Abstract
The objective of this study was to test a comprehensive model of biologic (pubertal status), family (communication and conflict), and psychological influences (behavioral autonomy) on diabetes management and glycemic control in a sample of youth (N = 226) with type 1 diabetes recruited during late childhood/early adolescence (ages 9–11 years). The study design was a prospective, multisite, multi-method study involving prediction of diabetes management and glycemic control 1 year post-baseline. The primary outcome measures included diabetes management behaviors based on the Diabetes Self-Management Profile (DSMP) administered separately to mothers and youth and glycemic control measured by glycated hemoglobin (HbA1c) obtained by blood samples and analyzed by a central laboratory to ensure standardization. Our hypothesized predictive model received partial support based on structural equation modeling analyses. Family conflict predicted less adequate glycemic control 1 year later (p < 0.05). Higher conflict predicted less adequate diabetes management and less adequate glycemic control. More advanced pubertal status also predicted less adequate glycemic control, but behavioral autonomy did not. Family conflict is an important, potentially clinically significant influence on glycemic control that should be considered in primary and secondary prevention in the management of type 1 diabetes in youth.
Keywords: Type 1 diabetes, Adherence, Self-management, Pediatrics, Glycemic control
Predicting diabetes management and glycemic control in youth with type 1 diabetes
The importance of adaptive self-management and treatment adherence in pediatric type 1 diabetes in improving glycemic control and reducing future complications is well recognized (Silverstein et al., 2005). The failure to achieve adequate glycemic control can have substantial consequences on long-term health outcomes and contribute to the development of early complications in multiple organ systems (DCCT, 1994; DCCT/EDIC Research Group, 2009). Moreover, improvement of glycemic control by as little as 1 % can result in significant risk reduction for future diabetes-related complications (DCCT, 1994; DCCT/EDIC Research Group, 2009). Nevertheless, a large body of research (Williams et al., 2009) and experiences in clinical care (Danne et al., 2001) have indicated that it is very difficult for children and adolescents with type 1 diabetes to manage their conditions in ways that sustain adequate treatment adherence and recommended levels of glycemic control.
Significant declines in the quality of diabetes management, treatment adherence, and glycemic control have been observed as children reach adolescence (Jacobsen et al., 1994, 1997; Kovacs et al., 1992; Morris et al., 1997). Moreover, diabetes management patterns that disrupt glycemic control can be established and sustained during early to mid-adolescence (Kovacs et al., 1992). Once established, these patterns may be very difficult to change, even with state-of-the-art behavioral intervention (Bryden et al., 2001). For example, Hampson et al.’s, (2001) meta-analysis of behavioral interventions with adolescents with type 1 diabetes reported negligible effects on metabolic control. A more recent meta-analysis found only a small effect of behavioral interventions on glycemic control for school-age children and adolescents with pediatric type 1 diabetes (Graves et al., 2010). Such data have indicated that more powerful interventions need to be developed to improve glycemic control. One way to enhance the impact of behavioral interventions on glycemic control is to target specific variables that have been shown to influence pediatric diabetes management and glycemic control during key developmental transitions (e.g., the onset of adolescence) in which glycemic control has been found to deteriorate. One of the critical issues in designing interventions is the need to strengthen the potential efficacy of interventions by tailoring them to modifiable risk factors that predict diabetes management and glycemic control during key transitions. These risk factors have not been established in available research.
For this reason, identification of factors that influence the course of treatment adherence and glycemic control among adolescents with type 1 diabetes over time are critical to the design of effective interventions with this population. However, it is well recognized that the negative changes in glycemic control that occur during adolescence reflect complex, multifactorial (including biologic and psychological) influences that occur at multiple levels (individual and family). Relevant biologic influences that include the influence of puberty on insulin resistance (Amiel et al., 1986; Moran et al., 2002); and need to be considered in studies of psychological and family influences on glycemic control.
Psychological influences involve developmental and family processes that disrupt diabetes management (Wysocki et al., 2006; Wysocki, 1993). For example, family level factors such as parental monitoring of diabetes management (Berg et al., 2008), involvement and emotional warmth (Anderson et al., 1997; Lewin et al., 2006), low conflict (Ingerski et al., 2010), and adaptive allocation of parent and child responsibilities for diabetes care (Anderson et al., 2002; Wysocki et al., 2006) have been shown to relate to better diabetes management and lower glycemic control in children and adolescents with type 1 diabetes.
One developmental process that may be important for adaptive self-management and glycemic control for adolescents with type 1 diabetes is the progression to behavioral autonomy in diabetes-related management (Dashiff & Bartolucci, 2002; Dashiff et al., 2008; Palmer et al., 2004; Wysocki et al., 2006). As children with type 1 diabetes reach adolescence, they assume increasing autonomy in managing their diabetes (Wysocki et al., 2006). Developmental progression toward autonomous and effective diabetes management may depend upon several factors, especially family influences. Cross-sectional research has suggested that autonomy in diabetes management is supported by a climate of positive family communication (Anderson et al., 2002) and disrupted by family conflict and criticism (Anderson & Coyne, 1991, 1993; Duke et al., 2008).
However, with some exceptions (Palmer et al., 2011) previous studies have not tested the interrelationships among specific family influences, behavioral autonomy, and glycemic control in a sample of early adolescents in the context of prospective, multisite study design, with large samples with multiple methods of assessment and informants. For example, the cross-sectional designs of most studies have limited causal inference. Many studies have been based on relatively small samples gathered at a single site, thus reducing their generalizability. Researchers have generally not used multiple methods of assessment (e.g., self-report, observational, and biomarker measures) in a single study. Finally, previous research has usually involved heterogeneous samples of adolescents across a wide age span, which makes it difficult to characterize influences on diabetes management and glycemic control during key developmental transitions, such as the transition to adolescence. Taken together, these methodological problems limit the scientific contribution of a number of previous studies from being able to make causal inferences and generalize results to youth who are undergoing the transition to adolescence.
