Abstract
Purpose
The purpose of this study is to examine whether physical activity is associated with less insomnia symptoms in the rural communities.
Methods
This study used cross-sectional data collected from a 2005 telephone survey for evaluation of a community walking trails intervention to promote physical activity in rural communities including 6 communities in the Missouri Ozark region and 6 communities in Arkansas and Tennessee (n=1,234). The exposure variable is self-reported regular current physical activity. The outcome includes symptoms of insomnia operationalized as having trouble falling asleep, staying asleep, and waking up too early nearly every day. Logistic regression was used to calculate prevalence odds ratios (PORs) and 95% confidence intervals (95% CI).
Findings
The study sample includes mostly white (95%), married (62%), overweight/obese (61%) women with a high school degree and a mean age of 54. Fourteen percent of participants reported having insomnia symptoms. Self-report of currently being physically active regularly was associated with decreased odds of insomnia symptoms (adjusted POR: 0.37; 95% CI, 0.14-0.99) among participants with under or normal body weight, after controlling for age, gender, education level, marital status, and chronic diseases. There was also a negative linear correlation between the number of days and total minutes of vigorous physical activity and insomnia symptoms.
Conclusions
In these rural communities, we observed a significant relationship between regular physical activity and decreased insomnia symptoms.
Keywords: exercise, insomnia, physical activity, rural communities
Insomnia is the most common of all sleep complaints and affects millions of people each year. Insomnia is characterized as difficulty in initiating and maintaining sleep and early morning waking with an inability to return to sleep.1 The prevalence of insomnia varies by the population being studied and the specific case definition used. Population-based studies drawn from different countries have indicated that approximately 30% of adults in the general population report one or more insomnia symptoms and 5%-10% have been diagnosed with specific insomnia disorders.2
Risk factors for insomnia include older age, female sex, concurrent depressive symptoms, and comorbid medical or psychiatric disorders.3-5 In addition, individuals who are separated or divorced, of black race, lower socioeconomic status, or who report higher chronic life stress also experience increased risk of insomnia.3,4 Insomnia, particularly chronic forms of insomnia, is a major public health problem because it is associated with significant psychological and physical impairment: insomnia increases psychiatric disturbances, decreases work productivity and quality of life, and may increase mortality.1,3 The economic costs of insomnia associated with increased health care use, workplace absenteeism, accidents, and increased alcohol consumption have been estimated to be between $35 billion and $107 billion a year.6
There are a wide range of pharmacologic and non-pharmacologic therapies available for the management of insomnia. The most common treatments are over-the-counter antihistamines, alcohol, and prescription medications with sedative-hypnotic effects, including benzodiazepine receptor agonists and various sedating antidepressants, antihistamines and antipsychotic medications.3,4 Of note, many drugs commonly prescribed for insomnia do not have a Food and Drug indication for treatment of insomnia, but rather, are used “off-label” for their hypnotic properties. Because the use of these medications has been associated with increased mortality, medication tolerance and dependence, and a number of negative side effects, they are seldom recommended for long-term use.7 Therefore, there is increasing interest in non-pharmacologic treatments such as cognitive and behavioral therapies.3 One behavioral therapy is physical activity, which is thought to promote sleep by increasing slow wave sleep and total sleep time, as well as decreasing sleep latency (the duration between initial attempts to fall asleep and onset of sleep).8
Studies based on self reports indicate that moderate and regular physical activity has sleep promoting benefits.8-11 Regular physical activity has been demonstrated to increase total sleep time, slow wave sleep, and rapid eye movement sleep latency, and reduce REM sleep.8,12,13 But evidence from experimental studies on the beneficial effect of physical activity is not as compelling.8,14,15 Discrepancies in the findings of existing studies may be attributed to small sample size,16 varying definitions and measurement of “physical activity,”9,17-19 and inclusion of mostly good or young sleepers, leaving little room for sleep improvement (ie, a ceiling effect).20 In addition, prior studies of physical activity and insomnia have generally refrained from exploring the relationship between the frequency and intensity (eg, moderate or vigorous) of physical activity and insomnia.
Although rural communities have higher rates of chronic illness and disability and poorer overall health status than urban communities,21-25 little is known about sleep patterns in rural populations. Many of the unique demographics and lifestyle characteristics of rural populations have the potential to contribute negatively to health outcomes including insomnia, given existing evidence of rural health disparities in heart disease, physical inactivity, alcohol abuse and tobacco dependence, and psychiatric symptoms.21,24,26
Prior research indicates a link between physical activity and insomnia in the general population. Specifically, regular physical activity may be protective against insomnia.8,14 However, this association is less firmly established among a rural, adult population. Since rural and urban communities vary in their characteristics, the unique rural culture may have bearing on the relationship between physical activity and insomnia.21 Therefore, the objective of our study is to determine the relationship between current regular physical activity and insomnia symptoms in a rural setting. Rural residents fare worse than their urban counterparts in regards to rates of obesity and physical inactivity, and overweight and obesity are associated with short sleep duration and sleep disturbances.27,28 Hence, the present study also evaluated body weight as an effect modifier in the association between current regular physical activity and insomnia symptoms.
