Abstract
Background
The optimal time to initiate venous thromboembolism pharmacoprophylaxis after blunt abdominal solid organ injury is unknown.
Methods
Postinjury coagulation status was characterized using thromboelastography (TEG) in trauma patients with blunt abdominal solid organ injuries; TEG was divided into 12-hour intervals up to 72 hours.
Results
Forty-two of 304 patients (13.8%) identified underwent multiple postinjury thromboelastographic studies. Age (P = .45), gender (P = .45), and solid organ injury grade (P = .71) were similar between TEG and non-TEG patients. TEG patients had higher Injury Severity Scores compared with non-TEG patients (33.2 vs 18.3, respectively, P < .01). Among the TEG patients, the shear elastic modulus strength and maximum amplitude values began in the normal range within the first 12-hour interval after injury, increased linearly, and crossed into the hypercoagulable range at 48 hours (15.1 ± 1.9 Kd/cs and 57.6 ± 1.6 mm, respectively; P < .01, analysis of variance).
Conclusions
Patients sustaining blunt abdominal solid organ injuries transition to a hypercoagulable state approximately 48 hours after injury. In the absence of contraindications, pharmacoprophylaxis should be considered before this time for effective venous thromboembolism prevention.
Keywords: Trauma, Blunt solid organ injury, Hypercoagulable
Critically injured trauma patients transition from an initial hypocoagulable state, characterized by protein C activation, fibrinolysis, and clotting factor dilution, depletion, and dysfunction,1 to an eventual hypercoagulable state, marked by an increased risk for venous thromboembolism (VTE). The most devastating complication of this hypercoagulable state is pulmonary embolism; the incidence of pulmonary embolism in trauma patients ranges from .13% to .55%, 2–9 with an associated mortality rate reported as high as 22%.2,4,8–10
Although the transition from a hypocoagulable to a hypercoagulable state in trauma patients has been recognized since the classic studies of Cannon in 1914,11 the precise timing of the change is unknown and is likely influenced by myriad variables, including injury severity, patient comorbidities, and surgical intensive care unit (ICU) therapies such as blood product transfusion. Pharmacoprophylaxis, most commonly with heparin or its derivatives, has been shown to decrease the incidence of VTE in trauma patients.12,13 Determination of the timing of initiation of VTE pharmacoprophylaxis involves weighing the risk for exacerbation of bleeding against that of VTE. This decision has become more complex in light of the trend toward nonoperative management of solid organ injuries. Typically, an arbitrary time period of 2 to 5 days is selected before the initiation of pharmacoprophylaxis in patients with blunt solid organ injuries who have been selected for nonoperative management, although this decision is currently not evidence based. The recently published Eastern Association for the Surgery of Trauma guidelines for nonoperative management of both hepatic14 and splenic15 injury concluded that there was insufficient literature to make recommendations regarding the timing of initiation of VTE pharmacoprophylaxis.
Recently, we have used whole-blood thromboelastography (TEG) as a comprehensive, point-of-care assessment of coagulation status in the surgical ICU. The potential benefits of TEG over traditional markers of the coagulation system such as activated partial thromboplastin, prothrombin time, and thrombin time are well described.16 The thromboelastographic tracing provides real-time information detailing all aspects of the coagulation system, including enzymatic function, thrombin generation, fibrin cross-linking, platelet function, and fibrinolysis. Importantly, the use of TEG gives clinicians the ability to diagnose hypercoagulability. Both our group17 and others18 have shown that hypercoagulability on TEG correlates with the likelihood of subsequent VTE in critically ill trauma patients.
The purpose of this study was to use serial thromboelastographic tracings to both identify and time a transition to a hypercoagulable state in patients with blunt abdominal solid organ injuries managed nonoperatively. This finding would aid in providing an evidenced-based recommendation for the timing of initiation of VTE pharmacoprophylaxis. Our hypothesis was that this transition occurs <72 hours after trauma.