To address these gaps in scientific knowledge, the present study was designed to test a comprehensive predictive model of biologic, family, and psychological influences on glycemic control using a prospective (1 year), multisite design. We studied a homogeneous (by age) sample of youth with type 1 diabetes recruited during late childhood/early adolescence (ages 9–11 years) in order to identify the influences that enhance or disrupt diabetes management and glycemic control as these youth transition to adolescence. Study methods included a biomarker (glycemic control), pubertal status, observational, and self-report measures based on multiple informants (mothers and youth). We tested a mediational model that focused on critical family factors: communication and conflict as primary predictor variables, youth autonomy and quality of diabetes management as mediators, pubertal status as a covariate, to predict glycemic control at 1 year following baseline assessment. The conceptual model that guided our work was based on theory and research and included three domains of potential influences on diabetes management: (1) family contextual, (2) individual psychological, and (3) biologic influences. Pubertal status was included as a primary covariate in the model based on research that has consistently underscored the impact of puberty in triggering insulin resistance (Amiel et al., 1986; Moran et al., 2002) that would be expected to reduce the effects of family context and psychological influences on the efficacy of diabetes management as it affects glycemic control.
We hypothesized that adaptive or positive family communication and low levels of family conflict would be associated with higher diabetes-related autonomy, which was expected to influence the level of diabetes management and glycemic control. We tested youth’s behavioral autonomy as a mediator between family conflict, support, and level of diabetes management and glycemic control (Anderson et al., 2002). Previous research has also suggested an alternative model of influence of family conflict (e.g., that conflict engenders stress that has a direct effect in decreasing the level of glycemic control) (Hanson et al., 1987). For this reason, we tested whether family conflict had a direct association with glycemic control.
Methods
Participants and procedures: baseline
Participants were youth with type 1 diabetes and their maternal caregivers who were followed at pediatric diabetes clinics at three university affiliated medical centers in the United States. Each site’s Institutional Review Boards approved the study. Data were collected as part of an ongoing, three-year longitudinal study. For the purpose of the present analysis, baseline predictors of 1 year outcome data were considered. Reports of baseline data are described in McNally et al. (2010), Rohan et al. (2011), and Hilliard et al. (2011). This is the first report from this study that focused on the prediction of glycemic control at 1 year post baseline.
Caregivers and children were recruited during a routine outpatient clinic visit. Potentially eligible participants were identified by clinic staff and then approached by research staff who explained the study procedures and verified eligibility. Inclusion criteria included duration of type 1 diabetes for at least 1 year, ages 9–11 at the time of recruitment, English speaking, no known plans to move out of the area within the next 3 years, and absence of secondary causes of a type 1 diabetes diagnosis (e.g., cystic fibrosis). Exclusionary criteria included current involvement in foster care, presence of severe psychiatric disorders or comorbid chronic conditions (e.g., renal disease) that required burdensome ongoing treatment regimens, or diagnosis of mental retardation.
Of the 361 eligible participants who were approached, 240 (66.5 %) consented and participated. Reasons for not participating included being too busy (n = 54), no transportation (n = 3), and other (n = 64). Signed informed consent was obtained from a parent or legal guardian, written assent from children 11 years old, and verbal assent from children less than 11 years according to the guidelines established by each site’s Institutional Review Boards. After enrollment, one child was diagnosed with monogenic diabetes of the young (MODY) (Hattersley et al., 2006), no longer treated with insulin, and hence removed from the study and analysis.
The 1 year follow-up yielded a sample of 226 youth (ages 10–13 years) with type 1 diabetes and their maternal caregivers. Drop-out from baseline to 1 year was 2.5 % (n = 6). Families chose not to participate in the study further for the following reasons: child and/or family no longer interested in research (n = 2), family moving out of the area (n = 1), changed endocrinologists and the doctor was not affiliated with the hospital (n = 1), family is too overwhelmed to participate in research (n = 1), and family would not schedule research visit and dropped by study personnel (n = 1). A small subsample of patients (n = 7) still enrolled in the study but did not complete the 1 year study visit because they were unavailable at the 1 year visit (e.g., did not return calls or schedule a study visit). There were no significant differences in baseline primary caregiver education, disease duration, race, baseline income, baseline household composition (one vs. two-parent), child’s gender, or 6 month HbA1c between those who participated in the 1 year follow-up (n = 226) and those who did not because they dropped out (n = 6) or did not complete the baseline measure (n = 7).
Sample characteristics: follow-up sample 1 year
The demographic and medical characteristics of our sample at 1 year follow-up are shown in Table 1. The sample (mean age of 11.59 years) had a comparable percentage of males (45.6 %) and females (54.4 %) and included a majority of non-Hispanic Caucasian youth (76 %), but higher than typical percentages of Hispanic Caucasian youth (11.1 %) in studies of type 1 diabetes. Recent studies of adolescents with type 1 diabetes (e.g., Helgeson et al., 2011; Ingerski et al., 2010) had 0.1 % Hispanic youth. The majority of the sample (66 %) received insulin via subcutaneous insulin infusion (i.e., insulin pump or pod).
Table 1.
Demographic and medical characteristics of sample at baseline and one year
| Baseline
|
One year
|
|||
|---|---|---|---|---|
| n (%) | Mean (SD); range | n (%) | Mean (SD); range | |
| Child age (years)a | – | 10.54 (0.94); 9.0–12.09 | – | 11.59 (0.97); 9.86–13.22 |
| Duration of diabetes (years) | – | 4.41 (2.46); 1–11 | – | 5.43 (2.49); 2–12 |
| Child gender | ||||
| Male | 109 (45.61) | – | 103 (45.6) | – |
| Female | 130 (54.39) | – | 123 (54.4) | – |
| Child ethnicity | ||||
| Non-Hispanic, Caucasian | 178 (74.5) | – | 171 (76) | – |
| Non-Hispanic, African-American | 11 (4.6) | – | 11 (4.9) | – |
| Non-Hispanic, multiple races | 13 (5.4) | – | 12 (5.3) | – |
| Non-Hispanic, other | 9 (3.8) | – | 5 (2.2) | – |
| Hispanic, Caucasian | 27 (11.3) | – | 25 (11.1) | – |
| Hispanic, other | 1 (0.4) | – | 1 (0.4) | – |
| Insulin regimen | ||||
| Conventional | 3 (1.3) | – | 7 (3.1) | – |
| Multiple daily injection | 104 (43.5) | – | 69 (30.5) | – |
| Pump | 126 (52.7) | – | 143 (63.3) | – |
| Pod | 6 (2.5) | – | 6 (2.7) | – |
| Maternal caregiver relationship | ||||
| Biological mother | 228 (97.4) | – | 207 (92) | – |
| Adoptive mother | 2 (0.9) | – | 2 (0.9) | – |
| Step-mother | 0 (0) | – | 1 (0.4) | – |
| Grandmother | 4 (1.7) | – | 4 (1.7) | – |
| Maternal caregiver educational level | ||||
| Unknown | – | 1 (0.5) | – | |
| Completed 9th–11th grade | – | 6 (2.8) | – | |
| High school diploma or equivalent | – | 37 (17.0) | – | |
| Some college | – | 51 (23.4) | – | |
| Associates degree | 31 (14.2) | |||
| Bachelor’s degree | – | 67 (30.7) | – | |
| Master’s degree | – | 22 (10.1) | – | |
| PhD/MD/JD degree | – | 3 (1.4) | – | |
Four children were recruited at age 11, but were not seen for baseline visits until after they turned 12 years of age due to study visit cancellations and reschedules
Measures: predictors
Family communication
Interaction Behavior Coding (IBC) System is a structured coding system that was used to code positive communication behaviors [absent (0)/present (1)] during a videotaped observation of common parent-adolescent diabetes-related problem-solving tasks that are identified by families (Wysocki et al., 2000). The IBC system has a positive communication domain that is rated separately for each individual in the family. For the purposes of this analysis, we utilized the Positive Communication Index, which included behaviors such as stating the other’s opinion, making suggestions, and praising. Higher scores reflected greater positive communication.