Materials and Methods
Study Design
This cross-sectional study was based on data from the Walk the Ozarks to Wellness (WOW) Project, a 4-year, quasi-experimental longitudinal study of an intervention to increase walking behaviors in rural communities. Detailed methods of the intervention are described elsewhere.29 Briefly, the intervention to promote physical activity included individually tailored newsletters, interpersonal activities that stressed social support and health provider counseling, and community-wide events such as “fun” walks. Study participants included individuals from 6 intervention communities in the Missouri Ozark region and 6 comparison communities in Arkansas and Tennessee, matched based on community size, race/ethnicity, and income level. Rural communities were defined as counties having populations less than 50,000 people and population density less than 1,000 people per square mile.30,31 Eligible households were those within a 2-mile radius of walking trails. Using computer-assisted random-digit dialing, a representative cohort was identified using a modified version of the Behavioral Risk Factor Surveillance System (BRFSS) interview protocol in 2003, 2004 and 2005.13,29,32 Data pertinent to this study were based on the third wave of data collection (July to September 2005) because of the inclusion of sleep habits in this phase of data collection. The study sample for the present study included a total of 1,258 non-institutionalized, predominantly non-Hispanic, white English-speaking adults age 18 years or older who completed the third wave of the WOW project survey from the 12 communities in Missouri, Tennessee, and Arkansas. The response rate for the interview was 65.2% as calculated using the method of the Council of American Survey Research Organizations.33 The current study was granted exemption by the Saint Louis University Institutional Review Board.
Measures
The WOW project survey instrument was developed using a combination of questions from the BRFSS, along with questions developed in the study sites.34-39 The survey instrument included 114 questions and the survey took approximately 30 minutes to complete. Trained interviewers administered the survey by telephone during the study period.
The primary exposure of interest for the present study was a binary status indicator of current regular physical activity. Current regular physical activity was assessed with a yes/no question, “I currently engage in regular physical activity.” “Regular” was defined as exercise for a total of 30 minutes per day and at least 5 days a week. In a secondary analysis, we also investigated the relationship between the intensity of physical activity and insomnia symptoms. Intensity and frequency of physical activity was measured by asking subjects the number of days per week they did moderate or vigorous physical activities. “Moderate” physical activities included brisk walking, bicycling, vacuuming, gardening, or anything causing small increases in breathing or heart rate whereas “vigorous” physical activities included running, aerobics, heavy yard work, or anything else causing large increases in breathing or heart rate. Most physical activity survey items from the WOW project have been evaluated for test-retest reliability.29,40 Yore and colleagues reported that test–retest reliability was 0.35–0.53 for moderate activity and 0.80–0.86 for vigorous activity.40
The outcome of interest for the present study was self-reported symptoms of insomnia. The following 3 questions were used to ascertain the frequency of insomnia symptoms subjects experienced each month: 1) “Having trouble falling asleep;” 2) “Wake up during the night and have difficulty getting back to sleep;” and 3) “Wake up too early in the morning and are unable to get back to sleep.” Insomnia was considered present if the subject reported “often (5-15 times a month)” or “almost always (16-30 times a month)” for all 3 of the symptoms described above. These questions are commonly used in population-based studies of insomnia symptoms.41,42 Body mass index (BMI), calculated as weight (kilograms) per height (square meters), was categorized as underweight (BMI, < 18.5 kg/m2), normal weight (BMI, 18.5 – 24.9 kg/m2), overweight (BMI, 25 – 29.9 kg/m2), or obese (BMI, ≥ 30 kg/m2). A binary indicator of BMI (underweight/normal weight vs overweight/obese) was also created to test weight as an effect modifier in the exposure-outcome relationship. Factors potentially associated with physical activity and insomnia symptoms were evaluated as potential confounders, including age, gender, marital status, education levels, and self-reported chronic disease history. Self-reported chronic disease was characterized by answering yes to any of the following conditions: heart disease, kidney disease, diabetes, high blood sugar, hypertension, elevated cholesterol, or arthritis; or self-reported history of cancer.
