Abstract
Endomorphin-1 (EM-1) and endomorphin-2 (EM-2) are two highly selective mu-opiate receptor agonists. We recently demonstrated that EM-1 and EM-2 have a saturable transport system from brain-to-blood in vivo. Since the endothelial cells are the main component of the non-fenestrated microvessels of the blood-brain barrier (BBB), we examined whether these endogenous tetrapeptides have a saturable transport system in cultured cerebral endothelial cells. EM-1 and EM-2 binding and transport were studied in a transwell system in which primary mouse endothelial cells were co-cultured with rat glioma cells. We found that binding of both endomorphins was greater on the basolateral than the apical cell surface. Flux of EM-1 and EM-2 occurred predominantly in the basolateral to apical direction, each showing self-inhibition with an excess of the respective endomorphin. Transport was not influenced by the addition of the P-glycoprotein inhibitor, cyclosporin A. Neither the mu-opiate receptor agonist DAMGO nor the delta-opiate receptor agonist DPDPE had any effect on the transport. Thus, the results show that a saturable transport system for EM-1 and EM-2 occurs at the level of endothelial cells of the BBB, and unlike β-endorphin and morphine, P-glycoprotein is not needed for the brain-to-blood transport. Cross-inhibition of the transport of each endomorphin by the other suggests a shared transport system that is different from mu- or delta-opiate receptors. As endormorphins are mainly produced in the CNS, the presence of the efflux system at the BBB could play an important role in pain modulation and neuroendocrine control.
Keywords: Endomorphin, Blood-brain barrier, P-glycoprotein, Endothelial cells
Introduction
The blood-brain barrier (BBB), formed by complex tight-junctions of the brain capillary endothelial cells, is an important regulatory system for maintaining the neural activity of the central nervous system (CNS). The BBB regulates transport of nutrients, hormones, and drugs between circulating blood and interstitial fluid.
Some peptides cross the BBB by saturable transport mechanisms while others cross by passive diffusion based on physicochemical properties (Davson and Segal 1996). The first saturable transport system across the BBB to be shown for a peptide was for Tyr-MIF-1 (Tyr-Pro-Leu-Gly-NH2) (Banks and Kastin 1984). Endomorphin-1 (EM-1) and endomorphin-2 (EM-2), are two endogenous peptides selectively activating mu-opiate receptors (Zadina et al. 1997; Goldberg et al. 1998; Gong et al. 1998; Hosohata et al. 1998). The endormorphins are structurally similar to Tyr-MIF-1. We have shown in mice that the brain-to-blood efflux of EM-1 and EM-2 is a saturable process showing characteristics of self-inhibition and cross-inhibition (Kastin et al. 2001). Although the P-glycoprotein transport system is responsible for the efflux of many therapeutic drugs out of the brain (Schinkel 1999; Terasaki and Hosoya 1999), P-glycoprotein is not required for the brain-to-blood transport of endomorphins in vivo (Kastin et al. 2002).
Efflux systems usually serve to restrict entry or eliminate substrates from the brain (Schinkel et al. 1994; Kusuhara and Sugiyama 2001). The physiological significance of the efflux system for endormorphins is not clear but probably is involved in pain modulation and neuroendocrine regulation. Endomorphins not only are potent analgesics but also can participate in immune modulation and behavioral changes (Bujdoso et al. 2001), as do other ligands of mu-opiate receptors. The immunoreactivity of endomorphins in the brain and spinal cord under conditions of basal and neuropathic pain has been mapped in our laboratory and others (Martin-Schild et al. 1998, 1999; Pierce et al. 1998; Smith et al. 2001; Wang et al. 2003a; b).
Endomorphins have short half-lives in blood and brain, where they are rapidly degraded (Gentry et al. 1999; Hau et al. 2002). In the studies described here, therefore, we use isolated brain capillary endothelial cells as a model to further characterize the transport system for endomorphins at the BBB, including the kinetics of transport and the effects of potential inhibitors. The results will provide a basis for identification of the transporter in our future studies.
Materials and methods
Reagents
EM-1and EM-2 were from American Peptide Company (Sunnyvale, CA, USA). Fetal bovine serum (FBS) was from Gibco (Rockville, MD, USA). DNase, L-1-chloro-3–7-amino-heptanone hydrochloride N-α-Tosyl-L-lysine chloromethyl ketone (TLCK), collagenase/dispase were purchased from Roche Molecular Biochemicals (Indianapolis, IN, USA), and 8-(4-chlorophenylthio)-adenosine 3′, 5′-cyclic monophosphate (CPT-cAMP) from Boehringer Mannheim (Indianapolis, IN, USA). Epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). RO-20-1724 was from Biomol Research Lab (Plymouth Meeting, PA, USA). DAMGO and DPDPE were from Multiple Peptide System (San Diego, CA, USA). The ABC Elite kit was obtained from Vector Laboratories (Burlingame, CA, USA). 125INa was purchased from Amersham – Pharmacia, (Piscataway, NJ, USA). C6 rat glioma cells were obtained from American Type Culture Collection (Manassas, VA, USA). All other antibodies, chemicals and reagents were purchased from Sigma (St. Louis, MO, USA).
