRx102 or triptolide for seven days. MRx102 groups 8, 9 and 10 received the low-dose at 0.5 mg/kg/day, mid-dose at 1.5 mg/kg/day, and high-dose at 3.0 mg/kg/day, respectively. Triptolide groups 11, 12 and 13 were given the low-dose at 0.05 mg/kg/day, mid-dose at 0.15 mg/kg/day, and high-dose at 0.3 mg/kg/day, respectively. The nominal dose volumes for the low, mid, and high dose groups were 0.5, 1.5, and 3.0 ml/kg, respectively. On Day 1 and Day 7, whole blood samples (0.5 ml/sample into tubes containing K3EDTA with 20 µL of 1 M citric acid solution) were collected by retroorbital puncture immediately pre-dose and 0.25, 0.5, 1, 2, and 24 hours post-dose. Each animal was bled no more than twice by using 3 of the 9 animals/sex/group at each time.
For the toxicokinetic component of the 7-day dog toxicology, the same animals were used. On Day 1 and Day 7, whole blood samples (1 ml/sample into blood collection tubes containing K3EDTA with 40 µL of 1 M citric acid solution) were collected from the jugular vein immediately pre-dose and 0.25, 0.5, 1, 2, and 24 hours post-dose.
For both rat and dog, plasma samples were stored at −70 °C until shipment to Frontage Laboratories, Inc. (Malvern, PA) for analysis of MRx102 and triptolide concentrations using a 2-in-1 qualified LC-MS/MS method. The lower limit of quantitation was 1.0 ng/ml for MRx102 and 2.0 ng/ml for triptolide. Averaged and composite concentration-time data were calculated, and any value reported as below the limit of quantification (BLQ) was assigned a value of zero. Non-compartmental pharmacokinetic analysis, using WinNonlin v5.3 (Pharsight, St. Louis, Missouri), was used to calculate the MRx102 and triptolide toxicokinetic parameters, including initial concentration (C0), maximal concentration (Cmax), the time of maximal concentration (Tmax), the area under the curve for the period of analysis (AUC0-inf), area under the curve from time zero to 24 hours (AUC0–24hr), half-life (T1/2,e), clearance (Cl), and volume of distribution (Vz).
Results
MRx102 and triptolide are cytotoxic for MV4–11 human AML cell line
Triptolide is a potent inducer of apoptosis and cytotoxicity in a variety of cancer cell lines [10–16]. MRx102 was compared to triptolide for cytotoxicity in MV4–11 AML cells. Both compounds were highly cytotoxic in MV4–11 cells (Table 1) as evidenced by the low nM IC50 measured with the Alamar Blue viability assay. For example, the 72 hr IC50 values were 5.6 nM and 16.2 nM for triptolide and MRx102, respectively. Triptolide was two- to three-fold more potent than MRx102 in these MV4–11 AML cell line assays. Similar comparative results were obtained with OCI-AML3, U937 and BaF3/ITD AML cell lines, as well as a number of human solid tumor cell lines (not shown).
Table 1.
In vitro cytotoxic activity with MV4–11 human AML cell line
| IC50 (nM) | |||
|---|---|---|---|
| 24 hrs. | 48 hrs. | 72 hrs. | |
| Triptolide | 24.1 | 7.29 | 5.6 |
| MRx102 | 74 | 15.2 | 16.2 |
| Ratio - MRx102 to Triptolide | 3.1 | 2.1 | 2.9 |
Cells of the MV4–11 human AML cell line were incubated in vitro in microcultures to assess the cytotoxic activity of MRx102. The IC50 for triptolide and MRx102, plus the ratio of the IC50 for MRx102 and triptolide activity, were calculated for the 24, 48 and 72 hr assays. These results are from a representative experiment among several that were conducted.
MRx102 and triptolide induce apoptosis in leukemic cells, including leukemia stem/progenitor cells from AML patients
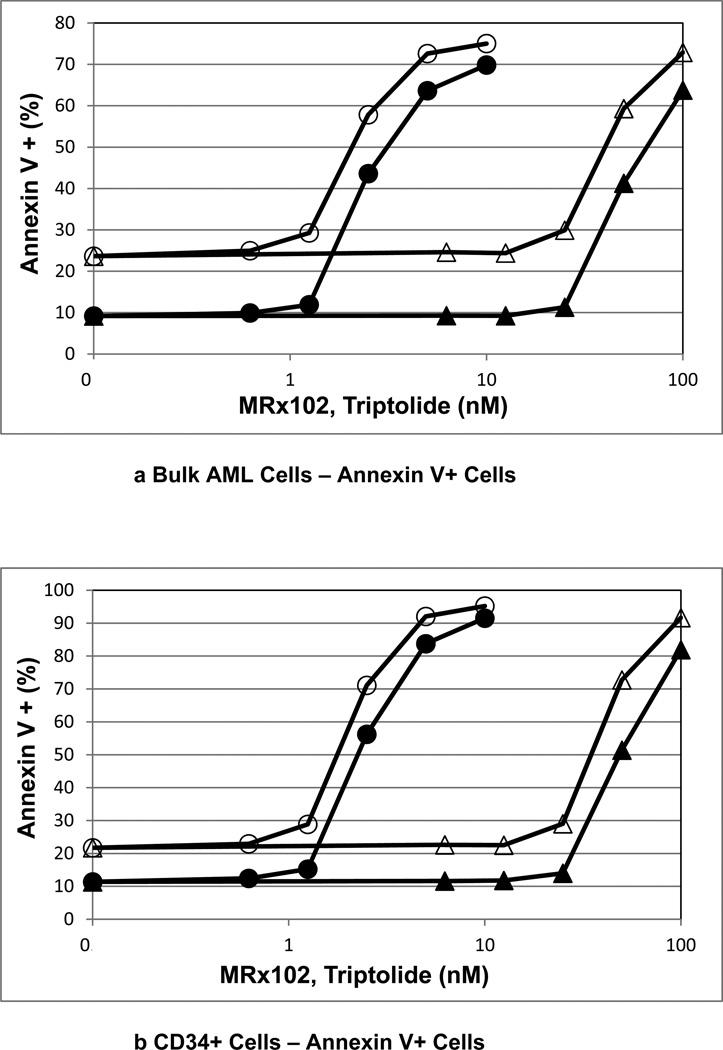
We reported previously that MRx102 induces apoptosis in leukemic cells including leukemic stem/progenitor cells from AML patients [17]. We compared the in vitro anti-leukemia activity of MRx102 to triptolide in samples from AML patients. MRx102 and triptolide at low nanomolar concentrations effectively induced apoptosis (Fig. 1, Table 2) and reduced cell counts (Table 2) in bulk mononuclear cells from AML patients. The concentration curves for MRx102 and triptolide shown in Fig. 1 are offset with lower triptolide concentrations being effective, as anticipated [17, unpublished results]. A comparison of the MRx102 and triptolide results (Table 2) indicates that MRx102 is less potent than triptolide.
Figure 1.
a-d Comparison of apoptosis with MRx102 and triptolide treatment of AML patient leukemic cells, including leukemic stem/progenitor cells
AML patient cells were incubated in vitro in microcultures to assess apoptosis induced by MRx102 (triangles) and triptolide (circles). AML cells were cultured alone (open symbols) or with MS-5 cells (closed symbols). The cells were classified flow cytometrically as bulk, CD34+, CD34+ 38−, and CD34+ 38+. The results for a representative patient sample are shown here as Annexin V+ Cells.
Circles – Incubation of AML cells with triptolide. Triangles – Incubation of AML cells with MRx102. Open symbols – AML cells cultured alone. Closed symbols – AML cells cultured with MS-5 cells.
Table 2.
