Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates many of the responses to toxic environmental chemicals such as TCDD or dioxin-like PCBs. To regulate gene expression, the AhR requires its binding partner, the aryl hydrocarbon receptor nuclear translocator (ARNT). ARNT is also required by the hypoxia-inducible factor-1α (HIF-1α), a crucial regulator of responses to conditions of reduced oxygen. The important role of ARNT in both the AhR and HIF-1α signaling pathways establishes a meaningful foundation for a possible crosstalk between these two vitally important signaling pathways. This crosstalk might lead to interference between the two signaling pathways and thus might play a role in the variety of cellular responses after exposure to AhR ligands and reduced oxygen availability. This review focuses on studies that have analyzed the effect of low oxygen environments and hypoxiamimetic agents on AhR signaling and conversely, the effect of AhR ligands, with a special emphasis on PCBs, on HIF-1α signaling. We highlight studies that assess the role of ARNT, elucidate the mechanism of the crosstalk, and discuss the physiological implications for exposure to AhR-inducing compounds in the context of hypoxia.
Keywords: ARNT, crosstalk, environmental toxicants, metabolism, oxygen sensing
Introduction
Humans are exposed to many environmental toxicants, including a broad range of aryl hydrocarbon receptor (AhR) ligands. Importantly, such exposures may portend human disease. For example, plasma concentrations of environmental toxicants, including AhR ligands such as certain dioxin-like polychlorinated biphenyls (PCBs), were recently found to be positively associated with future risks of stroke and type 2 diabetes in the elderly (Lee et al., 2011; Lee et al., 2012). Furthermore, exposure to certain chemicals may also be associated with an increased risk for cancer. For example, exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the prototypical AhR ligand, is associated with an increased incidence of papillomas in mice (Poland et al., 1982).
The importance of the AhR in mediating the adaptive metabolic responses to various endogenous compounds and environmental chemicals has been known for many years (Denison and Nagy, 2003). The AhR, a ligand-activated transcription factor that requires dimerization with the aryl hydrocarbon receptor nuclear translocator (ARNT), regulates the expression of numerous genes, many of which are essential components of cellular and xenobiotic metabolism (Beischlag et al., 2008).
Oxygen (O2) is essential for aerobic organisms as an important component of many cellular processes such as aerobic metabolism and energy balance. In addition, a variety of dioxygenase enzymes utilize molecular O2 as a cofactor to covalently oxygenate a variety of endogenous and exogenous substrates. However, under certain physiologic conditions or in certain disease states the amount of available oxygen in tissues can be reduced. Crucial factors that regulate the adaptive responses to reduced oxygen (hypoxia) are the hypoxia-inducible factors (HIFs). HIF-1α, one of the main regulators of responses to hypoxia, is a transcription factor that also requires ARNT as a dimerization partner (Kewley et al., 2004; Majmundar et al., 2010).
Interestingly, exposure to environmental toxicants in utero plays an important role during fetal development and may contribute to the developmental origins of adult disease. Notably, fetal development occurs in environments of relatively low oxygen (Dunwoodie, 2009). For example, in utero exposure to PCBs is associated with reduced fetal growth and developmental delays in humans (Yu et al., 1991; Hertz-Picciotto et al., 2005). Therefore, the crosstalk between these two important environmental sensing pathways may have physiological and pathological consequences and thus might predispose humans to various diseases.
Both the AhR and HIF-1α are members of the class I bHLH/PAS (basic helix-loop-helix/PER-ARNT-SIM) protein family. In general, bHLH proteins are important regulators of gene expression and must form heterodimers with other bHLH proteins in order to perform their respective functions. Class I bHLH/PAS proteins, including the AhR and HIF-1α, have to form obligate heterodimers with a member of the class II bHLH/PAS protein family to form functional DNA binding complexes. The different domains in bHLH/PAS proteins aid in various functions. The bHLH domain plays a role in dimerization and DNA binding. The PAS A and PAS B domains function as secondary dimerization domains and thus aid in interaction with ARNT. The transactivation region plays a role in interactions with other transcription coactivators and transcriptional transactivation. ARNT, a member of the class II bHLH/PAS protein family, is the common binding partner of both the AhR and HIF-1α which forms an important foundation for the crosstalk between these two signaling pathways at this axis (Kewley et al., 2004) (Figure 1).
Figure 1. Domain structures of various basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) proteins.
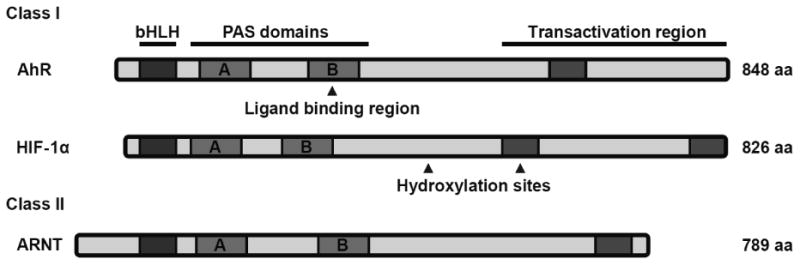
The AhR, HIF-1α (class I), and ARNT (class II) proteins each have three distinct domains that are crucial for their function. The bHLH domain plays a role in dimerization and DNA binding. The PAS A and PAS B domains function as secondary dimerization domains. The transactivation region plays a role in interactions with other transcription coactivators. aa = amino acids.
The role of ARNT in both the AhR and HIF-1α canonical signaling pathways is well established. The requirement of ARNT in both pathways, the importance of both pathways in cellular metabolism and the possibility for crosstalk have established an interesting foundation to study the interactions between these two signaling pathways. Numerous studies have assessed the crosstalk between low oxygen environments and AhR signaling and tried to elucidate the role of ARNT. Although there are non-classical mechanisms of action of the AhR that result in crosstalk and interference with other transcription factors and signaling pathways such as the estrogen receptor (ER) or NF-kappaB signaling pathways (for an excellent review we refer the reader to (Denison et al., 2011)), this review will focus on the signaling crosstalk between the canonical hypoxia and xenobiotic response pathways and elucidate the potential for such crosstalk to cause signal interference in biological responses.