Statistical Analyses
Differences in sample characteristics by regular physical activity status were assessed by using the Pearson Chi-square test (χ2) for categorical variables and t-test for continuous variables. Crude prevalence odds ratios (cPOR) and 95% confidence intervals (95% CI) for the relationship between current regular physical activity status and insomnia symptoms were calculated using a simple logistic regression model. Multivariate binary logistic regression models were used to estimate adjusted prevalence odds ratios (aPOR) and 95% CI for symptoms of insomnia controlling for potential confounders, including age, gender, marital status, education, and a composite indicator of chronic diseases. To evaluate if the binary BMI is an effect modifier, the Wald test was used to test if the regression coefficient of the interaction of recurrent regular physical activity and BMI was statistically different from zero. The aforementioned multivariable analysis was repeated in a sub-analysis including only subjects in the non-intervention group (n=657) to address the potential confounding effect of physical activity promotion media intervention on physical activity levels among participants. Binary multivariable logistic regression was also performed to evaluate the relationship between intensity of moderate or vigorous physical activity and insomnia symptoms. Due to the possibility of correlated data, all models were adjusted for potential clustering. All tests were 2-tailed and P < .05 was considered significant. All statistical analyses were performed with STATA (version 10.0, STATA Corp, College Station, Texas).
Results
The characteristics of study participants are summarized by their current regular physical activity status in Table 1. Compared to individuals who reported no current regular physical activity, subjects who reported currently engaging in regular physical activity had less frequent insomnia symptoms, higher education levels, were more likely to be normal weight or overweight, and were more likely to self-report a chronic disease. In bivariate analysis, we observed that engagement in current regular physical activity was associated with decreased odds of insomnia symptoms by 48% (cPOR: 0.52, 95% CI: 0.37-0.74) relative to subjects who reported no current regular physical activity (data not shown).
Table 1. Sample Characteristics: Rural Midwest, 2005 (n=1,258).
| Characteristics | Engaged in regular physical activity currently | P value | |
|---|---|---|---|
|
| |||
| Yes (n = 746) n(%) | No (n=512) n(%) | ||
| Insomnia | 78 (45.4) | 94 (54.7) | < .0001 |
| Gender (female) | 563 (58.2) | 404 (41.8) | .16 |
| Education level | <.0001 | ||
| < High school | 64 (42.1) | 88 (57.9) | |
| High school | 229 (60.6) | 149 (39.4) | |
| Some college | 199 (62.0) | 122 (38.0) | |
| ≥ college | 253 (62.5) | 152 (37.5) | |
| Marital status (unmarried) | 288 (60.1) | 191 (39.9) | .64 |
| Age mean (SD) | 54.9 (16.32) | 55.6 (15.59) | .43 |
| BMI | < .0001 | ||
| Normal | 337 (69.9) | 145 (30.1) | |
| Overweight | 246 (62.4) | 148 (37.6) | |
| Obese | 154 (42.4) | 209 (57.6) | |
| Chronic diseasesa | 502 (55.0) | 410 (45.0) | < .0001 |
self-reported history of any chronic disease by answering yes to any of the following conditions: heart disease, kidney disease, diabetes, high blood sugar, hypertension, cholesterol, or arthritis; and self-reported history of cancer
Because of the differences in the distribution of risk factors between the 2 study groups by regular physical activity status, we conducted multivariable analysis adjusting for variables potentially associated with outcomes of interest. In our multivariable analysis, we included the interaction of current regular physical activity and a binary BMI variable to evaluate BMI as an effect modifier in the association between current regular physical activity and insomnia. Because the regression coefficient of this interaction was statistically different from zero (P value = .04 for the Wald test), indicating that the association between current regular physical activity and insomnia symptoms varies by BMI, we subsequently stratified our multivariable analysis by BMI.
Among individuals with underweight or normal body weight, currently engaging in regular physical activity was associated with a 63% reduction (aPOR: 0.37, 95% CI: 0.14-0.99) in the likelihood of having insomnia symptoms compared to not having current regular physical activity, after controlling for gender, age, marital status, education levels, and a composite index of chronic diseases (Table 2). Among overweight or obese participants, engaging in current regular physical activity was also associated with decreased likelihood of insomnia symptoms; however, it was not statistically significant (Table 2). When the analysis sample was restricted to non-intervention group only, the association between current regular physical activity and insomnia symptoms remained significant and the effect was increased slightly (aOR: 0.15, 95% CI: 0.09-0.24, Table 2a) for under and normal weight subjects. For overweight/obese subjects, the association between physical activity and insomnia symptoms remained not significant. In Table 3, we detail the association between current regular physical activity and each individual insomnia symptom. Specifically, among subjects with under or normal weight, engaging in current regular physical activity was associated with reduced odds of difficulty falling asleep, continuity disturbances, and early morning awakening by 61%, 47%, and 54%, respectively, after controlling for confounders (Table 3). Among subjects who were overweight or obese, engaging in current regular physical activity was associated with reduced odds of continuity disturbances by 30% (Table 3).
