Abstract
Autocrine motility factor (AMF) enhances invasion by breast cancer cells, but how its secretion and effector signaling are controlled in the tumor microenvironment is not fully understood. In this study, we investigated these issues with a chimeric AMF that is secreted at high levels through a canonical ER/Golgi pathway. Using this tool, we found that AMF enhances tumor cell motility by activating AKT/ERK, altering actin organization and stimulating β-catenin/TCF and AP-1 transcription. EGF enhanced secretion of AMF through its casein kinase 2-mediated phosphorylation. RNAi-mediated attenuation of AMF expression inhibited EGF-induced invasion by suppressing ERK signaling. Conversely, exogenous AMF overcame the inhibitory effect of EGFR inhibitor gefitinib on invasive motility by activating HER2 signaling. Taken together, our findings show how AMF modulates EGF-induced invasion while affecting acquired resistance to cytotoxic drugs in the tumor microenvironment.
Keywords: Autocrine motility factor (AMF); phosphoglucose isomerase (PGI, GPI); epidermal growth factor (EGF); cell motility; invasion
Introduction
Autocrine motility factor (AMF) was originally isolated in the conditioned medium of melanoma cancer cells and stimulates both direct and random migration (1). Before identification of its primary sequence, it was also known by other names; Neuroleukin (NLK) promoting growth of embryonic spinal and sensory neurons, and maturation factor (MF) mediating differentiation of myeloid leukemia cells (2, 3). Several researchers independently identified that secreted AMF has the same sequence as phosphoglucose isomerase (PGI, GPI), which catalyzes the reversible isomerization of glucose-6-phosphate and fructose-6-phosphate in glycolysis and gluconeogenesis (4). AMF belongs to the orphan C-X-X-C cytokine family. Other members of the C-X-X-C family include oxidoreductases, e.g. macrophage migration inhibitory factor (MIF), and thioredoxin (Trx) (5, 6). These C-X-X-C proteins bind to more than one receptor or partner, e.g. MIF binds to CXCR2, CXCR4 and CD74 (7, 8). Likewise, AMF binds to AMFR/gp78 as a G-protein coupled receptor and HER2, leading to activation of PI3K/AKT and MAPK/ERK pathways in HER2-expressing breast cancer cells (9, 10). Moreover, C-X-X-C members lack a secretion leader sequence governing ER/Golgi-dependent secretion and might be secreted via non-conventional secretion. Of note, clinical observations have revealed an aberrant secretion of PGI/AMF into the blood and urine of cancer patients, as well as the circulation and synovial fluids of rheumatoid arthritis (RA) patients (4, 11).
Functional studies of AMF-transfected breast cancer cells (MCF10A) revealed the contribution of AMF to epithelial-to-mesenchymal transition (EMT) via downstream regulation of miRNA and the switch of EMT gene markers (12). Furthermore, silencing of AMF expression inhibits anchorage-independent growth of tumor cells and tumor growth in nude mice (13). Previously, AMF studies have addressed the molecular characteristics of its cytokine properties and downstream molecular networks, but failed to resolve its linkage to other tumor-associated growth factors facilitating oncogenic signaling pathways.
Cancer invasion is a coordinated process involving dynamic regulation of cell-cell adhesion, extracellular matrix (ECM) degradation and adhesion (14, 15). Extrinsic stimulation of growth factors, including EGF and TGF-β induces tumor cell invasion, although cancer cells have intrinsic and oncogenic mutations to drive tumor development (16). In this aspect of extrinsic modulation of cancer progression, it is meaningful to understand how endogenous AMF secretion is regulated and linked to growth factor-induced invasion in breast carcinoma cells, because therapeutically targeting a single signaling pathway is not completely effective in many cases (17, 18).
The objective of this study was to determine the secretory mechanisms of AMF upon micro environmental stimulus. We show here that AMF is secreted from human breast cancer cells following serine phosphorylation by CKII in response to EGF, and suggest that it cooperates with EGF in the induction of cell invasion.
Materials and methods
Cell culture and synchronization
T47D, MDA-MB-231, SKBR3 breast cancer and EBNA 293 cells (ATCC) were cultured at 37°C with 5% CO2 in DMEM (Invitrogen) supplemented with 10% FBS (Atlanta Biological). All experiments were performed at exponential growth and cell synchronization was achieved by serum-free medium for 16 hrs.
Chemicals and Antibodies
The BD Matrigel™ Basement Membrane Matrix, BD BioCoat™ BD Matrigel Invasion Chamber, and β-catenin were purchased from BD Transduction Laboratories. Monoclonal anti-PGI (12F9A6, Pfizer) and rabbit anti-PGI (H300, Santa Cruz) antibodies were used for Western blot analysis and immunoprecipitation. Anti-p-AKT (Ser473), AKT, p-EGFR, EGFR, and p-HER2 antibodies, Wortmannin (PI3 kinase inhibitor), and U0126 (MEK1/2 inhibitor) were from Cell Signaling. Anti-vimentin, c-jun, p-ERK (E-4), ERK1/2(MK1), HER2 antibodies, TBCA [(E)-3-(2, 3, 4, 5-Tetrabromophenyl) acrylic acid], Casein Kinase II Inhibitor I (TBB) were purchased from Santa Cruz. Anti-rabbit IgG-TRITC and anti-IgG-FITC antibodies, Phalloidin-TRITC (actin staining), and doxycycline were purchased from Sigma. EGF and Amicon centrifugal filter devices were purchased from Upstate Biotechnology.
Plasmids and transfection
We performed overlapping PCR after achievement of two fragments including signal peptide IgK fragment and Flag-fused human PGI/AMF product, followed by primer sets: EcoRI-signal-IgK-F: 5’-GAATTCGCCACCATGGAGACAGACACACTCCTGCTATGGGTAC-3’, IgK-signal-R: 5’-GTCACCAGTGGAACCTGGAACCCAGAGCAGCAGTACCCATAGCAGGAG-3’, Flag-hPGI-F: 5’-GGT TCCACTGGTGACGATTACAAGGATGACGACGATAAGGCCGCTCTCAC CCGGGAC-3’, hPGI-XbaI-R: 5’-GCTCTAGATTATTGGACTCTGGCCTCGCG-3’. The PCR products of sp-flag-AMF fragment were cloned into tet-on expression vector (Clontech). T47D cells were transfected with Lipofectamine® LTX Reagent (Invitrogen) and selected 3 weeks in antibiotics for mixed population of stable clones.
Western blot and immunoprecipitation
The cells were extracted in lysis buffer [20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and proteases inhibitors (Roche)]. After BCA protein assay (Pierce), 25~50 µg of total lysate was loaded and immunoblotted for regular Western blot. 500 εg of lysates were used for immunoprecipitation with appropriate antibodies for 16 hour at 4°C and washed with lysis buffer and were subjected to immunoblotting.