Videotapes were coded by independent raters who were blind to the purpose and hypotheses of the study. Discrepancies between two coders were resolved by a senior research assistant who coded the same video. Coders were instructed to watch the entire interaction at least two times while completing the ratings. Inter-rater reliability in previous studies has ranged from 0.81 to 0.86 and validity has been demonstrated (Wysocki et al., 2000). In this study, inter-rater reliability (Intraclass Correlation Coefficient: ICC) for positive communication was 0.87 (p < 0.01) for youth and 0.72 (p < 0.02) for maternal caregivers.
Family conflict
The Diabetes Family Conflict Scale-Revised (DFCS-R) (Hood et al., 2007) is a 19 item self-report measure that was completed by youth and parents and reflects the level of conflict within the family as a whole regarding specific tasks such as taking more or less insulin depending on results, remembering to check blood sugars. All items are rated on a three-point likert scale (1 = we never argue, 2 = we sometimes argue, and 3 = we always argue). Total possible scores on the measure range from 19 to 57 with higher scores representing higher levels of diabetes-specific conflict in the family. The normative sample means for the measure were 24.4 for youth reported conflict and 24.0 for caregiver reported conflict. This measure has demonstrated good internal consistency for youth (α = 0.85) and for parents (α = 0.81) (Hood et al., 2007). In the present sample, internal consistency (α) at baseline was 0.87 for the youth DFCS-R and 0.85 for the maternal caregiver DFCS-R.
Behavioral autonomy
An observational measure, the Autonomy and Intimacy Rating System (AIRS) (Maharaj et al., 2004) was used to assess youth autonomy in interactions with family members during the video-taped observation on diabetes-related problem-solving tasks used for the IBC coding procedure. The AIRS is a macro-analytic coding system that evaluates parent–child verbal and nonverbal communication concerning youth behavioral autonomy and parental support of autonomy in parent-adolescent relationships. Based on research with adolescents with diabetes (Hauser et al., 1986, 1987), dimensions on the AIRS reflect patterns of communication in parent–child transactions that reflect a balanced capacity for adolescent autonomy (Grotevant & Cooper, 1985), which has been shown to correlate with self-management among adolescents with diabetes (Anderson et al., 1997).
Videotaped observations of maternal caregiver-adolescent interactions during diabetes-related problem-solving tasks were rated by independent raters who were trained on the task, and blind to the purpose and hypotheses of the study. Previous research has shown excellent internal consistency (Kappa <0.90) and inter-rater reliability (Kappa <0.80) on subscales (Maharaj et al., 2001). Construct validity has also been demonstrated by Maharaj et al. (2001, 2004). Inter-rater reliability (ICC) for youth behavioral autonomy at baseline in the current sample was 0.64 (p <0.01).
Pubertal status
Pubertal status was assessed at 1 year based on physical exam conducted by physicians or nurse practitioners (Marshall & Tanner, 1969, 1970). Tanner stage was rated on a scale of 1–5, where one indicated pre-pubertal and five indicated full pubertal status. For females, pubertal status was the average of the breast development stage and pubic hair stage and for males pubertal status was the average of the pubic hair stage and testicular girth.
Measures: primary outcomes
Diabetes management
The Diabetes Self-Management Profile (DSMP) is a 25-item structured interview, which was administered independently to youth and their caregivers to assess diabetes-related management behaviors during their 1 year outcome visit (Harris et al., 2000). Questions were asked in an open-ended manner and addressed the following management domains: exercise, hypoglycemia management, diet, blood glucose monitoring, and insulin administration (e.g., how often do you (or your child) delay boluses or shots?). The DSMP is comprised of both dichotomous items (yes, no) and three- to five-point likert scale items that were coded based on how the child or caregiver responded to the open-ended questions. A total self-management score was calculated by summing all items, and subscale scores were obtained by summing items for each appropriate scale. Higher scores reflected better diabetes management behaviors. The DSMP total score has demonstrated good internal consistency (r = 0.76), moderate cross-informant validity for both parent and child report (r = 0.26), and strong inter-rater agreement (r = 0.94) (Harris et al., 2000). This measure also has demonstrated good predictive validity between parent and child reported diabetes management behaviors and glycemic control (Harris et al., 2000). In the present sample, internal consistency (α) was 0.64 for the youth DSMP and 0.68 for the maternal caregiver DSMP.
Glycemic control
Glycated hemoglobin (HbA1c) provided an estimate of glycemic control over the previous 4 weeks to 3 months. Blood samples were obtained at 1 year post-baseline by a finger stick during the study visit. Samples from each study site were analyzed by one central laboratory to ensure standardization of results across sites. Samples were analyzed using the TOSOH-G7 method (reference range 4.0–6.0 %).
Approach to statistical analysis
Descriptive statistics were first computed for all relevant variables, using both parametric and nonparametric measures of central tendency, variability, and association. Frequency counts and histograms were used for categorical as well as ordered categorical data. The study’s primary outcome variables, DSMP score and HbA1c, were tested for normality. Missing data were modest, ranging between 0.1 and 11 % of all data at baseline and 12 months. Simple imputation of medians for missing values based on non-missing observations for the same variable was used for relevant variables (Harrel, 2001). Separate analyses were then conducted with and without imputation to see if imputed values substantially affected the results, which they did not. Patterns of statistical significance/non-significance remained consistent with and without imputation, suggesting imputation of medians for the proportion of missing values was appropriate. No transformations were needed given the normal nature of the distributions. All computations were conducted using SAS v9.2 and MPlus v6.0.