Table 2. Adjusted Odds Ratio of Insomnia Stratified by Body Weight, Rural Midwest, 2005 (n=1,234).
| Characteristics | Underweight/Normalc (N=478) | Overweight/Obese (N=756) | ||
|---|---|---|---|---|
|
| ||||
| aPORa | 95% CI | aPOR | 95% CI | |
| Regular physical activity | 0.37 | (0.14-0.99) | 0.76 | (0.51-1.14) |
| Gender (1 = female) | 2.36 | (0.97-5.74) | 1.17 | (0.59-2.31) |
| Age | 0.99 | (0.96-1.01) | 0.98 | (0.97-1.00) |
| Marital status (1 = unmarried) | 1.88 | (1.30-2.74) | 1.03 | (0.62-1.71) |
| Education | ||||
| Less than high school | 4.37 | (1.65-11.56) | 5.76 | (2.72-12.21) |
| High school graduate | 1.56 | (0.99-2.47) | 3.05 | (2.25-4.13) |
| Some college | 1.14 | (0.70-1.86) | 3.27 | (2.01-5.33) |
| College and greater | reference | reference | reference | reference |
| Chronic diseasesb | 2.47 | (0.42-14.56) | 2.07 | (1.38-3.12) |
aPOR: adjusted prevalence odds ratio; 95% CI: 95% confidence interval
self-reported history of chronic disease by answering yes to any of the following conditions: heart disease, kidney disease, diabetes, high blood sugar, hypertension, cholesterol, or arthritis; and self-reported history of cancer
Interaction term of regular physical activity and BMI P value = .04; the significant interaction term was used to justify the stratified analysis.
Table 2a. Adjusted Odds Ratio of Insomnia Stratified by Body Weight Among Subjects in the Non-Intervention Group, Rural Midwest, 2005 (n=647).
| Characteristics | Underweight/Normalc (N=282) | Overweight/Obese (N=365) | ||
|---|---|---|---|---|
|
| ||||
| aPORa | 95% CI | aPOR | 95% CI | |
| Regular physical activity | 0.15 | (0.09-0.24) | 0.61 | (0.26-1.44) |
| Gender (1 = female) | 2.71 | (0.88-8.33) | 0.68 | (0.26-1.78) |
| Age | 1.00 | (0.96-1.03) | 1.00 | (0.96-1.03) |
| Marital status (1 = unmarried) | 2.27 | (1.36-3.80) | 1.19 | (0.49-2.93) |
| Education | ||||
| Less than high school | 4.43 | (0.84-23.40) | 4.17 | (0.84-20.77) |
| High school graduate | 1.67 | (0.56-5.00) | 3.40 | (1.85-6.25) |
| Some college | 0.81 | (0.21-3.15) | 3.41 | (1.66-7.00) |
| College and greater | reference | reference | reference | reference |
| Chronic diseasesb | 1.02 | (0.17-6.32) | 1.59 | (0.71-3.57) |
aPOR: adjusted prevalence odds ratio; 95% CI: 95% confidence interval
self-reported history of chronic disease by answering yes to any of the following conditions: heart disease, kidney disease, diabetes, high blood sugar, hypertension, cholesterol, or arthritis; and self-reported history of cancer
Interaction term of regular physical activity and BMI P value < .05; the significant interaction term was used to justify the stratified analysis.
Table 3. Adjusted Odds Ratio of the Effect of Regular Physical Activity on Individual Insomnia Symptom Stratified by Body Weight, Rural Midwest, 2005 (n=1,234).
| Characteristics | Underweight/Normal (N=478) | Overweight/Obese (N=756) | ||
|---|---|---|---|---|
|
| ||||
| aPORa | 95% CI | aPOR | 95% CI | |
| Difficulty falling asleep | 0.39 | (0.24-0.64) | 0.86 | (0.71-1.05) |
| Sleep continuity disturbances | 0.53 | (0.30- 0.94) | 0.70 | (0.58-0.84) |
| Early morning awakening | 0.46 | (0.18-0.14) | 0.83 | (0.60-1.15) |
aPOR: adjusted prevalence odds ratio; 95% CI: 95% confidence interval, controlling for age, gender, marital status, education, and a composite indicator of chronic diseases.
