Abstract
While sentinel lymph node biopsy (SLN) is a highly accurate and well-tolerated procedure for patients with cutaneous melanoma, the role of the completion lymph node dissection (CLND) for patients with positive SLN biopsy remains unknown. This study aimed to look at the prognostic value of a positive nonsentinel lymph node (NSLN). A prospectively maintained database identified 222 patients with cutaneous melanoma and a positive SLN biopsy, without evidence of distant disease. All of these patients underwent CLND, and 37 patients (17%) had positive NSLN. With median follow-up of 33 months, patients with negative NSLN had median survival of 104 months, while patients with positive NSLN had median survival of 36 months (p < 0.001). There were no survivors in the patients with positive NSLN beyond 6 years. When patients with an equal number of positive nodes were analyzed, the presence of a positive NSLN was still associated with worse melanoma-specific survival (66 months for NSLN– versus 34 months for NSLN+, p = 0.04). While increasing age, tumor thickness, and male sex were associated with an increased risk of death on multivariate analysis, a positive NSLN was the most important predictor of survival (hazard ratio 2.5). We conclude that positive NSLN is an independent predictor of disease-specific survival in patients with cutaneous melanoma.
Sentinel lymph node (SLN) biopsy as developed by Morton and colleagues is an accurate technique to assess the primary draining lymph node for metastatic disease.1 In addition, presence of metastatic disease in the draining lymph node is one of the most important predictors of survival in early melanoma.2 In the interim report of the Multicenter Selective Lymphadenectomy Trial (MSLT)-1 trial, presence of metastatic disease in the sentinel lymph node was associated with 5-year survival of 72%, while those patients that had negative SLN had 92% 5-year survival.3
As patients are followed over a longer time period, it is evident that there is a wide range of survival in patients with node-positive, or stage III, disease. Studies have demonstrated 5-year survival of 24–72%, depending on the primary tumor thickness, ulceration, mitotic rate, extent of nodal involvement by melanoma, and time of follow-up of the study.3–6 Traditionally patients with positive SLN have been offered CLND, where the incidence of detecting additional melanoma in the completion lymph node dissection (CLND) is 15–20%.7,8 This is based upon historical data which has suggested a therapeutic advantage to removing residual subclinical disease. Indeed, long-term follow-up of prospective randomized trials demonstrated a survival advantage with CLND, in a subgroup of patients with low-risk melanomas.9,10 In contrast, a retrospective multicenter study showed that patients followed expectantly after positive SLN biopsy had a slightly higher rate of regional recurrence, but a similar survival when compared with a nonrandomized cohort of patients who underwent CLND.7 The aim of the current study was to determine the prognostic significance of additional positive nonsentinel lymph nodes found at completion lymph node dissection in SLN-positive patients.
PATIENTS AND METHODS
Patients were identified from a prospectively maintained database at the Memorial Sloan-Kettering Cancer Center (MSKCC), and this study was approved by the institutional review board (IRB). A query was performed for all patients that had a positive SLN biopsy, and we identified a consecutive series of patients that spanned from 1991 to 2006. Patients with primary mucosal melanomas were excluded from the study. We also excluded patients who were found to have distant metastatic disease at the time of SLN biopsy, and those who did not undergo a completion lymph node dissection (CLND).
Patients were offered a SLN biopsy if the primary melanoma was ≥1 mm, or greater than 0.75 mm and Clark IV. Lymphatic mapping was performed at the time of surgery for the primary melanoma, using a combination of technetium sulfur colloid and isosulfan blue. Lymphoscintigraphy was performed the day of, or the afternoon prior to, surgery with an intradermal injection at the site of the primary melanoma. Draining lymph node basin(s) were identified with real-time and static images. At the time of surgery, under anesthesia, 0.5–1.0 ml isosulfan blue was injected into the dermis. A gamma probe was used along with the visual blue dye to identify the sentinel lymph node(s). The sentinel lymph nodes removed at the time of surgery included those identified on the preoperative lymphoscintigram. In addition, all blue nodes and all nodes in the nodal basin with >10% of the activity of the hottest SLN were designated sentinel nodes and were removed as previously described.11
The sentinel lymph nodes were placed in formalin for routine analysis of metastatic disease, although some patients had nodes evaluated by frozen section, and confirmation by permanent sectioning during the early portion of the study. The nodes were then paraffin-embedded and bivalved, and sections were taken for analysis. Hematoxylin and eosin (H&E) staining was performed and, if no evidence of metastatic melanoma was found, serial sections and immunohistochemistry (IHC) with the anti-sera to S-100 protein and HMB-45, and/or Melan A/Mart 1 were performed. The completion lymph node specimens were placed in formalin. After the removal of the individual lymph nodes from surrounding fat, nodes were bivalved, fixed, and paraffin-embedded. Sections from each lymph node were stained with H&E only.
