Abstract
Purpose
Cytogenetic abnormalities are currently the most important predictors of response and clinical outcome for patients with acute myeloid leukemia (AML) or advanced-stage myelodysplastic syndrome (MDS). Because clinical outcomes vary markedly within cytogenetic subgroups, additional biological markers are needed for risk stratification.
Experimental Design
We assessed the utility of measuring pretreatment proteasome chymotrypsin-like (Ch-L), caspase-like (Cas-L), and trypsin-like (Tr-L) activities in plasma to predict response and survival of patients with AML (n=174) or advanced-stage MDS (n=52).
RESULTS
All three enzymatic activities were significantly (P<0.001) increased in the plasma of patients with AML and MDS as compared with normal controls. Both Ch-L and Cas-L activities, but not Tr-L activity correlated with outcome. Ch-L and Cas-L activities, but not Tr-L activity predicted response in univariate analysis (P = 0.002). However, only Ch-L activity was independent predictor of response from age grouping (< vs ≥70 years), cytogenetics, and BUN in multivariate analysis. Similarly, both Ch-L and Cas-L activities, but not Tr-L activity were predictors of overall survival in univariate analysis (P <0.0001), but only Ch-L activity was independent of cytogenetics, age, performance status, BUN, and beta-2 microglobulin in multivariate Cox regression models. Ch-L activity was also a strong independent predictor of survival in patients with intermediate karyotype (n=124).
CONCLUSIONS
Measuring plasma chymotrypsin-like activity may provide a powerful biomarker for risk stratification in patients with AML and advanced-stage MDS, including those with normal karyotype.
Keywords: acute myeloid leukemia; myelodysplastic syndrome; proteasome, chymotrypsin-like activity; survival; response; nomogram
INTRODUCTION
Acute myeloid leukemia (AML) is a disease with significant morphologic, cytogenetic, and molecular heterogeneity. Management and therapeutic decisions are frequently based on multiple prognostic factors including age, karyotype, presence or absence of FLT3 and NPM1 mutations, white blood cell (WBC) count, comorbid conditions, underlying dysplasia, and other, less well-defined, factors. Despite the numerous predictive factors available, an effective and reliable means for risk stratification remains elusive. Often, analysis of available prognostic factors fails to answer the most important questions: which patients will not benefit from chemotherapy, and which should be considered for stem cell transplantation. Risk stratification is particularly problematic in patients with intermediate cytogenetics, including those with normal karyotype.
Recent studies suggested that gene-expression profiling may provide a new means to further refine risk stratification in patients with AML [1]. However, poor reproducibility and difficulties in adapting gene-expression profiling in clinical laboratories may limit the utility of such approaches. Additional challenges include variability from multiple sources, including post-transcriptional modification, sampling (i.e., inter-individual variation in the percentage of leukemic cells), and dilution effects from normal cells in the analyzed population of cells. Sorting leukemic cells or specifically dissecting the tumor can help avoid these problems, but both techniques are difficult and frequently require significant ex vivo manipulation, which may affect the internal signalling pathways and the original intracellular protein balance. Analysis of plasma or serum proteins may provide a solution to this dilemma; our search for such a biomarker led us to develop a plasma-based assay for proteasome activity.
The ubiquitin-proteasome system plays a major role in cell-cycle regulation and division, cell differentiation, response to stress and extracellular effectors, transcription regulation, and DNA repair [2–5]. This system also helps maintain the health of individual cells by recognizing and removing misfolded proteins. Through a complex interaction, ubiquitin is activated at the appropriate time, then binds the protein to be hydrolyzed and transfers it to the proteasome for degradation. The proteasome itself consists of a large, complex structure with three enzymatic activities—chymotrypsin-like (Ch-L), trypsin-like (Tr-L), and caspase-like (Cas-L)—that are responsible for digestion of proteins that are no longer needed [6–8]. A recent study indicates that serum levels of circulating proteasomes correlate with outcome in multiple myeloma [9]. We have also reported that proteasome activity can be monitored in the plasma and correlates with clinical behavior in patients with chronic lymphocytic leukemia (CLL) [10].
Here we used a simple plasma-based assay and measured the three enzymatic activities of proteasome and examined their ability to predict treatment response and survival in patients with AML or advanced myelodysplastic syndrome (MDS). We report that levels of Ch-L activity in plasma provide reliable prediction of response to standard chemotherapy and survival, even in patients with intermediate or normal cytogenetics, and discuss the implications of these findings for risk stratification.