In vitro activity of MRx102 and triptolide with AML patients’ samples
| EC50 - Annexin V + | EC50 -Cell Count | |||
|---|---|---|---|---|
| MRx102 | Triptolide | MRx102 | Triptolide | |
| (nM) | (nM) | (nM) | (nM) | |
| Alone | ||||
| Bulk | 40.6 | 2.13 | 36.2 | 2.19 |
| CD34+ | 40.8 | 2.13 | 35.8 | 2.15 |
| CD34+38− | 40.8 | 2.14 | 34.5 | 2.13 |
| CD34+38+ | 40.5 | 2.09 | 38.2 | 2.13 |
| + MS-5 | ||||
| Bulk | 40.6 | 2.34 | 45.4 | 2.33 |
| CD34+ | 40.8 | 2.35 | 46.9 | 2.38 |
| CD34+38− | 40.8 | 2.37 | 46.1 | 2.30 |
| CD34+38+ | 40.5 | 2.30 | 49.0 | 2.62 |
Primay samples from AML patients were treated for 24 h with a series of doses of triptolide or MRx102, and viability and apoptosis were assessed. The results were determined in bulk AML cells and in CD34+, CD34+CD38− and CD34+CD38+ AML cells characterized by flow cytometry. The EC50 for viable cells (Cell Count in M/ml) and the EC50 for apoptosis (percent Annexin V + cells) were determined from the triptolide and MRx102 concentrations. These representative results are shown here for one of two patients’ samples.
AML stem/progenitor cells are often more resistant to chemotherapy than bulk AML blasts [18], and are thought to be responsible for disease progression and resistance to therapy. AML patients’ cells treated with MRx102 or triptolide were assessed for primitive CD34+ AML cells, CD34+CD38− AML stem/progenitor cells and CD34+CD38+ AML cells. Both MRx102 and triptolide potently promoted apoptosis in all 3 subpopulations of AML cells (Table 2), showing that stem/progenitor cells were as sensitive to MRx102 as bulk AML blasts. MRx102 (EC50 = 40.8 nM) was less potent than triptolide (EC50 = 2.14 nM) in apoptosis induction in CD34+CD38− AML stem/progenitor cells (Table 2), a result reflected in the viable Cell Counts.
Both MRx102 and triptolide were effective inducing apoptosis in AML cells including all cell compartments (CD34+ cells, CD34+CD38− cells and CD34+CD38+ cells, Fig. 1) even when they were cocultured with MS-5 MSC (Table 2) or bone marrow derived MSC (not shown).
Development of a new preclinical formulation for MRx102 and triptolide
During earlier development of MRx102, studies were performed with MRx102 dissolved in a DMSO-based excipient. A clinically acceptable emulsion formulation was developed for use with both MRx102 and triptolide. The formulation was safe, had acceptable solubility (≥ 2 mg/ml MRx102), retained ≥ 95% of the nominal MRx102 concentration after filtration, displayed at least 7 days of chemical stability and showed triptolide compatibility (not shown). The emulsion was employed with MRx102 and triptolide in i.v. rat and dog toxicology studies.
7-Day Repeat Dose Rat Toxicology Comparison Study of MRx102 and Triptolide
This emulsion was used to compare MRx102 and triptolide in a 7-day i.v. repeat dose toxicology study in rats. Doses were selected based on escalating dose toxicology study results with i.v. single doses using the 15% DMSO/70% PEG 400/15% PBS excipient. MRx102 at 0.5, 1.5 and 3 mg/kg and triptolide at 0.05, 0.15 and 0.3 mg/kg were administered i.v. daily for seven days to male and female rats and a variety of parameters were evaluated (e.g., blood chemistry, hematology, histopathology, etc.). There were no definitive test article-related changes in hematology, coagulation or clinical chemistry parameters, other than decreased reticulocytes for both sexes in all dose groups treated with MRx102 or triptolide. Spleen weights were reduced for all MRx102− and triptolide-treated dose groups. Thymus weights were reduced for the 0.5 and 1.5 mg/kg MRx102 females.
Histopathology Assessment of 7-Day Repeat Dose Rat Toxicology Comparison Study
MRx102 had no histopathologic effect in tissues examined from males. In females, it was associated with lymphocytic depletion in the spleen and sporadic atrophy of the thymus. In the high dose group (3 mg/kg/day MRx102), necrosis and depletion of the bone marrow were noted. Triptolide given i.v. had no microscopic effect in tissues from females. In males it was associated with intraluminal cellular debris in the epididymis, consistent with cellular damage noted in the germinal epithelium of the testes (spermatid giant cells, cellular degeneration, vacuolation). These changes were observed in testes of both mid- and high-dose animals (0.15 and 0.3 mg/kg/day triptolide).
MTD and NOAEL of MRx102 and Triptolide in Rat Toxicology Comparison Study
The maximum tolerated dose (MTD) following a single i.v. injection of MRx102 to Sprague Dawley rats was 4.5 mg/kg for males and 3 mg/kg for females. The MTD for triptolide was 0.63 mg/kg for males and 0.317 mg/kg for females. The no observed adverse effect level (NOAEL) following 7-days of i.v. MRx102 administration at doses of 0.5, 1.5 and 3 mg/kg was 1.5–3.0 mg/kg. The NOAEL for triptolide at doses of 0.05, 0.15 and 0.3 mg/kg was 0.05–0.15 mg/kg. Comparing the low and high doses of these NOAEL ranges for MRx102 and triptolide indicates that MRx102 was between 20− and 60-fold safer than triptolide.
Toxicokinetic Assessment of MRx102 and Triptolide in the Rat
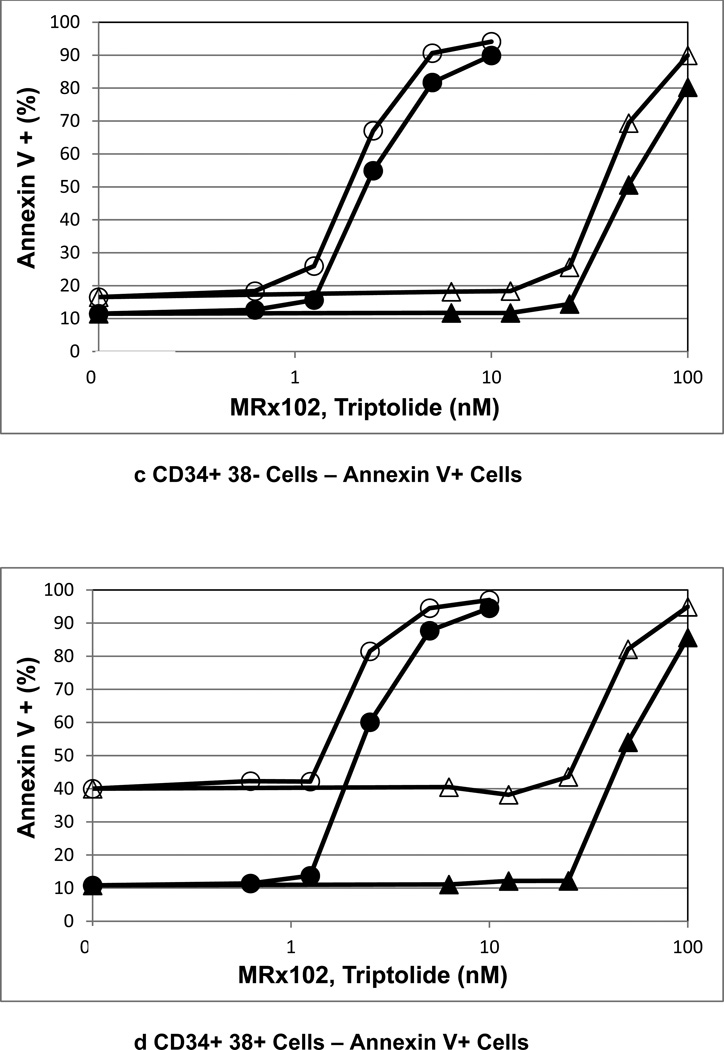
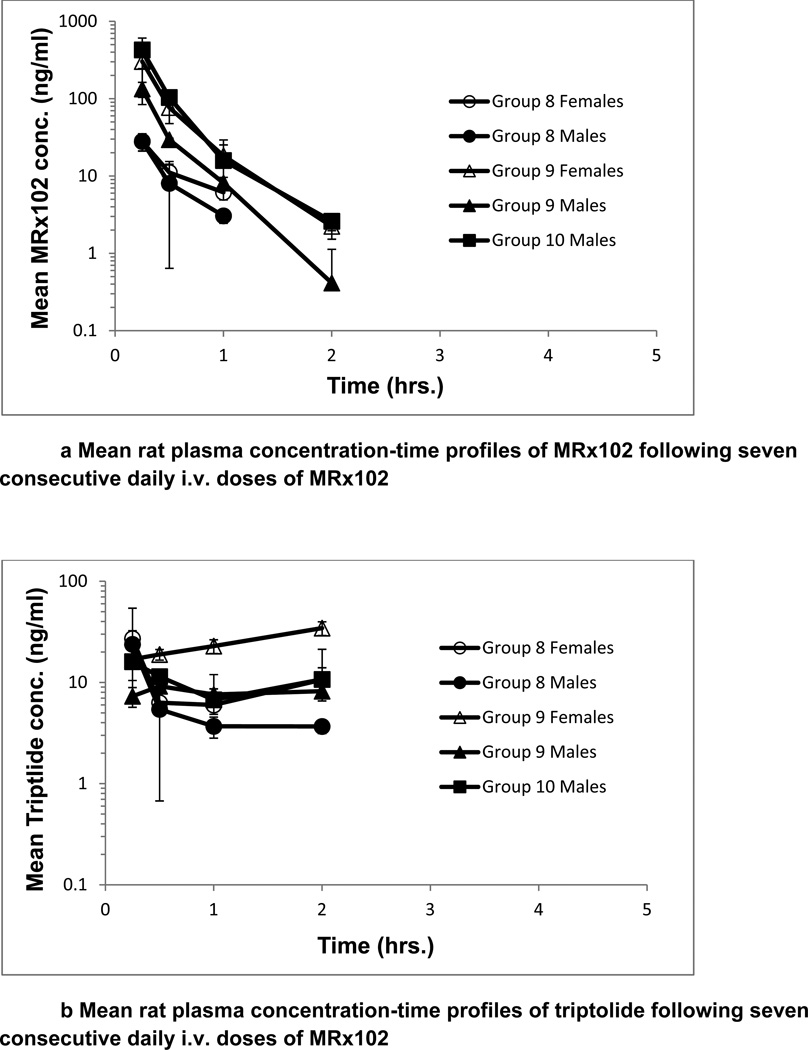