The aryl hydrocarbon receptor signaling pathway
The AhR is an ubiquitously expressed, latent signal-regulated transcription factor which belongs to the class I bHLH/PAS protein family and must heterodimerize with ARNT to function as a transcription factor (Kewley et al., 2004). The AhR is activated by exposure to various endogenous and exogenous ligands. The inactive, non-ligand-bound AhR exists in the cytoplasm bound to repressive factors such as heat shock protein 90 (hsp90) (Perdew, 1988), keeping the AhR in a quiescent (non-DNA binding) state. The ligand binding region of the AhR is contained in the PAS B region. Ligand binding to the AhR induces a conformational change, exposing the nuclear localization signal (NLS), which leads to nuclear translocation. In the nucleus, the AhR dimerizes with ARNT in order to form an active transcription factor complex (Fujii-Kuriyama and Mimura, 2005; Beischlag et al., 2008). ARNT, a class II bHLH/PAS protein, is constitutively expressed in the nucleus (Kewley et al., 2004) and is essential for AhR function (Tomita et al., 2000; Nukaya et al., 2010). After dimerization, the AhR:ARNT heterodimer induces the transcription of genes containing a xenobiotic response element (XRE) sequence in their promoter. The XRE core sequence has been identified as 5′–TNGCGTG–3′ (Whitlock et al., 1996) (Figure 2).
Figure 2. The aryl hydrocarbon receptor (AhR) signaling pathway.
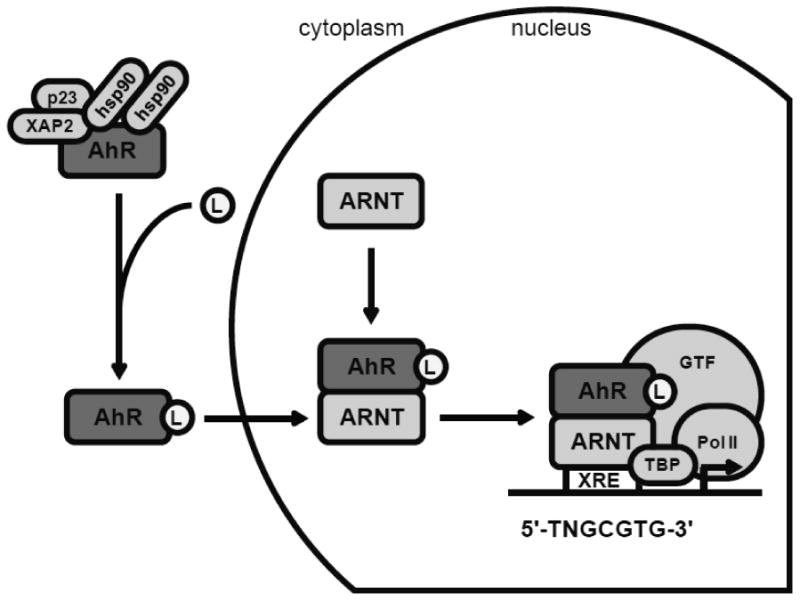
In its quiescent state, the AhR is bound to various repressive factors in the cytoplasm. Ligand (L) binding to the AhR activates the AhR and induces a conformational change which exposes a nuclear localization signal. After nuclear translocation, the AhR dimerizes with ARNT. The AhR: ARNT transcription factor complex binds to XRE sequences in target gene promoters. Interaction with multiple transcriptional coactivators is necessary to induce transcription. GTF: general transcription factors; hsp90: heat shock protein 90; PolII: RNA polymerase II; p23: prostaglandin E synthase 3/ hsp90 co-chaperone; TBP: TATA-box-binding protein; XAP2: AH receptor-interacting protein/ hepatitis B virus X-associated protein 2; XRE: xenobiotic response element.
Many endogenous and exogenous chemicals possess the ability to bind to and activate the AhR. Endogenous compounds that are thought to activate the AhR are bilirubin or various tryptophan metabolites. However, the majority of AhR activating compounds are synthetic in nature. Classical chemical compounds that activate the AhR include TCDD, benzo(a)pyrene (B(a)P), 3-methylcholanthrene (3MC), β-naphtoflavone (BNF) and the dioxin-like PCBs which have a coplanar conformation that allows binding to the AhR (Denison and Nagy, 2003). AhR target genes include phase I detoxifying enzymes such as the cytochrome P450 (CYP) 1 family members as well as phase II detoxifying enzymes such as aldehyde dehydrogenase 3 (ALDH3), UDP-glucuronosyl transferase (UGT1A1), and glutathione S-transferase (Beischlag et al., 2008).
The induction of drug metabolizing enzymes is an essential cellular defense mechanism of the cell against foreign substances. The initial metabolism of endogenous compounds, as well as foreign substances, primarily occurs in the liver via the heme-containing CYP monooxygenases. CYP enzymes play an important role in numerous endogenous signaling pathways and in drug metabolism. Among the 18 human CYP families, only the CYP 1-3 families, and to a lesser extent the CYP 4 family, are involved in drug metabolism (Nebert and Dalton, 2006). Each of these families is regulated by distinct receptor-mediated mechanisms (Waxman, 1999). The transcription of the CYP 1 family, which includes CYP1A1, CYP1A2, and CYP1B1, is controlled by the AhR and leads to tissue specific expression (Nebert and Dalton, 2006; Bieche et al., 2007). CYP1A1 gene expression is frequently used as readout for AhR induction. After ligand activation, the AhR binds to multiple XRE sequences within the CYP1A1 promoter which ultimately results in gene expression (Corchero et al., 2001; Ueda et al., 2006). Importantly, one of the functions of the CYP 1 enzymes is the metabolism of hydrophobic compounds such as PCBs (Nebert and Dalton, 2006). Although CYPs are primarily liver-expressed enzymes, the expression of CYP enzymes varies within the different zones of the liver. CYP expression is high in cells surrounding the central vein of the liver lobule (perivenous zone). Interestingly, the perivenous zone of the liver has a relatively low oxygen concentration (4% O2 and lower) compared to the periportal zone (13% O2) (Oinonen and Lindros, 1998). Furthermore, rats exposed to low doses of PCB 126, the most potent dioxin-like PCB, show induction of CYP1A1 mainly in the perivenous zone where oxygen is scarce (Chubb et al., 2004).