Methods
This was a retrospective review of trauma patients admitted to Denver Health Medical Center from 2009 to 2012. Denver Health Medical Center is an American College of Surgeons–verified and state-certified level 1 trauma center with approximately 500 annual admissions with Injury Severity Scores (ISS) > 15. The trauma registry was queried to identify surgical ICU patients who (1) sustained blunt injuries to ≥ 1 solid abdominal organ (spleen, kidney, and liver); (2) were selected initially for nonoperative management; and (3) had ≥ 2 thromboelastographic tracings obtained 12 to 24 hours apart after injury. During the study period, the decision to perform postinjury TEG was at the discretion of the surgical intensivist and outside of a preexisting protocol. Postinjury TEG was divided into 12-hour intervals, from 0 to 72 hours after injury. If > 1 thromboelastographic tracing was obtained within a 12-hour interval, the first tracing of the interval was used.
A computerized thromboelastographic coagulation analyzer (TEG model 5000; Haemoscope Corporation, Niles, IL) was used to obtain the thromboelastographic tracings. Quality control checks were completed within 8 hours of blood collection per the manufacturer. Within 4 minutes of obtaining the blood sample, 10 μL of r-TEG solution (consisting of 8% kaolin, human recombinant tissue factor, phospholipids, buffers, and stabilizers) was added to .35 mL of whole blood. The resulting specimens were then added to each cup, and the temperature setting was adjusted to that of the patient. The r-TEG was initiated and stopped after reaching complete tracings.
Coagulation variables provided by the thromboelastographic tracing are summarized in Fig. 1. The following variables were abstracted: (1) r-time (indicative of time to first clot formation; normal range, 0.3 to 0.7 minutes); (2) k-time and α angle (indicative of rate of clot strengthening via fibrin cross-linking; normal ranges, 0.5 to 2 minutes and 66° to 82°, respectively); (3) maximum amplitude (MA; indicative of platelet-fibrin interaction; normal range, 54 to 72 mm); (4) shear elastic modulus strength (G; indicative of overall clot strength; normal range, 5.2 to 12.4 Kd/cs); and (5) estimated percentage lysis (indicative of degree of fibrinolysis; normal range, <7%). The thromboelastographic G value is computer generated and reflects the complete strength of the clot from the initial fibrin burst through fibrinolysis. It is calculated from the amplitude, which begins at the bifurcation of the tracing. This is based on the curvilinear relationship G = (5,000 × MA)/(100 − MA). The MA measures the maximum clot strength and is the end result of maximal platelet-fibrin interaction via the glycoprotein IIb/IIIa receptors, which stimulate the end product of coagulation via the platelet plug. The MA is thus informative of platelet contribution to coagulation. The thromboelastographic G value reflects the contributions of both the enzymatic and platelet components of hemostasis19 and is associated with VTE events in postinjury patients.17
Figure 1.
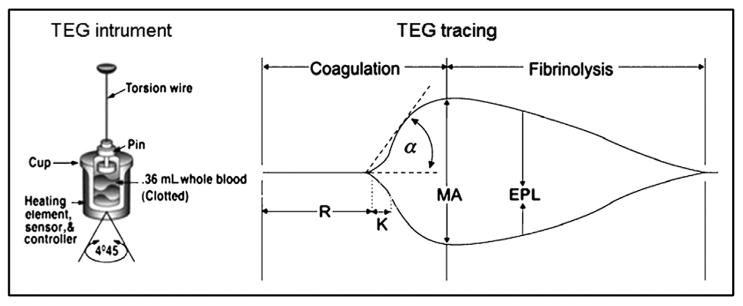
Thromboelastographic instrument and tracing. The instrument diagram depicts the cuvette where a whole-blood sample is placed and the pin attached to a torsion wire. Once the assay is initiated, a tracing is produced, and an initial linear segment (zone of pre-coagulation) extends from the beginning of the test to the formation of the first fibrin strand, causing the tracing to split (split point). The progressive divergence of the tracing reflects the formation of the clot. Reproduced with permission from Haemonetics Corporation. EPL = estimated percentage lysis.
The following clinical variables were abstracted: age (years), sex, maximum solid organ injury grade identified by computed tomographic scan,20,21 ISS, and time from admission to first pharmacoprophylaxis. In patients with ≥2 abdominal solid organ injuries, the highest grade was used for analysis. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). Differences in continuous variables were compared using Student's t tests. Differences in categorical variables were compared using chi-square tests. When expected cell counts were <10, Fisher's exact test was used. When comparing serial continuous thromboelastographic measurements over time, analysis of variance (ANOVA) was used. The α error level was set to .05, with P values <.05 being considered significant statistically. This study was approved by the Colorado Multiple Institutional Review Board.