METHODS
PATIENTS AND SAMPLES
All samples from patients and healthy volunteers were collected under an internal review board-approved protocol with written informed consent. Patients samples were collected during the period of 2001 and 2003 without selection from newly diagnosed patients prior to initiating therapy at MD Anderson. All patients were newly diagnosed, but majority were referred after diagnosis by their local physician within few days of their diagnosis. Diagnosis of AML and advanced MDS was made at MD Anderson Cancer Center and based on examination of peripheral blood and bone marrow samples. Blood counts, flow cytometry, and molecular study data were used for diagnosis. Plasma was separated from EDTA peripheral blood tubes by centrifuging at 1500 × g for 10 minutes at 4°C. Plasma samples obtained from apparently healthy volunteers were used as controls for each chip. Plasma samples were stored at −70°C.from EDTA peripheral blood and stored at −70 °C until analysis for chymotrypsin-like proteasome activity. This a retrospective study, all samples used in this study were frozen, and no fresh samples were used. Both AML and MDS patients were treated at MD Anderson Cancer Center with standard therapy based on idarubicine+ ara-C with minor variations (± topotecan or fludarabine). All patients with MDS had advance disease and were candidate for chemotherapy. Advanced MDS disease is defined by the presence of severe anemia (<8 g/dL), thrombocytopenia (<50 × 109/L), or >10% blasts. Of the MDS patients 65% had refractory anemia with excess blasts in transformation and can be classified as AML according to the World Health Organization (WHO) classification and the CMML patients had blast count >10%. The remaining 14 patients (table 1) had a score of 1 to 2 on the IPSS scoring system (International Prognostic Scoring System).
Table 1.
Characteristics of Patients with Acute Myeloid Leukemia or Myelodysplastic Syndrome.
| Characteristic | AML, n = 174 | MDS, n = 52 |
|---|---|---|
| Median age (range) | 64 (17–84) | 65 (23–75) |
| Performance Status | ||
| 0–1 | 131 (75%) | 51 (98%) |
| 2–4 | 43 (25%) | 1 (2%) |
| Cytogenetics | ||
| Favorable | 11 (6%) | 0 |
| Unfavorable | 66 (38%) | 25 (48%) |
| Intermediate | 97 (56%) | 27 (52%) |
| Median white blood cell count (range) ×109/L | 5.6 (0.4–228.0) | 2.85 (0.8–148.0) |
| Median Hemoglobin, g/dL (range) | 7.8 (3.4–13.1) | 7.7 (2.2–11.0) |
| Median Platelets ×109/L (range) | 50 (6–377) | 41.0 (10–270) |
| FAB classification | ||
| M0–2 | 106 (61%) | |
| M3 | 3 (2%) | |
| M4–5 | 47 (27%) | |
| M6/M7 | 18 (10%) | |
| RARS | 2 (4%) | |
| RAEB | 12 (23%) | |
| RAEB-T | 34 (65%) | |
| CMML | 4 (8%) |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; RARS, refractory anemia with ring sideroblasts; RAEB, refractory anemia with excess blasts; RAEB-T, refractory anemia with excess blasts in transformation.
MEASUREMENT OF PROTEASOME ENZYMATIC ACTIVITIES
Chymotrypsin-like, Cas-L, and Tr-L activities were assayed by continuously monitoring the production of 7-amino-4-methylcoumarin (AMC) from fluorogenic peptides as previously described [10]. Briefly, 45 µL plasma was first mixed with 5 µL 10% SDS at room temperature for 15 min to activate the plasma. The reaction wells contained 30 µL assay buffer (0.05% SDS in 25 mM HEPES), 10 µL activated plasma, and 10 µL of the prospective fluorogenic peptide-AMC substrate (Suc-LLVY-AMC for Ch-L, Z-LLE-AMC for Cas-L, and BZ-VGR-AMC for Tr-L). To measure the release of free AMC with time, the SpectraMax Gemini EM (Molecular Devices Corporation, Sunnyvale, CA) instrument was used with the following parameters: Exi = 380 nm; Emi = 460 nm; read interval = 1min; read length = 30 min at 37 °C. Enzymatic activities were quantitated by generating a standard curve of AMC (range, 0–8 µM). The slope of the AMC standard curve was then used as a conversion factor to calculate the absolute specific activity of each individual sample as pmol AMC/sec/mL plasma.
STATISTICAL METHODS
Clinical and biological characteristics were analyzed for their association with response and survival using log-rank test and multivariate Cox proportional hazards models. Estimates of survival curves (from the time of referral to MD Anderson Cancer Center) were calculated according to the Kaplan-Meier product-limit method [11]. All patients were newly diagnosed. However, most patients were referred to MD Anderson after diagnosis by their local physician and the possibility of few days lapsing before arriving to MD Anderson cannot be ruled out. Survival times were compared by means of the log-rank test [12]. Cox proportional hazards regression models [13] were used to assess the relationship between patient characteristics and survival, with goodness-of-fit assessed by martingale residual plots and likelihood ratio statistics. Univariate and multivariate Cox proportional hazard models were developed. Predictive variables in the Cox proportional hazards regression model were reviewed to assess the need for transformation based on smoothed martingale residual plots. Predictive variables with P values of less than 0.10 for the univariate Cox proportional hazards model were included in a multivariate model. We attempted to estimate optimal cutpoints for various covariates in this analysis. Since this dichotomization was based on an optimal cutpoint search, we adjusted the P value using the method of Schulgen et al. [14]. All computations were carried out on a DELL PC using the Windows NT operating system in SAS using standard SAS procedures (SAS Institute Inc, Cary, NC).