A toxicokinetic assessment of MRx102 and triptolide plasma levels was conducted following i.v. administration daily for 7 consecutive days. The plasma level results for MRx102 or triptolide were similar on days 1 and 7; day 7 results are presented in Fig. 2. The mean plasma concentration profiles for MRx102 are relatively steep at all dose levels, with the initial high concentration decreasing rapidly over the initial 2 hours (Fig. 2a). However, the mean plasma concentration profiles for triptolide after MRx102 administration (Fig. 2b) are different - the level of plasma triptolide leveled off at the low-dose of 0.05 mg/kg MRx102 and was at a consistent level or increased somewhat through 2 hrs at the mid- and high-doses of 1.5 and 3.0 mg/kg MRx102. The mean plasma concentration profiles for triptolide following triptolide injection are, like the MRx102 result after MRx102 injection, relatively steep at all dose levels with the concentration rapidly decreasing from a high level over the initial 2 hrs (Fig. 2c). In contrast to the triptolide result after triptolide injection, the mean plasma concentration-time profiles for triptolide after MRx102 injection show a more protracted level of this MRx102 active metabolite. This suggests a more consistent level of plasma exposure to triptolide during the initial 2 hours after MRx102 administration compared to that following the injection of triptolide.
Figure 2.
a-c Mean rat plasma concentration-time profiles of MRx102 and triptolide following seven consecutive daily i.v. doses of MRx102 or triptolide
These MRx102 and triptolide plasma concentration results are from rat plasma samples obtained on Day 7 following the last of 7 daily doses of MRx102 or triptolide. Similar results were obtained on Day 1 after a single i.v. dose of MRx102 or triptolide. The mean and standard deviation for plasma MRx102 and triptolide concentrations are shown.
Group 8 - 0.5 mg/kg/day MRx102
Group 9 - 1.5 mg/kg/day MRx102
Group 10 - 3.0 mg/kg/day MRx102
Group 11 - 0.05 mg/kg/day triptolide
Group 12 - 0.15 mg/kg/day triptolide
Group 13 - 0.30 mg/kg/day triptolide
Toxicokinetic results for MRx102 are summarized in Table 3a. Exposure to MRx102 was dose-dependent and appeared to be greater than dose-proportional. Plasma elimination phase half-life (T1/2,e) values ranged from ~0.24 to ~0.52 hours. Plasma clearance (Cl) and volume of distribution (Vz) appeared to decrease as the MRx102 dose increased; Cl values ranged from ~15 to ~21 L/hr after the MRx102 low dose (0.05 mg/kg), indicating a high clearance rate, and from ~1.5 to ~7.9 L/hr after the MRx102 high dose (3.0 mg/kg). Vz values ranged from ~7.1 to ~11 L after the MRx102 low dose, indicating a substantial tissue distribution for MRx102, and from ~0.68 to ~3.3 L after the MRx102 high dose. Exposure to, and elimination of, MRx102 were similar after single or multiple dosing, and were similar in male and female rats.
Table 3.
| a Toxicokinetic parameters for MRx102 following a single i.v. dose of MRx102 and following seven consecutive daily i.v. doses of MRx102 in rats | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group # |
Day | Sex | MRx102 Dose (mg/kg/day) |
C0 (ng/ml) |
Cmax (ng/ml) |
Tmax (hr.) |
AUC0-inf (ng*hr/ml) |
T1/2,e (hr) |
Cl (l/hr) |
Vz (l) |
| 8 | 1 | F | 0.5 | 113 | 35.4 | 0.25 | 33.20 | 0.52 | 15.1 | 11.3 |
| 9 | 1 | F | 1.5 | 442 | 157 | 0.25 | 127.7 | 0.41 | 11.7 | 6.9 |
| 10 | 1 | F | 3.0 | 9247 | 1920 | 0.25 | 1998 | 0.32 | 1.5 | 0.68 |
| 8 | 1 | M | 0.5 | 89.4 | 30.1 | 0.25 | 24.25 | 0.24 | 20.5 | 7.1 |
| 9 | 1 | M | 1.5 | 484 | 120 | 0.25 | 107.3 | 0.39 | 14.0 | 7.8 |
| 10 | 1 | M | 3.0 | 2696 | 723 | 0.25 | 607.6 | 0.28 | 4.9 | 2.0 |
| 8 | 7 | F | 0.5 | 70.6 | 28.0 | 0.25 | 24.49 | 0.36 | 20.4 | 10.7 |
| 9 | 7 | F | 1.5 | 1192 | 303 | 0.25 | 269.2 | 0.30 | 5.6 | 2.4 |
| 10 | 7 | F | 3.0 | a | a | a | a | a | a | a |
| 8 | 7 | M | 0.5 | 98.5 | 28.1 | 0.25 | 24.10 | 0.25 | 20.7 | 7.4 |
| 9 | 7 | M | 1.5 | 606 | 134 | 0.25 | 126.6 | 0.24 | 11.8 | 4.1 |
| 10 | 7 | M | 3.0 | 1763 | 428 | 0.25 | 380.4 | 0.29 | 7.9 | 3.3 |
| b Toxicokinetic parameters for triptolide following a single i.v. dose of MRx102 and following seven consecutive daily i.v. doses of MRx102 in rats | |||||||
|---|---|---|---|---|---|---|---|
| Group # |
Day | Sex | MRx102 Dose (mg/kg/day) |
Cmax (ng/ml) |
Tmax (hr) |
AUC0-inf (ng*hr/ml) |
T1/2,e (hr) |
| 8 | 1 | F | 0.5 | 11.8 | 0.25 | 22.03 | 1.6 |
| 9 | 1 | F | 1.5 | 26.6 | 1 | b | b |
| 10 | 1 | F | 3.0 | 41.1 | 1 | b | b |
| 8 | 1 | M | 0.5 | 32.5 | 0.25 | b | b |
| 9 | 1 | M | 1.5 | 7.83 | 0.25 | 12.66 | 1.1 |
| 10 | 1 | M | 3.0 | 17.7 | 0.25 | 269.69 | 1.4 |
| 8 | 7 | F | 0.5 | 27.1 | 0.25 | 50.12 | 2.8 |
| 9 | 7 | F | 1.5 | 34.5 | 2 | b | b |
| 10 | 7 | F | 3.0 | a | a | a | a |
| 8 | 7 | M | 0.5 | 23.8 | 0.25 | 15.97 | 0.85 |
| 9 | 7 | M | 1.5 | 9.17 | 0.5 | 164.8 | 13.1 |
| 10 | 7 | M | 3.0 | 16.0 | 0.25 | 66.12 | 3.7 |
| c Toxicokinetic parameters for triptolide following a single i.v. dose of triptolide and following seven consecutive daily i.v. doses of triptolide in rats | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group # |
Day | Sex | Triptolide Dose (mg/kg/day) |
C0 (ng/ml) |
Cmax (ng/ml) |
Tmax (hr) |
AUC0-inf (ng*hr/ml) |
T1/2,e (hr) |
Cl (l/hr) |
Vz (l) |
| 11 | 1 | F | 0.05 | 45.7 | 15.4 | 0.25 | 12.54 | 0.25 | 4.0 | 1.4 |
| 12 | 1 | F | 0.15 | 303 | 74.6 | 0.25 | 72.07 | 0.33 | 2.1 | 1.0 |
| 13 | 1 | F | 0.30 | 436 | 189 | 0.25 | 161.7 | 0.39 | 1.9 | 1.0 |
| 11 | 1 | M | 0.05 | 216 | 69.5 | 0.25 | 53.71 | 0.15 | 0.93 | 0.2 |
| 12 | 1 | M | 0.15 | 234 | 59.0 | 0.25 | 51.37 | 0.19 | 2.9 | 0.8 |
| 13 | 1 | M | 0.30 | 383 | 130 | 0.25 | 100.8 | 0.18 | 3.0 | 0.8 |
| 11 | 7 | F | 0.05 | 22.2 | 11.0 | 0.25 | 8.14 | 0.25 | 6.1 | 2.2 |
| 12 | 7 | F | 0.15 | 118 | 48.6 | 0.25 | 36.82 | 0.22 | 4.1 | 1.3 |
| 13 | 7 | F | 0.30 | a | a | a | a | a | a | a |
| 11 | 7 | M | 0.05 | 62.4 | 18.2 | 0.25 | 14.75 | 0.17 | 3.4 | 0.8 |
| 12 | 7 | M | 0.15 | 130 | 49.7 | 0.25 | 37.95 | 0.21 | 4.0 | 1.2 |
| 13 | 7 | M | 0.30 | 273 | 94.8 | 0.25 | 75.92 | 0.24 | 4.0 | 1.4 |
Could not be calculated or determined; due to toxicity, all Group 10 female animals were euthanized prior to Day 7.