Results
A total of 304 patients sustained blunt abdominal solid organ injuries from 2009 to 2012; 42 (13.8%) had ≥2 postinjury thromboelastograms drawn at 12-hour to 24-hour intervals, and the distribution over each time interval is depicted in Fig. 2. The distribution of patients by number of thromboelastograms at 12-hour to 24-hour intervals was as follows: 2 postinjury thromboelastograms, n = 42; 3 postinjury thromboelastograms, n = 30; 4 postinjury thromboelastograms, n = 19; and ≥5 postinjury thromboelastograms, n = 9.
Figure 2.
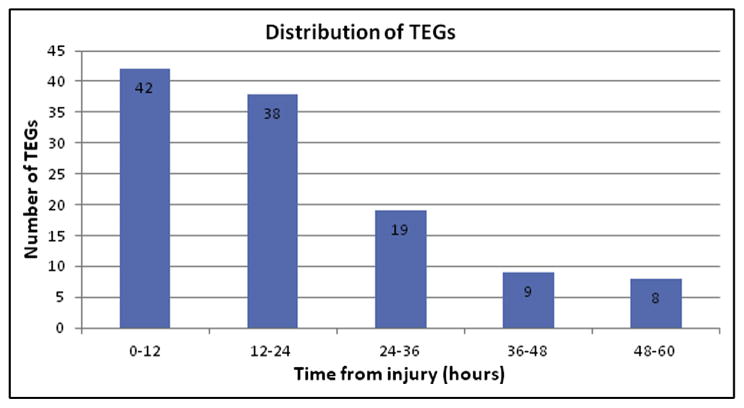
Distribution of thromboelastograms over each time interval.
Age (P = .45), gender (P =.45), and maximum solid organ injury grade (P = .71) were similar between the 42 TEG patients and 262 non-TEG patients. However, TEG patients had higher ISS compared with non-TEG patients (33.2 vs 18.3, respectively, P < .01; Table 1). No TEG patient failed nonoperative management, and no TEG patient received VTE pharmacoprophylaxis within the first 72 hours after injury.
Table 1. Patient demographics.
| Variable | Non-TEG (n = 262) | TEG (n = 42) |
|---|---|---|
| Age (y) | 35.1 ± 17.6 | 37.7 ± 16.6 |
| Men | 168 (64%) | 29 (69%) |
| Maximum solid organ injury grade | 3 (1–5) | 3 (1–5) |
| Injury Severity Score | 18.3 ± 10.2 | 33.2 ± 11.6 |
Data expressed as mean ± SD, number (percentage), or median (range).
At no point were patients noted to be hypocoagulable. By contrast, both the G and MA values began in the normal range and crossed into the hypercoagulable range at 48 hours (Figs. 3 and 4, respectively). Specifically, the G value began in the normal range within the first 12-hour interval after injury (7.4 ± 0.5 Kd/cs), increased linearly over each subsequent 12-hour interval, and rose to 15.1 ± 1.9 Kd/cs at 48 hours (P < .01, ANOVA). Similarly, the MA began at 57.6 mm, increased linearly over each 12-hour interval, and rose to 74.5 mm at 48 hours (P = .01, ANOVA). Both the k-time and α angle trended toward hypercoagulability, though neither crossed into to the hypercoagulable range during the study period, and values did not reach statistical significance. Neither r-time nor estimated percentage lysis appeared to differ with serial measurements.
Figure 3.
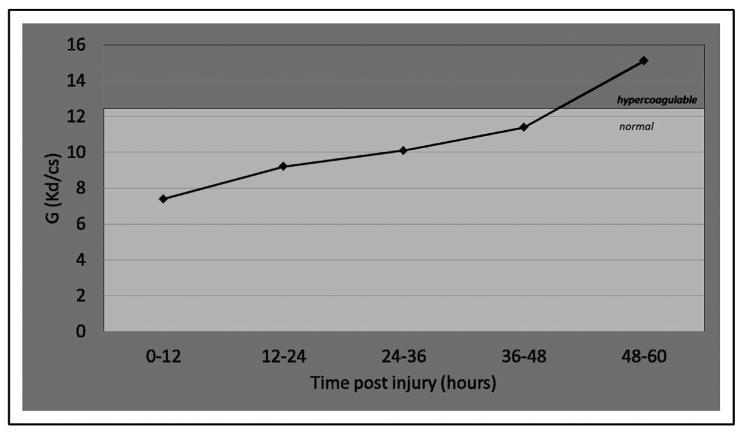
G values. The G value (normal range, 5.2 to 12.4 Kd/cs) crossed into the hypercoagulable range at 48 hours (15.1 ± 1.9 Kd/cs) (P < .01, ANOVA).