Iodination
EM-1 and EM-2 were labeled with 125INa by chloramine-T and separated from unlabeled peptide by high performance liquid chromatography (HPLC). The peak effluent was dried in a speed-vac, reconstituted in transport buffer, and frozen at −80°C in aliquots. The specific activities of both peptides were about 2,100 Ci/mmol. Human alpha-lactalbumin was labeled with 125INa by chloramine-T and purified by Sephadex G10 column chromatography.
Cell cultures
Brain microvessel fragments were isolated from 20–25 g male ICR/CD1 mice (Charles River, Wilmington, MA, USA) by the method of Abbot et al. (Abbott et al. 1992) with modifications. The mice were anesthetized with 40% urethane ip and used according to the NIH principles of laboratory animal care as approved by our Institutional Animal Care and Use Committee. After decapitation the neocortex was dissected into two hemispheres, the meningeal vessels were removed, and the tissue was transferred to buffer A (Hanks Balanced Salt Solution [HBSS] with 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin) with1% bovine serum albumin (BSA). The brain tissue was finely minced with a sterile blade and transferred to the homogenizing enzyme mix (collagenase/dispase 0.1%, DNase 20 U/ml, TLCK 0.147 μg/ml in buffer A), triturated with a pipette tip, and incubated 1 h at 37°C in a shaking water bath. Tissue pieces were separated from myelin by centrifugation in 25% BSA in buffer A, followed by a second digestion in the enzyme mix. Microvessels were separated from single cells and tissue fragments by centrifugation over a 50% Percoll gradient. The cells were applied to polyester transwell membranes (6.5 mm diameter, 0.4 μm pore size in 24 well plates; Corning, Corning, NY, USA), which were previously coated with 50 μg/ml rat tail collagen type I. At the same time, C6 rat glioma cells were plated on the bottom of the transwell system.
The microvessel fragments were cultured in a medium designed for microvascular endothelial cells (Dulbecco’s Modified Eagle Medium [DMEM] supplemented with 15% FBS, 1 ng/ml bFGF, 1 μg/ml hydrocortisone, 10 ng/ml EGF) at 37°C in a humidified 5% CO2 atmosphere with a change of medium every 2–3 days. In this polarized cell culture system, the basolateral side of the cells adhere to the collagen type I and the apical surface of the cells is apposed to the medium above. Therefore, the top (0.2 ml) of the transwell is called the luminal side and the bottom (1.0 ml), the abluminal side. At 70% confluence, the culture medium was supplemented with 250 μM CPT-cAMP and 17.5 μM of RO-20-1724 in order to get tight junction connections. Cell confluence was evaluated by measurement of transendothelial electrical resistance (TEER) across the cell monolayer with an Electrical Resistance System (World Precision Instruments, Sarasota, FL, USA). TEER was calculated as total resistance – cell free (blank) resistance. Binding and transport assays were performed when a consistent electrical resistance of 80–100 ohms [26.5–33.3 ohmscm2] (180–200 ohms [59.5–66.6 ohmscm2] total) was obtained, usually between days 9 and 12 after plating the cells into the transwell system.
Immunocytochemistry
Brain microvessel cells cultured on the transwell system were washed three times with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde. Endogenous peroxidase activity was blocked with 0.5% H2O2 for 1 h at room temperature, and the membranes were washed in PBS, treated with normal sera, and incubated with anti-factor VIII-related antigen (specific for endothelial cells) or anti-GFAP (specific for astrocytes) overnight at 4°C. After several washes in PBS, the cells were incubated with a biotinylated second antibody at room temperature. The immunochemical detection was visualized with Vectastain Elite ABC kit and DAB substrate kit for peroxidase, according to the manufacturer’s instructions.
Transport assays
Primary brain microendothelial cells (PBMEC) were plated in triplicate at a starting density of 2×105/ml cells onto the transwell system, and C6 cells were plated at a density of 105/ml cell on the bottom of the wells. After reaching confluence and high electrical resistance, the transwell membranes were moved to C6 cells-free wells, washed with transport buffer (DMEM + 0.1% BSA), and incubated for 30 min at 37°C with 5% CO2. For apical to basolateral experiments, at time zero, the media was removed from the luminal side and replaced with 200 μl transport buffer containing 125I-EM-1, 125I-EM-2 or 125I-lactalbumin (106cpm/0.2 ml). The abluminal side contained 1 ml of transport buffer. For basolateral to apical experiments, the labeled material was added to the abluminal side in 1 ml transport buffer, and the luminal side contained the transport buffer only. In some cases, the compounds of interest (unlabeled EM-1 and EM-2, DAMGO, DPDPE, or cyclosporin A) were mixed with the labeled peptides before addition to the wells.
The plates were incubated at 37°C with shaking. At 5, 10, 15, 20, 30, 45 and 60 min, 10 μl of sample was taken from the abluminal side (for apical transport) or from the luminal side (for basolateral transport) for analysis of radioactivity and immediately replaced with 10 μl transport buffer to maintain the same buffer volume throughout the experiment.
For the apical to basolateral experiments, the percent material transported at each time point was defined as [0.5 × cpm counted in the 10 μl sample taken from the abluminal side/all the cpm added to the luminal side] × 100. For the basolateral to apical experiments, the percent material transported at each time point was calculated as [0.05 × cpm counted in the 10 μl sample taken from the luminal side/all cpm added to the abluminal side] × 100. The percent of transported material was plotted against time, and the regression lines were determined by the least-squares method.