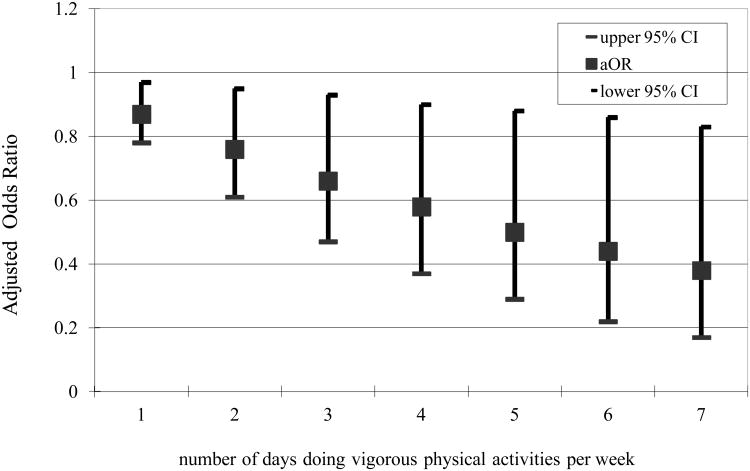
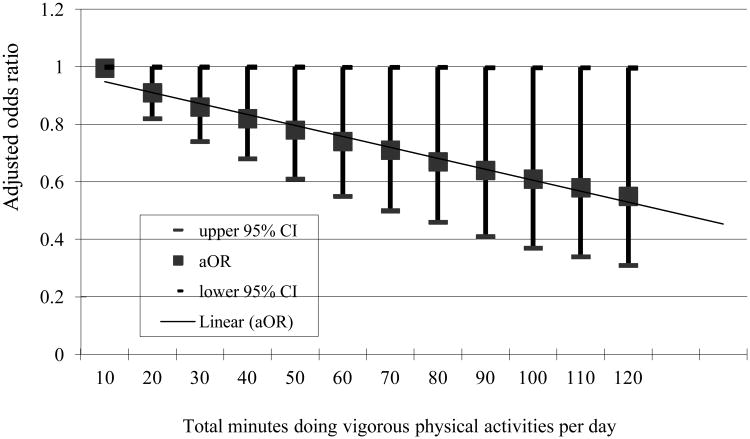
In Figure 1 and 2, we showed the relationship between intensity and frequency of physical activity and insomnia symptoms. The intensity and the volume of physical activity were operationalized by the number of days per week and number of minutes per day subjects reported doing moderate or vigorous physical activities. Moderate physical activity was not associated with reduced odds of insomnia symptoms in multivariable analyses. On the contrary, vigorous physical activity was associated with reduced odds of having symptoms of insomnia, after adjusting for gender, age, marital status, education level, and chronic diseases (Figures 1 and 2). Specifically, the more days per week or total minutes per day one was engaged in vigorous physical activities, the lower the likelihood of having insomnia symptoms.
Figure 1. Adjusted odds ratio for insomnia by the number of days subject doing vigorous physical activities per week (n=1,234).
Figure 2. Adjusted odds ratio for insomnia by total time subjects doing vigorous physical activities per day on days when subjects do vigorous physical activities (n=1,234).
Discussion
Our study observed that physical activity reduced insomnia symptoms. In addition, body weight modified the association between current regular physical activity and insomnia symptoms, with physical activity being beneficial mostly among participants with underweight or with normal body weight. We also observed that regular physical activity among overweight or obese individuals was associated with reduced odds of sleep continuity disturbances. Insomnia is a heterogeneous disorder with a wide range of underlying causes.43 The negative finding among overweight and obese subjects was unexpected. It may be due to residual confounding from lack of information on comorbid condition of other sleep disorders such as obstructive sleep apnea syndrome, which is more prevalent among overweight and obese individuals.44 Some reported insomnia symptoms from our study subjects indicate that they may have veiled sleep disorders. In addition, higher levels of psychological stress may also play a role in the reported insomnia symptoms in our rural study population, but this data was not collected in the present study.
Previous epidemiologic studies have suggested that physical activity promotes improved sleep quality and sleep efficiency.8-11 The hypothetical mechanisms for physical activity being beneficial to sleep have been driven by the energy conservation, body tissue restoration, and thermoregulation theories of sleep.45 It has been suggested that physical activities increase central nervous system temperature, which results in changes in sleep architecture.46 Although moderate or vigorous physical activity performed close to bedtime can prolong latency to sleep onset,47 physical activity may also have benefits for sleep through its anti-anxiety and antidepressant effects, and its tendency to raise levels of adenosine.48
Despite the general belief that physical activity promotes sleep, prior research on the effect of physical activity on sleep has presented conflicting results. In a meta analysis, Youngstedt and associates reported an average increase in total sleep time of 10 minutes in normal volunteers after exercise.15 In an experimental study of patients with insomnia, Guilleminault and colleagues compared the effects of nondrug sleep hygiene therapy in combination with either light therapy or moderate physical activity, but only sleep hygiene with light therapy showed statistically significant improvement in patients' sleep.49 To the contrary, acute, moderate intensity aerobic exercise has been observed to improve sleep in patients with chronic insomnia. Specifically, moderate intensity aerobic exercise reduced sleep onset latency and total wake time after sleep onset, and it increased total sleep time and sleep efficiency based on objective polysomnography data.50 In an older population, Morgan found that higher levels of physical activity were protective against incident and chronic insomnia.51 However, Youngstedt and associates reported no association between self-reported sleep and total daily physical activity among physically active, healthy college volunteers who were mostly good sleepers.52
The negative findings from prior research may be confounded by the good sleeper ceiling effect.20 Differences in findings from prior research may also be due to the different definitions and the frequency and intensity of physical activity used in each study. The lack of association in moderate physical activity and insomnia symptoms observed in the present study were in line with the findings of Guilleminault and colleagues, but they were in contrast to those from Passos and associates. Nevertheless, the differences in study designs preclude a direct comparison with our study findings.