Figure 4.
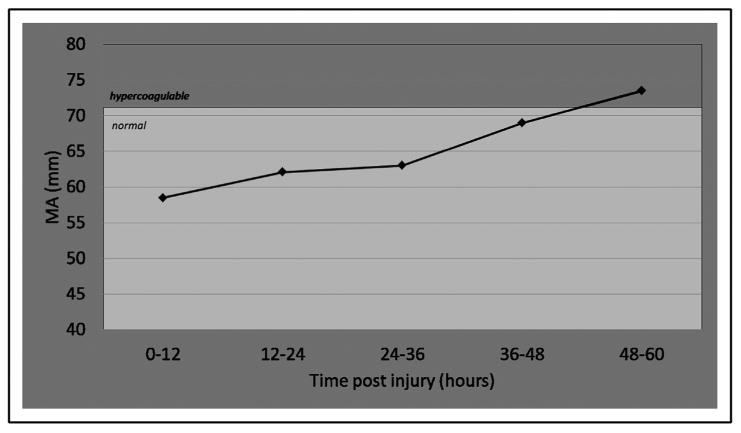
Serial MA values. The MA (normal range, 54 to 71.0 mm) crossed into the hypercoagulable range at 48 hours (73.5 ± 0.7 mm) (P < .01, ANOVA).
To assess for the possibility of attrition bias, we repeated the analysis for both G and MA using only the 9 patients who had ≥5 postinjury thromboelastograms drawn at 12-hour to 24-hour intervals. Results for both serial G and MA mirrored those of the entire sample. Both G (P = .03, ANOVA) and MA (P = .02, ANOVA) began in the normal range and crossed into the hypercoagulable range after 48 hours. At no time interval were these 9 patients noted to be hypocoagulable as measured by TEG.
Comments
In this study, we found a time-dependent transition to hypercoagulability in patients with blunt abdominal solid organ injury managed nonoperatively. When analyzing serial MA and G values, we found identical patterns: values were normal initially, rose steadily toward a hypercoagulable range, and ultimately transitioned into this range at approximately 48 hours. This pattern was not influenced by administration of VTE pharmacoprophylaxis, because no patient received such therapy within 72 hours of injury. Of the myriad thromboelastographic variables, G is believed to be the most indicative of overall clotting propensity and has been associated with the development of VTE after trauma.17 These results suggest that, left without VTE pharmacoprophylaxis, patients with blunt abdominal solid organ injuries become hypercoagulable by 48 hours after injury. This finding has therapeutic implications with respect to the timing of initiation of postinjury VTE pharmacoprophylaxis.
Virchow's triad of stasis, endothelial injury, and hypercoagulability predisposes post-trauma patients to VTE, and pulmonary embolism in patients with major trauma may occur as early as 48 hours after injury.4,6,22 Although several groups have documented hypercoagulability after trauma, our data represent the first to our knowledge to delineate the timing of a transition from normal to hypercoagulable states using TEG. In a porcine model of animals subjected to polytrauma and hemorrhagic shock, TEG identified a hypercoagulable state between 4 and 72 hours after the onset of injury, with statistically significant increases in hypercoagulability found at 16 and 48 hours.23 Park et al24 evaluated patients admitted to the ICU with TEG and showed that patients were in an overall hypercoagulable state for the first 7 days after injury. Our data represent a refinement of these findings, pinpointing the transition to between 36 and 48 hours after injury.
Although the decision to initiate VTE pharmacoprophylaxis in a patient with blunt solid organ injury must be individualized, it is helpful to base this decision on generalizable, objective evidence. Routine serial thromboelastographic analysis in all patients with blunt abdominal solid organ injuries may not be cost effective, and many traumatologists at the present time remain unfamiliar with interpreting thromboelastographic tracings. Moreover, initiating VTE pharmacoprophylaxis only after evidence of hypercoagulability on TEG may already be too late. Thus, barring other contraindications, we propose that patients with blunt abdominal solid organ injuries managed non-operatively begin VTE pharmacoprophylaxis within 48 hours of injury.