PGI Enzymatic Activity
The enzymatic activities of immunoaffinity-purified AMFs were measured as described previously (19). Briefly, we pre-incubated a reaction mixture consisting of 0.1 M Tris (pH 8.5), 4 mM fructose 6-phosphate, 0.5 mM NADPH, and 1 unit of glucose-6-phosphate dehydrogenase for 10 min, and purified AMF was added to the mixture and its enzymatic activity was immediately monitored at A340 nm using a Shimadzu spectrophotometer.
AMF secretion in conditioned media
Confluent cells were cultured for 3 days in completed medium, 10% FBS, and then were starved with serum-free medium with inhibitors or EGF. The supernatant was filtered through a 30-kDa cut-off filter (Millipore). AMF secretions were measured in Western blot, after normalization and loading, dependent on cell number.
Luciferase assay
Reporter assays were performed as previously mentioned (20). Briefly, cells were plated onto six-well plates (21). Two days following transfection, luciferase and Renilla activity of lysates was measured with Dual-Glo luciferase reagents (Promega). Luciferase activities were normalized against Renilla activity, and relative ratios for each transfection were calculated and represented. Experiments were performed on at least two independent occasions, and error bars indicate SE.
Transwell assay
Transwell (Corning Costar) was used for migration assay and BD BioCoat™ BD Matrigel Invasion Chamber was used for invasion assay. A total of 5 × 104 cells in serum-free medium were introduced into the upper compartment of Transwell chambers (8 εm pore) and were allowed to migrate in lower chambers with 10% FBS, Matrigel or EGF (100 ng/ml) for 24 hours. Migrated or invaded cells were fixed and stained with Hema-3 stain kit (Fisher). Each condition was assayed in triplicate and each experiment was repeated 3 times.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked in 1% bovine serum albumin/PBS, and incubated with primary antibodies, then incubated with secondary antibodies. After each antibody treatment, cells were washed three times with 0.1% bovine serum albumin/PBS each. Next, cells were stained with DAPI (4′, 6-diamidino-2-phenylindole), washed and mounted on a glass slide with 80% glycerol. Fluorescent images were acquired using a color Sony camera connected to a fluorescence Olympus microscope.
Statistical Analysis
Data from triplicate experiments are expressed as mean ± SD or SE. Comparisons between the groups were determined by using one-way analysis of variance test. P<0.05 was considered statistically significant.
Results
Signal peptide-fused AMF enhances cell motility via activation of PI3K/AKT and MAPK/ERK
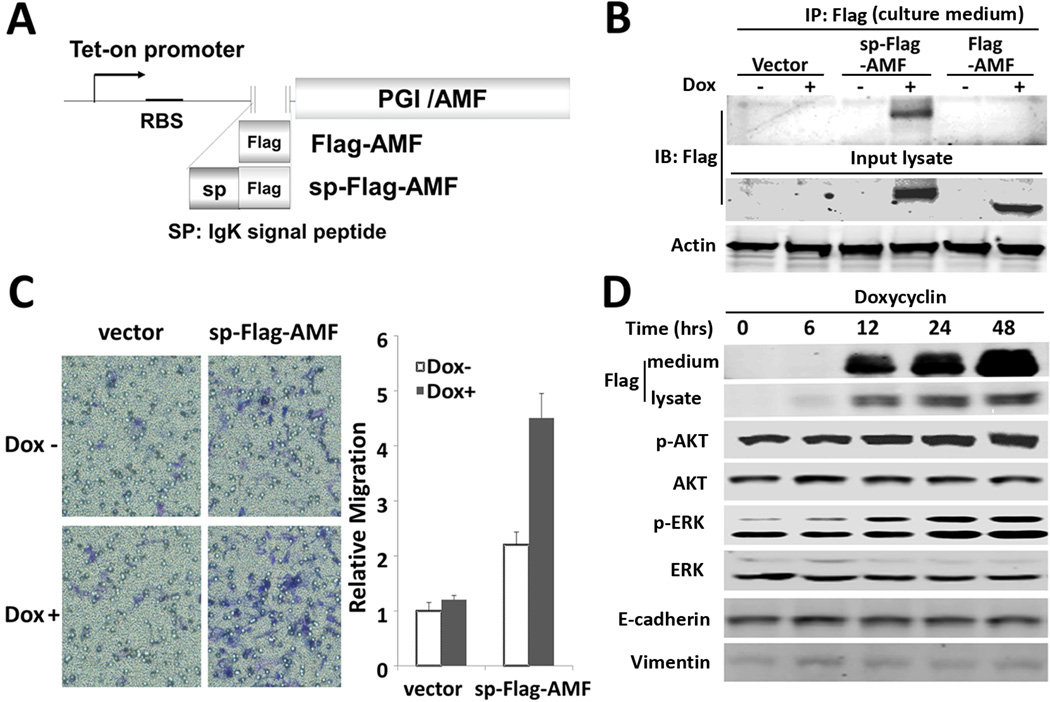
AMF plays a role in glucose metabolism and enhances cell migration. In order to differential between intracellular and extracellular AMF, we have developed a chimeric AMF construct needed for AMF over-secretion and dependent on canonical pathway by adding a secretion leader sequence (sp) (Fig. 1A). In addition, an inducible promoter (tet-on system) was used to avoid a permanently adapted phenotype, which might be generated by constitutive promoter-driven AMF expression during the selection and cell cloning processes. T47D breast cancer cells were chosen, as they are AKT/ERK activated by purified AMF (10). We have T47D cells stably transfected with sp-Flag-AMF and its counterpart of Flag-AMF (Fig. 1A) and migration was tested thereafter. We have detected a significant secretion of sp-Flag-AMF (Fig. 1B) as compared with Flag-AMF transfectants, which fail to secret AMF under the conditions tested. Cell motility was enhanced in cells transfected with chimeric AMF, indicating no difference from endogenous AMF, regardless of the route of their secretion (Fig. 1C). To examine the activation of cellular signaling pathways by chimeric AMF secretion as observed in endogenous AMF-treated cells (10), a time course experiment was performed and showed that overexpression of chimeric AMF activates AKT and ERK (Fig. 1D), which was not activated with doxycycline in control cells (Data not shown), indicating a similar function of chimeric AMF and endogenous AMF in migration and AKT/ERK activation.
Figure 1.