Few research studies have investigated the effect of the frequency and intensity of physical activity on sleep or insomnia. In a meta-analysis, the duration of physical activity was one of the most important factors modulating the effect of exercise on sleep.15 Specifically, as the duration of physical activity increased to more than 1 hour per day, total sleep time became progressively greater.15 In the present study, we observed an inverse linear correlation between the number of days per week as well as the total minutes per day of vigorous physical activity and insomnia symptoms. The greater the number of days and total minutes participants engaged in vigorous physical activity, the lower the odds of reported insomnia symptoms. However, the protective effect of vigorous physical activity on insomnia symptoms from our analyses is smaller than what has been observed in the literature.15 This may result from recall bias in the duration and intensity of physical activity in the present study.
Our finding that moderate physical activity was not significantly associated with insomnia symptoms was unexpected. We believe the observed negative findings may be a result of the lack of variation in moderate physical activity levels in our study sample due to the physical activity promotion media intervention. Vigorous physical activity levels were significantly lower among subjects with insomnia symptoms compared to those without symptoms, regardless of intervention status. To the contrary, levels of moderate physical activity were lower among subjects with insomnia symptoms in the non-intervention group whereas in the intervention group, high levels of moderate physical activity were observed in subjects with insomnia symptoms. However, neither observation for moderate physical activity and insomnia symptoms reached statistical significance.
Study Strengths and Limitations
A major strength of our study was the large population-based sample in a unique population and the evaluation of body weight as an effect modifier. In addition, we were able to delineate the association between the frequency and intensity of physical activity and insomnia symptoms. There are also some noteworthy limitations in our study. Data from the present study were based on self report. The potential non-differential misclassification of the measures may have biased our estimated effect of physical activity toward the null value. This measurement error may partially explain the lack of association among overweight/obese individuals. The cross-sectional study design also limits our ability to make causal inferences from our study findings. Our study sample was drawn from a rural population, providing additional insight into the relationship between physical activity and insomnia symptoms in a unique population. However, our study findings may not be generalizable to urban or suburban populations, or to other rural populations such as rural Southerners. We also acknowledge the lack of assessment of daytime sleepiness, fatigue or daytime dysfunction in our insomnia measurement. Furthermore, we were unable to control for other relevant potential confounders such as time of day of physical activity.
Conclusion
There have been few studies of sleep and sleep disorders in a rural population. We found that insomnia is common in this Midwestern rural population. Insomnia is associated with substantial medical, psychiatric, personal, and societal consequences.1 It adversely affects individuals' quality of life from comorbid conditions and can lead to impaired interpersonal relationships.1 Insomnia has been associated with depressive symptoms, anxiety and pain in a rural population.5 In our study, regular physical activity was associated with fewer insomnia symptoms in this rural population. Considering physical activity as a possible treatment alternative for sleep problems is appealing due to its low cost, easy access, and few adverse side effects. Objective measurement of intensity and duration of physical activity in randomized trials of physical activity for treatment of insomnia are needed to better understand the relationship between physical activity and insomnia. Nevertheless, given our findings and prior epidemiologic and experimental evidence, physicians and health care professionals can suggest regular physical activity, particularly vigorous physical activity, as a means of improving sleep to individuals with symptoms of insomnia.
Acknowledgments
This study was supported by the National Institutes of Health grant NIDDK #5 R18 DK061706 and Cooperative Agreement Number U48/DP000060 from the Centers for Disease Control and Prevention, Prevention Research Centers Program. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
There is no conflict of interest among all authors related to this study.