AUC0-inf - area under the curve from 0 to infinity. C0 – estimated plasma concentration at time zero, Cl - plasma clearance, Cmax – maximum observed concentration, time when maximum concentration was observed, AUC0-inf - maximum observed concentration, T1/2,e – plasma elimination phase half-life, Vz - volume of distribution.
Could not be calculated or determined; due to toxicity, all Group 10 female animals were euthanized prior to Day 7.
Could not be calculated or determined.
Could not be calculated or determined; due to toxicity, all Group 13 female animals were euthanized prior to Day 7.
Exposure to triptolide following MRx102 administration was evident (Table 3b), and dose-dependent in only the female animals as shown by increases in Cmax and AUC0-inf values with increases in MRx102 doses. These increases appeared to be less than dose-proportional. T1/2,e values ranged from ~0.85 to ~3.7 hours with one large half-life value of 13.1 hours (1.5 mg/kg/day males, Day 7). Exposure to, and elimination of, triptolide were similar in male and female rats after single or multiple MRx102 dosing, with the exception of the 3.0 mg/kg/day MRx102 females, which were euthanized prior to Day 7.
Exposure to triptolide following triptolide administration was dose-dependent as shown by increases in Cmax and AUC0-inf values with increases in triptolide doses (Table 3c). These increases appeared to be near dose-proportional. T1/2,e values ranged from ~0.15 to ~0.39 hours. Cl values ranged from ~0.93 to ~6.1 L/hr indicating a lower clearance rate than for MRx102. Vz values ranged from ~0.2 to ~2.2 L, considerably lower than the lower dose results with MRx102, suggesting less extensive tissue distribution. Exposure to, and elimination of, triptolide was similar after single or multiple dosing, and the less than unity accumulation index values in all triptolide dose groups suggest decreased total exposure to triptolide after 7 daily doses. Exposure to, and elimination of, triptolide were similar in male and female rats with the exception that all 0.3 mg/kg/day females were euthanized prior to Day 7 due to triptolide toxicity.
The triptolide Cmax values with MRx102 injections at 0.5, 1.5 and 3.0 mg/kg ranged from 11.8 to 41.1 ng/ml for females, and from 7.83 to 32.5 ng/ml for males, with a median Cmax of 23.8 ng/ml (Table 4). By comparison, triptolide Cmax values with triptolide injections at 0.05, 0.15 and 0.30 mg/kg ranged from 11.0 to 189 ng/ml for females and from 18.2 to 130 ng/ml for males, with a median Cmax of 59 ng/ml. These results show that significantly higher triptolide levels (Cmax, P<0.05) were observed after triptolide than after MRx102 administration (Fig. 1b and c), even though the triptolide doses were lower than those for MRx102. The median AUC0-inf of MRx102 after MRx102 injection was 126.6 ng*hr/ml. The triptolide median AUC0-inf was 29.69 ng*hr/ml for animals receiving MRx102 compared to the higher median of 51.37 ng*hr/ml after triptolide injection, showing a greater systemic exposure to triptolide after triptolide administration than after MRx102. The median T1/2,e for MRx102 following MRx102 administration was 0.30 hours. The triptolide median T1/2,e was 1.6 hours for animals receiving MRx102, indicating a more extended systemic exposure to triptolide compared to the median T1/2,e of 0.22 hours for rats given triptolide. Triptolide plasma T1/2,e values for MRx102-injected rats were significantly greater than the values for triptolide-injected rats (P < 0.05). These results show clearly that higher triptolide levels (Cmax values) were observed after triptolide than after MRx102 administration, with higher AUC0-inf values as well. There was a more extended exposure to triptolide, however, when MRx102 was given, as indicated by elevated T1/2,e values. Triptolide had a higher Cmax and AUC0-inf as well as a shorter T1/2,e after triptolide than following MRx102 injection.
Table 4.
Summary of toxicokinetic parameters for triptolide following i.v. dosing with triptolide and MRx102 in rat and dog studies
| Rat Study | Treatment | Cmax (ng/ml) | AUC0-inf (ng*hr/ml) | T1/2,e (hr) | |||
|---|---|---|---|---|---|---|---|
| Range | Median | Range | Median | Range | Median | ||
| MRx102 in plasma | MRx102 | 28.0 – 1920 | 134 | 24.10 – 1998 | 126.6 | 0.28 – 0.83 | 0.55 |
| Triptolide in plasma | MRx102 | 7.83 – 41.1 | 23.8 | 12.66 – 66.12 | 29.69 | 0.85 – 13.1 | 1.6 |
| Triptolide | 11.0 – 189 | 59 | 8.14 – 75.92 | 51.37 | 0.15 – 0.39 | 0.22 | |
| Dog Study | Treatment | Cmax (ng/ml) | AUC0–24hr (ng*hr/ml) | T1/2,e (hr) | |||
| Range | Median | Range | Median | Range | Median | ||
| MRx102 in plasma | MRx102 | 4.38 – 593 | 26.3 | 3.96 – 554 | 55.91 | 0.28 – 13.1 | 0.83 |
| Triptolide in plasma | MRx102 | 2.0 – 49.0 | 3.44 | 1.50 – 212.0 | 34.83 | a | a |
Animals in the rat and dog studies were injected with triptolide or MRx102, and the toxicokinetic parameters for triptolide in the rat (from Table 3) and in the dog (from Table 5) are presented The range and median are for Day 1 and Day 7 data used together.
Triptolide plasma Cmax values for MRx102-injected rats were significantly less than the values for triptolide-injected rats (p< 0.05). Triptolide plasma T1/2,e values for MRx102-injected rats were significantly greater than the values for triptolide-injected rats (P< 0.05). AUC0–24hr is cited for the dog study because AUC0-inf could not be calculated for triptolide.
Could not be calculated or determined.