Signal peptide-fused AMF enhances cell motility via activation of PI3K/AKT and MAPK/ERK. A, Illustration of IgK signal peptide (sp) fused to human PGI/AMF. B, Intracellular expression and secretion of sp-Flag-AMF and Flag-AMF were determined in culture medium, after immunoprecipitation (IP) with anti-Flag antibodies were carried out in stable cells which were treated with doxycycline (Dox, 1 εg/ml) for 24 hrs. C, Transwell migration assay in T47D/sp-Flag-AMF cells. Cells were induced with Dox for 24 hrs. and then subjected to migration assay for 24 hrs. Data are shown as mean ± SE of three independent experiments. D, Phosphorylation of AKT and ERK, expression of EMT markers in T47D/sp-Flag-AMF cells which were treated with Dox at the time points indicated. Total cell lysates were separated by SDS-PAGE and immunoblotted.
Enzyme activity of PGI/AMF is not indispensable for AKT/ERK activation
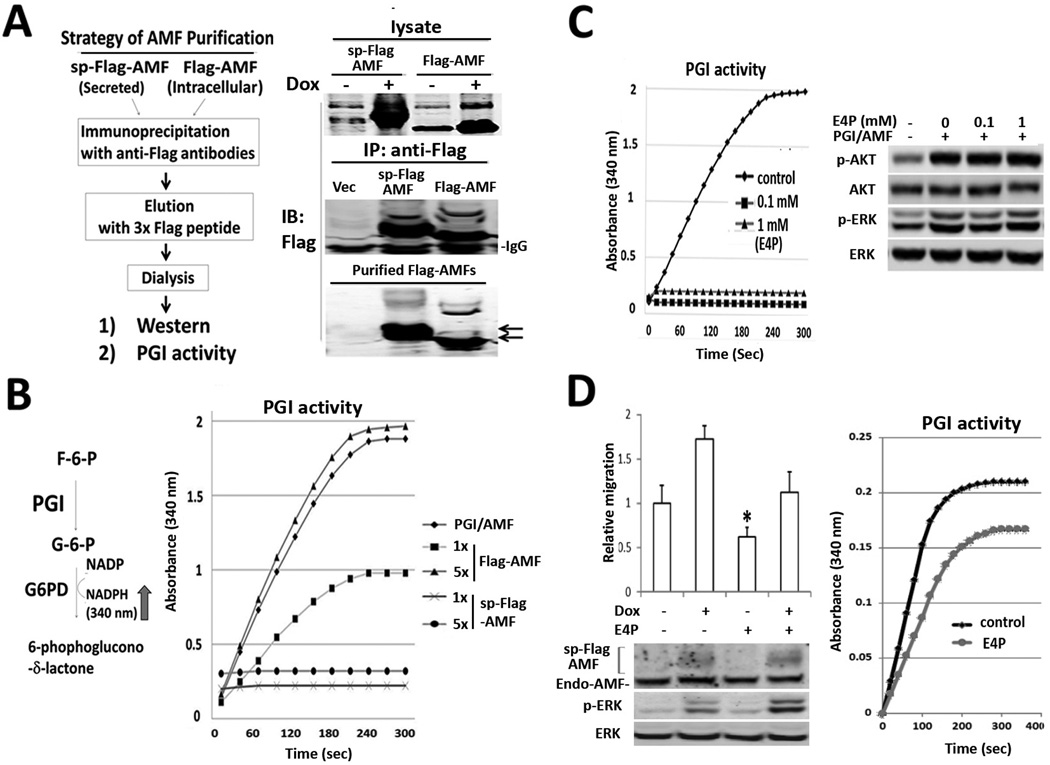
Previously, it was suggested that AMF-induced cell motility required an intact of the sugar binding domain of AMF for its cytokine function based on the data of migration suppression by erythrose 4-phosphate (E4P) which binds to active site of GPI isomerase (1). However, mutant constructs of CXXC motif of AMF lose isomerase activity but maintain characteristic of enhanced motility (22). Therefore, we questioned whether canonically secreted sp-Flag-AMF retains the PGI isomerase activity. To rule out the possible background of endogenously secreted AMF, we purified sp-Flag-AMF by affinity chromatography with anti-Flag antibodies (Fig. 2A) and found a loss of isomerase activity in sp-Flag-AMF, unlike Flag-AMF (Fig. 2B), implying that the isomerase activity of chimeric AMF is not indispensable for AKT/ERK activation. We returned to main question whether extracellular isomerase activity of endogenous AMF is not essential to AKT/ERK activation. We pretreated purified AMF with erythrose 4-phosphate (E4P) to inhibit isomerase activity as it previously reported to be competitive inhibitor against Glucose-6-Phosphate (Fig. 2C, left). Interestingly, E4P-pretreated AMF still stimulates cells, leading to p-AKT/p-ERK elevation, indicating no effect of E4P on AKT/ERK activation (Fig. 2C, right). To clarify the effect of E4P on cell migration, we performed migration assay in T47D/sp-Flag-AMF cells due to loss of enzymatic activity of sp-Flag-AMF. The results exhibit inhibitory effect of E4P upon non-induced condition as well as the condition expressing sp-Flag-AMF, indicating indirect effect of E4P on cell migration (Fig. 2D, left). We addressed a raising possibility whether E4P inhibits intracellular PGI/AMF activity in glycolytic metabolism instead of E4P effect on extracellular AMF. After E4P-treated cells were severally washed to avoid E4P contamination in lysate, intracellular G6P isomerase activity was measured (Fig. 2D, right). In conclusion, the carbohydrate-binding motif of AMF is indispensable for AKT/ERK activation and E4P at least inhibits migration via intracellular AMF binding. However, we cannot still exclude the possibility that AMF enhances migration, dependent on carbohydrate binding motif, because migration is dynamic and complex process, not only dependent on kinase activation such as AKT/ERK.
Figure 2.
Enzyme activity of PGI/AMF is not indispensable for AKT/ERK activation. A. Strategy of Flag-AMF purification (left). Products are subjected to immunoblotting analysis during serial steps of purification (right). B, Enzyme activity of glucose-6-phosphate isomerase was analyzed by spectrophotometer, which detects NADPH production by coupling reaction of glucose-6-phosphate dehydrogenase (G6PD) and PGI (left). 2.5 ng (1×) of Flag-AMF and sp-Flag-AMF were measured for their activities (right). C, Effect of E4P on PGI activity and AKT/ERK activation. 2.5 ng of purified PGI/AMF were preincubated with 0, 0.1, 1 mM of E4P at tube, respectively and subjected to enzymatic spectrophotometer assay (left). T47D parental cells were induced with E4P-pretreated PGI/AMF and analyzed (right). D, Effect of E4P on cell motility and intracellular PGI/AMF enzyme activity in T47D/sp-Flag-AMF cells. The cells migrated to bottom wells contained compounds as indicated for 16 hrs. PGI activity of lysate (25 εg) was measured in T47D/sp-Flag-AMF cells treated with 1 mM E4P for 16 hrs.