Nomograms for overall survival and response were developed as described by Kattan et al. [15], using patient characteristics found to be predictive of these two outcomes in the Cox proportional hazards model for survival and logistic regression for response. Overall survival time and response were also predicted via the use of a Visual Basic for Applications (VBA) computer application developed within Microsoft® Excel. This application was developed as a clinical aid and can be considered a computer-based analog to the nomogram. The core construction was based on the Cox proportional hazard model given in the multivariate analysis. To develop this VBA application, we first obtained base hazard rate and parameter estimates using the SAS® phreg procedure (for overall survival) and SAS® logistic procedure (for response). Estimates from these models were then used to obtain estimated survival and response probabilities given the patient’s covariates. The program makes use of statistical models to create a graphical representation of a given patient’s predicted survival curve.
RESULTS
CHARACTERISTICS OF PATIENTS
The 97 control subjects ranged in age from 22 to 72 years, with a median of 47. Complete clinical data for AML and MDS patients were recorded at the time of diagnosis at MD Anderson (Table 1). Patients with advanced MDS were treated with AML therapy. There was also no significant difference in response or survival between the AML and the MDS patients. Few AML patients had good cytogenetics [inv16, t(8;21), or t(15;17)] and about one-third had poor cytogenetics (−5, −7, and complex abnormalities); the majority of the AML and MDS patients had intermediate cytogenetics (diploid and other cytogenetics). Since all MDS patients had aggressive disease, only the AML classification of cytogenetics was used. Most of the MDS patients had refractory anemia with excess blasts in transformation (RAEB-T). Few had CMML with increased blasts (>10%) (Table 1).
PROTEASOME ACTIVITIES IN PLASMA OF PATIENTS WITH AML AND MDS
All three enzymatic activities (CH-L, Cas-L, and Tr-L) were significantly higher in AML and MDS patients than in control subjects (P <0.001) (Table 2). The median for Ch-L activity was 2.0 pMol/sec/ml in AML and 1.4 pMol/sec/ml in MDS as compared with 0.8 pMol/sec/ml in controls. The difference between the AML and MDS group was not significant (P = 0.62). The median for Tr-L activity was 2.5 pMol/sec/ml in AML and 2.1 pMol/sec/ml in MDS compared to 0.8 pMol/sec/ml in control and the difference between the AML and MDS group was not significant (P=0.2). As for Cas-L activity, the median was 3.6 pMol/sec/ml in AML and 2.1 pMol/sec/ml in MDS compare to 0.9 pMol/sec/ml in control, but the difference between MDS and AML was significant (P = 0.0006) (Table 2). There was significant direct correlation between Cas-L activity and Ch-L activity in AML (R=0.55) and MDS (R= 0.51). In contrast there was negative correlation between Tr-L and Ch-L activities in AML (R = −0.57) and MDS (R = −0.60). There was no correlation between Tr-L and Cas-L activities in AML (R = 0.21) and MDS (R = 0.17).
Table 2.
Higher levels of Proteasome activities in AML and MDS
| Variable | AML (N=188) |
MDS (N=58) |
Normal (N=97) |
P- value vs Normal |
P-value AML vs MDS |
|---|---|---|---|---|---|
| Ch-L | 2.0±1.9 | 1.4±0.9 | 0.8±0.4 | <.0001 | 0.62 |
| Tr-L | 2.5±2.5 | 2.1±2.6 | 0.8±1.1 | <.0001 | 0.2 |
| Cas-L | 3.6±3.5 | 2.1±1.2 | 0.9±0.6 | <.0001 | 0.0006 |
CLINICAL CORRELATES OF PROTEASOME ACTIVITIS
As shown in table 3, there was significant correlation between levels of Ch-L and Cas-L activities and beta-2 microglobulin in AML and MDS. Only Cas-L activity correlated with WBC in both AML and MDS. LDH correlated with Cas-L activity in both AML and MDS and only with Ch-L activity in AML. Blast count in peripheral blood correlated with Cas-L activity in both AML and MDS and with Ch-L activity in MDS. Interstingly, lymphocyte count in peripheral blood correlated negatively with Cas-L activity.
Table 3.