7-Day Repeat Dose Dog Toxicology Study of MRx102
A 7-day i.v. repeat dose toxicology study was conducted in dogs using the emulsion formulation. Doses were selected based on escalating dose toxicology study results with i.v. single doses using the 15% DMSO/70% PEG 400/15% PBS excipient. MRx102 at doses of 0.1, 0.3 and 1.0 mg/kg was administered i.v. daily for seven days to male and female beagle dogs. There were no definitive changes in hematology parameters for the males and females dosed up to 0.3 mg/kg MRx102 or males at 1 mg/kg. The 1 mg/kg female alive for the day 8 terminal sacrifice had increased white blood cells, red blood cells, hemoglobin, hematocrit, neutrophils, and monocytes and decreased lymphocytes, eosinophils, basophils and reticulocytes (one female in this group was moribund sacrificed on Day 7). There were no test article related changes in red blood cell morphology, and no definitive test article related clinical chemistry changes on Day 8.
Lesions of consequence occurred in the intestine (large and small), including necrosis of the epithelium and hemorrhage, of one male (found dead on Day 8) and one female (moribund sacrificed on Day 7) receiving 1 mg/kg. Animals that survived to the scheduled sacrifice had no intestinal lesions of consequence. The intestinal damage cannot be unequivocally attributed to MRx102, as these lesions were not consistently observed in all animals at this dose. The testes of all males were immature; spermatogenesis had not begun. However, a discernible degeneration of the maturing germinal epithelium in both 0.3 and 1.0 mg/kg males was evident, apparently caused by MRx102.
The MTD following a single MRx102 i.v. dose to male and female beagle dogs was 2.0 mg/kg. The NOAEL following MRx102 i.v. administration daily for 7 days at doses of 0.1, 0.3 and 1.0 mg/kg was 0.3 mg/kg.
Toxicokinetic Assessment after MRx102 Injection in the Dog
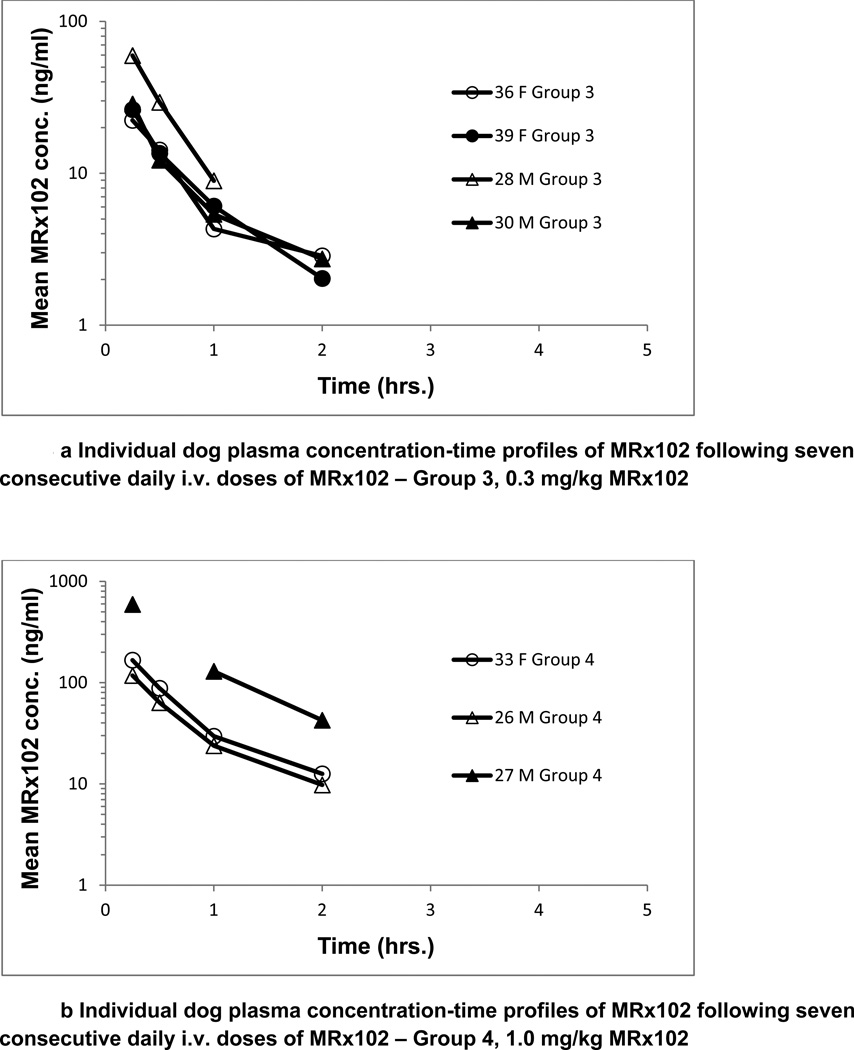
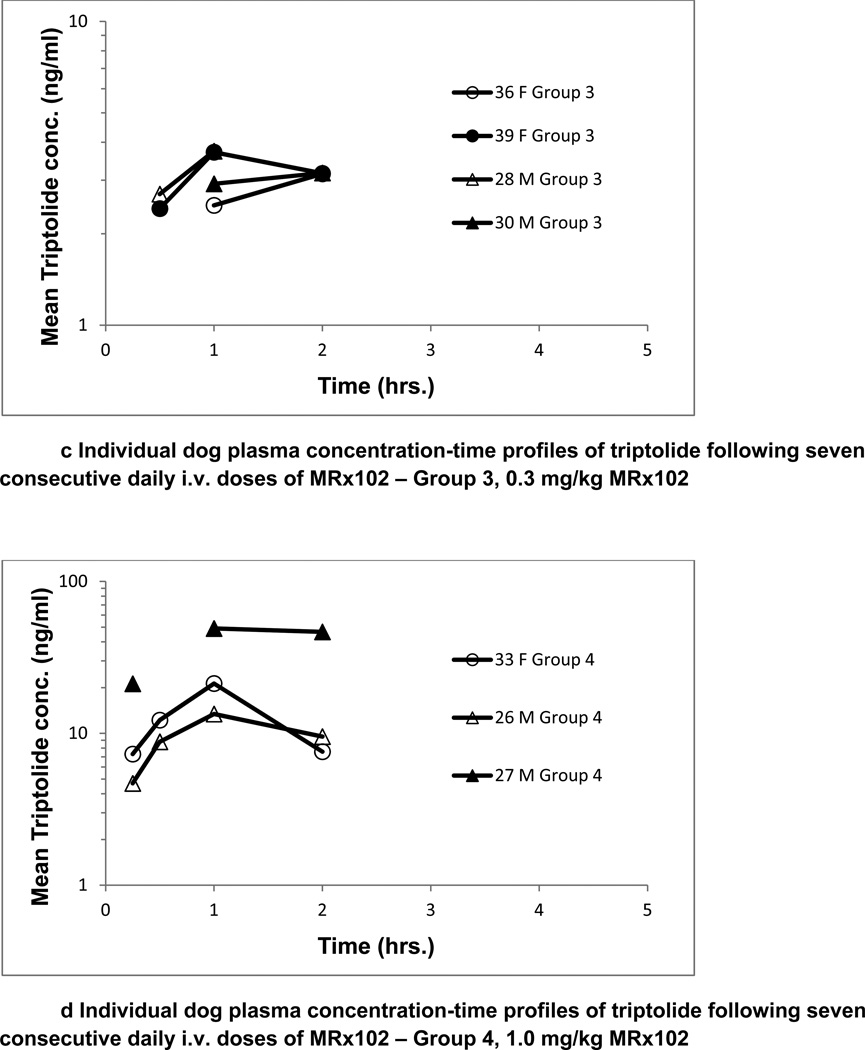
Toxicokinetic parameters for MRx102 were determined following a single dose of MRx102 and after seven daily consecutive MRx102 doses to dogs. Exposure to MRx102 was dose-dependent as shown by increases in Cmax and AUC values with dose. The results were similar for analysis of plasma levels after the injection of MRx102 on days 1 and 7; the individual animal results for day 7 are presented in Fig. 3. The plasma concentration profiles for MRx102 were relatively steep at all dose levels, with the initial high concentration decreasing by over a full log during the initial 2 hrs (Fig. 3a). However, the plasma concentration profiles for triptolide after MRx102 administration (Fig. 3b) were different – the level of plasma triptolide increased from the earliest time point (15 minutes) and was still above the initial level at 2 hours. There was a more constant triptolide concentration with little diminution than shown in the profiles for MRx102, indicating a more consistent level of plasma exposure to triptolide during the initial 2 hours after MRx102 administration.