Chimeric AMF activates β-catenin/TCF and AP-1 transcription, a marker of motility and invasion
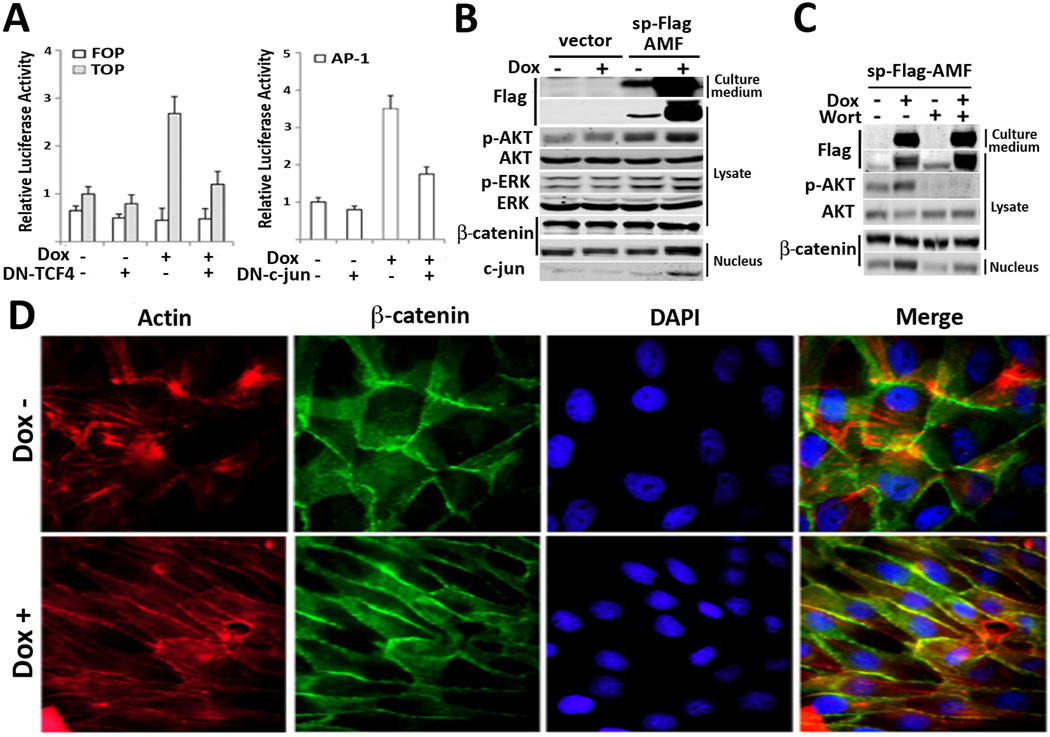
Next, we questioned whether chimeric AMF affects gene expression, which is indeed associated with cell migration. As ectopic expression of AMF disrupts cell-cell adherent junctions via dissociation of E-cadherin/β-catenin complex in MCF10A cells (12), we used TOPFlASH reporter of Wnt signaling in which nuclear translocation of β-catenin initiates Wnt-responsive gene expression after associating with TCF in the nucleus (23). In addition, we investigated Activating protein 1 (AP-1) transcription which is composed of the c-jun/c-fos regulator complex, because it is mainly regulated by the MAPK signaling pathway and promotes the expression of metalloproteases such as MMP1 and MMP3 required for extracellular matrix degradation (24). T47D/sp-Flag-AMF cells were co-transfected with reporters and/or dominant negative TCF4 (DN-TCF) and DN-c-jun. The results revealed that AMF induced both TOPFLASH and AP-1 activities but the transcriptions were suppressed by cotransfection of dominant negative constructs (Fig. 3A). Next, a supporting experiment was performed for confirming elevated levels of nucleus β-catenin and c-jun (Fig. 3B). As EGF/EGFR can activate β-catenin via receptor tyrosine kinase-PI3K/AKT pathway in invasion and metastasis of cancer cells (25) and chimeric AMF can also induce PI3K/AKT, we addressed whether EGF/EGFR and/or PI3K/AKT is associated with AMF-activated β-catenin pathway. The results showed that EGFR inhibitor did not affect AMF-induced translocation of β-catenin (supplemental Fig. 1) and Wortmannin, covalent inhibitor of phosphoinositide 3-kinase (PI3K) showed significant reduction of translocated β-catenin along with abrogation of p-AKT in chimeric AMF expressed cells (Fig. 3C). In conclusion, sp-Flag-AMF induces cell motility through AKT/ERK signaling pathways as well as β-catenin/TCF and AP-1 transcriptional activations. Next, immunocytochemistry was executed to further analyze the process of cell motility induced by AMF (Fig. 3D). AMF-secreting cells along with Dox induction showed straight organization of actin filaments along with lateral stretching cell shape, compared to the round shape of control cells. But, nuclear β-catenin and loss of cell-to-cell contact were not significant, indicates that AMF alone is not sufficient for inducing EMT, showing cell-scattering effect (15). Thus, the data suggested that AMF plays a role of modifier in the EMT process and might coordinate with extrinsic growth factors relative to EMT and invasion (26).
Figure 3.
Chimeric AMF activates β-catenin/TCF and AP-1 transcription, a marker of motility and invasiveness. A, Transcriptional reporter assays in T47D/sp-flag-AMF cells which were transiently transfected with TCF response (TOPFlASH) or AP-1 response (AP-1 Luc) reporters or dominant negative (DN)-TCF and DN-c-jun. Doxycycline was simultaneously treated with transfection. Data are displayed as mean ± SD of three independent experiments. B, T47D/sp-Flag-AMF cells were induced by Dox for 24hrs and then lysates, nuclear fraction were immunoblotted for expression levels of nuclear β-catenin and c-jun. C, T47D/sp-Flag-AMF cells were treated with Wortmannin inhibitor for 24 hrs to analyze its effect on β-catenin translocation. D, Immunofluorescence images of T47D/sp-Flag-AMF cells with Dox induction for 96 hrs. Phalloidin and DAPI were used for Actin and nucleus staining respectively. Lateral extension of cells is shown upon Dox stimulus (original magnification, ×100).