Spearman rank (R-value) Correlations between proteasome activities and various laboratory findings:
| Age | B2-M | WBC | % PB Blasts | % PB Lymphocytes | Platelets | HGB | BUN | Creatinine | LDH | |
|---|---|---|---|---|---|---|---|---|---|---|
| AML | ||||||||||
| Tr-L | 0.03 | −0.03 | 0.17 | 0.09 | −0.14 | 0.20 | 0.03 | −0.10 | −0.17 | 0.14 |
| Cas-L | 0.02 | 0.39 | 0.41 | 0.33 | −0.43 | 0.07 | −0.09 | 0.13 | 0.16 | 0.59 |
| Ch-L | 0.05 | 0.30 | 0.15 | 0.14 | −0.16 | −0.10 | −0.05 | 0.22 | 0.24 | 0.33 |
| MDS | ||||||||||
| Tr-L | 0.03 | −0.01 | 0.04 | −0.18 | −0.02 | 0.17 | 0.03 | −0.10 | −0.08 | 0.08 |
| Cas-L | −0.04 | 0.36 | 0.44 | 0.31 | −0.38 | 0.02 | −0.08 | −0.14 | −0.10 | 0.44 |
| Ch-L | 0.01 | 0.29 | 0.16 | 0.27 | −0.16 | 0.03 | −0.08 | −0.02 | 0.00 | 0.16 |
Abreviation: B2-M, beta-2 microglobulin; WBC, white blood count; PB, peripheral blood; HGB, haemoglobin; BUN, blood urea nitrogen; LDH, lactate dehydrogenase.
CORRELATION WITH RESPONSE
Response to therapy was similar in the AML and MDS patients (54% vs 53%, respectively). Overall, only 53% of the AML and MDS patients responded to therapy. Because AML and advanced MDS patients had similar outcomes, we pooled these groups for subsequent analyses.
Significant predictors of response in univariate logistic regression modeling were cytogenetic grouping, BUN, percentages of blasts and lymphocytes in peripheral blood, and both Cas-L and Ch-L activities as a continuous variables (Table 4). There was no correlation between Tr-L and response. Multivariate models based on the univariate results yielded only three independent predictors of non-response: Ch-L activity (continuous), age (dichotomized by age 70), and cytogenetic grouping (Table 2). Ability of Cas-L activity to predict response was redundant to that of Ch-L and was not independent in the multivariate model. Identical results were obtained when the AML patients and MDS patients were analyzed as separate groups (not shown).
Table 4.
Logistic Regression Modeling Results for Predicting Response in Patients with Acute Myeloid Leukemia and advanced Myelodysplastic Syndrome.
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Univariate | ||
| Beta-2 Microglobulin | 1.07 (0.98–1.18) | 0.122 |
| Platelet Count | 0.999 (0.995–1.004) | 0.818 |
| White Blood Cell Count | 1.01 (0.995–1.014) | 0.334 |
| Age (<70 vs ≥ 70 yr) | 1.92 (1.06–3.48) | 0.032 |
| Cytogenetics (Unfavorable Vs other) | 2.20 (1.28–3.78) | 0.004 |
| Performance Status (<2 vs ≥2) | 1.65 (0.86–3.19) | 0.135 |
| % Blasts in Blood | 1.01 (1.00–1.02) | 0.043 |
| % Monocytes in Blood | 1.00 (0.98–1.02) | 0.877 |
| % Lymphocytes in Blood | 0.99 (0.98–1.00) | 0.040 |
| Hemoglobin | 0.88 (0.75–1.03) | 0.120 |
| % Blasts in Bone Marrow | 1.01 (0.99–1.02) | 0.363 |
| % Monocytes in Bone Marrow | 1.02 (0.98–1.06) | 0.315 |
| % Lymphocytes in Bone Marrow | 1.01 (0.98–1.04) | 0.558 |
| Blood Urea Nitrogen | 1.04 (1.01–1.07) | 0.014 |