Binding assays
To assess 125I-EM-1 or 125I-EM-2 binding on the apical or basolateral cell surface, binding assays were carried out with either 125I-EM-1 or 125I-EM-2 and PBMEC culture. Binding of 125I-EM-1 or 125I-EM-2 was done at 4°C in transport buffer (DMEM + 0.1% BSA) to minimize endocytosis and intracellular processing of the hormones. Cells were washed three times in ice-cold transport buffer and then incubated with 105cpm/ml labeled peptide added either to the apical or the basolateral chamber for 4 h. To differentiate bound cpm from nonspecific background binding, binding of the labeled peptide was carried out in the presence or absence of excess unlabeled EM-1 or EM-2 (1 μg/ml). After incubation, the membranes were washed three times with ice-cold transport buffer, cut from the transwell with a scalpel, and counted in a γ-counter. The number of cpm bound above the background was defined as: [total cpm bound – cpm bound in the presence of cold peptide].
Degradation analysis
To determine the degree to which transported cpm represented intact EM-1 or EM-2, trichloroacetic acid (TCA) precipitation was carried out on media collected from chambers contralateral to that to which radiolabeled ligand was added. At the end of the transport experiment, 100 μl of media were collected from each chamber and mixed with 1 ml 30% TCA dissolved in brine NaCl. After incubation for 5 min at room temperature, the tubes were centrifuged at 1,500 g for 10 min at 4°C. The supernatant was decanted to another tube and both tubes measured in a γ-counter. The percent of intact peptide was calculated as: [cpm pellet/cpm pellet + supernatant] × 100. Controls consisted of samples taken from the radiolabeled stock. To further verify that intact EM-1 or EM-2 were present in the transported media, 104 cpm in 100 μl media was injected onto a C18 column and separated by HPLC. The percentage of the peak effluent was compared with the control, intact radiolabeled stock.
Statistical analysis
Groups were compared by analysis of variance (ANOVA) followed by Newman-Keuls’s multiple comparison tests. Regression lines were determined by the least-squares method and the differences between slopes compared by GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA).
Results
Cell culture
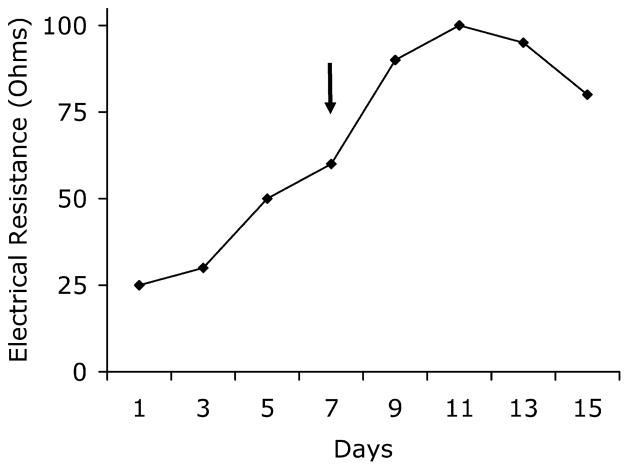
The mouse brain microvessel fragments became attached to the collagen-coated transwell membrane within 24 h and spread slowly onto the surface over the first week, becoming a confluent monolayer by 9–13 days of culture. At days 7–9, the medium was supplemented with CPT-cAMP (a cell membrane-permeable cAMP analogue) and RO-20-1740 (a phospohodiesterase inhibitor) in order to get tight junctions on the monolayer, which increased the TEER across the PBMEC+C6 glioma co-culture (Fig. 1). The electrical resistance measured reached a plateau between days 9 and 12, when the resistance was 95–98 ± 4 ohms [31.5–32.5 ± 1.3 ohmscm2]. After day 13, the TEER started to decline and the variance became larger among the wells (80±6.2 ohms [26.5±2.1 ohmscm2]). Based on the TEER measurements, which correspond with the permeability of the monolayer and the light microscopic appearance of the endothelial cells, we decided to do the experiments between days 9 and 12, when the TEER was the highest and the cells were confluent.
Fig. 1.
Transendothelial electrical resistance on successive days after seeding (Day 0) of PBMEC on transwell system. TEER is expressed as total resistance – blank (cell free) resistance. Total resistance is 180–200 ± 8 ohms, blank resistance is 102±2 ohms. The arrow indicates the addition of culture medium supplemented with 250 μM CPT-cAMP and 17.5 μM of RO-20-1724. Values represent the mean of three measurements
Immunocytochemistry
The immunocytochemical analysis of representative cultures with an endothelial cell-specific marker (factor VIII-related antigen) showed that about 95% of the cells were endothelial. Very few, if any, cells were stained with anti-GFAP, indicating a pure endothelial cell culture.