The hypoxia signaling pathway
The hypoxia pathway is a crucial sensor of changes in the oxygen environment which can occur during various physiological and pathological conditions. The response to reduced oxygen availability is mediated by hypoxia-inducible factors (HIFs), a class of proteins that bind to ARNT to initiate hypoxia-induced gene expression. There are three known isoforms of the hypoxia-inducible factors which all belong to the class I bHLH/PAS protein family: HIF-1α, HIF-2α and HIF-3α (collectively HIF-α) (Kewley et al., 2004). While HIF-1α and HIF-2α both function as transcription factors, less is known about HIF-3α, which is thought to function as negative regulator of HIF-1α and HIF-2α. Each of the isoforms shows distinct expression patterns in human tissues. Whereas HIF-1α is ubiquitously expressed in all cells, HIF-2α and HIF-3α are only selectively expressed in certain cell types including endothelial cells (Majmundar et al., 2010).
Regulation of HIF-1α occurs at the post-translational level. Under normal oxygen conditions (21% oxygen), HIF-1α proteins are targeted for proteasomal degradation by prolyl hydroxylase domain-containing enzymes (PHDs). PHD enzymes hydroxylate HIF-1α in an oxygen-dependent manner at two proline residues, P564 and P402, which leads to subsequent ubiquitination by von Hippel-Lindau (VHL) proteins and ultimately proteasomal degradation (Ohh et al., 2000; Kewley et al., 2004; Dery et al., 2005). Further hydroxylation occurs via factor inhibiting HIF-1 (FIH-1) at the asparagine residue N803 which prevents the recruitment of cofactors and subsequent transactivation. In contrast, conditions with a limited oxygen supply diminish PHD function and thus lead to HIF-1α accumulation due to the impaired hydroxylation step. Non-hydroxylated HIF-1α translocates to the nucleus where it dimerizes with ARNT (Kewley et al., 2004). Similar to AhR:ARNT heterodimers, HIF-1α:ARNT complexes activate gene transcription through a specific cognate sequence (5′–G/ACGTG–3′) termed the hypoxia response element (HRE) (Mole et al., 2009; Majmundar et al., 2010) (Figure 3).
Figure 3. The hypoxia-inducible HIF-1α signaling pathway.
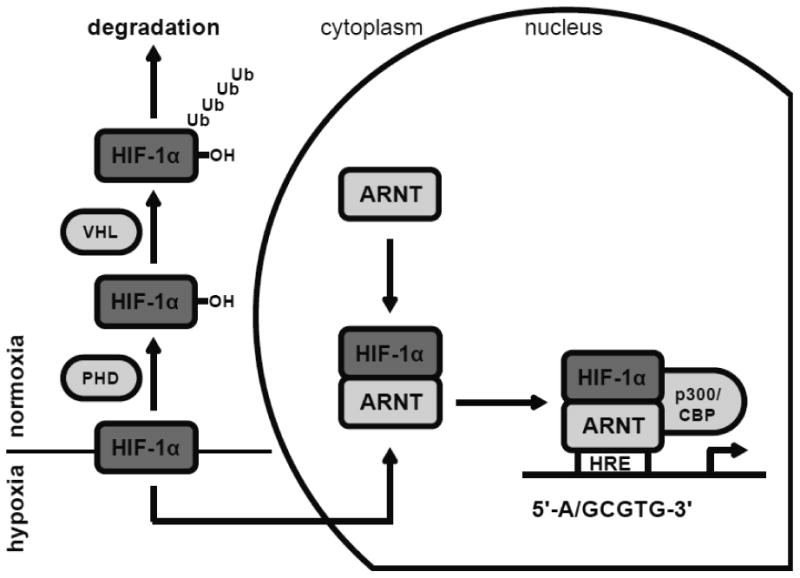
Under normal oxygen conditions (21% oxygen), HIF-1α proteins are proline hydroxylated by PHDs, ubiquitinated by VHL proteins and ultimately degraded via the proteaseome. Hypoxic conditions (< 6% oxygen) diminish PHD function and thus lead to HIF-1α stabilization. Non-hydroxylated HIF-1α translocates to the nucleus and dimerizes with ARNT. The HIF-1α:ARNT transcription factor complex binds to HRE sequences in target gene promoters. HRE: hypoxia response element; PHD: prolyl hydroxylase domain-containing enzymes; p300/CBP: histone actelytransferase p300/CREB-binding protein; VHL: von Hippel-Lindau tumor suppressor.
HIF-1α target genes include those genes that promote glucose metabolism and cell survival under hypoxic conditions (Rankin and Giaccia, 2008; Majmundar et al., 2010). Elevated levels of HIF-1α and HIF-2α are often found in malignant tumors and are thought to play a role in tumorigenesis and poor patient outcome (Rankin and Giaccia, 2008). For example, increased HIF-1α expression in tumors has been shown to promote resistance to radiotherapy (Moeller et al., 2005).