| Creatinine | 0.95 (0.76–1.20) | 0.671 |
| Ch-Like Activity | 1.41 (1.14–1.76) | 0.002 |
| Cas-L Activity | 1.21 (1.07–1.36) | 0.002 |
| Multivariate | ||
| Chymotrypsin-Like Activity | 1.44 (1.15–1.81) | 0.002 |
| Cytogenetics | 2.36 (1.34–4.16) | 0.003 |
| Age (<70 vs ≥70) | 2.03 (1.08–3.79) | 0.028 |
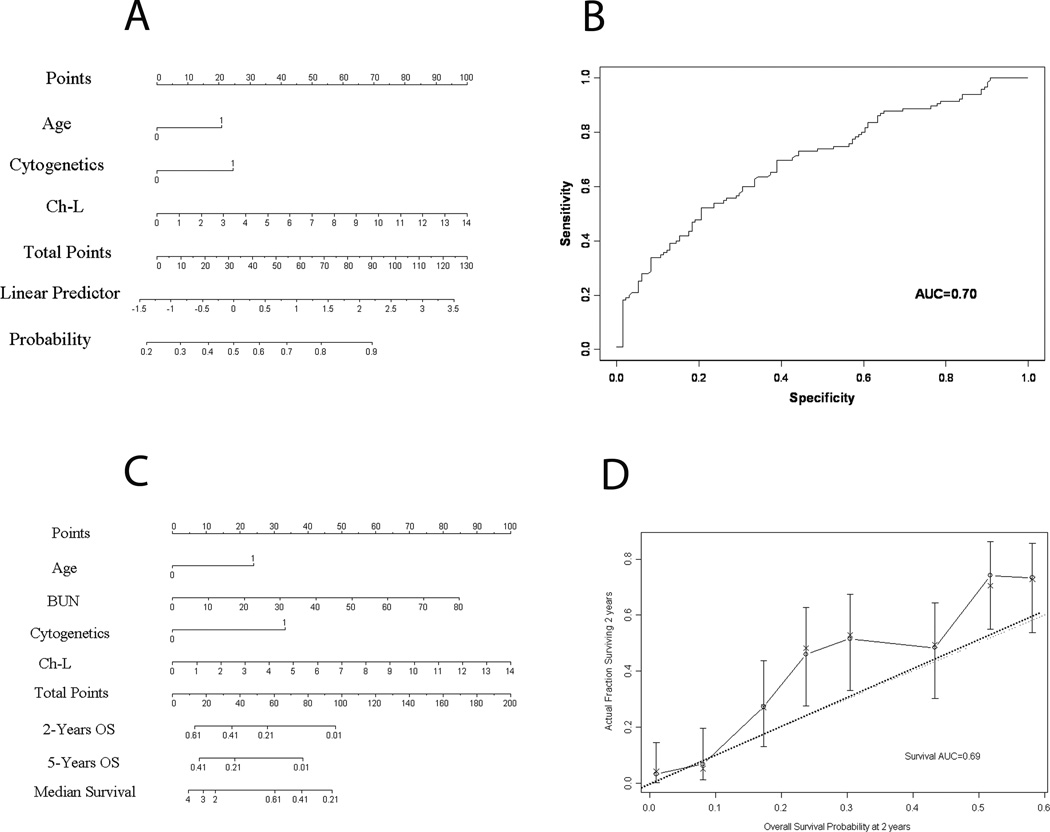
Using the three independent covariates detected in the multivariate model, we developed a nomogram (Figure 1 A) to predict the odds of non-response for individual patients. Comparison of nomogram-predicted outcomes versus actual outcomes in 126 test patients yielded an AUC of 0.70 (Figure 1 B).
Figure 1. Estimating probability of non-response (Panels A and B) and survival (Panels C and D) in previously untreated patients with acute myeloid leukemia or and advanced myelodysplastic syndrome.
Panel A illustrates the nomogram for non-response, which is used by totaling the points identified on the top scale for each independent variable. The age is dichotomized as <70 (0 points). Cytogenetics are also dichotomized (24 points, unfavorable; 0 point, intermediate). Chymotrypsin-like proteasome activity is scored on a continuous scale. The total is then used to determine the estimated probability of non-response. Panel B shows the receiver operating characteristics (ROC) curve generated from 126 patients, in which nomogram-predicted outcomes were retrospectively compared with actual outcomes. Panel C shows the nomogram for survival. Interpretation is similar to that describe in Figure 1, but poor cytogenetics are scored as 33 and blood urea nitrogen levels are scored on a continuous scale. The probability of 2- and 5-year survival are shown. Panel D shows the calibration curve for 2-year survival based on a retrospective comparison of nomogram-predicted versus actual outcomes for 126 patients. Solid line represents performance of present nomogram with 95% confidence intervals; dashed line, actual survival of the patients.
When we limited the analysis to patients with intermediate cytogenetic abnormalities (n = 124), response was associated with age group, percent of peripheral blood lymphocytes, BUN, and Ch-L and Cas-L activities as a continuous variable (each P<0.01), but not with peripheral blood blasts, or performance status. Ch-L, but not Cas-L activity predicted response when we considered only patients with poor cytogenetics (N = 91; P = 0.02).
PREDICTORS OF SURVIVAL
During the follow-up period, 134 (77%) patients with AML and 44 (84%) with advanced MDS died. Univariate Cox regression modeling was used to determine overall hazard ratios for survival in the combined group of MDS and AML patients. Unfavorable cytogenetics, performance status <2, age <70 yr, and higher values of beta 2-microglobulin, BUN, and both Ch-L and Cas-L activities were associated with increased risk of death (Table 5). Tr-L activity did not correlate with survival.
Table 5.