Figure 3.
a-d Individual dog plasma concentration-time profiles of MRx102 and triptolide following seven consecutive daily i.v. doses of MRx102
These MRx102 and triptolide plasma concentration results are from dog plasma samples obtained on Day 7 following the last of 7 daily doses of MRx102. Similar results were obtained on Day 1 after a single i.v. dose of MRx102. On days 1 and 7, the triptolide plasma levels were below the level of detection for all but one sample in group 2 receiving 0.1 mg.kg MRx102; the group 2 results are not shown.
Group 3 - 0.3 mg/kg/day MRx102
Group 4 - 1.0 mg/kg/day MRx102
Exposure to MRx102 in dogs was dose-dependent as shown for increases in Cmax and AUC values (Table 4), which appeared to be near to and greater than dose-proportional. T1/2,e values ranged from ~0.30 to ~0.83 hours. Clearance and volume of distribution decreased slightly as the MRx102 dose increased. Cl values ranged from ~7.9 to ~25 L/hr after the MRx102 low dose (0.1 mg/kg) and from ~1.7 to ~13 L/hr after the MRx102 high dose (1.0 mg/kg). Vz values ranged from ~5.4 to ~13 L after the MRx102 low dose and Vz values ranged from ~1.2 to ~10 L after the MRx102 high dose. Exposure to, and elimination of, MRx102 were similar after single or multiple dosing, and were similar in male and female dogs, with the exception of the female dog (Group 4, # 37) that was moribund sacrificed on Day 7.
Exposure to triptolide was evident and MRx102-dose-dependent as shown by increases in triptolide Cmax and AUC values with MRx102 dose, and appeared greater than dose-proportional. Nearly all of the plasma samples from animals dosed at 0.1mg/kg/day MRx102 were BLQ for triptolide. AUC0-inf and T1/2,e could not be calculated due to the triptolide concentration-time data; AUC0–24hr is provided in Tables 4 and 5a and b for comparison purposes. Exposure to, and elimination of, triptolide after MRx102 injection was similar after single or multiple MRx102 dosing, and there appeared to be no major sex differences.
Table 5.
| a Toxicokinetic parameters for MRx102 following a single i.v. dose of MRx102 and following seven consecutive daily i.v. doses of MRx102 in dogs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | Sex | Dog # |
Dose (mg/kg/day) |
C0 (ng/ml) |
Cmax (ng/ml) |
Tmax (hr) |
AUC0–24hr (ng*hr/ml) |
AUC0-inf (ng*hr/ml) |
T1/2,e (hr) |
Cl (l/hr) |
Vz (l) |
| 2 | 1 | F | 35 | 0.1 | 23.6 | 9.90 | 0.25 | 8.26 | 8.11 | 0.31 | 12.3 | 5.4 |
| 2 | 1 | F | 38 | 0.1 | 24.5 | 9.29 | 0.25 | 7.86 | 7.72 | 0.30 | 13.0 | 5.7 |
| 2 | 1 | M | 25 | 0.1 | 17.1 | 8.82 | 0.25 | 19.99 | 9.015 | 0.79 | 11.1 | 12.7 |
| 2 | 1 | M | 29 | 0.1 | 15.8 | 7.79 | 0.25 | 6.68 | 6.67 | 0.36 | 15.0 | 7.8 |
| 3 | 1 | F | 36 | 0.3 | 50.6 | 26.0 | 0.25 | 49.17 | 24.08 | 0.67 | 12.5 | 12.1 |
| 3 | 1 | F | 39 | 0.3 | 46.9 | 26.1 | 0.25 | 51.39 | 28.47 | 0.63 | 11.8 | 10.6 |
| 3 | 1 | M | 28 | 0.3 | 110 | 58.1 | 0.25 | 103.9 | 53.60 | 0.60 | 5.6 | 4.9 |
| 3 | 1 | M | 30 | 0.3 | 69.8 | 32.0 | 0.25 | 67.55 | 31.28 | 0.83 | 9.6 | 11.5 |
| 4 | 1 | F | 33 | 1.0 | 163 | 90.8 | 0.25 | 144.1 | 78.27 | 0.55 | 12.8 | 10.0 |
| 4 | 1 | F | 37 | 1.0 | 283 | 153 | 0.25 | 214.5 | 214.5 | 0.43 | 4.7 | 2.9 |
| 4 | 1 | M | 26 | 1.0 | 260 | 113 | 0.25 | 139.6 | 94.98 | 0.44 | 10.5 | 6.7 |
| 4 | 1 | M | 27 | 1.0 | 139 | 86.6 | 0.25 | 169.7 | 169.8 | 0.51 | 5.9 | 4.4 |
| 2 | 7 | F | 35 | 0.1 | 10.8 | 6.07 | 0.25 | 5.63 | 5.96 | 0.48 | 16.8 | 11.7 |
| 2 | 7 | F | 38 | 0.1 | 5.01 | 4.38 | 0.25 | 3.96 | 4.01 | 0.36 | 25.0 | 12.6 |
| 2 | 7 | M | 25 | 0.1 | 18.6 | 12.3 | 0.25 | 27.95 | 12.64 | 0.66 | 7.9 | 7.5 |
| 2 | 7 | M | 29 | 0.1 | 12.3 | 6.88 | 0.25 | 5.76 | 5.72 | 0.33 | 17.5 | 8.3 |
| 3 | 7 | F | 36 | 0.3 | 35.0 | 22.3 | 0.25 | 62.95 | 62.95 | 0.71 | 4.8 | 4.9 |
| 3 | 7 | F | 39 | 0.3 | 50.9 | 26.3 | 0.25 | 45.91 | 25.15 | 0.56 | 11.9 | 9.6 |
| 3 | 7 | M | 28 | 0.3 | 140 | 59.6 | 0.25 | 146.7 | 47.59 | 0.28 | 6.3 | 2.6 |
| 3 | 7 | M | 30 | 0.3 | 67.0 | 28.6 | 0.25 | 55.51 | 28.16 | 0.73 | 10.6 | 11.2 |
| 4 | 7 | F | 33 | 1.0 | 317 | 167 | 0.25 | 301.2 | 301.2 | 0.56 | 3.3 | 2.7 |
| 4 | 7 | F | 37 | 1.0 | a | a | a | a | a | a | a | a |
| 4 | 7 | M | 26 | 1.0 | 218 | 118 | 0.25 | 237.6 | 237.6 | 0.58 | 4.2 | 3.5 |
| 4 | 7 | M | 27 | 1.0 | 985 | 593 | 0.25 | 554.0 | 579.6 | 0.47 | 1.7 | 1.2 |
| b Toxicokinetic parameters for triptolide following a single i.v. dose of MRx102 and following seven consecutive daily i.v. doses of MRx102 in dogs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Day | Sex | Dog # |
Dose (mg/kg/day) |
Cmax (ng/ml) |
Tmax (hr) |
AUC0–24hr (ng*hr/ml) |
AUC0-inf (ng*hr/ml) |
T1/2,e (hr) |
| 2 | 1 | F | 35 | 0.1 | a | a | a | a | a |
| 2 | 1 | F | 38 | 0.1 | a | a | a | a | a |
| 2 | 1 | M | 25 | 0.1 | 2.22 | 2 | 25.53 | c | c |
| 2 | 1 | M | 29 | 0.1 | a | a | a | a | a |
| 3 | 1 | F | 36 | 0.3 | 2.00 | 1 | 1.5 | c | c |
| 3 | 1 | F | 39 | 0.3 | 2.74 | 2 | 33.54 | c | c |
| 3 | 1 | M | 28 | 0.3 | 2.85 | 2 | 34.83 | c | c |
| 3 | 1 | M | 30 | 0.3 | 2.34 | 2 | 26.91 | c | c |
| 4 | 1 | F | 33 | 1.0 | 8.35 | 1 | 95.13 | c | c |
| 4 | 1 | F | 37 | 1.0 | 8.76 | 2 | 212.2 | c | c |
| 4 | 1 | M | 26 | 1.0 | 113 | 1 | 89.11 | c | c |
| 4 | 1 | M | 27 | 1.0 | 9.16 | 2 | 114.7 | c | c |
| 2 | 7 | F | 35 | 0.1 | a | a | a | a | a |
| 2 | 7 | F | 38 | 0.1 | a | a | a | a | a |
| 2 | 7 | M | 25 | 0.1 | a | a | a | a | a |
| 2 | 7 | M | 29 | 0.1 | a | a | a | a | a |
| 3 | 7 | F | 36 | 0.3 | 3.15 | 2 | 38.09 | c | c |
| 3 | 7 | F | 39 | 0.3 | 3.71 | 1 | 40.03 | c | c |
| 3 | 7 | M | 28 | 0.3 | 3.74 | 1 | 44.96 | c | c |
| 3 | 7 | M | 30 | 0.3 | 3.17 | 2 | 38.65 | c | c |
| 4 | 7 | F | 33 | 1.0 | 21.1 | 0.25 | 301.2 | c | c |
| 4 | 7 | F | 37 | 1.0 | b | b | b | b | b |
| 4 | 7 | M | 26 | 1.0 | 13.4 | 1 | 124.0 | c | c |
| 4 | 7 | M | 27 | 1.0 | 49.0 | 1 | 78.28 | c | c |
Could not be calculated or determined; animal found dead after dosing on Day 7.