Transport assays
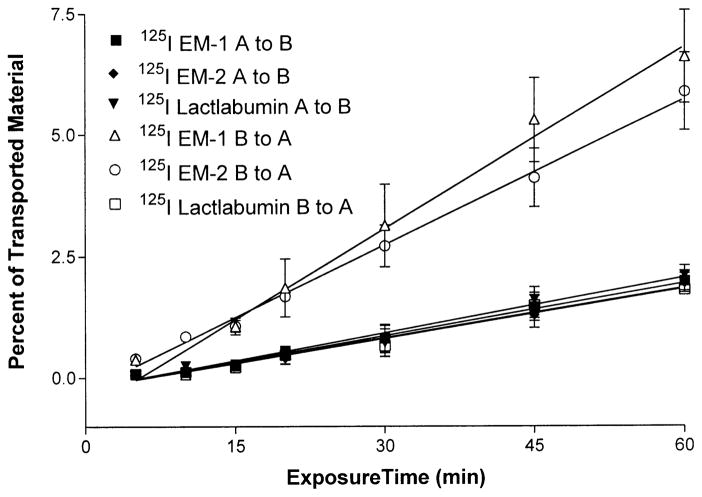
Figure 2 shows that the transport of 125I-EM-1 and 125I-EM-2 is unidirectional, from basolateral to apical surface. The Ki of 125I-EM-1 is 0.0359±0.0014%/min from apical to basolateral (influx) and 0.1248±0.072%/min from basolateral to apical sides (efflux). 125I-EM-2 showed a similar pattern, with a Ki of 0.0341±0.0017%/min from apical to basolateral and 0.0997±0.003%/min from basolateral to apical sides. 125I-lactalbumin showed a slow influx and efflux rate of 0.0378±0.0026%/min and 0.0339±0.0024%/min, respectively, which is significantly (P<0.001) different from the efflux rate of both peptides, indicating a good barrier function with low non-specific permeability.
Fig. 2.
Comparison of 125I-EM-1, 125I-EM-2, and 125I-lactalbumin transport from the apical to basolateral (A to B) and from the basolateral to apical (B to A) side. There was a significant efflux of both endomorphins compared with the efflux rate of lactalbumin (p<0.001). Each data point represents the mean ± SEM of three determinations in triplicate
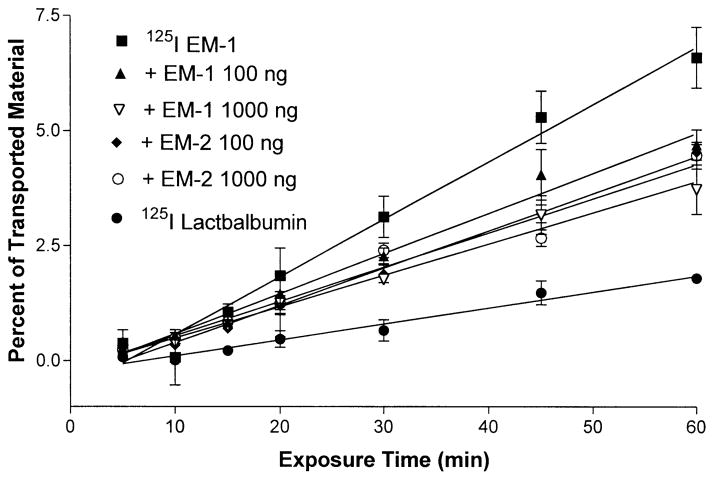
The addition of unlabeled, excess EM-1 or EM-2 decreased the efflux rate of 125I-EM-1 (Fig. 3) significantly (p<0.001), resulting in a Ki of 0.087±0.0048%/min in the presence of an excess of 100 ng/ml EM-1, 0.068±0.0036%/min with 1,000 ng/ml EM-1 (p<0.01 between the two doses), 0.08±0.0025%/min with 100 ng/ml EM-2, and 0.074±0.0059%/min with 1,000 ng/ml EM-2 (no significant change between doses).
Fig. 3.
Transport of 125I-EM-1 and 125I-lactalbumin from the basolateral to apical direction on mouse endothelial cells grown on collagen-coated transwell. In some experiments, unlabeled EM-1 or EM-2 (100 ng/ml or 1,000 ng/ml) was added to the transport buffer. This resulted in significant self-inhibition characteristic of a saturable system. Each data point represents the mean ± SEM of three determinations
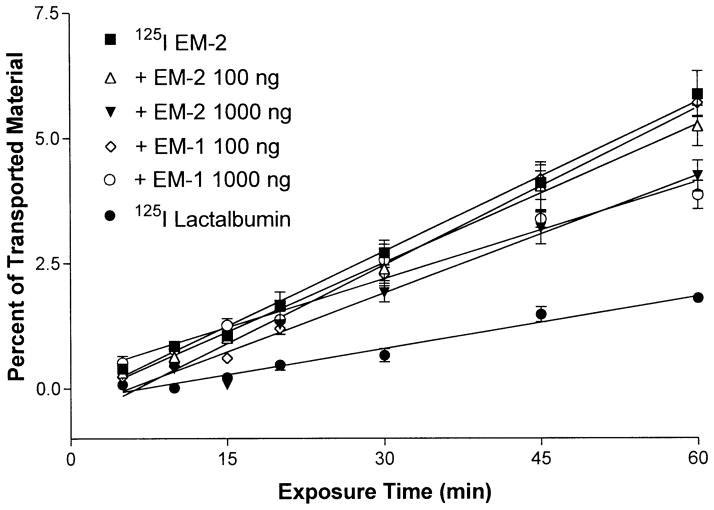
The efflux rate of 125I-EM-2 also was reduced by the addition of each of the unlabeled peptides to the labeled material (Fig. 4). The Ki was reduced from 0.0997±0.03%/min to 0.092±0.0024%/min by the addition of 100 ng/ml EM-2, a nonsignificant change, while the inclusion of 1,000 ng/ml EM-2 resulted in a significant (p<0.001) decrease of the efflux rate (0.078±0.0069%/min).