In the inferential phase of the study, two distinct sets of models were specified and tested, one with diabetes management (DSMP score) at 12 months as the outcome and a second, more complete model, with hemoglobin A1c at 12 months as the outcome, incorporating DSMP score as a mediator. An initial model that tested a latent variable model of diabetes management including parent and child report of the DSMP was not upheld. For this reason, two distinct models were hypothesized and tested, one for youth and one for mothers. All analyses were conducted using a series of structural equation models (SEM) based on the complexity of the hypothesized models, the need to use parallel sources of data across respondents, and the need to test mediators of glycemic control. All parameter estimates were treated as fixed effects. With respect to model fit indices, four different fit statistics (Hu & Bentler, 1999) were used to estimate statistical significance, including: traditional Chi-square goodness of fit test, root mean square error of approximation (RMSEA), standardized root mean residual (SRMR), and comparative fit index (CFI). No adjustments for multiplicity were conducted, and the criterion for statistical significance was held constant at the nominal α = 0.05 level across all models.
Decision concerning covariation of baseline values of outcomes
It should be noted that in planning our analysis, we decided not to covary baseline values of glycemic control or self-management. The reason for this decision was that the focus of our study was on the prediction of outcomes at 1-year follow-up rather than on the prediction of change in these measures. One year is not a sufficient period to observe change. It should also be noted that analyses that covaried baseline values did not result in statistically significant models.
Foundational model
Prior to testing the more complex latent variable, meditational model, a simpler, foundational model was tested with diabetes management (i.e., DSMP) as the outcome. Two latent factors: Positive Communication and Family Conflict were derived from manifest variables obtained at baseline. In both cases, errors were allowed to correlate for observed variables within a factor. In anticipation of a statistically significant relationship between the two aforementioned latent factors, a path coefficient was estimated to account for the relationship between the Positive Communication factor and the Family Conflict factor. In addition to estimating the direct effects between these latent factors and DSMP scores, both models included a single potentially confounding covariate, pubertal status (tanner score) measured at 12 months. To test our primary hypotheses, an additional variable: behavioral autonomy was initially included as testable covariate, alone and as a mediator between our latent variables and DSMP score, but failed to contribute to the predictive power of the current or subsequent (with HbA1c) model and was dropped from further consideration.
Complete model
The models illustrated in Figs. 1 and 2 incorporated DSMP scores as a mediator of glycemic control and tested direct and indirect effects of the latent Positive Communication and Family Conflict factors on glycemic control as measured by HbA1c. In these models, pubertal status was hypothesized to affect glycemic control directly.
Fig. 1.
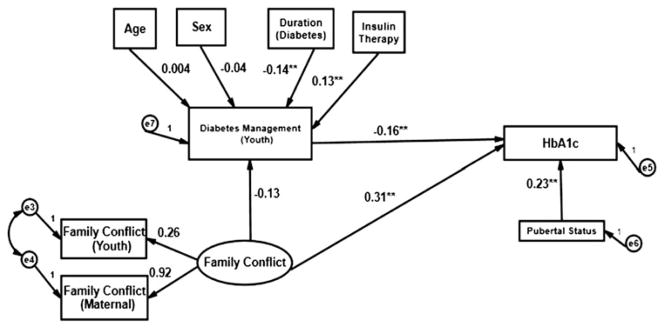
Prediction of glycemic control (youth report).
**p < 0.01
Fig. 2.
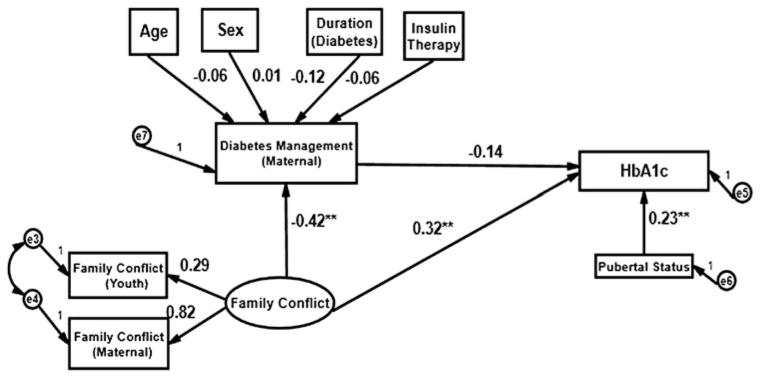
Prediction of glycemic control (maternal report).
**p < 0.01
Several analyses were tested for mediation, as suggested by Barron and Kenny (1986), Holmbeck (1997, 2002), and Kraemer et al. (2008). Our approach followed Holmbeck’s (1997, 2002) recommendations most closely. More specifically, the complete model was decomposed into the following sub-models and analyses and evaluated with respect to significance and directionality: (a) effect of latent factors on HbA1c scores, (b) effect of the latent factors on DSMP scores, and (c) effect of DSMP scores on HbA1c scores. Similar to the approach recommended by Holmbeck (1997), statistical significance of the mediator was then tested using likelihood ratio tests comparing the full model with and without the direct effects from the latent factors to the HbA1c scores constrained to zero. As an added measure of verification that the indirect effect of latent variable factors on HbA1c were not purely a function of sample specific phenomena, a series of simulated mediational effects using boot-strapping were computed. Finally, an additional analysis of the potential clinical significance of the relationship of family conflict to glycemic control was conducted.
Results
Descriptive statistics
Descriptive statistics for all relevant variables are shown in Table 2 and a table of intercorrelations of variables used in modeling is provided in Table 3. The mean level of glycemic control in this sample was higher (M = 8.31) than what is recommended by the American Diabetes Association (2010) for youth (<8.0 for age 12 and under M < 7.5 for 13 years and above) but comparable to other clinic samples of adolescents (M = 8.67) (Danne et al., 2001). Youth-reported (M = 26.2) and mother-reported (M = 24.7) conflict were comparable to the normative sample for the DFCS-R (Hood et al., 2007) that is 24.4 and 24.0 respectively.