HIF-1α, a major mediator of responses to reduced oxygen, plays an important role in cellular metabolism and homeostasis. As an adaptive response, HIF-1α reprograms cellular metabolism to ensure cell survival (Majmundar et al., 2010). Cancer cells have an increased demand for metabolic energy which is met by a shift to non-oxidative forms of glucose metabolism. Numerous HIF-1α-regulated target genes, including glucose transporters and glycolytic enzymes, are upregulated or changed in their expression profile in cancer cells and thus affect glucose uptake and metabolism (Gordan et al., 2007; Majmundar et al., 2010). Furthermore, HIF-1α has an effect on the pentose phosphate pathway and thus can affect the use of glucose as a building block for RNA and DNA (Majmundar et al., 2010). HIF-1α has been shown to induce the expression of pyruvate dehydrogenase kinase (PDK1) which inhibits the citric acid cycle and thus reduces mitochondrial oxygen consumption (Kim et al., 2006; Papandreou et al., 2006).
Glucose uptake into the cell is regulated by glucose transporters. The glucose transporters GLUT1 and GLUT3 are targets of HIF-1α and have been shown to be upregulated in cancer (Macheda et al., 2005). In human adipocytes GLUT1 has been shown to be upregulated after exposure to a range of reduced oxygen concentrations (Wood et al., 2011). Hypoxic regions are found in solid tumors and upregulation of GLUT3 in these regions might affect tumor growth (Maxwell et al., 1997).
Overall, HIF-1α is a critical sensor of changes in oxygen availability and is important to ensure cell survival. Furthermore, HIF-1α is a crucial regulator of cellular metabolism and thus plays an important role in cancer.
Crosstalk between the AhR and hypoxia signaling pathways
Both the AhR and HIF-1α proteins are important sensors of changes in the environment. The AhR mediates responses to exposure to environmental chemicals whereas HIF-1α regulates responses to low oxygen conditions. To promote their respective functions, both proteins have to form a heterodimer with their binding partner ARNT (Kewley et al., 2004). ARNT has been shown to bind to the nucleotide sequence 5′–GTG–3′ which is present in both the XRE and HRE core sequence (Bacsi et al., 1995). Because ARNT is a central regulator of these pathways, the possibility for signaling crosstalk exists at this axis (Figure 4). The antagonistic relationship between AhR and HIF-1α signaling via ARNT may result in changes in AhR- and HIF-1α-mediated responses. Hypoxic conditions may cause HIF-1α to sequester ARNT and thus inhibit the activation of a robust AhR transcriptional response in response to an AhR ligand. Conversely, exposure to AhR ligands may attenuate HIF-1α-mediated responses in hypoxic environments. Numerous studies have assessed the crosstalk between the AhR and hypoxia response and tried to elucidate the role of ARNT.
Figure 4. Crosstalk at the AhR-ARNT-HIF1α signaling node.
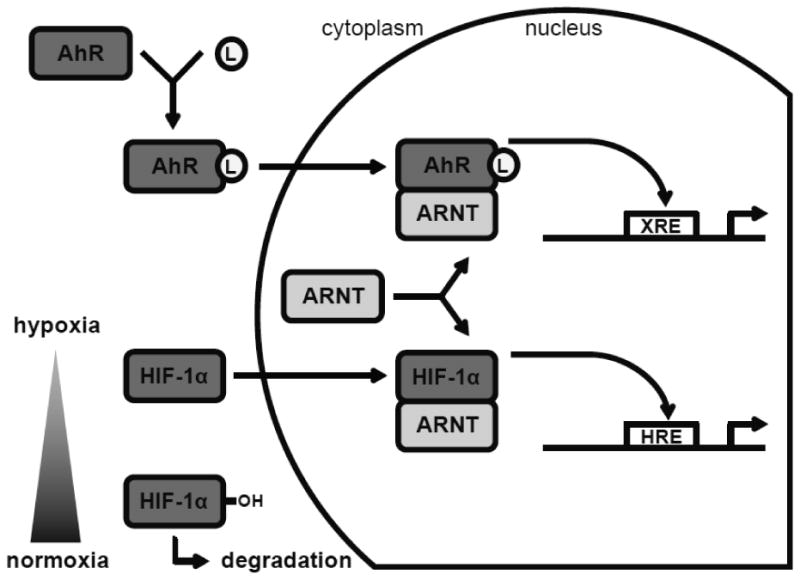
Top of figure: the AhR is quiescent in the cytoplasm. Ligand (L) binding activates the AhR and initiates translocation to the nucleus. In the nucleus the AhR binds to ARNT to induce transcription of XRE-containing genes. Bottom of figure: normoxic conditions lead to HIF-1α hydroxylation and degradation. In contrast, hypoxic environments cause HIF-1α stabilization and translocation to the nucleus. In the nucleus HIF-1α binds to ARNT to induce transcription of HRE-containing genes.
The uses of ARNT deficient cell lines and conditional ARNT knockout mice have been useful tools in assessing the role of ARNT in both the AhR and hypoxia pathways. In a study by Wood et al., ARNT deficient Hepa1c1c7 cells were unable to induce HIF-1α transcriptional activity in hypoxia. They further showed that each of the basic, helix-loop-helix, and PAS domains were necessary for ARNT function (Wood et al., 1996). In a similar study, Gassmann et al. showed that ARNT deficient Hepa1c4 cells were unable to respond to hypoxia suggesting that ARNT is necessary for HIF-1α function. Furthermore, they showed that hypoxia inhibited AhR-mediated CYP1A1 mRNA expression in Hepa1 cells (Gassmann et al., 1997). Importantly, ARNT is not only crucial for HIF-1α function, but also for the function of the AhR. Tomita et al. showed that in conditional ARNT knockout mice, TCDD was unable to induce various AhR target genes. Furthermore, cobalt chloride (CoCl2), an important chemical inducer of HIF-1α which thus functions as a hypoxia mimetic, could not induce the HIF-1α target gene GLUT1 in these mice suggesting a critical role of ARNT in both AhR and HIF-1α signaling (Tomita et al., 2000).