Fig. 4.
Transport of 125I-EM-2 and 125I-lactalbumin from the basolateral to apical side in mouse endothelial cells grown on collagen-coated transwell. In some experiments, unlabeled EM-1 or EM-2 (100 ng/ml or 1,000 ng/ml) was added to the transport buffer. This resulted in self-inhibition characteristic of a saturable system. P<0.001 for the addition of 1,000 ng/ml EM-1 or EM-2. The inclusion of 100 ng/ml EM-1 or EM-2 resulted in a nonsignificant change of transport. Each data point represents the mean ± SEM of three determinations
The addition of unlabeled, excess EM-1 to the 125I-EM-2 containing transport media caused similar changes. There was a nonsignificant change of the efflux rate with the addition of 100 ng/ml EM-1 (0.1048±0.0054%/min). However, 1,000 ng/ml cold EM-1 decreased the efflux (0.064±0.0051%/min) of 125I-EM-2 significantly (p<0.001).
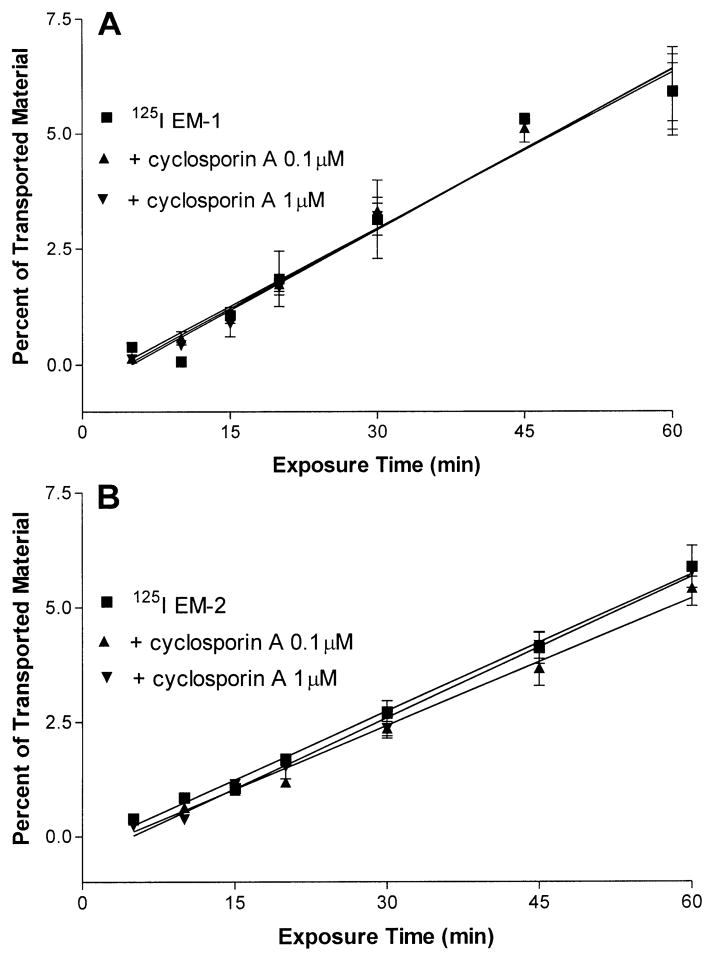
In the next experiments, we added excess cyclosporin A (a P-glycoprotein inhibitor) to the labeled peptides to determine whether the transport was mediated by the P-glycoprotein system. The doses chosen, 0.1 or 1 μM, are known to inhibit the P-glycoprotein system without affecting the TEER (van der Sandt et al. 2001). The inclusion of cyclosporin A to 125I-EM-1 or 125I-EM-2 containing media did not change the efflux of either peptides (Fig. 5A, B).
Fig. 5.
A, B The presence of 0.1 or 1 μM of cyclosporine A, a P-glycoprotein inhibitor, did not change the efflux of 125I-EM-1 (A) or 125I-EM-2 (B) on cultured endothelial cells. Each data point represents the mean ± SEM of three determinations
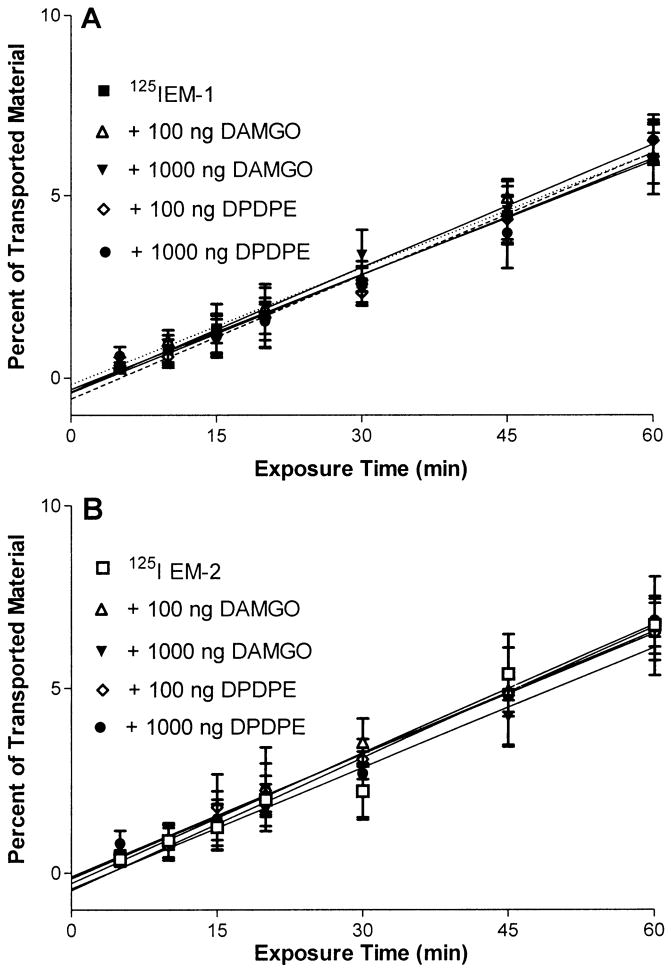
Neither the mu-opiate receptor agonist DAMGO nor the delta-opiate receptor agonist DPDPE had a significant effect on the transport of either EM-1 or EM-2 (Fig. 6A, B).