Statistical analysis was performed with the SPSS software (SPSS version 12, Chicago, IL). Clinicopathological descriptive features were analyzed for their presence in both the nonsentinel lymph node positive (NSLN+) and nonsentinel lymph node negative (NSLN–) groups. Comparisons were analyzed using chi-square (χ2) and Student's t-test. Clinicopathologic factors analyzed were age, sex, tumor thickness, site of the primary, ulceration, number of positive nodes, and NSLN status for their predictive value in disease-specific survival. Age and thickness were analyzed as continuous variables. The independent influence of a positive NSLN on survival was determined through a multivariate analysis with Cox regression analysis. A p-value of < 0.05 was considered significant. Estimated survival rates were calculated by Kaplan–Meier analysis. Patients were followed on a regular basis for disease recurrence and death.
RESULTS
Median follow-up for the entire study population was 33 months. The characteristics of patients with a positive SLN biopsy are shown in Table 1. Of those patients undergoing a SLN biopsy, 17% had additional melanoma detected in the completion lymph node specimen. When comparing the NSLN– and NSLN+ groups, there was no significant difference detected in age, sex or site of the primary melanoma. The group with a positive NSLN had thicker primary lesions and a higher incidence of ulceration.
TABLE 1.
Patient and tumor characteristics in 222 patients with a positive SLN biopsy
| Factor | NSLN– (n =185) | NSLN+ (n =37) | p-Value | |
|---|---|---|---|---|
| Age | Mean (range) | 55 (7–90) | 56 (10–86) | 0.56 |
| Sex | Female | 64 (35%) | 12 (32%) | 0.85 |
| Male | 121 (65%) | 25 (68%) | ||
| Site | Extremity | 71 (39%) | 20 (54%) | 0.187 |
| Head and neck | 15 (8%) | 5 (14%) | 0.496 | |
| Truncal | 98 (53%) | 12 (32%) | 0.062 | |
| T stage | T1 | 11 (6%) | 0 (0%) | 0.256 |
| T2 | 56 (30%) | 4 (11%) | 0.021 | |
| T3 | 62 (34%) | 18 (47%) | 0.158 | |
| T4 | 55 (30%) | 16 (42%) | 0.359 | |
| Thickness (mean) | 3.7 (mm) | 5.5 (mm) | 0.004 | |
| Ulceration | Present | 82 (45%) | 24 (63%) | 0.011 |
| Absent | 85 (46%) | 8 (21%) | ||
| Unknown | 17 (9%) | 6 (16%) |
Table 2 presents the characteristics of the nodes removed. The median number of SLNs removed in the NSLN– and NSLN+ groups was two; the median number of NSLNs removed was 16. As expected, there were more total positive nodes in the NSLN+ group, with a median of three positive nodes in the NSLN+ group and only one positive node in the NSLN– group.
TABLE 2.