Based on these studies it is clear that ARNT is necessary for both the AhR and HIF-1α pathways. To further elucidate the role of ARNT, numerous studies have assessed the crosstalk between the two pathways. Nie et al. used the iron chelating compounds desferrioxamine (DFX) and the hypoxia-mimetic agent CoCl2 to induce hypoxia-like responses in various cell lines. Hypoxia-like conditions induced in this manner reduced AhR-mediated CYP1A1 enzyme activity and reporter gene expression after TCDD treatment. Conversely, TCDD inhibited hypoxia-mediated reporter gene activity suggesting that there is crosstalk between the two pathways via ARNT (Nie et al., 2001). Reduced TCDD-induced CYP1A1 promoter activity in hypoxia-like conditions was also shown in a study by Kim and Sheen in which mouse cells were treated with various HIF-inducing chemicals prior to TCDD treatment (Kim and Sheen, 2000). Hofer et al. also observed an inhibitory effect of hypoxic responses, induced by exposing rats to 0.1% carbon monoxide (CO), on TCDD-induced CYP1A1 mRNA expression levels. In contrast, rats treated with TCDD did not show negative effects on VEGF mRNA expression after CO treatment (Hofer et al., 2004). In another study, hypoxia reduced TCDD-induced CYP1A1 mRNA in fish cells. In the same study, however, the authors found that TCDD did not affect the hypoxia-induced target genes heme oxygenase (Prasch et al., 2004). Supporting the inhibitory effect of AhR ligands, Kraemer and Schulte showed that PCB 77, another dioxin-like PCB, decreased hypoxia-induced glycolytic enzyme activity in fish (Kraemer and Schulte, 2004). Furthermore, Terzuoli et al. showed inhibition of HIF-1α target gene expression under physiologic hypoxia in human cells treated with the AhR ligand aminoflavone (Terzuoli et al., 2010). In a surgically-induced ischemia model, Ichihara et al. showed that AhR null mice had a stronger HIF-1α response compared to wild-type mice. AhR null mice showed increased VEGF expression and angiogenesis in ischemic conditions which might be due to an increased abundance of HIF-1α:ARNT heterodimers (Ichihara et al., 2007). A study by Legendre et al. showed that hypoxic conditions, induced by different mechanisms, inhibited various drug metabolizing enzymes which might ultimately compromise the effectiveness of chemotherapy in certain cancer patients (Legendre et al., 2009).
Induction of the AhR and the crosstalk with the hypoxia pathway seem to be very sensitive. Hendon et al. showed that CYP1A1 mRNA expression could be induced by the weak AhR agonist pyrene, which induced CYP1A1 mRNA in a time- and dose-dependent manner in fish (Hendon et al., 2008). Matson et al. showed that reduced oxygen conditions of 7% O2 were sufficient to reduce B(a)P or BNF-induced CYP1A1 activity in zebrafish (Matson et al., 2008). Furthermore, human cells exposed to TCDD in 5% O2 conditions showed reduced HIF-1α stabilization and HRE-mediated promoter activity (Seifert et al., 2008). Takacova et al. showed that TCDD reduced the induction of the HIF-1α-regulated target gene carbonic anhydrase IX in human cells in hypoxic conditions of 2% O2 (Takacova et al., 2009). Interestingly, a study by Zhang and Walker showed that CYP1A1 constitutive mRNA expression was reduced by hypoxia even in the absence of exogenous ligand (Zhang and Walker, 2007). Using mouse wild type and HIF-1α null hepatocytes, Allen et al. assessed the role of HIF-1α in AhR function. In their study the authors showed that HIF-1α-deficient cells showed a reduced response to hypoxia. Cotreatment with hypoxia and 3-methylcholanthrene did not affect the expression of various HIF-1α target genes in either cell line. Furthermore, CYP1A1 expression and enzyme activity were inhibited by hypoxia even in HIF-1α null hepatocytes suggesting that HIF-1α might not be directly involved in the negative regulation of AhR signaling (Allen et al., 2005).
These studies strongly suggest that there is indeed a crosstalk between the AhR and hypoxia pathways. However, the mechanism of this crosstalk and whether there is competition for ARNT is still unclear. To clarify the role of ARNT and to assess the binding of the AhR to ARNT in hypoxia, Pollenz et al. assessed the formation of AhR:ARNT heterodimer complexes in normoxia and hypoxia and found no effect on heterodimer formation suggesting there is no competition for ARNT. They further observed that ARNT protein levels were stable in normoxic and hypoxic oxygen environments. Therefore, ARNT was thought not to be a limiting factor in the crosstalk. In the same study, however, the authors did observe an inhibitory effect of hypoxia on CYP1A1 protein levels induced by TCDD in mouse and rat cells (Pollenz et al., 1999). In contrast to the finding by Pollenz et al., a study by Schults et al. showed in similar experiments in human cells that AhR:ARNT formation was reduced in CoCl2-induced hypoxia-like conditions and that, conversely, B(a)P inhibited the formation of HIF-1α:ARNT heterodimers. The authors further showed that B(a)P treatment reduced HIF-1α target mRNA and that hypoxia-like conditions, induced via CoCl2, reduced CYP1A1 and CYP1B1 mRNA levels (Schults et al., 2010). Reduced AhR:ARNT formation after TCDD exposure was also observed by Gradin et al. in human cells in which HIF-1α was overexpressed. In contrast, HIF-1α:ARNT formation was not affected by TCDD suggesting that HIF-1α has a higher affinity for ARNT. Furthermore, they observed an inhibitory effect of CoCl2-induced hypoxia-like conditions on 2,3,7,8-tetrachlorodibenzofuran (TCDF)-induced AhR responses (CYP1A1 mRNA and luciferase reporter activity) (Gradin et al., 1996). In another study, hypoxia also reduced CYP1A1 mRNA and protein expression after TCDD treatment in human cells (Khan et al., 2007). Overexpression of ARNT did not abolish this inhibitory effect. Furthermore, using chromatin immunoprecipitation, the authors showed that AhR localization to the nucleus and AhR binding to the CYP1A1 promoter was not reduced in CoCl2-induced hypoxic conditions (Khan et al., 2007).