EGF enhances endogenous AMF secretion in breast cancer cells
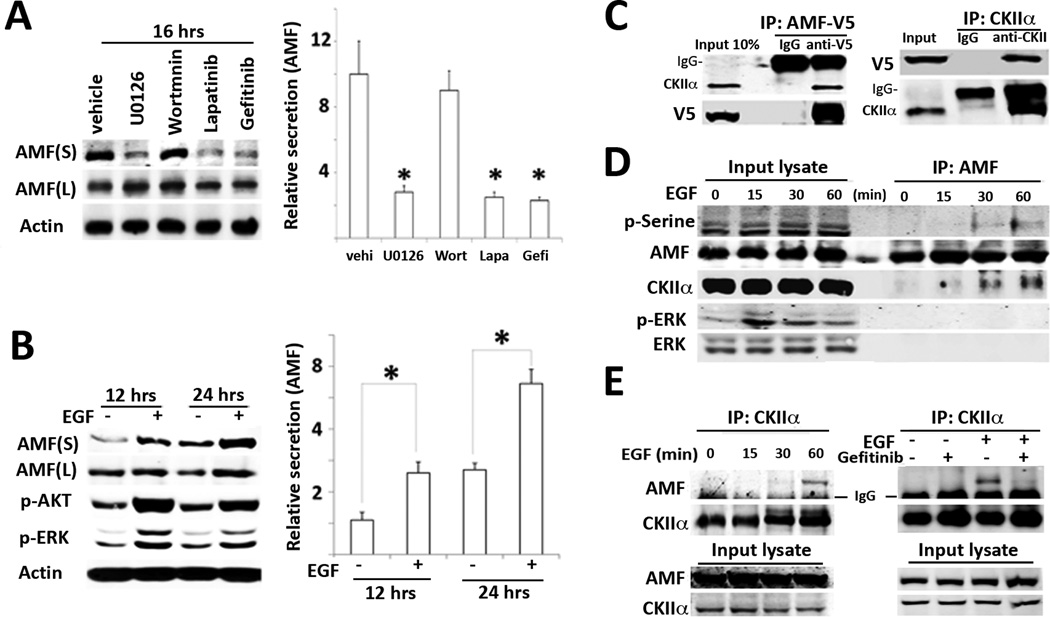
While the above overexpression and chimeric studies delineate some of the biochemical function of AMF, it remains unknown whether the mechanism underlying AMF secretion is linked to any other growth factor(s), which is implicated in EMT and cell invasion processes in the tumor microenvironment. Previously, we have reported that HER2 (ErBB2) knockdown and Herceptin (anti-HER2 antibodies) inhibit endogenous AMF secretion, served as a clue suggesting that AMF secretion might be regulated by HER receptor pathway and/or its downstream signaling pathways including the dual pathways of PI3K/AKT and MAPK/ERK (27). Therefore, we performed pharmacological inhibition studies of cell signal transduction to clarify the signaling cascade responsible for AMF secretion in HER2-overexpressing SKBR3 cells. As shown in Fig. 4A, AMF secretion was significantly reduced by the MEK inhibitor (U0126), Lapatinib (EGFR, HER2 dual inhibitor), and Gefitinib (EGFR inhibitor), indicating that the intracellular MEK pathway of HER family downstream is responsible for AMF secretion. As these inhibitors usually affect cell growth status, we wanted to know whether AMF secretion is positively up-regulated by MEK kinase relative to HER family signal transduction. We employed EGF ligand and examined its effect on AMF secretion in AMF low-secreting T47D cells, resulting in enhancement of AMF secretion (Fig. 4B). Meanwhile, this inhibition and increase of AMF secretions were unlikely to be affected by cell numbers and cell growth, because of our measuring AMF secretion within a 24 hrs. timeframe and similar cell numbers in each group.
Figure 4.
EGF enhances CKII-mediated AMF secretion. A, HER2-overexpressing SKBR3 cells were starved with serum-free medium and treated with inhibitors for 16 hrs. respectively. 100 nM Wortmannin (PI3K inhibitor), 20 εM U0126 (MAPK inhibitor), 10 εM Lapatinib (dual EGFR/HER2 inhibitor), and 10 εM Gefinitib (EGFR specific inhibitor) were used. Culture supernatant (S) was concentrated with 30-kDa cut-off membrane filters. After viable cell counting, culture supernatant is subjected to immunoblotting. S and L indicate culture medium and lysate respectively. B, T47D cells were stimulated with EGF (100 ng/ml) as indicated time points. P-AKT/p-ERK is positive control in response to EGF. Data are representative as the mean ± SE of three independent experiments. * P > 0.05 significantly different from vehicle. C, Lysates were prepared from EBNA293 cells transiently transfected with AMF-V5 and immunoprecipitated with indicated antibodies. IgG indicates heavy chain of IgG. D, T47D cells were stimulated with EGF after serum starvation as indicated time course. IP were performed with anti-AMF antibodies. E. IP with anti-CKII antibodies in T47D cells stimulated with EGF at indicated time points (left). The cells were pretreated with Gefitinib (10 εM) for 2 hours and induced with EGF for 1hr.