Logistic Regression Modeling Results for Predicting Survival in Patients with Acute Myeloid Leukemia and advanced Myelodysplastic Syndrome.
| Variable | Hazards Ratio (95% CI) | P Value |
|---|---|---|
| Univariate | ||
| Beta-2 Microglobulin | 1.07 (1.04–1.10) | <0.001 |
| Platelet Count | 0.999 (0.996–1.001) | 0.347 |
| White Blood Cell Count | 1.00 (0.997–1.007) | 0.486 |
| Age (<70 vs ≥ 70) | 2.09 (1.51–2.89) | <0.001 |
| Cytogenetics (Unfavorable vs other) | 2.30 (1.70–3.13) | <0.001 |
| Performance Status (<2 vs ≥2) | 2.14 (1.51–3.03) | <0.001 |
| Peripheral Blood Blasts | 1.00 (0.997–1.01) | 0.372 |
| Peripheral Blood Monocytes | 1.00 (0.99–1.01) | 0.870 |
| Peripheral Blood Lymphocytes | 0.997 (0.99–1.00) | 0.289 |
| Hemoglobin | 0.96 (0.89–1.05) | 0.397 |
| Bone Marrow Blasts | 1.00 (0.995–1.007) | 0.772 |
| Bone Marrow Monocytes | 1.00 (0.98–1.02) | 0.894 |
| Bone Marrow Lymphocytes | 1.01 (0.998–1.03) | 0.092 |
| Blood Urea Nitrogen | 1.05 (1.03–1.06) | <0.001 |
| Creatinine | 1.00 (0.91–1.11) | 0.939 |
| Ch-Like Activity | 1.21 (1.11–1.33) | <0.001 |
| Cas-L Activity | 1.12 (1.07,1.18) | <.001 |
| Multivariate | ||
| Cytogenetics (unfavorable Vs Other) | 2.35 (1.72–3.22) | <0.001 |
| Age (<70 vs ≥70) | 2.00 (1.44–2.79) | <0.001 |
| Performance Status (<2 vs ≥2) | 1.844 (1.29–2.63) | 0.001 |
| Blood Urea Nitrogen* | 1.026 (1.01–1.04) | 0.001 |
| Ch-Like Activity* | 1.20 (1.10–1.32) | <0.001 |
Used as continuous variables
In multivariate Cox proportional hazards models for survival incorporating the above covariates, cytogenetics, age, and performance status grouping as well as BUN level and chymotrypsin-like activity as continuous variables were independent prognostic factors for survival (Table 5). Identical results were obtained when AML and MDS patients were considered separately (not shown).
We also used the four independent variables identified in the multivariate analysis to develop a nomogram to predict survival for individual patients (Figure 1 C). Based on the total points, the 2- and 5-year probabilities of survival can be estimated, in addition to median survival. Comparison of nomogram-predicted survival versus actual survival in 126 test patients yielded an AUC of 0.69 (Figure 1 D).
Considering only patients with intermediate cytogenetic abnormalities (n = 124), survival correlated with performance status (P <0.001), beta 2-microglobulin level (P <0.001), BUN level (P = 0.001), and both Ch-L and Cas-L activities (P < 0.001) in univariate analysis. In multivariate analysis, however, only Ch-L activity, beta 2-microglobulin level, and performance status remained as independent predictors of survival. Ch-L and Cas-L activities were also strong predictor of survival when only patients with poor cytogenetics (N = 91) (P=0.002 and 0.01, respectively). Similarly Ch-L and Cas-L activities correlated strongly with survival in patients with normal karyotype (N = 84) (P = 0.001 and P = 0.01, respectively).
Univariate models for event-free survival showed similar findings, with age, performance status, and cytogenetic grouping in addition to beta 2-microglobulin, BUN, and both Ch-L and Cas-L activities as strong predictors (see Table 1 in Supplementary Appendix to this article); all but beta 2-microglobulin remained as significant predictors of event-free survival in multivariate analysis (see Table 1 in Supplementary Appendix).
Discussion
Proteasomes are intracellular complexes, and their presence in the plasma as functional structures is intriguing. Based on multiple paper, we speculate that their presence in plasma results from turnover of tissue cells (bone marrow) and not necessarly circulating tumor cells [16–22]. Data suggests that leukemic cells have high turnover rates and do not go through the routine programmed cell death and cleaning of the cell debris by the reticuloendothelial system, especially in patients with hematopoietic disease [16]. Thus, plasma is enriched by tumor-specific products and is less affected by the dilution effect of normal residual cells than are cell samples obtained from bone marrow [17–23]. However, further studies are needed to fully understand the mechanisms responsible for the presence of the leukemic cells proteasomes in plasma. Circulating proteasome levels have been measured in serum and plasma samples by enzyme-linked immunosorbent assay (ELISA) techniques, and are elevated in patients with various types of malignant diseases [9]. Recent work showed that serum proteasome levels were significantly elevated during active disease in patients with multiple myeloma, and decreased significantly in post-therapy samples of responders but not non-responders [9].