In contrast to the many studies using TCDD and other AhR ligands, only a few studies have assessed the effect of PCBs on the crosstalk between the AhR and hypoxia pathways. A study by Fleming et al. used fish cells to assess the role of ARNT in AhR signaling induced by various AhR ligands, including PCB 126, with and without hypoxia. They showed that PCBinduced reporter gene activity was reduced in hypoxia. Importantly, this effect was abolished by overexpression of ARNT which strongly suggests that AhR and HIF-1α crosstalk is dependent on ARNT. Further data showed that PCB 126 and benzo[k]fluoranthene (BkF) could not, but B(a)P could, inhibit HIF-1α reporter activity (Fleming et al., 2009). These data support the findings by Gradin et al., suggesting that HIF-1α dominates over AhR when competing for ARNT. In our own recent studies we showed that the activation and function of the AhR as well as CYP1A1 expression induced by PCB 126 were significantly inhibited by hypoxia in human skin and liver-derived cells. In our studies, overexpression of ARNT relieved the inhibitory effect of hypoxia which suggests that the mechanism of interference between the AhR and hypoxia signaling pathways in those cell types might at least in part be dependent on ARNT (Vorrink et al., 2014).
The complexity of the crosstalk between the AhR and hypoxia signaling pathways has been demonstrated in various studies. Chan et al. showed that exposure to TCDD inhibited HIF-1α reporter gene activity in cell culture systems. Conversely, CoCl2-induced hypoxia-like conditions inhibited the activity of an AhR-regulated reporter gene. Interestingly, concomitant treatment resulted in an additive effect on the HIF-1α target gene erythropoietin (EPO) (Chan et al., 1999). This effect was also observed in vivo in rats treated with TCDD and exposed to CO (Hofer et al., 2004). In addition, similar effects were seen in a study by Yu et al. in which grouper fish were treated with B(a)P. Hypoxia had no effect on B(a)P-induced AhR target genes. However, concomitant treatment with hypoxia and B(a)P had an additive effect on EPO expression (Yu et al., 2008). These data suggest a more complex regulation of certain genes, especially genes which have both HRE and XRE sequences in or near their promoter (Chan et al., 1999; Hofer et al., 2004; Yu et al., 2008).
The genomic regulation of genes is based on a complex relationship between AhR and HIF proteins as well as the regulatory response elements and other DNA motifs involved in gene regulation (Lee et al., 2006). Although the importance of ARNT in both AhR and HIF-1α signaling is well established, many of the observed inhibitory effects seem to be dependent on the cell type, the exposure agent and the duration of exposure.
Because the responses assessing the crosstalk between the AhR and HIF-1α pathways are inconsistent between studies, more research is needed to elucidate responses of the crosstalk. The importance of both pathways in various cellular functions establishes the need to further study the effect of the antagonistic relationship between AhR and HIF-1α signaling via ARNT in human cells.
Summary and Conclusions
The AhR and HIF-1α proteins are critical factors that sense and respond to chemical exposures or changes in the oxygen environment, respectively. Through their common cofactor ARNT a regulatory crosstalk exists that can affect AhR- and HIF-1α-mediated responses. The regulatory crosstalk between these two pathways has been studied for many years. Numerous research groups have assessed the antagonistic relationship between AhR and hypoxia signaling using a broad range of AhR ligands and agents to induce hypoxic responses (Table 1). The studies highlighted in this review show that ARNT is indeed a crucial factor in both AhR and HIF-1α signaling. Low oxygen environments in which HIF-1α is stabilized can affect cellular responses mediated by the AhR. Conversely, activation of the AhR can interfere with HIF-1α-mediated responses.
Table 1. List of publications relevant to the crosstalk between the AhR and hypoxia signaling pathways.
AF: aminoflavone; AhR: aryl hydrocarbon receptor; B(a)P: benzo(a)pyrene; BkF: benzo[k]fluoranthene; BNF: β-naphtoflavone; CO: carbon monoxide; CoCl2: cobalt chloride; DFX: desferrioxamine; HIF: hypoxia-inducible factor; ICZ: indolo[3,2-b]carbazole; NA: not applicable; PA: picolinic acid; PCB: polychlorinated biphenyl; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDF: 2,3,7,8-tetrachlorodibenzofuran; 3MC: 3-methylcholanthrene; ↑: increase; ↓: decrease; ↔: no change.
| AhR Ligand | HIF Inducer | Main Observation | References |
|---|---|---|---|
| TCDF | CoCl2 | ↓ AhR, ↔ HIF | Gradin et al. (1996) |
| ICZ | 1% oxygen | ↓ AhR, ↔ HIF | Gassmann et al. (1997) |
| TCDD, BNF | CoCl2 | ↓ AhR, ↓/↑ HIF | Chan et al. (1999) |
| TCDD | 1% oxygen | ↓ AhR | Pollenz et al (1999) |
| TCDD | CoCl2, DFX, PA | ↓ AhR | Kim and Sheen (2000) |
| TCDD | CoCl2, DFX | ↓ AhR, ↓ HIF | Nie et al. (2001) |
| TCDD | CO | ↓ AhR, ↔/↑ HIF | Hofer et al (2004) |
| PCB 77 | Hypoxic water | ↓ HIF | Kraemer and Schulte (2004) |
| TCDD | Hypoxic water | ↓ AhR, ↔ HIF | Prasch et al. (2004) |
| 3MC | 1% oxygen | ↓ AhR | Allen et al (2005) |
| NA | Ischemia | ↑ HIF | Ichihara et al (2007) |
| TCDD | 1% oxygen, CoCl2 | ↓ AhR | Khan et al. (2007) |
| NA | 2.5-1% oxygen | ↓ AhR | Zhang and Walker (2007) |
| Pyrene | NA | ↑ AhR | Hendon et al. (2008) |
| B(a)P, BNF | 7.3% oxygen | ↓ AhR | Matson et al. (2008) |
| TCDD | 5% oxygen | ↓ HIF | Seifert et al. (2008) |
| B(a)P | Hypoxic water | ↔ AhR, ↑ HIF | Yu et al. (2008) |
| B(a)P, PCB 126, BkF | 1% oxygen | ↓ AhR, ↔/↓ HIF | Fleming et al. (2009) |
| NA | 1% oxygen, CoCl2, DFX | ↓ AhR | Legendre et al. (2009) |
| TCDD | 2% oxygen | ↓ HIF | Takacova et al. (2009) |
| B(a)P | CoCl2 | ↓ AhR, ↓ HIF | Schults et al. (2010) |
| AF | 1% oxygen | ↓ HIF | Terzuoli et al. (2010) |
| PCB 126 | 1% oxygen | ↓ AhR, ↓ HIF | Vorrink et al. (2014) |
As introduced above, the signaling crosstalk between these important environmental sensing pathways may be consequential to humans. Humans continue to be exposed to a broad range of environmental chemicals, among which are numerous compounds that are ligands for the AhR. Exposure to these toxicants can have many adverse health effects, such as an increased future risk of stroke or type 2 diabetes (Lee et al., 2011; Lee et al., 2012). In addition, both normal physiologic conditions and numerous medical conditions and disease states such as diabetes, can lead to hypoxic environments in the human body. Given the possible significance to human health, it is important to understand the effects and possible outcomes of the crosstalk between these two significant environmental sensing pathways in the context of exposure of human cells. Finally, environmental exposure to AhR ligands during fetal or early life development may have long term effects on oxygen sensing pathways that contribute to later onset diseases. Clearly, further studies are needed to specifically elucidate biological outcomes and physiological significance of regulatory crosstalk and interference at this important signaling node.