Table 2.
descriptive statistics for variables included in SEM models
| Mean | SD | Range | Possible range | |
|---|---|---|---|---|
| Diabetes family conflict (baseline) | ||||
| Youth total score | 26.20 | 5.74 | 19–49 | 19–57 |
| Maternal total score | 24.69 | 4.65 | 19–56 | 19–57 |
| Positive communication (IBC coding: baseline) | ||||
| Youth positive communication | 0.15 | 0.15 | 0–0.64 | 0–1 |
| Maternal positive communication | 0.35 | 0.20 | 0–0.86 | 0–1 |
| Behavioral autonomy (AIRS coding: baseline) | ||||
| Youth behavioral autonomy | 2.43 | 1.72 | 0–6 | 1–6 |
| Pubertal status (1 year) | ||||
| Tanner stage | 2.47 | 1.19 | 1–5 | 1–5 |
| Diabetes management: youth report (1 year) | ||||
| Exercise | 5.59 | 2.43 | 1–12 | 0–12 |
| Hypoglycemia management | 8.61 | 1.46 | 4–11 | 0–11 |
| Blood glucose monitoring | 22.23 | 3.96 | 11–31 | 0–32 |
| Diet | 12.70 | 3.01 | 2–17 | 0–17 |
| Insulin administration | 11.57 | 3.10 | 0–16 | 0–16 |
| Total score | 60.69 | 8.25 | 36–83 | 0–88 |
| Diabetes management: maternal report (1 year) | ||||
| Exercise | 6.81 | 2.65 | 1–12 | 0–12 |
| Hypoglycemia management | 8.73 | 1.58 | 3–11 | 0–11 |
| Blood glucose management | 23.72 | 4.00 | 9–31 | 0–32 |
| Diet | 12.44 | 2.90 | 4–17 | 0–17 |
| Insulin administration | 11.38 | 3.45 | 0–16 | 0–16 |
| Total score | 63.08 | 9.05 | 35–82 | 0–88 |
| Glycemic control (baseline) | ||||
| HbA1c | 8.20 | 1.37 | 5.7–16.8 | 5 % + |
| Glycemic control (1 year) | ||||
| HbA1c | 8.31 | 1.38 | 5.6–14.5 | 5 % + |
Table 3.
Bivariate correlations for observed variables
| Variables | Communication maternal |
Age | Baseline values
|
12-month values
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conflict youth |
Conflict maternal |
Autonomy youth |
DSMP youth |
Duration therapy |
DSMP youth |
DSMP maternal |
Tanner | HbA1c | |||
| Baseline | |||||||||||
| Communication youth | 0.54* | −0.04 | −0.01 | 0.01 | 0.28* | 0.06 | −0.06 | 0.07 | 0.06 | −0.11 | −0.07 |
| Communication maternal | 0.17 | −0.05 | 0.07 | 0.29* | 0.05 | −0.01 | 0.06 | 0.06 | −0.16* | −0.10 | |
| Age | −0.01 | 0.17 | −0.03 | 0.06 | 0.05 | −0.02 | −0.15 | 0.50 | 0.16 | ||
| Conflict youth | 0.24* | −0.15* | −0.17* | 0.05 | −0.14* | −0.15* | 0.03 | 0.16* | |||
| Conflict maternal | −0.22* | −0.01 | 0.22 | −0.15* | −0.37* | 0.07 | 0.32* | ||||
| Autonomy youth | 0.07 | −0.08 | 0.25* | 0.19* | −0.07 | −0.19* | |||||
| DSMP youth | −0.05 | 0.51* | 0.30* | −0.01 | −0.21* | ||||||
| Duration therapy | −0.15 | −0.23* | −0.01 | −0.01 | |||||||
| 12-month | |||||||||||
| DSMP youth | 0.55* | −0.08 | −0.23* | ||||||||
| DSMP maternal | −0.09 | −0.31* | |||||||||
| Tanner | 0.26* | ||||||||||
p < 0.05
Prediction of diabetes management
The predictive model (see Fig. 1) based on youth report of the DSMP represented a good fit based on an observed (p = 0.16) and three additional supporting fit statistics (CFI = 0.98, RMSEA = 0.04, SRMR = 0.03). Maternal and child positive communication scores were statistically significant contributors (p < 0.01) to the Communication factor while mother (p = 0.05) and child (p = 0.06) Family Conflict scores were on the threshold of statistical significance (using α = 0.05 level as the criterion). Contrary to our prediction, individually and collectively, the latent Positive Communication factor did not predict diabetes management (DSMP score) at 12 months. As expected, DSMP score at baseline was a statistically significant predictor of DSMP score at 12 months (p < 0.01). Pubertal status (tanner score) did not predict DSMP score alone or after adjusting for DSMP baseline value.
The hypothesized predictive model (see Fig. 2) based on maternal report of the DSMP also represented a reasonably good fit as evidenced by an observed (p = 0.72) and three supporting fit statistics (CFI = 0.99, RMSEA = 0.00, SRMR = 0.00). The R2 for DSMP at 12 months in the adjusted model was 0.40. In contrast to the model based on youth report, the two Positive Communication scores and the two Family Conflict scores were all statistically significant contributors to their respective latent factors (p < 0.01). Consistent with our hypotheses, the relationship of Family Conflict to diabetes management (DSMP) at 12 months was statistically significant (p = 0.01). Higher conflict was associated with less adequate diabetes management. Tanner score did not contribute to the model.
Prediction of glycemic control
The hypothesized predictive model of glycemic control (HbA1c) based on youth report (see Fig. 1) was deemed a good fit (see Hu and Bentler, 1999) based on an observed (p = 0.16) and three supporting fit statistics (CFI = 0.95, RMSEA = 0.04, SRMR = 0.02). The R2 values for the endogenous variables in the adjusted models, DSMP at 12 months and glycemic control, were 0.06 and 0.20, respectively. Mother and child family conflict scores were statistical significant contributors (p < 0.01) to the Family Conflict factor. The direct effect of the Family Conflict factor derived from baseline data on glycemic control at 12 months was statistically significant (p < 0.01) Higher family conflict was associated with higher HbA1c (or less adequate glycemic control). For the youth report, diabetes management at 12 months (p = 0.01) as well as Tanner score were statistically significant predictors of glycemic control (p < 0.01) after adjusting for age, sex, duration of diabetes, and type of insulin therapy (pump versus multiple daily injections). While more advanced pubertal status was associated with higher HbA1c, more adequate diabetes management was associated with less adequate glycemic control. Age, sex of the patient, duration of diabetes, and type of insulin therapy did not have a confounding effect on the DSMP score at 12 months. Finally, the indirect effect of Family Conflict factors on glycemic control mediated by diabetes management was not statistically significant, suggesting no mediation effect of diabetes management on glycemic control. The simulated model using the bootstrap method also failed to reflect any possible mediator effect.