All designated samples were BLQ for triptolide
Could not be calculated or determined; animals found dead after dosing on Day 7
Could not be calculated or determined
AUC0–24hr - area under the curve from time zero to 24 hours
The MRx102 Cmax values with MRx102 injections at 0.1, 0.3 and 1.0 mg/kg ranged from 4.38 to 156 ng/ml for females, and 6.88 to 593 ng/ml for males, with a median Cmax value of 23.8 ng/ml (Table 4). By comparison, triptolide Cmax values ranged from 2.0 to 21.1 ng/ml for females and 2.22 to 49.0 ng/ml for males, with a median Cmax value of 3.44 ng/ml. These results show that higher levels were observed for MRx102 than triptolide after MRx102 administration (Fig. 1b and c). The median AUC0–24hr of MRx102 was 55.91 ng*hr/ml, whereas the median AUC0–24hr result for triptolide was 34.83 ng*hr/ml, indicating a greater systemic exposure to MRx102 than triptolide. The median T1/2,e value for MRx102 was 0.55 hours. The triptolide median T1/2,e could not be calculated. There was a more extended systemic exposure to triptolide but at a lower level than to MRx102, as indicated by the lower Cmax and AUC0–24hr for triptolide compared to MRx102.
Conclusions
To realize the therapeutic potential of triptolide-based therapies, we view the development of a safer triptolide as essential. The unequivocal efficacy in AML reported for the triptolide prodrug F60008 was tempered by the deleterious and even lethal side effects that appeared to derive from suboptimal pharmacokinetics, with inconsistent conversion leading to widely divergent circulating triptolide levels and overexposure in the most severely affected patients [6]. We show here that, when administered i.v. in a preclinical rat toxicology study using a novel emulsion formulation developed by MyeloRx, the new lipophilic triptolide prodrug derivative, MRx102, displayed a 20− to 60-fold reduction in toxicity in direct comparison to triptolide, and MRx102 exhibited an improved toxicokinetic profile as well.
Triptolide and MRx102 were preclinically evaluated for in vitro efficacy, toxicology and toxicokinetics. A new emulsion formulation was developed by MyeloRx to address issues with drug solubility and side effects, and was used in the toxicology comparison of MRx102 to triptolide. MRx102 administration using the emulsion vehicle produced altered toxicokinetic parameters that show a more extended, consistent level of exposure to triptolide than with triptolide injection. Consistent with this result are the T1/2,e values for triptolide after MRx102 injection that were over 7-fold greater than after triptolide (medians of 1.6 and 0.22 hours [i.e., 96 and 13 minutes], respectively). Furthermore, the more extended triptolide exposure was achieved without a high Cmax. Distinctly higher triptolide Cmax levels were reached following triptolide injection than after MRx102 administration, even though doses of triptolide were lower than MRx102 (a ten-fold difference by mg/kg and six-fold lower on a molar basis). Emulsification of more hydrophobic MRx102 in the new formulation may have resulted in lower, more extended exposure to the circulation than with triptolide.
Administration of MRx102 produced a toxicokinetic profile for the injected compound that had relatively high Cmax and low T1/2,e values, similar to the triptolide profile following triptolide injection. However, the toxicokinetic profile for plasma triptolide levels after MRx102 administration was distinct – a moderate Cmax and more extended exposure as reflected in the greater T1/2,e values. High plasma concentrations of triptolide (Cmax) [11, 16] as well as anti-cancer chemotherapeutic agents have been associated with increased toxic side effects. We would expect a reduced Cmax and more extended triptolide exposure to be associated with reduced toxic side effects, resulting in increased safety of MRx102 in the emulsion formulation. We are developing MRx102 to optimize the activity of this triptolide prodrug derivative for clinical application, and the combination of MRx102 and the emulsion formulation appear to constitute an improved and safer triptolide therapeutic agent.
In this comparative rat toxicology study of MRx102 and triptolide, MRx102 was 20− to 60-fold safer than triptolide based on the histopathology endpoints reflected in the NOAELs. The toxicokinetics analysis suggests that this safety margin may derive from the toxicokinetic profile of MRx102. When injected i.v., MRx102 is consistently and nearly completely converted to triptolide, the active moiety, which maintained a relatively constant blood level through 2 hours. The NOAEL of MRx102 was 1.5–3.0 mg/kg, considerably higher than the 0.05–0.15 mg/kg NOAEL for triptolide. The MTD of MRx102 was 4.5 mg/kg for males and 3 mg/kg for females. Clearly, the dose of MRx102 that is well-tolerated is considerably greater than that for triptolide, with an MTD for triptolide in rats of 0.63 mg/kg for males and 0.317 mg/kg for females.
Similar to the rat toxicokinetics results, a more consistent level of plasma exposure to triptolide than MRx102 was observed in dogs during the initial 2 hours after MRx102 injection. The MRx102 blood concentration rapidly decreased after injection, whereas the triptolide level did not drop over the initial 2 hours with a lower Cmax value.
The F60008 prodrug requires conversion by serum esterases to express in vitro activity, whereas MRx102 does not require exposure to serum for in vitro efficacy (Table 1) [17]. There is a similar requirement for the triptolide prodrug Minnelide that is inactive without conversion by alkaline phosphatase [19]. In vitro activity would be more difficult to display with this compound, as with F60008.
Binding to the XPB (ERCC3) [20] target of triptolide produces rapid, potent and irreversible inhibition of RNA Pol II activity and RNA Pol II proteosomal degradation [20–24]. The sequellae include inhibition of mRNA transcription initiation, degradation of short-lived mRNA, and the apoptotic response of tumor cells that are oncogene- addicted. The decrease in the major component of RNA POL II is closely correlated with the cytotoxic activity of triptolide [24]. Triptolide decreases mRNA and protein levels of XIAP and Mcl-1 in myeloid leukemia cells [3], and we showed that MRx102 also decreased XIAP and Mcl-1 protein levels and inhibited RNA synthesis in leukemia cells [17].
There were earlier attempts to modify the pharmacokinetic/toxicokinetic characteristics of triptolide so as to improve the side effect profile and reduce toxicity. Solid lipid nanoparticles used to deliver triptolide produced an increased AUC, a lengthened Tmax and mean retention times, and a decreased Cmax compared to free triptolide [25]. Solid lipid nanoparticles [26] and high alcohol microemulsion ethosomes [27] for topical delivery showed enhanced triptolide skin permeability but mixed results in anti-inflammatory activity [26, 27]. Triptolide absorption with a slow release character was promoted by the nanoformulation, suggesting that toxicokinetic changes were an important mechanism for enhanced efficacy. Triptolide-loaded micelles showed an enhanced AUC and altered biodistribution [28]. New compounds have been derived from triptolide, with the same intention. In contrast to MRx102 that is considerably more lipophilic than triptolide, minnelide was designed to increase triptolide water solubility [19]. This is a goal similar to F60008, which displayed inconsistent conversion, widely divergent triptolide blood levels and a dangerously high triptolide Cmax in some patients [5, 6]. Unlike MRx102, F60008 requires conversion by serum esterases to express in vitro activity (Table 1) [17]. There is a similar requirement for minnelide, which is inactive without conversion by alkaline phosphatase [19]. In vitro activity would be more difficult to display with this compound, as with F60008.