Highlights.
The xenobiotic response and hypoxia response pathways intersect
Exposure to one stimulus can squelch the biological response to the other stimulus
The signaling crosstalk between these pathways is frequently reciprocal
Biological effectors of the responses, AhR and HIF1a, both use ARNT as a partner
Normal xenobiotic responses may be perturbed under physiological hypoxia
Acknowledgments
The authors thank Dr. Larry Robertson for critical review of the manuscript. This work was supported by National Institutes of Health (NIH) grant P42 ES013661-5099. SUV received salary support from the Superfund Research Program Training Core P42 ES013661-5110. SUV further received a SRP K.C. Donnelly Externship Administrative Supplement. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- B(a)P
benzo(a)pyrene
- bHLH/PAS
basic helix-loop-helix/PER-ARNT-SIM
- CoCl2
cobalt chloride
- CYP1A1
cytochrome P450 1A1
- HIF-1α
hypoxia-inducible factor-1α
- HRE
hypoxia response element
- PCB
polychlorinated biphenyl
- PCB 126
3,3′,4,4′,5-pentachlorobiphenyl
- PHD
prolyl hydroxylase domain-containing enzyme
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- XRE
xenobiotic response element
Footnotes
Conflict of Interest Statement: The authors declare that no conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JW, Johnson RS, Bhatia SN. Hypoxic inhibition of 3-methylcholanthrene-induced CYP1A1 expression is independent of HIF-1alpha. Toxicol Lett. 2005;155:151–159. doi: 10.1016/j.toxlet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bacsi SG, Reisz-Porszasz S, Hankinson O. Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence. Mol Pharmacol. 1995;47:432–438. [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17:731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- Chubb LS, Andersen ME, Broccardo CJ, Legare ME, Billings RE, Dean CE, Hanneman WH. Regional induction of CYP1A1 in rat liver following treatment with mixtures of PCB 126 and PCB 153. Toxicol Pathol. 2004;32:467–473. doi: 10.1080/01926230490483306. [DOI] [PubMed] [Google Scholar]
- Corchero J, Pimprale S, Kimura S, Gonzalez FJ. Organization of the CYP1A cluster on human chromosome 15: implications for gene regulation. Pharmacogenetics. 2001;11:1–6. doi: 10.1097/00008571-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Fleming CR, Billiard SM, Di Giulio RT. Hypoxia inhibits induction of aryl hydrocarbon receptor activity in topminnow hepatocarcinoma cells in an ARNTdependent manner. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:383–389. doi: 10.1016/j.cbpc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Kvietikova I, Rolfs A, Wenger RH. Oxygen- and dioxin-regulated gene expression in mouse hepatoma cells. Kidney Int. 1997;51:567–574. doi: 10.1038/ki.1997.81. [DOI] [PubMed] [Google Scholar]
- Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin K, McGuire J, Wenger RH, Kvietikova I, fhitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendon LA, Carlson EA, Manning S, Brouwer M. Molecular and developmental effects of exposure to pyrene in the early life-stages of Cyprinodon variegatus. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:205–215. doi: 10.1016/j.cbpc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology. 2005;16:648–656. doi: 10.1097/01.ede.0000173043.85834.f3. [DOI] [PubMed] [Google Scholar]
- Hofer T, Pohjanvirta R, Spielmann P, Viluksela M, Buchmann DP, Wenger RH, Gassmann M. Simultaneous exposure of rats to dioxin and carbon monoxide reduces the xenobiotic but not the hypoxic response. Biol Chem. 2004;385:291–294. doi: 10.1515/BC.2004.024. [DOI] [PubMed] [Google Scholar]
- Ichihara S, Yamada Y, Ichihara G, Nakajima T, Li P, Kondo T, Gonzalez FJ, Murohara T. A role for the aryl hydrocarbon receptor in regulation of ischemiainduced angiogenesis. Arterioscler Thromb Vac Biol. 2007;27:1297–1304. doi: 10.1161/ATVBAHA.106.138701. [DOI] [PubMed] [Google Scholar]
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Khan S, Liu S, Stoner M, Safe S. Cobaltous chloride and hypoxia inhibit aryl hydrocarbon receptor-mediated responses in breast cancer cells. Toxicol Appl Pharmacol. 2007;223:28–38. doi: 10.1016/j.taap.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Sheen YY. Inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-stimulated Cyp1a1 promoter activity by hypoxic agents. Biochem Pharmacol. 2000;59:1549–1556. doi: 10.1016/s0006-2952(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kraemer LD, Schulte PM. Prior PCB exposure suppresses hypoxia-induced upregulation of glycolytic enzymes in Fundulus heteroclitus. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:23–29. doi: 10.1016/j.cca.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–1784. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Background exposure to persistent organic pollutants predicts stroke in the elderly. Environ Int. 2012;47:115–120. doi: 10.1016/j.envint.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Lee KA, Burgoon LD, Lamb L, Dere E, Zacharewski TR, Hogenesch JB, LaPres JJ. Identification and characterization of genes susceptible to transcriptional cross-talk between the hypoxia and dioxin signaling cascades. Chem Res Toxicol. 