References
- 1.Mai E, Buysse DJ. Insomnia: Prevalence, Impact, Pathogenesis, Differential Diagnosis, and Evaluation. Sleep Med Clin. 2008;3(2):167–174. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999 May 1;22(Suppl 2):S347–353. [PubMed] [Google Scholar]
- 3.National Institute of Health. Manifestations and Management of Chronic Insomnia in Adults. NIH Consensus Statement. 2005 Jun 13-15;22(2) [Google Scholar]
- 4.Buysse DJ. Chronic insomnia. Am J Psychiatry. 2008 Jun;165(6):678–686. doi: 10.1176/appi.ajp.2008.08010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartz AJ, Daly JM, Kohatsu ND, Stromquist AM, Jogerst GJ, Kukoyi OA. Risk factors for insomnia in a rural population. Ann Epidemiol. 2007 Dec;17(12):940–947. doi: 10.1016/j.annepidem.2007.07.097. [DOI] [PubMed] [Google Scholar]
- 6.Chilcott LA, Shapiro CM. The socioeconomic impact of insomnia. An overview. Pharmacoeconomics. 1996;10(Suppl 1):1–14. doi: 10.2165/00019053-199600101-00003. [DOI] [PubMed] [Google Scholar]
- 7.National Institutes of Health. Drugs and Insomnia: The Use of Medications to Promote Sleep NIH Consensus Development Conference Statement. 1983 Nov 15-17;4(10) [Google Scholar]
- 8.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000 Aug;4(4):387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 9.Sherrill DL, Kotchou K, Quan SF. Association of physical activity and human sleep disorders. Arch Intern Med. 1998 Sep 28;158(17):1894–1898. doi: 10.1001/archinte.158.17.1894. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Sun Z, Uchiyama M, Shibui K, Kim K, Okawa M. Prevalence and correlates of sleep problems in Chinese schoolchildren. Sleep. 2000 Dec 15;23(8):1053–1062. [PubMed] [Google Scholar]
- 11.Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000 Feb 1;23(1):41–47. [PubMed] [Google Scholar]
- 12.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005 Apr;24(2):355–365. xi. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Remington PL, Smith MY, Williamson DF, Anda RF, Gentry EM, Hogelin GC. Design, characteristics, and usefulness of state-based behavioral risk factor surveillance: 1981-87. Public Health Rep. 1988 Jul-Aug;103(4):366–375. [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor PJ, Youngstedt SD. Influence of exercise on human sleep. Exerc Sport Sci Rev. 1995;23:105–134. [PubMed] [Google Scholar]
- 15.Youngstedt SD, O'Connor PJ, Dishman RK. The effects of acute exercise on sleep: a quantitative synthesis. Sleep. 1997 Mar;20(3):203–214. doi: 10.1093/sleep/20.3.203. [DOI] [PubMed] [Google Scholar]
- 16.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997 Jan 1;277(1):32–37. [PubMed] [Google Scholar]
- 17.Ohayon MM, Zulley J, Guilleminault C, Smirne S, Priest RG. How age and daytime activities are related to insomnia in the general population: consequences for older people. J Am Geriatr Soc. 2001 Apr;49(4):360–366. doi: 10.1046/j.1532-5415.2001.49077.x. [DOI] [PubMed] [Google Scholar]
- 18.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001 Jun 15;24(4):425–430. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997 Jan;45(1):1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 20.Youngstedt SD. Ceiling and floor effects in sleep research. Sleep Med Rev. 2001 Feb;5(1):79–81. doi: 10.1053/smrv.2000.0137. [DOI] [PubMed] [Google Scholar]
- 21.Hartley D. Rural health disparities, population health, and rural culture. Am J Public Health. 2004 Oct;94(10):1675–1678. doi: 10.2105/ajph.94.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers JR. National surveillance of occupational fatalities in agriculture. Am J Ind Med. 1990;18(2):163–168. doi: 10.1002/ajim.4700180208. [DOI] [PubMed] [Google Scholar]
- 23.Quality Through Collaboration: The Future of Rural Health Care. The National Academies Press; 2005. Care CoTFoRH. [Google Scholar]
- 24.Eggebeen DJ, Lichter DT. Health and well-being among rural Americans: variations across the life course. The Journal of rural health: official journal of the American Rural Health Association and the National Rural Health Care Association. 1993 Spring;9(2):86–98. doi: 10.1111/j.1748-0361.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow N, Beale CL. Rural elderly in demographic perspectives. Rural Development Perspectives. 1985;2(1):22–26. [Google Scholar]
- 26.Probst JC, Laditka SB, Moore CG, Harun N, Powell MP, Baxley EG. Rural-urban differences in depression prevalence: implications for family medicine. Fam Med. 2006 Oct;38(9):653–660. [PubMed] [Google Scholar]
- 27.Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. The Journal of rural health: official journal of the American Rural Health Association and the National Rural Health Care Association. 2004 Spring;20(2):151–159. doi: 10.1111/j.1748-0361.2004.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Bixler EO, Chrousos GP, Pejovic S. Obesity and sleep disturbances: meaningful sub-typing of obesity. Archives of physiology and biochemistry. 2008 Oct;114(4):224–236. doi: 10.1080/13813450802521507. [DOI] [PubMed] [Google Scholar]
- 29.Brownson RC, Hagood L, Lovegreen SL, Britton B, Caito NM, Elliott MB, Emery J, Haire-Joshu D, Hicks D, Johnson B, McGill JB, Morton S, Rhodes G, Thurman T, Tune D. A multilevel ecological approach to promoting walking in rural communities. Prev Med. 2005 Nov-Dec;41(5-6):837–842. doi: 10.1016/j.ypmed.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of the Census. Urban and Rural Definitions. 1995 Oct 10; http://www.census.gov/population/censusdata/urdef.txt.
- 31.Cromartie J, B S. Defining the “Rural” in Rural America. Amber Waves. 2008 [October 10, 2010]., http://www.ers.usda.gov/AmberWaves/June08/Features/RuralAmerica.htm.
