Abstract
Context
Poisoning is the second leading cause of injury-related fatality in the United States. An elevated serum lactate concentration identifies medical and surgical patients at risk for death; however, its utility in predicting death in drug overdose is controversial and unclear.
Objective
We aimed to evaluate the prognostic utility of serum lactate concentration for fatality in emergency department (ED) patients with acute drug overdose.
Materials and Methods
This was a case–control study at two urban university teaching hospitals affiliated with a regional poison control center. Data were obtained from electronic medical records, poison center data, and the office of the chief medical examiner. Controls were consecutive acute drug overdoses over a 1-year period surviving to hospital discharge. Cases were subjects over a 7-year period with fatality because of drug overdose. Serum lactate concentration was obtained from the initial blood draw in the ED and correlated with fatality.
Results
During the study period, 873 subjects were screened with 50 cases and 100 controls included. Drug exposures and baseline characteristics were similar between groups. Mean lactate concentration (mmol/L) was 9.88 ± 6.7 for cases and 2.76 ± 2.9 for controls (p < 0.001). The receiver operating characteristic area under the curve for prediction of fatality was 0.87 (95% CI: 0.81–0.94). The optimal lactate cutpoint was 3.0 mmol/L (84% sensitivity, 75% specificity), which conferred a 15.8-fold increase in odds of fatality (p < 0.001).
Conclusion
In this derivation study, serum lactate concentration had excellent prognostic utility to predict drug-overdose fatality. Prospective validation in the ED evaluation of drug overdoses is warranted.
Keywords: Overdose, Fatality, Acute poisoning
Introduction
Each year, there are over 1.5 million drug-related emergency department (ED) visits and over 2.4 million poison exposures reported to the American Association of Poison Control Centers in the United States.1–2 Poisoning is defined as injury resultant from exposure to drugs, chemicals, or natural toxins and is currently the second leading cause of injury-related fatality in the United States.3 Although poisoning is an infrequent cause of cardiac arrest in elderly patients, it is the leading cause of cardiac arrest in victims <40 years of age.2–4
The prognostic and diagnostic utility of a serum lactate concentration in the initial evaluation of drug overdose is historically controversial.5 Lactate concentration is a useful prognostic indicator for mortality in both medical and surgical patients including those with sepsis,6–9 trauma,10–11 myocardial infarction with cardiogenic shock,12 and in undifferentiated intensive care unit (ICU) patients.13 Current guidelines for the initial approach to management of the patient with a drug overdose do not include routine evaluation of serum for a lactate concentration.14–16 However, lactate concentration is an established prognostic marker for the evaluation of patients with elevated anion gap metabolic acidosis,17 as well as selected drug overdoses (e.g., metformin, acetaminophen),18–19 selected chronic drug toxicities (e.g., stadivudine),20 and chemical poisoning (e.g., cyanide).21 Despite this evidence, specific indications to obtain a serum lactate concentration in drug-overdose patients remain unclear and studies to clarify the role of serum lactate concentration in the evaluation of patients with drug-overdose emergencies are needed.
We designed a case–control study to explore the prognostic significance of serum lactate concentration in acute drug-overdose emergencies. We aimed to calculate the diagnostic test characteristics of serum lactate concentration for drug-overdose fatality and to assign an optimal cutpoint for prediction of mortality. We hypothesized that an elevated serum lactate concentration following acute drug overdose would have excellent prognostic utility for drug-overdose fatality.
Materials and methods
Study design
This was a case–control study to evaluate the prognostic utility of serum lactate concentration for acute drug-overdose emergencies. Cases were identified retrospectively and controls were enrolled prospectively.
Setting
EDs from two urban, tertiary-care hospitals were used for enrollment of controls. Both EDs have annual visit volumes in excess of 50,000 and are staffed 24 h per day with board-certified emergency physicians. The regional Poison Control Center (PCC) used for enrollment of cases in the study has an annual referral volume of 70,000. The study protocol was approved by the Institutional Review Board (IRB) for all participating institutions.