CKII kinase interacts with AMF under EGF stimulus
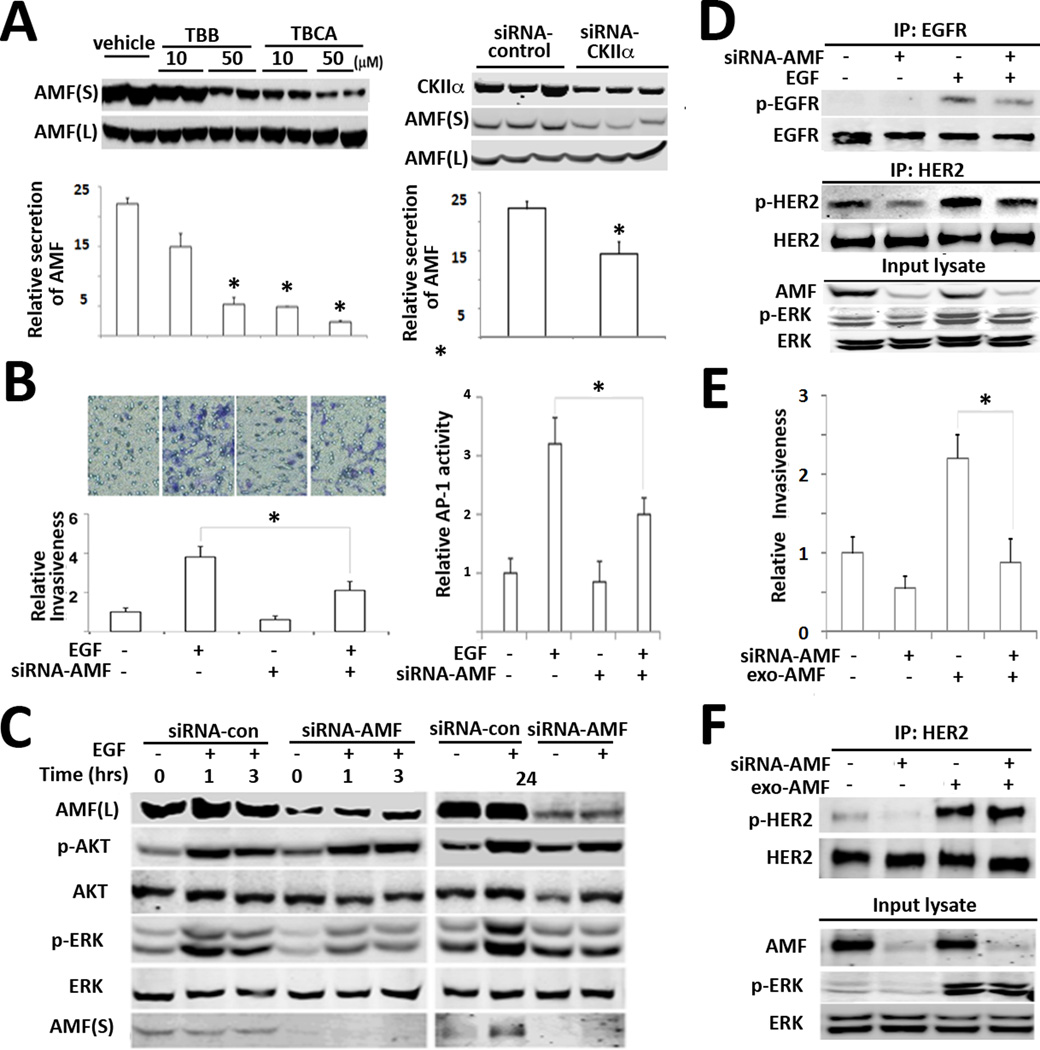
Previously, in vitro kinase assay showed that CKII serine/threonine kinase phosphorylates AMF (28). Of note, it was reported that CKII is ubiquitously expressed and activated in response to various growth-factor stimuli including EGF (29). Therefore, we hypothesized that CKII endogenously interacts with AMF and promotes its secretion following EGF stimulus. Initially, AMF-V5 was transfected into EBNA293 cells to examine interaction of AMF-V5 and CKII. Expectedly, reciprocal immunoprecipitation (IP) studies showed that AMF-V5 binds to CKII (Fig. 4C). The results encouraged us to explore endogenous interaction of AMF and CKII upon EGF stimulus. Obviously, time-course IP with anti-AMF antibodies showed that CKII interacts with AMF in a time dependent manner, resulting in serine phosphorylation of AMF (Fig. 4D). Reciprocally, it makes for convincing data that EGF induces interaction of CKII with AMF and Gefitinib, EGFR inhibitor abolished interaction of CKII and AMF (Fig. 4E). Next, we wondered whether CKII is responsible for AMF secretion. We employed AMF high-secreting MDA-MB 231 cells to examine inhibition effect of CKII on AMF secretion. We expected the reduction of AMF secretion under pharmacological inhibition of CKII, resulting in inhibition of AMF secretion by CKII inhibitors. Also, reduction of AMF secretion was observed under CKII knockdown (Fig. 5A). Because CKII-mediated AMF secretion occupies a position downstream of EGF (an invasion inducer), we wanted to determine the endogenous contribution of AMF secretion to EGF-induced invasion. Interestingly, AMF knockdown suppresses EGF-induced invasion and EGF-induced AP-1 transcription, indicating AMF impact and downstream modulation of EGF/EGFR (Fig. 5B). To understand how AMF knockdown affects the downstream signaling pathways of EGF/EGFR, we investigated the change of p-AKT/p-ERK levels. In response to EGF, we did not find significant differences of p-AKT in AMF-knockdown cells (Fig. 5C), implying the influence of PI3K constitutive mutation (H1047R) in T47D cells (30). However, we observed that p-ERK half-life is shortened upon AMF-knockdown in EGF-stimulated cells. Next, we performed immunoprecipitation to delineate how AMF knockdown influences on EGF-activated EGFR as well as HER2. The results exhibits that AMF knockdown reduces p-HER2 without EGF stimulus, which is not completely overridden with EGF stimulus (Fig. 5D). It indicates that EGF-induced invasion requires HER2 activation by AMF. To distinguish whether knockdown of intracellular AMF or extracellular AMF affects EGF-induced invasion, we performed add-back experiment with exogenous AMF. The results showed that exogenous AMF did not completely rescue cell motility in AMF-knockdown cells (Fig. 5E), regardless of restoration of HER2 and ERK activation (Fig. 5F). It is consistent with the observation of intracellular AMF suppression by E4P (Fig. 2E). Intracellular PGI/AMF activity or protein level might be prerequisite for cell motility. It is unclear whether or not reduced PGI/AMF isomerase activity affects glucose metabolism and energy generation during migration. In conclusion, we suggest the EGF-AMF axis for positive feedback loop of AKT/ERK activation, in which exogenous EGF ligands stimulate cancer cells and thereby increase AMF secretion. AMF activates more AKT/ERK pathway via HER2 in autocrine manner during cancer invasion.
Figure 5.
AMF knockdown suppresses EGF-induced invasiveness. A, MDA-MB 231 cells were treated with CKII inhibitor (TBB or TBCA) as indicated concentration for 16 hrs. Intracellular and extracellular AMF levels were determined in immunoblot (left). The cells were treated with siRNA-CKIIα and then secreted AMF level was analyzed (right). Bars indicate the mean ± SE of three independent experiments (* p > 0.05). B, after treatment with siRNA-AMF for 48 hrs., T47D cells were counted and loaded into upper side of transwell and chemoattractant of EGF (100 ng/ml) was added at bottom wells (left). Minus (−) indicates vehicle or siRNA-control. AP-1 transcriptional activity was determined in T47D cells, which were pretreated with siRNA-AMF for 24 hrs and then transfected with AP-1 reporter for 24 hrs. (right). Values are expressed as the mean ± SE of three replicates (* p> 0.05). C, Effect of AMF knockdown on EGF-induced p-AKT/p-ERK. After T47D cells were transfected with siRNA-AMF and starved in serum-free medium for 16 hrs. EGF was treated as indicated time points. D, IP with anti-EGFR or HER2 in MDA-MB-231 cells transfected with siRNA-AMF or control siRNA (−) for 48hr and starved, stimulated with EGF for 5 min. E. Rescue experiment of invasion with exogenous AMF in AMF-knockdown MDA-MB-231 cells. The cells were transfected with siRNA-AMF for 48 hrs and subjected to transwell assay. D. AMF-knockdown cells were stimulated with purified AMF and then IP were performed to analyze p-HER2 level.