The finding of possible platelet hypercoagulability at 48 hours as evidenced by MA raises the possibility that antiplatelet agents, such as aspirin, may reduce further the risk for VTE when initiated in this time window. Further prospective evaluation of the impact of antiplatelet therapy in trauma patients with evidence of platelet hypercoagulability is ongoing at our institution.
The present study had several limitations. Because the decision to check a postinjury thromboelastogram was at the discretion of the treating team, selection bias is possible. This is corroborated by the significantly higher ISS in the TEG patients compared with the non-TEG patients. Less severely injured patients may demonstrate a different postinjury pattern on TEG. This issue is being addressed by ongoing collection of routine postinjury thromboelastograms in all patients with blunt solid abdominal organ injuries at our institution, regardless of ISS. The retrospective nature of the data collection may have masked additional reasons that TEG was ordered in our cohort of patients, such as continued transfusion requirements or clinical evidence of ongoing coagulopathy. Furthermore, because no TEG patient received VTE pharmacoprophylaxis within 72 hours of injury, we were unable to determine if initiation of such therapy within 48 hours increased either the likelihood or rate of bleeding. However, several authors have reported that initiation of VTE prophylaxis within 48 hours in patients with blunt abdominal solid organ injury managed nonoperatively is not associated with an increased likelihood of bleeding.25,26 Last, the relationship between thromboelastographic variables and risk for VTE remains incompletely understood. Elevation of both G17 and r-time18 have been associated with an increased likelihood of VTE after injury. However, it is unclear if normalization of thromboelastographic variables through the use of pharmacoprophylaxis decreases the risk for subsequent VTE. Furthermore, the relationship of the thromboelastographic end point of prophylaxis (eg, normalization of thromboelastographic tracing vs migration into the hypocoagulable range) and risk for VTE remains incompletely understood. We have recently shown that doubling the routine dose of VTE pharmacoprophylaxis did not affect clot strength as measured by TEG27; much larger doses or even continuous infusions may be necessary to influence significantly both thromboelastographic parameters and risk for subsequent VTE.
Conclusions
Patients with blunt solid organ injuries managed non-operatively appear to become progressively hypercoagulable on serial thromboelastographic tracings after injury. Transition of both G and MA values into the hypercoagulable range occurs at approximately 48 hours after injury. In the absence of contraindications, we recommend initiation of VTE pharmacoprophylaxis before this time point. Ongoing directions in this line of research at our institution include the impact of early initiation of pharmacoprophylaxis on TEG tracings, risk for bleeding, and risk for subsequent VTE in the postinjury period.
Discussion
Bellal Joseph, M.D. (Tucson, AZ): Good morning. First and foremost I want to thank the Southwest Surgical and the authors for the privilege to make comments on this research.
Congratulations to Dr Chapman and his colleagues for a job well done, a well written manuscript, and the oral presentation we all just heard.
Although the title states when to start anticoagulation, the study was undertaken to define the period of hypercoagulable state based on the results of TEG.
The concept and search for appropriate point of care devices in trauma however often leads to more questions than it answers, leaving us to try to figure out what to do with the data.
After reviewing your manuscript, I have a few questions.
Previous studies have reported a single TEG MA value >65 is predictive of a PE. Patients in your study, the mean MA was lower than this. As the patients progressed over the next few hours or days, did you look at the 48 to 72 hour MA value to see if they were predictive of a clinical PE or DVT? Did you do a regression to see if the 48 hr 72 hour MA value had any predictive role?
In the study of your methodology you did not distinguish between different grades of the number of different organs involved. I'm not sure we can compare a grade 4 liver with a grade 2 renal laceration. Did you look at the results by organ or grade of injury by organ? Also did you look at other factors in your patients such as chest injury or TBIs? We know those are higher instances of PE in those patients.
Would you recommend TEG for every trauma patient? At our institution only patients who are enrolled in research are having TEGs performed due to the cost. Have you looked at a cost to benefit analysis?