The hypothesized predictive model of glycemic control based on maternal report of DSMP (see Fig. 2) represented a good fit based on a , p = 0.16; CFI = 0.96, RMSEA = 0.04, and SRMR = 0.03. The R2 values in the adjusted models for both DSMP at 12 months and glycemic control were both 0.23. As was the case in the youth report model, mother and child conflict scores were statistically significant contributors (p < 0.01) to the Family Conflict factor. The direct effect of Family Conflict measured at baseline on glycemic control at 12 months represented a statistically significant relationship (p < 0.01). The relationship of Tanner score to glycemic control was positive and statistically significant (p < 0.01).
No confounding effects of age, sex, duration of diabetes or type of insulin therapy on maternal DSMP score at 12 months were observed. While the effect of Family Conflict on DSMP score at 12 months was statistically significant, the effect of the latter (DSMP score) on glycemic control at 12 months was not statistically significant. Hence, the indirect effect of Family Conflict on glycemic control via diabetes management (DSMP score) as a mediator was not statistically significant for maternal report. As with the youth report model, simulated results using the bootstrap method corroborated the findings of no possible mediator effect of diabetes management on glycemic control at 12 months.
Additional analysis: potential clinical significance of relationship of family conflict to glycemic control
Based on Hilliard et al. (2011), we estimated the potential clinical significance of the predictive relationship of family conflict to glycemic control. This variable was derived by defining a clinically meaningful HbA1c change score (e.g., 1.0 %) and dividing this score by the standardized beta for the independent variable’s effect on the dependent variable (i.e., Family Conflict to HbA1c). The standardized beta for the relationship between the Family Conflict factor and glycemic control for youth (β = 0.43) and maternal report (β = 0.36) represents an increase in a conflict score of 2.33 (for youth report) and 2.78 (maternal report) that would account for an increase of 1 % in HbA1c, which is associated with an increase in diabetes-related complications (DCCT, 1994).
Discussion
To our knowledge, this study was one of the first to test a comprehensive predictive model of biologic, family, and psychological influences on diabetes management and glycemic control measured 1 year later in a sample of youth with type 1 diabetes. Strengths of the study included a relatively large and homogenous age range of youth recruited at the onset of adolescence, a prospective, multisite study design, measures that included multiple methods (observational, self-report, and biomarkers) and multiple informants (e.g., mothers and youth).
Our findings provided partial support for our hypothesized model. Specifically, based on both youth and maternal report of diabetes management, frequency of family conflict but not positive communication at baseline predicted the quality of diabetes management 12 months later. These findings are consistent with other studies (Ingerski et al., 2010) but extend the findings to a sample of youth 10–13 years old at follow-up with type 1 diabetes in a multisite, 1 year prospective study. Family conflict can interfere with the quality of collaborative maternal-youth problem-solving concerning diabetes management based on the following (Wysocki et al., 2000). Pediatric diabetes management requires a high level of daily family collaboration and decision making involving multiple tasks (e.g., insulin management, exercise, etc.) that can be disrupted by family conflict. The presence of family conflict may have also disrupted the family’s affective climate as well as youth’s perceptions of their parents’ interactions with them (Anderson & Coyne, 1991).
A second primary finding was that family conflict predicted higher levels (or less adequate) glycemic control 1 year later. This finding may reflect the influence of family conflict on other relevant variables, such as individual or family stress, which was not measured in this study. In support of this notion, Williams et al. (2009) found a relationship between diabetes family conflict and psychological stress in children and adolescents with type 1 diabetes. Hanson et al. (1987) found that chronic family stress had a direct effect on glycemic control in adolescents with type 1 diabetes. More recent research has documented multiple effects of family conflict on children’s psychological adaptation and autonomic nervous system functioning (El-Sheikh & Erath, 2011). Consistent with previous research that has documented increased insulin resistance with the onset of puberty (Amiel et al., 1986; Moran et al., 2002), pubertal status also had a negative relationship with glycemic control. More advanced pubertal status was associated with worse glycemic control but did not relate to diabetes management.
Our findings did not support the hypothesized mediation of behavioral autonomy on the effects of family variables on diabetes management. It was surprising that behavioral autonomy did not relate to diabetes management as was expected. It is possible that the small sample of observations of the diabetes-related problem solving task was too limited to capture the full range of behavioral autonomy that is expressed in day to day diabetes management. In addition, it should be noted that behavioral autonomy was assessed at one point in this study. Changes in youth behavioral autonomy over time may be a more sensitive predictor. As a related point, youth in this sample may have been too young to demonstrate the salient changes in behavioral autonomy. Moreover, lower autonomy may relate to less adequate self-management primarily when parents are less involved.
Our findings for the youth report were consistent with previous research (Ingerski et al., 2010; Lewin et al., 2006) that found that treatment adherence mediated the impact of family conflict on glycemic control. However, maternal report did not demonstrate such mediation. These discrepant findings could be explained by differences in participants’ ages (the current sample was younger and more homogenous in age than previous research (Hilliard et al., 2011; Palmer et al., 2004), and discrepant measures of diabetes management (e.g., use of frequency of blood glucose monitoring as a measure of adherence versus the DSMP, a comprehensive measure of diabetes management based on parent and youth report), differences in study design (cross-sectional versus prospective), and length of follow-up. In contrast, our findings underscored the importance of family conflict as a predictor of glycemic control in its own right for both the maternal and youth reports. The consistency of this finding across informants lends support to validity.
Several limitations should be considered when interpreting our findings. While the homogeneity of our sample is a strength because of its developmental specificity, it also limits the generalizability of our findings. Similarly, the demographics of our sample that included a majority of Caucasian, highly educated families also limit generalizability of study findings. In addition, the findings were also limited to 1 year follow-up. Findings did not indicate significant prediction of changes in self-management and glycemic control after controlling for baseline status. The 1 year follow-up period may have been too short to identify significant prediction of change. For this reason, studies that evaluate individual psychological and family processes that predict changes in glycemic control over a longer period of time are needed.