In this study we not only confirm that proteasome enzymatic activities can be measured in the plasma of patients with AML and advanced MDS, but also demonstrate that these activities, especially Ch-L activity, can be used as a unique tumor marker for predicting therapeutic response and survival. There is significant overlap between Ch-L and Cas-L activities and their levels correlate with each other, buth differ significantly from Tr-L activity in AML and MDS. Ch-L activity overshadows the biological value of Cas-L activity in predicting clinical behavior in AML and MDS patients. Our data showes that chymotrypsin-like activity of proteasomes a a strongest independent marker of all previously well-established prognostic markers in AML and MDS. To date, cytogenetics, age, and performance status remain the most important prognostic factors in patients with AML or advanced MDS. However, a significant proportion of patients have intermediate cytogenetic abnormalities, which are associated with highly variable outcomes. Additional markers are thus needed to distinguish aggressive from less-aggressive disease in this group of patients. Multiple markers have been reported to be relevant for predicting clinical behavior for this group of patients, including FLT3 (fms-related tyrosine kinase 3 gene) mutations, NPM1 (nucleophosmin gene) mutations, BAAL (brain and acute leukemia gene) expression, CEBPA (CCAAT/enhancer-binding protein alpha gene) mutation, and gene-expression profiling [24]. However, most are not well-established or require sophisticated or cost-prohibitive technology [25]. In contrast, the chymotrypsin-like proteasome activity assay described herein is relatively simple and reproducible, and can be performed on peripheral blood plasma.
Moreover, our findings suggest that chymotrypsin-like activity is an independent marker for predicting response to standard chemotherapy when combined with cytogenetics and age grouping. The nomogram developed in this study for prediction of non-response is a promising tool; prospective clinical trials are needed to evaluate the utility of this approach for guiding treatment decisions.
Chymotrypsin-like activity can be used to predict overall and event-free survival. The data presented here show that chymotrypsin-like activity is a strong predictor of survival, independent of cytogenetics, age grouping, beta-2 microglobulin level, and performance status. The second nomogram developed in this study holds potential for use in predicting overall survival for individual patients at 2 and 5 years. Again, prospective studies are needed to verify the predictive values of these nomograms.
Together, our findings indicate that measurement of proteasome chymotrypsin-like activity in plasma provides a powerful biomarker for predicting response and survival in patients with AML or advanced MDS. The demonstration that the ubiquitin-proteasome system is particularly important in this group of patients not only provides valuable information on the biology of AML and advanced MDS, but also opens the door for potential therapeutic approaches that incorporate proteasome inhibitors that specifically target the most important enzymatic activity of the proteasome in AML. Preclinical studies showed encouraging effects of the proteasome inhibitor bortezomib on AML cells [26]. However, clinical trials showed that unlike multiple myeloma, bortezomib when added to the standard combination therapy idarubicin and cytarabine in treating AML did not significantly improve response rate [27]. While proteasome enzymatic activities are increased in various diseases, direct comparison of the profile of activities between various diseases is needed. Differences in the enzymatic profile between multiple myeloma and AML may explain the difference in the response to bortezomib. These information need to be considered in the context of the new generation of proteasome inhibitors that target different enzymatic activities and further studies are clearly needed to fully take advantage of the information provided in this paper in treating patients with AML.
The data presented here have the potential to allow us to stratify individual patients for therapeutic approaches. Patients with a high probability of not responding to standard chemotherapy should be given the choice of stem cell transplant or other clinical trials that investigating new therapeutic approaches. The value of such stratification should be investigated in prospective clinical trials and data on overall survival and quality of life should guide future decisions for the use of this approach.
Supplementary Material
Translational Relevance statement.
We demonstrate that measuring proteasome enzymatic activities in the plasma provides new biomarkers for the prediction of clinical behaviour in patients with acute myeloid leukaemia and advanced stage myelodysplastic syndrome. We show that the three enzymatic activities of the proteasomes (Chymotrypsin-like, trypsin-like, and caspase like) are highly elevated in the plasma of patients with AML and MDS. Using multivariate regression model we show that levels of the chymotrypsin-like activity is strong predictor of response and survival that is independent of cytogenetic grouping and all other known prognostic factors. We believe that this new biomarker, which is easily and reliably measured in the peripheral blood plasma, can be used to stratify patients for therapeutic approaches (chemotherapy vs. transplant or other experimental therapy). The use of plasma levels of proteasome activities as biomarkers is a novel approach and has the potential to be used in other cancers.