And finally, we are pretty aggressive with blunt solid organ injury patients to get them up and moving around. Did you look at the effects of SCDs and early ambulation on the TEG results?
Thank you.
Brandon C. Chapman, M.D. (Denver, CO): Thank you Dr Joseph for taking the time to review our manuscript and for your thoughtful questions. For your first question, the MA value has been shown to be predictive of thromboembolic events. Unfortunately, in our particular cohort of patients we did not specifically address the issue of whether they developed a symptomatic DVT or pulmonary embolism. This is a great question and we plan to evaluate patients prospectively in the near future. However, we have shown in a previous study of 152 critically patients at our institution, 67% were hypercoagulable characterized by an elevated G-value and 19% percent of these patients suffered a thromboembolic event and 12% were predicted based on prior r-TEG.17
For your second question, we did not distinguish between the grade or number of organs involved. There were patients in our analysis that had more than 1 organ involved and we used the highest grade of injury for analysis. But I do think it is an important to distinguish in future studies. It seems intuitive that a patient with a greater degree of trauma with a more pronounced inflammatory response would elicit a greater hypercoagulable response. We also did not look at specific injuries including chest injury or traumatic brain injuries and their association with hypercoagulability or venous thromboembolic disease.
To answer your third question regarding whether we recommend a TEG on every trauma patient, I think it is too early to recommend routine surveillance TEGs on all trauma patients and we have not performed a cost-benefit analysis. We think that the next step is a prospective trial following serial TEGs in blunt solid organ injury patients. After this, the next study would be a comparison of a “TEG-guided” initiation of VTE prophylaxis versus current standard of care. These trials are currently ongoing by our group.
For your last question, unfortunately, we did not evaluate the effect of SCDs and early ambulation on the TEG parameters. This is a very important question to answer because trauma patients with blunt abdominal organ injury may be at a higher risk of bleeding and we need to be cautious with the use of anticoagulation to minimize this risk. Mechanical prophylaxis has been shown to reduce the risk of venous thromboembolic events and it would be interesting to evaluate if mechanical prophylaxis reduces hypercoagulability in trauma patients identified on TEG. The most recent CHEST Guidelines recommend mechanical prophylaxis when not contraindicated by lower extremity injury and pharmacologic prophylaxis should be added when the risk of bleeding diminishes. Unfortunately, many trauma patients may be excluded from non-pharmacologic forms of prophylaxis by the nature of their injuries. For example, bilateral full leg compression devices cannot be used with casts or external fixators. Additionally, patients are not always compliant with wearing SCDs and they may discourage ambulation.
Dan Vargo, M.D. (Salt Lake City, UT): It is a nice presentation. It is another presentation on TEG, trying to figure out exactly how this fits in to management of trauma patients. I look at this similar to the MRI debate earlier this morning. Is this a technology that we are trying to identify a use for? If you look at our guidelines, as far as trauma patients go, we are supposed to initiate anticoagulation as quickly as possible. My question is: where do you see this fitting into the grand scheme of taking care of a trauma patient—somebody who we know that we are going to drive towards trying to give some sort of chemical prophylaxis to as quickly as possible?