AMF overcomes Gefitinib-suppressed invasiveness in breast cancer cells
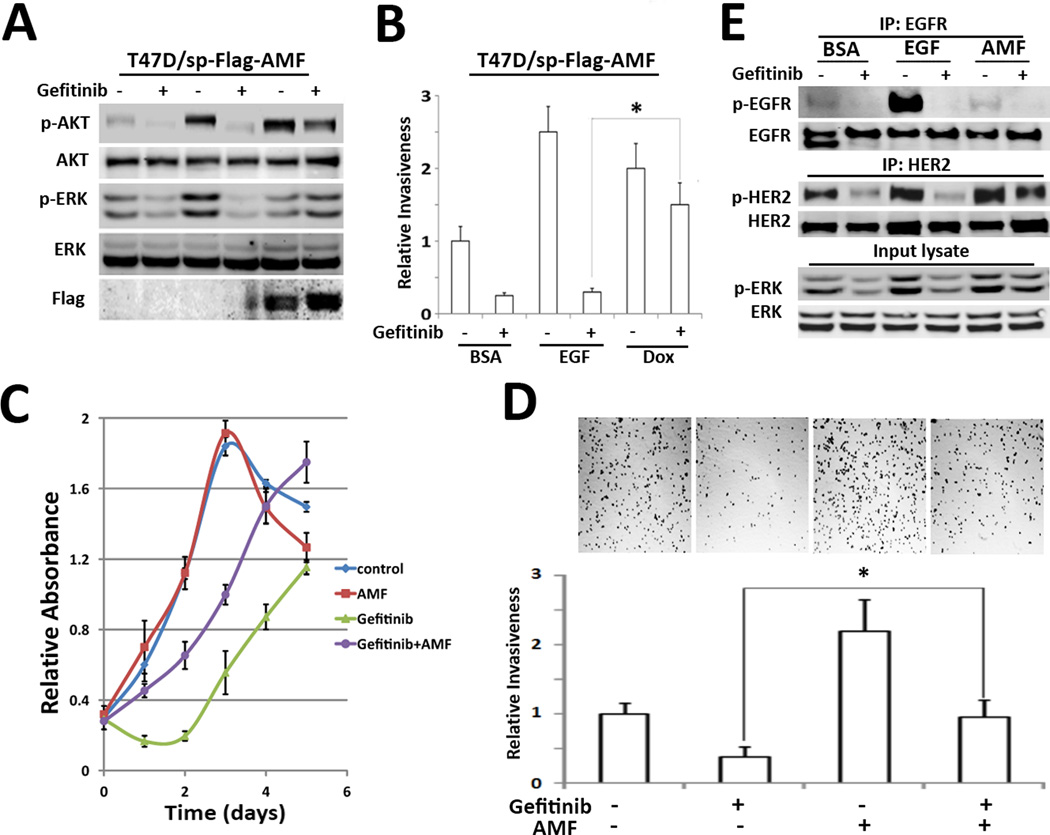
Breast cancer cells frequently exhibit intrinsic and acquired resistance to tyrosine kinase inhibitors in HER family and since AMF secretion is regulated by CKII kinase, which can be activated by other receptor tyrosine kinases and growth factors, while AMF can activate HER2, resulting in PI3K activation (10), it might be involved in the resistance against the tyrosine kinase inhibitor of EGFR (Gefitinib). Thus, to establish whether AMF plays a role in bypassing EGFR in therapy with Gefitinib, we treated T47D/sp-Flag-AMF with Gefitinib. We found that Gefitinib suppressed EGF-induced p-AKT/p-ERK, but chimeric AMF over-secretion overcame Gefitinib-inhibited AKT/ERK activation (Fig. 6A). Consistently, chimeric AMF over-secretion recovered invasiveness reduced by Gefitinib (Fig. 6B). Next, we addressed that these findings were reproducible at purified AMF treatment. These results highlight that AMF partially restore the growth and invasion in MDA-MB-231 cells inhibited by Gefitinib (Fig. 6C, D) and that HER2 activation is in part, responsible for restoration effect of AMF (Fig. 6E).
Figure 6.
AMF overexpression overcomes Gefitinib-inhibited invasion. A, Effect of AMF on Gefitinib-pretreated T47D/sp-Flag-AMF cells. After pretreatment with Gefitinib (10 µM) for 2 hrs, Dox and EGF were treated for 24 hrs. P-AKT/p-ERK statuses were analyzed. B, Gefitinib-pretreated cells were subjected to transwell assay with EGF or Dox induction. C, the growth of MDA-MB-231 cells was measured under Gefitinib and AMF treatment, using MTT assay. D, MDA-MB-231 cells were subjected to transwell assay. Each bottom side contained Gefitinib or purified AMF as indicated. Photographs show settle-down cells in bottom wells after cell invasion (* p> 0.05). E. MDA-MB-231cells were pretreated with Gefitinib and then stimulated with EGF or AMF for 5 min respectively. phosphorylation of EGFR and HER2 were analyzed with IP.
Discussion
Previously, the experimental attempts focused on biochemical characteristics and cytokine activity of AMF, which displays enhancement of migration in several cancer cells. However, since cancer cell motility, EMT, and invasion are a coordinated process of metastasis, in which the signaling regulation is highly complex, involving activation of numerously different pathways. That process frequently involves microenvironment crosstalk between Wnt, EGF, TGF-β, and Notch etc.(16, 31, 32). Thus, we addressed here the role of AMF and regulation of its secretion through oncogenic networks and crosstalk with their extrinsic factors in breast cancer cells. We demonstrated that AMF secretion is regulated by EGF/EGFR through CKII interaction and thereby promotes EGF-induced invasiveness. Our studies suggest a possible mechanism underlying the positive feedback of AMF on extrinsic EGF-induced invasion to enhance PI3K/MAPK signaling pathways in autocrine manner of AMF during tumor invasion and metastasis. Moreover, it is an attractive observation that AMF is able to overcome Gefitinib-inhibited invasion, although AMF secretion is partially dependent on EGF signaling and CKII-mediation. Especially, non-small-cell lung cancer (NSCLC) are frequently associated with EGFR genetic alteration (17, 33). However, PTEN mutation or c-Met amplification is proposed to provide initial or acquired resistance to Gefitinib (17, 33). Given that AMF over-secreting cells show resistance to Gefitinib in breast cancer cells, AMF might be involved in resistance to EGFR inhibitor in lung cancers. Accordingly, it might be important to examine the relationship of endogenous AMF secretion geared at Gefitinib-resistant NSCLC. In respect to cancer targeting, CKII is a candidate to block AMF secretion. It is doubtful because CKII is a ubiquitous kinase and has been shown to regulate numerous growth-related proteins as intracellular substrates in both normal and cancer cells (29). It may be argued that Gefitinib is sufficient for blocking AMF secretion. However, Gefitinib treatment clinically shows application limitation and resistance (17). Indeed, because AMF secretion is partially downregulated by Gefitinib, it remains elusive which microenvironment signals enhance AMF secretion. Therefore, AMF targeting can offer therapeutic benefits against the Gefitinib-resistant phenomena in combination therapy.