Targeted cancer therapy has shown striking activity in some cases, but the efficacy of the agents is often lost as resistance rapidly develops. A therapeutic agent like MRx102 acting through inhibition of mRNA synthesis [20, 21, 23, 24, 29, 30], targeting signaling molecules and pathways that are most sensitive to depletion of short-lived mRNA or protein, would retain activity since it would be difficult for resistance to develop. It is not unexpected that triptolide is efficacious with a wide variety of cell lines and various types of cancer, since the activity is not dependent on a single oncogenic signaling molecule or pathway.
In conclusion we believe that MRx102 is a “safer” triptolide, i.e., a version of triptolide that may obviate some of the toxicities observed with the native compound as well as other prodrugs previously used clinically. This is due to the novel emulsion formulation as well as the conversion kinetics. For this reason MRx102 should be advanced to clinical trials and evaluated in patients with liquid or solid tumors.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (P01 CA055164 and MD Anderson’s Cancer Center Support Grant CA016672) and by the Paul and Mary Haas Chair in Genetics to MA and NCI SBIR Contract HHSN261200900061C to MyeloRx LLC with JMF as the Principal Investigator. The efforts of Jill Fogelman and Steven Sloneker in the toxicology and toxicokinetics studies at Calvert Laboratories, Inc., and of Steve Noonan and Henry Lopez in the studies with triptolide and MRx102 at Murigenics, Inc., are acknowledged.
Footnotes
Conflict of interest
JMF and JA are employees of MyeloRx LLC. The other authors have no conflict of interest.
Contributor Information
John M. Fidler, MyeloRx LLC, 941 Railroad Avenue, Vallejo, CA 94592, USA
Jinhua An, MyeloRx LLC, 941 Railroad Avenue, Vallejo, CA 94592, USA.
Bing Z. Cater, Section of Molecular Hematology and Therapy, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
Michael Andreeff, Section of Molecular Hematology and Therapy, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA.
References
- 1.Kupchan SM, Court WA, Dailey RG, Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194–7195. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 2.Su D, Song Y, Li R. Comparative clinical study of rheumatoid arthritis treated by triptolide and an ethyl acetate extract of Tripterygium wilfordii. Zhong Xi Yi Jie He Za Zhi Mar. 1990;10:144–146. [PubMed] [Google Scholar]
- 3.Song HX, Gong J, Chen W. Effect of triptolide on urinary monocyte chemoattractant protein-1 in patients with diabetic nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:416–418. [PubMed] [Google Scholar]
- 4.Lu LH, Lian YY, He GY, Lin SP, Huan SH, Chen ZZ, Dend HX, Zheng YL. Clinical study of triptolide in treatment of acute leukemia. Clin Exp Investig Hematol. 1992;3:1–3. [Google Scholar]
- 5.Harousseau JL, Dombret H, Pigneux A, Michellet M, Brandely M. Phase 1 study of F60008, a triptolide derivative, in patients with refractory or relapsing leukemia. Haematologica. 2008;93:14. (abstract 0038) [Google Scholar]
- 6.Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L, Ter Steeg J, Brandely M, Puozzo Ch, Verweij J. Phase 1 dose escalation study of F60008, a novel apopotosis inducer in patients with solid tumors. Eur J Cancer. 2009;45:1764–1772. doi: 10.1016/j.ejca.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K, Friel J, Kluge N, Kina T, Kondo-Takaori A, Kawamata S, Uchiyama T, Ostertag W. A novel hematopoietic multilineage clone, Myl-D-7, is stromal cell-dependent and supported by an alternative mechanism (s) independent of stem cell factor/c-kit interaction. Blood. 1996;87:3218–3228. [PubMed] [Google Scholar]
- 8.Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- 9.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stroma cells prevent apoptosis of AML cells by upregulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 10.Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, Evans RL, Andreeff M. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630–637. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998;133:169–175. doi: 10.1016/s0304-3835(98)00222-5. [DOI] [PubMed] [Google Scholar]
- 12.Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666–2674. [PubMed] [Google Scholar]
- 13.Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R, Chaichana S, Tuchinda P, Cleason P, Reutrakul V. Evaluation of the mutagenic, cytotoxic, and antitumor potential of triptolide, a highly oxygenated diterpene isolated from tripterygium wilfordii. Cancer Lett. 1997;112:113–117. doi: 10.1016/S0304-3835(96)04554-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–13455. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 15.Chang WT, Kang JJ, Lee KY, Wei K, Anderson E, Gotmare S, Ross JA, Rosen GD. Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. J Biol Chem. 2001;276:2221–2227. doi: 10.1074/jbc.M009713200. Epub 2000 Oct 26. [DOI] [PubMed] [Google Scholar]
- 16.Mak DH, Schober WD, Chen W, Konopleva M, Cortes J, Kantarjian HM, Andreeff M, Carter BZ. Triptolide induces cell death independent of cellular responses to imatinib in blast crisis chronic myelogenous leukemia cells including quiescent CD34+ primitive progenitor cells. Mol Cancer Ther. 2009;8:2509–2516. doi: 10.1158/1535-7163.MCT-09-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter BZ, Mak DH, Shi Y, Fidler JM, Chen R, Ling X, Plunkett W, Andreeff M. MRx102, a triptolide derivative, has potent antileukemic activity in vitro and in a murine model of AML. Leukemia. 2012;26:443–450. doi: 10.1038/leu.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fasinduced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- 19.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y, Smeaton M, Demain AL, Miller PS, Kugel JF, Goodrich JA, Liu JO. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7:182–188. doi: 10.1038/nchembio.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vispe S, DeVries L, Creancier L, Besse J, Breand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet C, Guilbaud N, Barret JM, Bailly C. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of shortlived mRNA. Mol Cancer Ther. 2009;8:2780–2790. doi: 10.1158/1535-7163.MCT-09-0549. [DOI] [PubMed] [Google Scholar]
- 22.Leuenroth SJ, Crews CM. Triptolide-induced transcriptional arrest is associated with changes in nuclear substructure. Cancer Res. 2008;68:5257–5266. doi: 10.1158/0008-5472.CAN-07-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bensaude O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription. 2011;2:103–108. doi: 10.4161/trns.2.3.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzo SG, Zhou ZL, Wang YQ, Marinello J, He JX, Li YC, Ding J, Capranico G, Miao ZH. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012;72:5363–5373. doi: 10.1158/0008-5472.CAN-12-1006. [DOI] [PubMed] [Google Scholar]
- 25.Xue M, Zhao Y, Li XJ, Jiang ZZ, Zhang L, Liu SH, Li XM, Zhang LY, Yang SY. Comparison of toxicokinetic and tissue distribution of triptolide-loaded solid lipid nanoparticles vs free triptolide in rats. Eur J Pharm Sci. 2012;47:713–717. doi: 10.1016/j.ejps.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Mei Z, Chen H, Weng T, Yang Y, Yang X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56:189–196. doi: 10.1016/s0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen JG, Liu YF, Gao TW. Preparation and anti-inflammatory activity of triptolide ethosomes in an erythema model. J Liposome Res. 2010;20:297–303. doi: 10.3109/08982100903544144. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Wen XS, Di W. In vitro and in vivo evaluation of Triptolide-loaded pluronic P105 polymeric micelles. Arzneimittelforschung. 2012;62:340–344. doi: 10.1055/s-0032-1312602. [DOI] [PubMed] [Google Scholar]
- 29.Vispe S, DeVries L, Creancier L, Besse J, Bréand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet C, Guilbaud N, Barret JM, Bailly C. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol Cancer Ther. 2009;8:2780–2790. doi: 10.1158/1535-7163.MCT-09-0549. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Lu JJ, He L, Yu Q. Triptolide (TPL) inhibits global transcription by inducing proteasome-dependent degradation of RNA polymerase II (Pol II) PLoS One. 2011;6:e23993. doi: 10.1371/journal.pone.0023993. doi: 10.1371/journal.pone.0023993. [DOI] [PMC free article] [PubMed] [Google Scholar]