Lymph node characteristics in patients
| NSLN– (n = 185) | NSLN+ (n = 37) | |
|---|---|---|
| SLN removed (median) | 2 | 2 |
| Nodes in CLND (median) | 16 | 16 |
| Number of positive nodes (median) | 1 (1–4) | 3 (2–10) |
The median number of lymph nodes removed at SLN biopsy and CLND were equal among the groups As anticipated, the number of positive nodes was higher in the group of patients with a positive NSLN
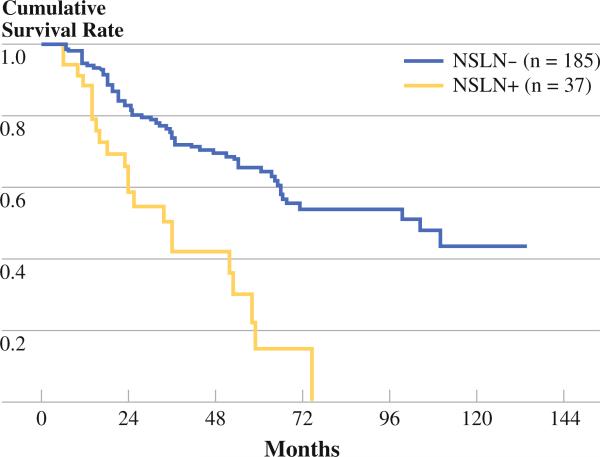
Disease-specific survival analysis for the patients with positive and negative NSLN is shown in Fig. 1. Patients who did not have additional disease detected in the completion lymph node specimen had median survival of 104 months. Those patients with a positive NSLN had median survival of 36 months (p < 0.001). The 5-year disease-specific survival for the patients with a negative NLSN was 65% and for a positive NSLN was 15%. There were no survivors beyond 6 years in the positive NSLN group.
FIG. 1.
DSS in patients with a positive and negative NSLN. Median survival in the NSLN– group was 104 months, while median survival in the NSLN+ group was 36 months (p < 0.001)
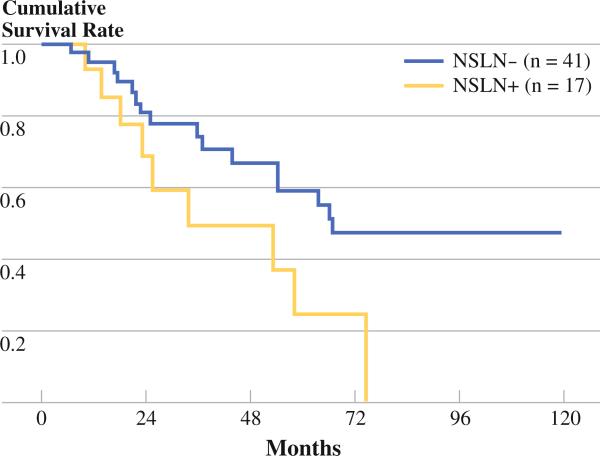
In order to determine if decreased survival observed in NSLN+ patients was merely due to an increase in the number of total positive nodes, a group of patients with an equal number of positive lymph nodes were analyzed. We selected patients that had two positive lymph nodes. The first group (n = 41), had two positive SLNs and a negative NSLN. The second group (n = 17) had one positive lymph node at the time of SLN biopsy, and one additional positive node detected in the CLND. As shown in Fig. 2, the trend for an overall poor prognosis in patients with a positive NLSN persisted in this group. Those patients with a negative NSLN had median survival of 66 months, while those with a positive NSLN had median survival of 34 months (p = 0.04).
FIG. 2.
DSS in patients with two positive nodes. The NSLN– group had two positive sentinel nodes. The NSLN+ group had one positive SLN and one positive NSLN. Median survival of the NSLN– group was 66 months, while median survival of the NSLN+ group was 34 months (p = 0.04)
As shown in Table 3, on univariate analysis, increasing age, tumor thickness, presence of ulceration, number of positive nodes and positive NSLN status correlated with poor disease-specific survival. We also performed a multivariate analysis to confirm that the decrease in survival was not merely due to an increase in the number of positive nodes. In a Cox model with stepwise selection, age, thickness, and nonsentinel positive node were the only significant factors (all p < 0.01). In contrast, number of positive nodes was not significant in the final model (p = 0.12). Patients with a positive NSLN had an increased relative risk of death from melanoma compared with those with negative NSLN.
TABLE 3.