Our findings have several important implications for clinical care. First, the impact of family conflict on diabetes management and glycemic control underscores the need to assess family conflict in ongoing clinic-based diabetes management. In addition, parental and youth perceptions of family conflict can be assessed reliably, validly, and quickly (5–10 min) using self-report measures. Such data can be used to inquire about the specific impact of family conflict on diabetes management and target preventative intervention to family conflict that can affect specific areas of diabetes management and would be expected to predict level of glycemic control. Providing support to families and encouraging conflict resolution and problem solving could reduce the level of family conflict and enhance glycemic control.
The fact that glycemic control is also monitored routinely in clinical care for type 1 diabetes also presents opportunities for secondary prevention. For example, youth whose levels of glycemic control that are consistently above the recommended target range (ADA, 2010) and whose families demonstrate high levels of family conflict in diabetes management would benefit from referral for more intensive treatments such as Behavioral Family Systems Therapy (BFST). This intervention model has been shown to be effective in reducing family conflict, enhancing diabetes management, and improving glycemic control among adolescents with type 1 diabetes (Wysocki et al., 2006), including those with poor glycemic control (Harris et al., 2009).
Acknowledgments
The work reported in this article was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (1R01 DK069486). The HbA1c data was analyzed by the Diabetes Diagnostic Laboratory (DCD) at the University of Missouri Columbia Health Sciences Center. Data collection and management of this study were facilitated by a talented group of research assistants, including Claire Peterson, Michelle Eakin, Danielle Rosnov, Daniela Fernandez, Jennifer Hernandez, Katharina Wetterau, Erica Sood, Megan Miller, and Andrea Perry. We very much appreciated the efforts of a talented group of undergraduate research assistants who coded the videotapes of the problem-solving interactions, as well as, the support from Dr. Larry Dolan and Grafton Reeves, who were the endocrinologists that collaborated on the study. Finally, the efforts of study participants who gave their time and energy to this work are gratefully acknowledged.
Contributor Information
Dennis Drotar, Email: dennis.drotar@cchmc.org, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA. Department of Psychology, University of Cincinnati, Cincinnati, OH, USA.
Richard Ittenbach, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Jennifer M. Rohan, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA. Department of Psychology, University of Cincinnati, Cincinnati, OH, USA
Resmi Gupta, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Jennifer Shroff Pendley, Division of Behavioral Health, Alfred I. duPont Hospital for Children, Wilmington, DE 19803, USA.
Alan Delamater, Department of Pediatrics, University of Miami, Miami, FL 33136, USA.
References
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty: A contributing factor to poor glycemic control in adolescents with diabetes. New England Journal of Medicine. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Coyne JC. “Miscarried helping” in the families of children and adolescents with chronic diseases. In: Johnson JH, Johnson SB, editors. Advances in child health psychology. Gainesville: University of Florida; 1991. pp. 167–177. [Google Scholar]
- Anderson BJ, Coyne JC. Family context and compliance behavior in chronically ill children. In: Krasnegor NA, Epstein L, Johnson SB, Yaffe SJ, editors. Developmental aspects of health compliance behavior. Hillsdale, NJ: Erlbaum; 1993. pp. 77–89. [Google Scholar]
- Anderson BJ, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Vongsness L, Connell A, Butler D, Goebel-Fabbis A, Laffel LMB. Family conflict, adherence and glycemic control in youth with short duration type 1 diabetes. Diabetes Medicine. 2002;19:635–642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- Barron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berg CA, Butler J, Osborn P, King G, Palmer D, Butner J, et al. The role of parental monitoring in understanding the benefits of parental acceptance on adolescent adherence and metabolic control of type 1 diabetes. Diabetes Care. 2008;31:678–683. doi: 10.2337/dc07-1678. [DOI] [PubMed] [Google Scholar]
- Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: A longitudinal cohort study. Diabetes Care. 2001;24:1536–1540. doi: 10.2337/diacare.24.9.1536. [DOI] [PubMed] [Google Scholar]
- Danne T, Mortensen HB, Hougaard P, Lynggaard H, Aanstoot H, Chiarelli F, et al. Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidore Study Group. Diabetes Care. 2001;24:1342–1347. doi: 10.2337/diacare.24.8.1342. [DOI] [PubMed] [Google Scholar]
- Dashiff C, Bartollucci A. Autonomy development in adolescents with insulin dependent diabetes mellitus. Journal of Pediatric Nursing. 2002;17:96–105. doi: 10.1053/jpdn.2002.124127. [DOI] [PubMed] [Google Scholar]
- Dashiff C, Vance D, Abdullatif H, Wallander J. Parenting and self-care of adolescents with type 1 diabetes. Child: Care, Health and Development. 2008;35:79–88. doi: 10.1111/j.1365-2214.2008.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Journal of Pediatrics. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- DCCT/EDIC Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983–2005) Archives of Internal Medicine. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke DC, Geffken GR, Lewin AB, Williams LB, Storch EA, Silverstein JH. Glycemic control in youth with type 1 diabetes: Family predictors and mediators. Journal of Pediatric Psychology. 2008;33:719–727. doi: 10.1093/jpepsy/jsn012. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath S. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves MM, Roberts MC, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology. 2010;35:368–382. doi: 10.1093/jpepsy/jsp072. [DOI] [PubMed] [Google Scholar]
- Grotevant HD, Cooper CR. Patterns of interaction in family relationships and the development of identity exploration in adolescence. Child Development. 1985;56:415–428. [PubMed] [Google Scholar]
- Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: A systematic review. Health Technology Assessment. 2001;5:1–77. doi: 10.3310/hta5100. [DOI] [PubMed] [Google Scholar]
- Hanson CL, Henggeler SW, Burghen GA. Model of associations between psychosocial variables and health-outcome measures of adolescents with IDDM. Diabetes Care. 1987;10:752–758. doi: 10.2337/diacare.10.6.752. [DOI] [PubMed] [Google Scholar]