- 32.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, Marks JS, Trowbridge FL. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985 Nov-Dec;1(6):9–14. [PubMed] [Google Scholar]
- 33.CASRO Task Force on Completion Rates. Special Report. Council of American Survey Research Organizations; New York: 1982. On the definitions of response rates. [Google Scholar]
- 34.Saelens BE, Sallis JF, Frank LD. Environmental correlates of walking and cycling: findings from the transportation, urban design, and planning literatures. Ann Behav Med. 2003 Spring;25(2):80–91. doi: 10.1207/S15324796ABM2502_03. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth BE, Bassett DR, Jr, Strath SJ, Swartz AM, O'Brien WL, Thompson RW, Jones DA, Macera CA, Kimsey CD. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S457–464. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kirtland KA, Porter DE, Addy CL, Neet MJ, Williams JE, Sharpe PA, Neff LJ, Kimsey CD, Jr, Ainsworth BE. Environmental measures of physical activity supports: perception versus reality. Am J Prev Med. 2003 May;24(4):323–331. doi: 10.1016/s0749-3797(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 37.Brownson RC, Eyler AA, King AC, Shyu YL, Brown DR, Homan SM. Reliability of information on physical activity and other chronic disease risk factors among US women aged 40 years or older. Am J Epidemiol. 1999 Feb 15;149(4):379–391. doi: 10.1093/oxfordjournals.aje.a009824. [DOI] [PubMed] [Google Scholar]
- 38.Brownson RC, Baker EA, Housemann RA, Brennan LK, Bacak SJ. Environmental and policy determinants of physical activity in the United States. Am J Public Health. 2001 Dec;91(12):1995–2003. doi: 10.2105/ajph.91.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoehner CM, Brennan Ramirez LK, Elliott MB, Handy SL, Brownson RC. Perceived and objective environmental measures and physical activity among urban adults. Am J Prev Med. 2005 Feb;28(2 Suppl 2):105–116. doi: 10.1016/j.amepre.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Yore MM, Ham SA, Ainsworth BE, Kruger J, Reis JP, Kohl HW, 3rd, Macera CA. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007 Aug;39(8):1267–1274. doi: 10.1249/mss.0b013e3180618bbe. [DOI] [PubMed] [Google Scholar]
- 41.Baldwin CM, Ervin AM, Mays MZ, Robbins J, Shafazand S, Walsleben J, Weaver T. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010 Apr 15;6(2):176–183. [PMC free article] [PubMed] [Google Scholar]
- 42.Silva GE, An MW, Goodwin JL, Shahar E, Redline S, Resnick H, Baldwin CM, Quan SF. Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: the Sleep Heart Health Study (SHHS) Sleep. 2009 Aug 1;32(8):1049–1057. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buysse DJ, Reynolds CF, 3rd, Kupfer DJ, Thorpy MJ, Bixler E, Manfredi R, Kales A, Vgontzas A, Stepanski E, Roth T, et al. Clinical diagnoses in 216 insomnia patients using the International Classification of Sleep Disorders (ICSD), DSM-IV and ICD-10 categories: a report from the APA/NIMH DSM-IV Field Trial. Sleep. 1994 Oct;17(7):630–637. doi: 10.1093/sleep/17.7.630. [DOI] [PubMed] [Google Scholar]
- 44.Lindberg E, Taube A, Janson C, Gislason T, Svardsudd K, Boman G. A 10-year follow-up of snoring in men. Chest. 1998 Oct;114(4):1048–1055. doi: 10.1378/chest.114.4.1048. [DOI] [PubMed] [Google Scholar]
- 45.Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav. 2007 Feb 28;90(2-3):229–235. doi: 10.1016/j.physbeh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horne JA. The effects of exercise upon sleep: a critical review. Biol Psychol. 1981 Jun;12(4):241–290. doi: 10.1016/0301-0511(81)90001-6. [DOI] [PubMed] [Google Scholar]
- 47.Browman CP, Tepas DI. The effects of presleep activity on all-night sleep. Psychophysiology. 1976 Nov;13(6):536–540. doi: 10.1111/j.1469-8986.1976.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 48.Youngstedt SD. The exercise -sleep mystery. international Journla of Sport Psychology. 2000;30:241–255. [Google Scholar]
- 49.Guilleminault C, Clerk A, Black J, Labanowski M, Pelayo R, Claman D. Nondrug treatment trials in psychophysiologic insomnia. Arch Intern Med. 1995 Apr 24;155(8):838–844. [PubMed] [Google Scholar]
- 50.Passos GS, Poyares D, Santana MG, Garbuio SA, Tufik S, Mello MT. Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med. 2010 Jun 15;6(3):270–275. [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan K. Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003 Sep;12(3):231–238. doi: 10.1046/j.1365-2869.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 52.Youngstedt SD, Perlis ML, O'Brien PM, Palmer CR, Smith MT, Orff HJ, Kripke DF. No association of sleep with total daily physical activity in normal sleepers. Physiol Behav. 2003 Mar;78(3):395–401. doi: 10.1016/s0031-9384(03)00004-0. [DOI] [PubMed] [Google Scholar]