Analysis of factors predictive of disease-specific survival
| Univariate P | Multivariate P | Hazard ratio | 95% CI | |
|---|---|---|---|---|
| Age | 0.003 | 0.003 | 1.0 | 1.01–1.04 |
| Sex | 0.292 | |||
| Thickness | <0.001 | <0.001 | 1.1 | 1.06–1.16 |
| Number of positive nodes | <0.001 | 0.100 | 1.1 | 0.97–1.30 |
| Positive NSLN | <0.001 | <0.001 | 2.5 | 1.51–4.26 |
DISCUSSION
In this paper, we demonstrate the prognostic significance of metastatic disease in the completion lymph node dissection specimen. The presence of a positive NSLN was an independent predictor of melanoma-specific mortality. Indeed, there were no long-term survivors beyond 6 years. Clearly, increasing number of lymph nodes also portends worse prognosis, as reflected in the current staging system.4 While it is difficult to completely separate increasing tumor burden from NSLN status, the presence of a positive NSLN, even among a group of patients with an equal number of positive nodes, was still associated with worse survival.
Previous studies have looked at predictors of positive NSLN and their correlation with survival in patients with a positive SLN. As shown in Table 4, increasing thickness of the primary tumor, and size of the tumor burden in the SLN lymph node, are associated with a worse outcome.12–16 The tumor burden was not uniformly characterized over the time of the study, and therefore could not be included in our analysis. Future studies will investigate the contribution of the tumor burden in this population. However, other studies have also corroborated the importance of the NSLN status. As shown in Table 5, a recent paper from Roka et al. demonstrated 97% survival in patients with a negative NSLN, and 59% survival in patients with a positive NSLN, with median follow-up of 31 months.16 An additional study by Cascinelli et al. had similar results to our study, and in the group with a positive NSLN, there were no survivors beyond 8 years.17
TABLE 4.
Predictors of survival in patients with a +SLN
| Study | Age | Ulceration | Gender | Primary site | Breslow thickness | SLN tumor burden | DDC | No. of + nodes | NSLN status | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Starz 200112 | +* | N/A | – | +* | +* | +* | N/A | N/A | N/A | S3 class most important on MV analysis |
| Carlson 200313 | – | – | N/A | – | +* | +* | N/A | + | N/A | Size > 2 mm in SLN |
| Ranieri 200214 | – | – | – | – | N/A | +* | N/A | – | N/A | Size > 3 mm in SLN |
| Cochran 200415 | N/ | A | N/A | N/A | N/A | +* | +* | +* | N/A | N/A |
| Roka 200816 | N/ | A | + | – | N/A | + | + | N/A | – | + |
| Current study | + | – | – | N/A | +* | N/A | N/A | + | +* | +NSLN most important on MV analysis |
DDC density of dendritic cells/mm2; MV multivariate; SLN sentinel lymph node; NSLN nonsentinel lymph node; +, significant on univariate analysis; +*, significant on multivariate analysis; N/A not analyzed
TABLE 5.
Summary of survival in patients with positive NSLN
The reasons why NSLN status is so predictive of outcome are not clear. One potential explanation for the poor survival in the NSLN–positive patients is the method of detection. These patients had melanoma in NSLNs detected by H&E staining alone, and therefore represent a group of patients with clinically significant tumor volume. If the NSLNs had been examined as rigorously as the SLNs with serial sectioning and IHC, presumably the yield of microscopically positive NSLNs would be higher. In that case, while the survival of NSLN positive patients would be higher, the prognostic value of the NSLN might be lost. However, in studies where IHC was performed on all CLND specimens, the CLND positivity rate was only as high as 21–24%, and in some cases, no additional disease was detected by the addition of IHC to routine H&E staging.15,18 Therefore, the maximal potential incremental yield of 5–8% by IHC detection, over the rate of 16% demonstrated in the large MSLT-I trial, is unlikely to completely account for the prognostic importance of a positive NSLN noted in this study.
Alternative explanations for the importance of a NSLN address the importance of tumor biology. On the one hand, a positive NSLN may be a reflection of a more aggressive tumor phenotype. In this case, lymph node disease is merely a surrogate of the distant disease that will likely become manifest clinically at some point in the future, which is represented by a positive NSLN. Alternatively, the metastatic phenotype may allow the spread of melanoma to follow an orderly progression of events. In this case, the SLN is the first step along that pathway, and the NSLN is the second step along this pathway. If this is true, then the biologic events that allow escape beyond the first-order nodes may be distinct.