Case adjudication
Patients with a fatal drug overdose reported to the regional PCC over a 7-year period (2000–2006) were eligible for inclusion as cases. All human deaths from a single large metropolitan city that were attributed to poisoning were analyzed regardless of age. To be included, deaths were adjudicated as either poison-related fatality (PRF) or not by an adjudication committee composed of three medical toxicologists with a range of clinical experience (3–25 years practicing clinical toxicology, all board certified by either the American Board of Emergency Medicine or the American Osteopathic Board of Emergency Medicine). After review of the medical record, each adjudicator was asked to determine the relationship between poisoning and the cause of death according to one of the following five categories: 1) probable/definite, 2) possible, 3) unclear, 4) improbable, or 5) definitely unrelated. For each case, these five determinations were then dichotomized into PRF (1 or 2) versus non-PRF (3, 4, or 5) in accordance with validated adjudication methods in the epidemiology literature.22 Dichotomized data from two adjudicators were initially used to decide if the death was drug-overdose related. Disagreements were settled by a third adjudicator. Adjudicators were blinded to the current study purpose and hypothesis.
Exclusion criteria were the following: no serum lactate concentration obtained in the ED, alternative diagnosis (per adjudication), chronic presentation (i.e., not acute), nondrug overdose (e.g., plant), dermal or inhalational exposures only, age <18 years, anaphylaxis, and subjects with incomplete data.
Selection of controls
Control data were prospectively collected over a 1-year period (April 1, 2007–March 31, 2008) with IRB approval. Control patients were selected from consecutive ED patients presenting with acute drug overdose who were severe enough to warrant bedside consultation with the medical toxicology service but were not complicated by in-hospital fatality. Exclusion criteria were exactly the same as that for cases (see above) with the additional exclusion of in-hospital fatality (five subjects). Fatalities excluded as controls were not included as cases.
Control patients were referred from the EDs of two urban, university teaching hospitals. All controls underwent consultation from the PCC-affiliated medical toxicology service consisting of at least one clinical fellow supported by a board-certified medical toxicologist. All control patients had evaluation for drug-overdose emergencies including bedside history, physical examination, and screening laboratory tests while in the ED.
Data collection and processing
Electronic and paper medical records were provided for all cases and controls with IRB approval. Standardized data collection was performed by a single blinded abstractor and data were stored in a de-identified electronic database. Sources of data included hospital medical records (primary source), PCC electronic records, death reports or cause of death data from the Office of the Chief Medical Examiner (when available), and any additional paper notes (e.g., toxicology consultation service note). Using a standardized data collection instrument, demographics (age, gender), history of exposure (intent, timing, medications, etc.), and drug exposure information (using data from both history and toxicology testing) were recorded. Drug exposures were categorized into standard classes based on mechanism of action (e.g., benzodiazepines, sympathomimetics) to facilitate subgroup analysis. In addition to confirmation of exposure by history, laboratory confirmation of exposures using routine toxicology screens was recorded, if available. A blood gas (either arterial or venous) was performed on patients as was clinically necessary per the clinicians’ judgment. Serum toxicology (acetaminophen, salicylate, ethanol, and rarely, selected drug concentrations on an individual basis per clinicians’ judgment) and urine toxicology screens (most commonly included amphetamines, opioids, benzodiazepines, cocaine metabolite, barbiturates, phencyclidine, tetrahydrocannabinol, tricyclics) were performed per clinicians’ judgment.
Methods of measurement
Venous serum lactate concentration was drawn at the bedside for all control patients. The decision to measure serum lactate was made at the discretion of the treating physician as part of clinical care, and results were readily available to the clinicians in real time. Only the initial ED lactate was used in the analysis for both cases and controls; as such, subsequent lactates even if changed or abnormal were not included in the analysis. Serum was analyzed using amperometric electrodes with enzymatic membranes, and run using Radiometer ABL™ 700 analyzers. According to the manufacturer, the range of normal values for venous serum lactate concentration is 1.0–2.5 mmol/L.