Fig. 6.
A, B The addition of 100 ng/ml or 1,000 ng/ml of the mu-opiate receptor agonist DAMGO or the delta-opiate receptor agonist DPDPE to the 125I-EM-1 (A) or 125I-EM-2 (B) containing media did not result in a significant change in the efflux rate of these peptides. Each data point represents the mean ± SEM of three determinations
Binding assays
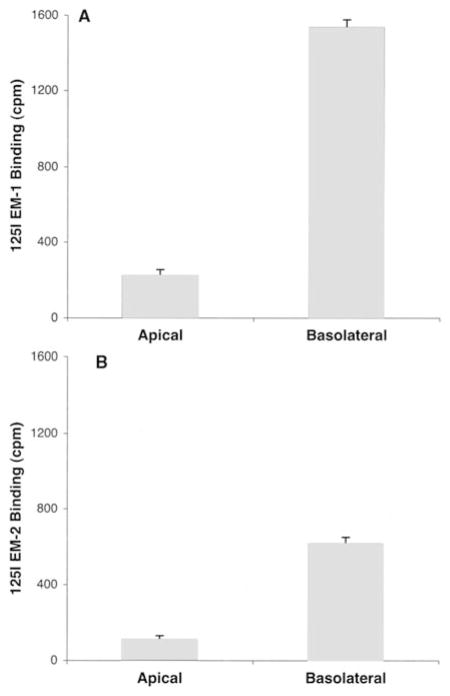
Because PBMEC cells exhibit differentiated plasma membranes when grown on the substrate, we next tested whether the binding of 125I-EM1 or125I-EM-2 might be polarized to either the apical or basolateral cell surface. By measuring binding to PMBEC at 4°C, we found that both labeled peptides showed significantly higher binding to the basolateral surface compared with the apical surface (Fig. 7). 125I-EM-1 bound to the basolateral surface eight times higher than to the apical side, while 125I-EM-2 showed six times higher binding to the basolateral side than to the apical.
Fig. 7.
A, B Number of cpm 125I-EM-1 (A) or 125I-EM-2 (B) bound to either the apical or basolateral surface of cultured brain endothelial cells from mice. 125I-EM-1 or 125I-EM-2 was added to either the apical or the basolateral chamber for 4 h at 4°C. Cells were then washed and counted in a γ-counter. Values represent the mean ± SEM of three measurements
Degradation analysis
To determine the amount of transported radioactivity, which represented intact EM-1 or EM-2, we first performed TCA precipitation on media taken from the basolateral or apical chamber at the end of the experiments (60 min). We found that 84±3.6% of the transported EM-1 and 81±4.5% of the transported EM-2 were precipitated by TCA. Almost all (95±0.8% for EM-1 and 93±2.1% for EM-2) of the radioactivity in the stock 125I-EM-1and 125I-EM-2 was precipitated by TCA.
Media taken from the basolateral or apical chamber of the transwell system at 60 min was then subjected to HPLC analysis. It showed that 76±4.3% and 71±3.5% of the radioactivity recovered represented intact 125I-EM-1 and 125I-EM-2, respectively. The groups receiving the labeled peptides and various concentrations of unlabeled EM-1 or EM-2 did not have a significant decrease in the percent of intact 125I-EM-1 and 125I-EM-2, showing that addition of excess cold peptides did not influence the rate of degradation.
Discussion
We have established a primary mouse brain endothelial culture system on membranes that serves as a model of the in vitro BBB. This system was used for assessment in vitro of the transport of EM-1 and EM-2. Immunohistochemical staining showed that the culture system consisted of pure endothelial cells. In vitro models are generally more permeable than the BBB in vivo, but permeability can be decreased by co-culturing the cells with astrocytes (Rubin et al. 1991) or by the use of various compounds that cause an elevation of cAMP levels, thereby increasing barrier function (Rubin and Staddon 1999). With these approaches, we were able to obtain relatively high TEER. The lack of lactalbumin transport and the significantly different transport of endomorphins across the cells in the abluminal to luminal direction as compared with the opposite direction established the functionality of the system.