References
- 1.Bullinger L, Döhner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 2.Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 3.Reinstein E, Ciechanover A. Narrative review: protein degradation and human diseases: the ubiquitin connection. Ann Intern Med. 2006;145:676–684. doi: 10.7326/0003-4819-145-9-200611070-00010. [DOI] [PubMed] [Google Scholar]
- 4.Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- 5.Tatebe H, Yanagida M. Cut8, essential for anaphase, controls localization of 26S proteasome, facilitating destruction of cyclin and Cut2. Curr Biol. 2000;10:1329–1338. doi: 10.1016/s0960-9822(00)00773-9. [DOI] [PubMed] [Google Scholar]
- 6.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 7.Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 8.Rock KL, Gramm C, Rothstein L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 9.Jakob C, Egerer K, Liebisch P, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109:2100–2105. doi: 10.1182/blood-2006-04-016360. [DOI] [PubMed] [Google Scholar]
- 10.Ma W, Kantarjian H, O'Brien S, Jilani I, Zhang X, Estrov Z, Ferrajoli A, et al. Enzymatic activity of circulating proteasomes correlates with clinical behavior in patients with chronic lymphocytic leukemia. Cancer. 2008 doi: 10.1002/cncr.23301. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. American Statistical Association. 53:457–481. [Google Scholar]
- 12.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- 14.Schulgen G, Lausen B, Olsen JH, Schumacher M. Outcome-oriented cutpoints in analysis of quantitative exposures. American Journal of Epidemiology. 1994;140:172–184. doi: 10.1093/oxfordjournals.aje.a117227. [DOI] [PubMed] [Google Scholar]
- 15.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 16.Giles FJ, Albitar M. Plasma-based testing as a new paradigm for clinical testing in hematologic diseases. Expert Rev Mol Diagn. 2007;7:615–623. doi: 10.1586/14737159.7.5.615. [DOI] [PubMed] [Google Scholar]
- 17.Rogers A, Joe Y, Manshouri T, Dey A, Jilani I, Giles F, Estey E, Freireich E, Keating M, Kantarjian H, Albitar M. Relative increase in leukemia-specific DNA in peripheral blood plasma from patients with acute myeloid leukemia and myelodysplasia. Blood. 2004;103:801–818. doi: 10.1182/blood-2003-06-1840. [DOI] [PubMed] [Google Scholar]
- 18.Manshouri T, Do KA, Wang X, Giles FJ, O'Brien SM, Saffer H, Thomas D, Jilani I, Kantarjian HM, Keating MJ, Albitar M. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101:2507–2513. doi: 10.1182/blood-2002-06-1639. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed M, Giles F, Joe Y, Weber DM, Jilani I, Manshouri T, Giralt S, De Lima M, Keating M, Albitar M. Use of plasma DNA in detection of loss of heterozygosity in patients with multiple myeloma. Eur J Haematol. 2003;71:174–178. doi: 10.1034/j.1600-0609.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 20.Jilani I, Estey E, Manshuri T, Caligiuri M, Keating M, Giles F, Thomas D, Kantarjian H, Albitar M. Better detection of FLT3 internal tandem duplication using peripheral blood plasma DNA. Leukemia. 2003;17:114–119. doi: 10.1038/sj.leu.2402743. [DOI] [PubMed] [Google Scholar]
- 21.Ma W, Kantarjian H, Jilani I, Gorre M, Bhalla K, Ottmann O, Giles F, Albitar M. Heterogeneity in detecting Abl kinase mutations and better sensitivity using circulating plasma RNA. Leukemia. 2006;20:1989–1991. doi: 10.1038/sj.leu.2404355. [DOI] [PubMed] [Google Scholar]
- 22.Ma W, Kantarjian H, Zhang X, Sun W, Buller AM, Jilani I, Schwartz JG, Giles F, Albitar M. Higher detection rate of JAK2 mutation using plasma. Blood. 2008;111:3906–7. doi: 10.1182/blood-2008-02-139188. [DOI] [PubMed] [Google Scholar]
- 23.Jilani I, Kantarjian H, Faraji H, Gorre M, Cortes J, Ottmann O, Bhalla K, O'Brien S, Giles F, Albitar M. An immunological method for the detection of BCR-ABL fusion protein and monitoring its activation. Leuk Res. 2008;32:936–943. doi: 10.1016/j.leukres.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mrózek K, Bloomfield CD. Chromosome aberrations, gene mutations and expression changes, and prognosis in adult acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2006:169–177. doi: 10.1182/asheducation-2006.1.169. [DOI] [PubMed] [Google Scholar]
- 26.Colado E, Alvarez-Fernández S, Maiso P, Martín-Sánchez J, Vidriales MB, Garayoa M, Ocio EM, Montero JC, Pandiella A, San Miguel JF. The effect of the proteasome inhibitor bortezomib on acute myeloid leukemia cells and drug resistance associated with the CD34+ immature phenotype. Haematologica. 2008;93:57–66. doi: 10.3324/haematol.11666. [DOI] [PubMed] [Google Scholar]
- 27.Attar EC, De Angelo DJ, Supko JG, D'Amato F, Zahrieh D, Sirulnik A, Wadleigh M, Ballen KK, McAfee S, Miller KB, Levine J, Galinsky I, Trehu EG, Schenkein D, Neuberg D, Stone RM, Amrein PC. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008;14:1446–1454. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.