It should be appreciated that it has been difficult to delineate AMF therapeutic potential with an in vivo. Therefore, we have constructed a secretion leader sequence-fused to AMF cDNA in order to enhance secretion via the classical secretion pathway, resulting in AKT/ERK activation and augmentation of cell motility, similar to endogenous AMF function. For this reason, a classical secretion-dependent construct might be useful tool to study its therapeutic potential in a xenograft model injected with cancer cells containing the chimeric AMF as well as other cytokines and interleukins that are secreted via a nonclassical mode.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH/National Cancer Institute R01 CA51714 (A. Raz).
Footnotes
There are no potential conflicts to declare.
References
- 1.Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996;56:2960–2963. [PubMed] [Google Scholar]
- 2.Gurney ME. Neuroleukin: basic biology and functional interaction with human immunodeficiency virus. Immunol Rev. 1987;100:203–223. doi: 10.1111/j.1600-065x.1987.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Seiter K, Feldman E, Ahmed T, Chiao JW. The differentiation and maturation mediator for human myeloid leukemia cells shares homology with neuroleukin or phosphoglucose isomerase. Blood. 1996;87:4502–4506. [PubMed] [Google Scholar]
- 4.Fairbank M, St-Pierre P, Nabi IR. The complex biology of autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and its receptor, the gp78/AMFR E3 ubiquitin ligase. Mol Biosyst. 2009;5:793–801. doi: 10.1039/b820820b. [DOI] [PubMed] [Google Scholar]
- 5.Bertini R, Howard OM, Dong HF, Oppenheim JJ, Bizzarri C, Sergi R, et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, et al. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 7.Schwertassek U, Balmer Y, Gutscher M, Weingarten L, Preuss M, Engelhard J, et al. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz V, Kruttgen A, Weis J, Weber C, Ostendorf T, Lue H, et al. Role for CD74 and CXCR4 in clathrin-dependent endocytosis of the cytokine MIF. Eur J Cell Biol. 2012;91:435–449. doi: 10.1016/j.ejcb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Nabi IR, Watanabe H, Raz A. Identification of B16-F1 melanoma autocrine motility-like factor receptor. Cancer Res. 1990;50:409–414. [PubMed] [Google Scholar]
- 10.Kho DH, Nangia-Makker P, Balan V, Hogan V, Tait L, Wang Y, et al. Autocrine motility factor promotes HER2 cleavage and signaling in breast cancer cells. Cancer Res. 2013;73:1411–1419. doi: 10.1158/0008-5472.CAN-12-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, Muraki Y, Yasukochi T, Zhang H, Kori Y, Wakamatsu E, et al. Immunoglobulin G from anti-glucose-6-phosphate isomerase antibodies positive patient with rheumatoid arthritis induces synovitis in cynomolgus monkeys. Autoimmun Rev. 2005;4:475–478. doi: 10.1016/j.autrev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Funasaka T, Hogan V, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Research. 2009;69:5349–5356. doi: 10.1158/0008-5472.CAN-09-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funasaka T, Hu A, Yanagawa T, Hogan V, Raz A. Down-regulation of phosphoglucose isomerase/autocrine motility factor results in mesenchymal-to-epithelial transition of human lung fibrosarcoma cells. Cancer Research. 2007;67:4236–4243. doi: 10.1158/0008-5472.CAN-06-3935. [DOI] [PubMed] [Google Scholar]
- 14.Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cellular and Molecular Life Sciences. 2013;70:1335–1356. doi: 10.1007/s00018-012-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craene BD, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature Reviews Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vecchione L, Jacobs B, Normanno N, Ciardiello F, Tejpar S. EGFR-targeted therapy. Experimental Cell Research. 2011;317:2765–2771. doi: 10.1016/j.yexcr.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 19.Yanagawa T, Funasaka T, Tsutsumi S, Raz T, Tanaka N, Raz A. Differential regulation of phosphoglucose isomerase/autocrine motility factor activities by protein kinase CK2 phosphorylation. Journal of Biological Chemistry. 2005;280:10419–10426. doi: 10.1074/jbc.M409457200. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Bae JA, Seo YW, Kho DH, Sun EG, Lee SE, et al. Glycoprotein 90K, downregulated in advanced colorectal cancer tissues, interacts with CD9/CD82 and suppresses the Wnt/β-catenin signal via ISGylation of β-catenin. Gut. 2010;59:907–917. doi: 10.1136/gut.2009.194068. [DOI] [PubMed] [Google Scholar]
- 21.Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Seo YW, et al. KITENIN recruits Dishevelled/PKCd to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut. 2009;58:509–519. doi: 10.1136/gut.2008.150938. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi S, Gupta SK, Hogan V, Tanaka N, Nakamura KT, Nabi IR, et al. The enzymatic activity of phosphoglucose isomerase is not required for its cytokine function. FEBS letters. 2003;534:49–53. doi: 10.1016/s0014-6793(02)03773-0. [DOI] [PubMed] [Google Scholar]
- 23.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer and Metastasis Reviews. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 24.Verde P, Casalino L, Talotta F, Yaniv M, Weitzman JB. Deciphering AP-1 function in tumorigenesis: Fra-ternizing on target promoters. Cell Cycle. 2007;6:2633–2639. doi: 10.4161/cc.6.21.4850. [DOI] [PubMed] [Google Scholar]
- 25.Hu T, Li C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer and Metastasis Reviews. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 27.Yarden Y, Pines G. The ERBB network: At last, cancer therapy meets systems biology. Nature Reviews Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 28.Haga A, Niinaka Y, Raz A. Phosphohexose isomerase/autocrine motility factor/neuroleukin/maturation factor is a multifunctional phosphoprotein. Biochimica et Biophysica Acta - Protein Structure and Molecular Enzymology. 2000;1480:235–244. doi: 10.1016/s0167-4838(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 29.Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochemical Journal. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cellular Signalling. 2010;22:1369–1378. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Research. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giaccone G, Rodriguez JA. EGFR inhibitors: What have we learned from the treatment of lung cancer? Nature Clinical Practice Oncology. 2005;2:554–561. doi: 10.1038/ncponc0341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.