Our results show that EM-1 and EM-2 have a saturable efflux system. This was established by self-inhibition of the transport of each radioactively labeled peptide by an excess of the corresponding peptide. There also was cross-inhibition of the rate of efflux of each endomorphin by the other: the excess of EM-2 inhibited the efflux of EM-1, and an excess of EM-1 inhibited the efflux of EM-2. This indicates shared components for the brain-to-blood transport of these peptides. These findings are consistent with our in vivo results (Kastin et al. 2001).
Several investigators have shown that the P-glycoprotein transport system is responsible for the efflux of many therapeutic drugs out of the brain (Schinkel 1999; Terasaki and Hosoya 1999). However, in our in vitro experiments, the rates of efflux of EM-1 and EM-2 were not influenced by cyclosporin A, a P-glycoprotein inhibitor, indicating that, in contrast to endorphin (King et al. 2001), the endomorphins do not require P-glycoprotein for transport out of the brain. This supports our previous in vivo finding (Kastin et al. 2002) of a saturable efflux system for endomorphins in P-glycoprotein knockout mice. However, this is in contrast with the findings of Oude Elferink and Zadina (2001) who showed that P-glycoprotein transports endogenous opiate peptides including EM-1 and EM-2. This discrepancy from our results might be explained by different in vitro systems. They used a transfected non-small cell lung cancer cell line SW1573/SI, while our system consists of primary brain microvessels, which is more closely related to the BBB in vivo.
The addition of the mu-opiate receptor agonist DAMGO, or the delta-opiate receptor agonist DPDPE, had no effect on the efflux of either peptide, indicating no involvement of these receptors in the transport. Similar results were found on crude microvessel preparations, where DAMGO, DPDPE and the kappa-opiate receptor agonist U50488 did not change 125I-Tyr-MIF-1 binding (Maresh et al. 1999). Thus, the transporter of EM-1 and EM-2 is different from its binding protein, the mu-opiate receptor.
The P-glycoprotein transporter system is expressed on brain capillaries and localized on the luminal membrane (Cordon-Cardo et al. 1989). Multidrug resistance-associated proteins (MRPs), transporting conjugated metabolites such as glutathione- and glucuronide-conjugates (Loe et al. 1996; Kusuhara et al. 1998), are expressed and localized on the basal membrane of testis Sertolli cells and the basolateral membrane of lung epithelial cells (Wijnholds et al. 1998; Wright et al. 1998) but no expression of MRPs was detected on isolated human brain capillaries (Seetharaman et al. 1998) and no significant staining was found in mouse brain (Wijnholds et al. 2000). Breast Cancer Related Protein (BCRP) is located at the BBB, mainly at the luminal surface of microvessel endothelium (Cooray et al. 2002). The preferential binding of EM-1 and EM-2 on the basolateral membrane in our polarized cell model suggests an efflux transporter located on the abluminal side of the BBB. However, it is possible that other efflux systems may share transport capabilities with the endomorphin system.
In summary we have shown that (1) EM-1 and EM-2 have a unidirectional transport in vitro; (2) the transport is saturable, as shown by the inhibition of transport of trace amounts of these peptides by an excess of the corresponding peptide; (3) EM-1 and EM-2 have shared transport, indicated by cross-inhibition of the rate of efflux of each endomorphin by the other; (4) the P-glycoprotein transport system is not involved because the addition of a P-glycoprotein inhibitor did not change the transport; (5) the transporter is not related to the mu- or delta-opiate receptors because agonists for these receptors do not inhibit transport; and (6) the brain-to blood transporter of EM-1 and EM-2 may represent a different type of mechanism in which basolateral binding is a rate-limiting step in the efflux.
Acknowledgments
Supported by the Department of Veterans Affairs and NIH (AA12865 and DK54880).