2006;19:1284–1293. doi: 10.1021/tx060068d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre C, Hori T, Loyer P, Aninat C, Ishida S, Glaise D, Lucas-Clerc C, Boudjema K, Guguen-Guillouzo C, Corlu A, Morel F. Drug-metabolising enzymes are down-regulated by hypoxia in differentiated human hepatoma HepaRG cells: HIF-1alpha involvement in CYP3A4 repression. Eur J Cancer. 2009;45:2882–2892. doi: 10.1016/j.ejca.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CW, Timme-Laragy AR, Di Giulio RT. Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish. Chemosphere. 2008;74:149–154. doi: 10.1016/j.chemosphere.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Nie M, Blankenship AL, Giesy JP. Interactions between aryl hydrocarbon receptor (AhR) and hypoxia signaling pathways. Environ Toxicol Pharmacol. 2001;10:17–27. doi: 10.1016/s1382-6689(01)00065-5. [DOI] [PubMed] [Google Scholar]
- Nukaya M, Walisser JA, Moran SM, Kennedy GD, Bradfield CA. Aryl hydrocarbon receptor nuclear translocator in hepatocytes is required for aryl hydrocarbon receptor-mediated adaptive and toxic responses in liver. Toxicol Sci. 2010;118:554–563. doi: 10.1093/toxsci/kfq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Oinonen T, Lindros KO. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem J. 1998;329(Pt 1):17–35. doi: 10.1042/bj3290017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263:13802–13805. [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982;300:271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Davarinos NA, Shearer TP. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol Pharmacol. 1999;56:1127–1137. doi: 10.1124/mol.56.6.1127. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and hypoxia signaling pathways in zebrafish: hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schults MA, Timmermans L, Godschalk RW, Theys J, Wouters BG, van Schooten FJ, Chiu RK. Diminished carcinogen detoxification is a novel mechanism for hypoxia-inducible factor 1-mediated genetic instability. J Biol Chem. 2010;285:14558–14564. doi: 10.1074/jbc.M109.076323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert A, Katschinski DM, Tonack S, Fischer B, Navarrete Santos A. Significance of prolyl hydroxylase 2 in the interference of aryl hydrocarbon receptor and hypoxia-inducible factor-1 alpha signaling. Chem Res Toxicol. 2008;21:341–348. doi: 10.1021/tx7001838. [DOI] [PubMed] [Google Scholar]
- Takacova M, Holotnakova T, Vondracek J, Machala M, Pencikova K, Gradin K, Poellinger L, Pastorek J, Pastorekova S, Kopacek J. Role of aryl hydrocarbon receptor in modulation of the expression of the hypoxia marker carbonic anhydrase IX. Biochem J. 2009;419:419–425. doi: 10.1042/BJ20080952. [DOI] [PubMed] [Google Scholar]
- Terzuoli E, Puppo M, Rapisarda A, Uranchimeg B, Cao L, Burger AM, Ziche M, Melillo G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1alpha expression in an AhR-independent fashion. Cancer Res. 2010;70:6837–6848. doi: 10.1158/0008-5472.CAN-10-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- Ueda R, Iketaki H, Nagata K, Kimura S, Gonzalez FJ, Kusano K, Yoshimura T, Yamazoe Y. A common regulatory region functions bidirectionally in transcriptional activation of the human CYP1A1 and CYP1A2 genes. Mol Pharmacol. 2006;69:1924–1930. doi: 10.1124/mol.105.021220. [DOI] [PubMed] [Google Scholar]
- Vorrink SU, Severson PL, Kulak MV, Futscher BW, Domann FE. Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines. Toxicol Appl Pharmacol. 2014;274:408–416. doi: 10.1016/j.taap.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- Whitlock JP, Jr, Okino ST, Dong L, Ko HP, Clarke-Katzenberg R, Ma Q, Li H. Cytochromes P450 5: induction of cytochrome P4501A1: a model for analyzing mammalian gene transcription. FASEB J. 1996;10:809–818. doi: 10.1096/fasebj.10.8.8666157. [DOI] [PubMed] [Google Scholar]
- Wood IS, Stezhka T, Trayhurn P. Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflugers Arch. 2011;462:469–477. doi: 10.1007/s00424-011-0985-7. [DOI] [PubMed] [Google Scholar]
- Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J Biol Chem. 1996;271:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- Yu ML, Hsu CC, Gladen BC, Rogan WJ. In utero PCB/PCDF exposure: relation of developmental delay to dysmorphology and dose. Neurotoxicol Teratol. 1991;13:195–202. doi: 10.1016/0892-0362(91)90011-k. [DOI] [PubMed] [Google Scholar]
- Yu RM, Ng PK, Tan T, Chu DL, Wu RS, Kong RY. Enhancement of hypoxia-induced gene expression in fish liver by the aryl hydrocarbon receptor (AhR) ligand, benzo[a]pyrene (BaP) Aquat Toxicol. 2008;90:235–242. doi: 10.1016/j.aquatox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang N, Walker MK. Crosstalk between the aryl hydrocarbon receptor and hypoxia on the constitutive expression of cytochrome P4501A1 mRNA. Cardiovasc Toxicol. 2007;7:282–290. doi: 10.1007/s12012-007-9007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