It will be important to examine the characteristics of these positive SLN and NSLN nodes in the future, to determine if distinct genetic and/or immunologic changes allow the melanoma cells to escape beyond these first-order nodes. It has been previously documented that an active process of lymphangiogenesis accompanies the progression to a positive SLN, and that SLNs express increasing inflammatory and overall immunosuppressed phenotype when compared with tumor negative NSLNs.19–21
In conclusion, this study demonstrates that NSLN status is an independent predictor of disease-specific survival in patients with melanoma metastatic to the SLN. The findings in this paper suggest that NSLN status provides important prognostic information. Only the prospective, randomized MSLT-II trial can comment on the therapeutic impact of a CLND.
REFERENCES
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 2.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17(3):976–83. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–11. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 5.Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97(6):1488–98. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 6.Francken AB, Shaw HM, Thompson JF, et al. The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Ann Surg Oncol. 2004;11(4):426–33. doi: 10.1245/ASO.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006;13(6):809–16. doi: 10.1245/ASO.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 8.McMasters KM, Wong SL, Edwards MJ, et al. Frequency of nonsentinel lymph node metastasis in melanoma. Ann Surg Oncol. 2002;9(2):137–41. doi: 10.1007/BF02557364. [DOI] [PubMed] [Google Scholar]
- 9.Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351(9105):793–6. doi: 10.1016/s0140-6736(97)08260-3. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol. 2001;8(2):101–8. doi: 10.1007/s10434-001-0101-x. [DOI] [PubMed] [Google Scholar]
- 11.McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol. 2001;8(3):192–7. doi: 10.1007/s10434-001-0192-4. [DOI] [PubMed] [Google Scholar]
- 12.Starz H, Balda BR, Kramer KU, Buchels H, Wang H. A micromorphometry-based concept for routine classification of sentinel lymph node metastases and its clinical relevance for patients with melanoma. Cancer. 2001;91(11):2110–21. [PubMed] [Google Scholar]
- 13.Carlson GW, Murray DR, Lyles RH, Staley CA, Hestley A, Cohen C. The amount of metastatic melanoma in a sentinel lymph node: does it have prognostic significance? Ann Surg Oncol. 2003;10(5):575–81. doi: 10.1245/aso.2003.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Ranieri JM, Wagner JD, Azuaje R, et al. Prognostic importance of lymph node tumor burden in melanoma patients staged by sentinel node biopsy. Ann Surg Oncol. 2002;9(10):975–81. doi: 10.1007/BF02574515. [DOI] [PubMed] [Google Scholar]
- 15.Cochran AJ, Wen DR, Huang RR, Wang HJ, Elashoff R, Morton DL. Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol. 2004;17(7):747–55. doi: 10.1038/modpathol.3800117. [DOI] [PubMed] [Google Scholar]
- 16.Roka F, Mastan P, Binder M, et al. Prediction of non-sentinel node status and outcome in sentinel node-positive melanoma patients. Eur J Surg Oncol. 2008;34(1):82–8. doi: 10.1016/j.ejso.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Cascinelli N, Bombardieri E, Bufalino R, et al. Sentinel and nonsentinel node status in stage IB and II melanoma patients: two-step prognostic indicators of survival. J Clin Oncol. 2006;24(27):4464–71. doi: 10.1200/JCO.2006.06.3198. [DOI] [PubMed] [Google Scholar]
- 18.Scolyer RA, Li LX, McCarthy SW, et al. Immunohistochemical stains fail to increase the detection rate of micrometastatic melanoma in completion regional lymph node dissection specimens. Melanoma Res. 2004;14(4):263–8. doi: 10.1097/01.cmr.0000136708.90534.71. [DOI] [PubMed] [Google Scholar]
- 19.Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18(9):1232–42. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 20.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6(9):659–70. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 21.Torisu-Itakura H, Lee JH, Scheri RP, et al. Molecular characterization of inflammatory genes in sentinel and nonsentinel nodes in melanoma. Clin Cancer Res. 2007;13(11):3125–32. doi: 10.1158/1078-0432.CCR-06-2645. [DOI] [PubMed] [Google Scholar]