References
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103:23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. A brain-to-blood carrier-mediated transport system for small, N-tyrosinated peptides. Pharmacol Biochem Behav. 1984;21:943–946. doi: 10.1016/s0091-3057(84)80077-5. [DOI] [PubMed] [Google Scholar]
- Bujdoso E, Jaszberenyi M, Tomboly C, Toth G, Telegdy G. Behavioral and neuroendocrine actions of endomorphin-2. Peptides. 2001;22:1459–1463. doi: 10.1016/s0196-9781(01)00466-1. [DOI] [PubMed] [Google Scholar]
- Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H, Segal M. Physiology of the CSF and Blood-Brain Barriers. CRC Press; New York: 1996. [Google Scholar]
- Gentry CL, Egleton RD, Gillespie T, Abbruscato TJ, Bechowski HB, Hruby VJ, Davis TP. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20:1229–1238. doi: 10.1016/s0196-9781(99)00127-8. [DOI] [PubMed] [Google Scholar]
- Goldberg IE, Rossi GC, Letchworth SR, Mathis JP, Ryan-Moro J, Leventhal L, Su W, Emmel D, Bolan EA, Pasternak GW. Pharmacological characterization of endomorphin-1 and en-domorphin-2 in mouse brain. J Pharmacol Exp Ther. 1998;286:1007–1013. [PubMed] [Google Scholar]
- Gong J, Strong JA, Zhang S, Yue X, DeHaven RN, Daubert JD, Cassel JA, Yu G, Mansson E, Yu L. Endomorphins fully activate a cloned human mu opioid receptor. FEBS Lett. 1998;439:152–156. doi: 10.1016/s0014-5793(98)01362-3. [DOI] [PubMed] [Google Scholar]
- Hau VS, Huber JD, Campos CR, Lipkowski AW, Misicka A, Davis TP. Effect of guanidino modification and proline substitution on the in vitro stability and blood-brain barrier permeability of endomorphin II. J Pharm Sci. 2002;91:2140–2149. doi: 10.1002/jps.10202. [DOI] [PubMed] [Google Scholar]
- Hosohata K, Burkey TH, Alfaro-Lopez J, Varga E, Hruby VJ, Roeske WR, Yamamura HI. Endomorphin-1 and endomorphin-2 are partial agonists at the human mu-opioid receptor. Eur J Pharmacol. 1998;346:111–114. doi: 10.1016/s0014-2999(98)00117-4. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Fasold MB, Smith RR, Horner KA, Zadina JE. Saturable brain-to-blood transport of endomorphins. Exp Brain Res. 2001;139:70–75. doi: 10.1007/s002210100736. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Fasold MB, Zadina JE. Endomorphins, Met-enkephalin, Tyr-MIF-1, and the P-glycoprotein efflux system. Drug Metab Dispos. 2002;30:231–234. doi: 10.1124/dmd.30.3.231. [DOI] [PubMed] [Google Scholar]
- King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 1) Drug Discov Today. 2001;6:150–156. doi: 10.1016/s1359-6446(00)01632-9. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Suzuki H, Sugiyama Y. The role of P-glycoprotein and canalicular multispecific organic anion transporter in the hepatobiliary excretion of drugs. J Pharm Sci. 1998;87:1025–1040. doi: 10.1021/js970100b. [DOI] [PubMed] [Google Scholar]
- Loe DW, Deeley RG, Cole SP. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer. 1996;32A:945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Maresh GA, Kastin AJ, Brown TT, Zadina JE, Banks WA. Peptide transport system-1 (PTS-1) for Tyr-MIF-1 and Metenkephalin differs from the receptors for either. Brain Res. 1999;839:336–340. doi: 10.1016/s0006-8993(99)01755-2. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Endomorphin-2 is an endogenous opioid in primary sensory afferent fibers. Peptides. 1998;19:1783–1789. doi: 10.1016/s0196-9781(98)00136-3. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- Oude Elferink RP, Zadina J. MDR1 P-glycoprotein transports endogenous opioid peptides. Peptides. 2001;22:2015–2020. doi: 10.1016/s0196-9781(01)00564-2. [DOI] [PubMed] [Google Scholar]
- Pierce TL, Grahek MD, Wessendorf MW. Immunoreactivity for endomorphin-2 occurs in primary afferents in rats and monkey. Neuroreport. 1998;9:385–389. doi: 10.1097/00001756-199802160-00005. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Seetharaman S, Barrand MA, Maskell L, Scheper RJ. Multidrug resistance-related transport proteins in isolated human brain microvessels and in cells cultured from these isolates. J Neurochem. 1998;70:1151–1159. doi: 10.1046/j.1471-4159.1998.70031151.x. [DOI] [PubMed] [Google Scholar]
- Smith RR, Martin-Schild S, Kastin AJ, Zadina JE. Decreases in endomorphin-2-like immunoreactivity concomitant with chronic pain after nerve injury. Neuroscience. 2001;105:773–778. doi: 10.1016/s0306-4522(01)00228-7. [DOI] [PubMed] [Google Scholar]
- Terasaki T, Hosoya K. The blood-brain barrier efflux transporters as a detoxifying system for the brain. Adv Drug Deliv Rev. 1999;36:195–209. doi: 10.1016/s0169-409x(98)00088-x. [DOI] [PubMed] [Google Scholar]
- van der Sandt IC, Gaillard PJ, Voorwinden HH, de Boer AG, Breimer DD. P-glycoprotein inhibition leads to enhanced disruptive effects by anti-microtubule cytostatics at the in vitro blood-brain barrier. Pharm Res. 2001;18:587–592. doi: 10.1023/a:1011016923346. [DOI] [PubMed] [Google Scholar]
- Wang QP, Zadina JE, Guan JL, Kastin AJ, Shioda S. Electron microscopic examination of the endomorphin 2-like immunoreactive neurons in the rat hypothalamus. Brain Res. 2003a;969:126–134. doi: 10.1016/s0006-8993(03)02290-x. [DOI] [PubMed] [Google Scholar]
- Wang QP, Zadina JE, Guan JL, Shioda S. Morphological evidence of endomorphin as an agonist for the mu-opioid receptor in the rat spinal cord. Neurosci Lett. 2003b;341:107–110. doi: 10.1016/s0304-3940(03)00182-4. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, Borst P. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med. 1998;188:797–808. doi: 10.1084/jem.188.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, deLange EC, Scheffer GL, van den Berg DJ, Mol CA, van der Valk M, Schinkel AH, Scheper RJ, Breimer DD, Borst P. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Invest. 2000;105:279–285. doi: 10.1172/JCI8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SR, Boag AH, Valdimarsson G, Hipfner DR, Campling BG, Cole SP, Deeley RG. Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res. 1998;4:2279–2289. [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]