- Harrel FE. Regression modeling strategies with application to linear models, logistic regression and survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- Harris MA, Freeman KA, Beers M. Family therapy for adolescents with poorly controlled diabetes: Initial test of clinical significance. Journal of Pediatric Psychology. 2009;34:1097–1107. doi: 10.1093/jpepsy/jsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MA, Wysocki T, Sadler M, Wilkinson K, Harvey LM, Buckloh LM, et al. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care. 2000;23:1301–1304. doi: 10.2337/diacare.23.9.1301. [DOI] [PubMed] [Google Scholar]
- Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K. International Society for Pediatric and Adolescent Diabetes, ISPAD: Clinical practice consensus guidelines 2006–2007: The diagnosis and management of monogenic diabetes in children. Pediatric Diabetes. 2006;7:352–360. doi: 10.1111/j.1399-5448.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- Hauser ST, Jacobson AM, Wertlieb D, Weiss-Perry B, Follansbee D, Wolfsdorf J, et al. Children with recently diagnosed diabetes: Interactions within their families. Health Psychology. 1986;5:273–296. doi: 10.1037//0278-6133.5.3.273. [DOI] [PubMed] [Google Scholar]
- Hauser ST, Powers SI, Weiss-Perry B, Follansbee D, Rajapark DC, Green WM. Family Constraining and Enabling Coding System (CECS) manual. Harvard Medical School; 1987. Unpublished manuscript. [Google Scholar]
- Helgeson VS, Honcharuk E, Becker D, Escobar O, Siminerio L. A focus on blood glucose monitoring: Relation to glycemic control and determinants of frequency. Pediatric Diabetes. 2011;12:25–30. doi: 10.1111/j.1399-5448.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Guilfoyle SM, Dolan LM, Hood KK. Predictions of adolescents’ glycemic control 1 year after diabetes-specific family conflict: The mediating role of blood glucose monitoring adherence. Archives of Pediatrics and Adolescent Medicine. 2011a;165:624–629. doi: 10.1001/archpediatrics.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Rohan JM, Carle AC, Pendley JS, Delamater A, Drotar D. Fathers’ involvement in preadoles-cents’ diabetes adherence and glycemic control. Journal of Pediatric Psychology. 2011b;36:911–922. doi: 10.1093/jpepsy/jsr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literature. Journal of Consulting and Clinical Psychology. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Post hoc probing of significant moderational and meditational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hood KK, Butler DA, Anderson BJ, Laffel LMB. Updated and revised diabetes family conflict scale. Diabetes Care. 2007;30:1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Ingerski LM, Anderson BJ, Dolan LM, Hood KK. Blood glucose monitoring and glycemic control in adolescence: Contribution of diabetes-specific responsibility and family conflict. Journal of Adolescent Health. 2010;47:191–197. doi: 10.1016/j.jadohealth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AM, Hauser ST, Lavori P, Willett JB, Cole CF, Wolfsdorf JI, et al. Family environment and glycemic control: A four year prospective study of children and adolescents with insulin-dependent diabetes mellitus. Psychosomatic Medicine. 1994;56:401–409. doi: 10.1097/00006842-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Hauser ST, Lavori P, Willett JB, Cole CF, Wolfsdorf JI, et al. Psychological adjustment to IDDM: 10-year follow-up of an onset cohort of child and adolescent patients. Diabetes Care. 1997;20:811–818. doi: 10.2337/diacare.20.5.811. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Goldston D, Obrosky S, Iyengar S. Prevalence and predictors of pervasive noncompliance with medical treatment among youths with insulin-dependent diabetes mellitus. Journal of the Academy of Child and Adolescent Psychiatry. 1992;31:1112–1119. doi: 10.1097/00004583-199211000-00020. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychology. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin AB, Heidgerken AD, Geffken GR, Williams LB, Storch EA, Gelfand KM, et al. The relation between family factors and metabolic control: The role of diabetes adherence. Journal of Pediatric Psychology. 2006;31:174–183. doi: 10.1093/jpepsy/jsj004. [DOI] [PubMed] [Google Scholar]
- Maharaj S, Daneman D, Olmsted M, Rodin G. Metabolic control in adolescent girls: Links to relationality and the female sense of self. Diabetes Care. 2004;27:709–715. doi: 10.2337/diacare.27.3.709. [DOI] [PubMed] [Google Scholar]
- Maharaj S, Rodin G, Connolly J, Olmsted M, Daneman D. Eating problems and the observed quality of mother-daughter interactions among girls with Type 1 diabetes. Journal of Consulting and Clinical Psychology. 2001;69:950–958. [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159–1162. doi: 10.2337/dc09-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Jacobs DR, Jr, Steinberger J, Cohen P, Hong CP, Prineas R, et al. Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. Journal of Clinical Endocrinology and Metabolism. 2002;87:4817–4820. doi: 10.1210/jc.2002-020517. [DOI] [PubMed] [Google Scholar]
- Morris AD, Boyle DI, McMahon AD, Greene SA, MacDonald TM, Newton RW. Adherence to insulin treatment, glycaemic control and ketoacidosis in insulin-dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes audit and research in Tayside Scotland. Medicines monitoring unit. Lancet. 1997;350:1505–1510. doi: 10.1016/s0140-6736(97)06234-x. [DOI] [PubMed] [Google Scholar]
- Palmer DL, Berg CA, Wiebe DJ, Beveridege RM, Korbel CD, Upchurch R, et al. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. Journal of Pediatric Psychology. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- Palmer DL, Osborn P, King P, Berg CA, Butler J, Butner J, et al. The structure of parental involvement and relations in disease management for youth with type 1 diabetes. Journal of Pediatric Psychology. 2011;35:591–605. doi: 10.1093/jpepsy/jsq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan JM, Delamater A, Pendley JS, Dolan L, Reeves G, Drotar D. Identification of self-management patterns in pediatric type 1 diabetes using cluster analysis. Pediatric Diabetes. 2011;12:611–618. doi: 10.1111/j.1399-5448.2010.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Williams LB, Laffel LMB, Hood KK. Diabetes specific family conflict and psychological distress in paediatric type 1 diabetes. Diabetes Medicine. 2009;26:908–914. doi: 10.1111/j.1464-5491.2009.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T. Associations among parent-adolescent conflict, metabolic control and adjustment to diabetes in adolescents. Journal of Pediatric Psychology. 1993;18:441–452. doi: 10.1093/jpepsy/18.4.441. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Wilkinson K, Sadler M, Mauras N, et al. Self-care autonomy and outcomes of intensive therapy or usual care in youth with type 1 diabetes. Journal of Pediatric Psychology. 2006;31:1036–1045. doi: 10.1093/jpepsy/jsj017. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Miller KM, Harvey LM, Taylor A, Elder-Danda C, McDonell K, et al. Behavior therapy for families of adolescents with diabetes: Effects on directly observed family interactions. Behavior Therapy. 2000;30:507–525. [Google Scholar]