Dr Chapman: Thank you Dr Vargo for your question. I believe TEG has the opportunity to benefit the patient immediately upon arrival to the emergency room. Patients initially present in a hypocoagulable state that is characterized by protein C activation, fibrinolysis, and clotting factor dilution, depletion and dysfunction. TEG provides real time analysis of thrombostatic function and allows for accurate, goal directed transfusion therapy. However, patients ultimately transition to a hypercoagulable state and our present study suggests that this transition occurs within the first 48 hours of injury. Previous studies have shown that a hypercoagulable state identified on TEG is predictive of subsequent thromboembolic events. Routine TEG surveillance in postinjury trauma patients may help identify a precise transition point at which patients become hypercoagulable. Pharmacologic prophylaxis should be initiated at this time if not contraindicated by bleeding risk. However, we need to have a large prospectively randomized controlled study to confirm our findings.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Pieracci FM, Kashuk JL, Moore EE. Postinjury hemotherapy and hemostasis. In: Mattox KL, Moore EE, Feliciano DV, editors. Trauma. 7th. New York: McGraw-Hill; 2013. pp. 216–35. [Google Scholar]
- 2.Knudson MM, Ikossi DG, Khaw L, et al. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–8. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stawicki SP, Grossman MD, Cipolla J, et al. Deep venous thrombosis and pulmonary embolism in trauma patients: an overstatement of the problem? Am Surg. 2005;71:387–91. [PubMed] [Google Scholar]
- 4.Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. J Trauma. 2007;63:620–4. doi: 10.1097/TA.0b013e31812f60aa. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle-Newhall JE, Rutledge R, Hultman CS, et al. Statewide, population-based, time-series analysis of the frequency and outcome of pulmonary embolus in 318,554 trauma patients. J Trauma. 1997;42:90–9. doi: 10.1097/00005373-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Owings JT, Kraut E, Battistella F, et al. Timing of the occurrence of pulmonary embolism in trauma patients. Arch Surg. 1997;132:862–7. doi: 10.1001/archsurg.1997.01430320064010. [DOI] [PubMed] [Google Scholar]
- 7.Knudson MM, Gomez D, Haas B, et al. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;245:625–32. doi: 10.1097/SLA.0b013e3182300209. [DOI] [PubMed] [Google Scholar]
- 8.Menaker J, Stein DM, Scalea TM. Pulmonary embolism after injury: more common than we think? J Trauma. 2009;67:1244–9. doi: 10.1097/TA.0b013e31818c173a. [DOI] [PubMed] [Google Scholar]
- 9.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl):132S–75S. doi: 10.1378/chest.119.1_suppl.132s. [DOI] [PubMed] [Google Scholar]
- 10.Knudson MM, Collins JA, Goodman SB, et al. Thromboembolism following multiple trauma. J Trauma. 1992;92:2–11. doi: 10.1097/00005373-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Cannon WB, Fraser J, Cowell E. The preventative treatment of wound shock. JAMA. 1918;70:618–21. [Google Scholar]
- 12.Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335:701–7. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 13.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(suppl):318S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 14.Stassen NA, Bhullar I, Cheng JD, et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(suppl):S288–93. doi: 10.1097/TA.0b013e318270160d. [DOI] [PubMed] [Google Scholar]
- 15.Stassen NA, Bhullar I, Cheng JD, et al. Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(suppl):S294–300. doi: 10.1097/TA.0b013e3182702afc. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez E, Pieracci FM, Moore EE, et al. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36:723–37. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–72. doi: 10.1016/j.surg.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Van PY, Cho SD, Underwood SJ, et al. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009;66:1509–15. doi: 10.1097/TA.0b013e3181a51e33. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen VG, Geary BT, Baird MS. Evaluation of the contribution of platelets to clot strength by thromboelastography in rabbits: the role of tissue factor and cytochalasin D. Anesth Analg. 2000;91:35–9. doi: 10.1097/00000539-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Moore EE, Cogbill TH, Jurkovich GJ, et al. Organ injury scaling: spleen and liver (1994 revision) J Trauma. 1995;38:323–4. doi: 10.1097/00005373-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Moore EE, Shackford SR, Pachter HL, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma. 1989;29:1664–6. [PubMed] [Google Scholar]
- 22.O'Malley KF, Ross SE. Pulmonary embolism in major trauma patients. J Trauma. 1990;30:748–50. doi: 10.1097/00005373-199006000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Mulier KE, Greenberg JG, Beilman GJ. Hypercoagulability in porcine hemorrhagic shock is present early after trauma and resuscitation. J Surg Res. 2012;174:e31–5. doi: 10.1016/j.jss.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as abetter indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–75. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberle BM, Schnüriger B, Inaba K, et al. Thromboembolic prophylaxis with low-molecular-weight heparin in patients with blunt solid abdominal organ injuries undergoing nonoperative management: current practice and outcomes. J Trauma. 2011;70:141–6. doi: 10.1097/TA.0b013e3182032f45. [DOI] [PubMed] [Google Scholar]
- 26.Alejandro KV, Acosta JA, Rodriguez PA. Bleeding manifestations after early use of low-molecular-weight heparins in blunt splenic injuries. Am Surg. 2003;69:1006–9. [PubMed] [Google Scholar]
- 27.Harr JN, Moore EE, Chin TL, et al. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74:756–65. doi: 10.1097/TA.0b013e3182826d7e. [DOI] [PMC free article] [PubMed] [Google Scholar]