Primary data analysis
Categorical and continuous variables were assessed using the chi-squared and t-test, respectively, with two-tailed alpha equal to 0.05. Odds ratios with 95% confidence intervals were calculated to evaluate associations between individual drug classes and hyperlactatemia. Receiver operating characteristic (ROC) curves were created to determine the diagnostic test characteristics and the optimal cutpoint for serum lactate concentration. The optimal cutpoint was defined as the serum lactate concentration which maximized the sum of sensitivity plus specificity rounded to the nearest integer. Computer analysis was performed using SPSS version 17 software (SPSS, Inc., Chicago, IL, USA).
Sample size and power
With 50 cases available to be analyzed in the PCC electronic database, and assuming that the control group would have a mean serum lactate concentration of 2 mmol/L, we estimated the need to analyze data from 100 consecutive control subjects. This would yield 90% power to detect a twofold difference (i.e., clinically meaningful) in mean serum lactate concentration using the t-test.
Results
Subject enrollment
During the study period, a total of 873 subjects were screened. Application of inclusion and exclusion criteria resulted in analysis of 50 cases (acute drug-overdose fatalities) and 100 controls (acute drug-overdose survivors). For cases, mortality occurred at a median of hospital day 3 (mean 5.6; range day 1–71).
PCC referral fatalities over the study period (n = 414) were eligible if deemed to be drug-related deaths by toxicologist adjudication (n = 227), at which point application of exclusion criteria [no lactate drawn (n = 94), insufficient data (n = 23), nondrug overdose (n = 18), age <18 (n = 15), chronic presentation (n = 14), dermal/inhalational (n = 6), alternative diagnosis (n = 6), anaphylaxis (n = 1)] yielded 50 cases for analysis.
Eligible controls (n = 459) over the study period were excluded if any of the following criteria were met: deaths (n = 5), no lactate obtained (n = 187), chronic presentation (n = 57), nondrug overdose (n = 37), age <18 (n = 33), alternative diagnosis (n = 21), dermal/inhalational exposure (n = 12), insufficient data (n = 6), anaphylaxis (n = 1). In total, 359 subjects were excluded, leaving 100 controls for data analysis.
Baseline characteristics
Demographics (age, gender) and comorbidities (hypertension, diabetes, congestive heart failure, chronic obstructive pulmonary disease, coronary disease) were similarly distributed between cases and controls (p = NS). Table 1 summarizes baseline characteristics of all subjects in the study.
Table 1.
Baseline characteristics of 50 cases and 100 control subjects
| Baseline characteristic | Cases (N = 50) % or mean ± SD | Controls (N = 100) % or mean ± SD |
|---|---|---|
| Demographics | ||
| Age | 41.6 ± 17 | 37.9 ± 15.8 |
| Males | 50 | 63 |
| Comorbidities | ||
| Hypertension | 16 | 7 |
| Diabetes mellitus | 10 | 3 |
| Coronary artery disease | 6 | 3 |
| Congestive heart failure | 2 | 1 |
| COPD | 2 | 2 |
| Permanent pacemaker | 2 | 0 |
COPD, chronic obstructive pulmonary disease; N, number of subjects; SD, standard deviation.
Drug exposures
Proof of at least one drug exposure (either serum drug concentrations or urine toxicology screens) was obtained in 80 and 83% of cases and controls, respectively. Exposure information comparing cases and controls is summarized in Table 2. Aside from ethanol co-ingestion (n = 40), the most common drug exposures in all 150 subjects were acetaminophen (n = 39), opioids (n = 26), and benzodiazepines (n = 26). No drug exposure categories were significantly associated with increased risk of hyperlactatemia (defined as a serum lactate concentration >2.5 mmol/L per assay). Benzodiazepines were the only category significantly associated with the decreased risk of hyperlactatemia (OR 0.37; CI 0.15–0.91). Drug exposure categories were similar between groups and the most common drug exposures with odds of hyperlactatemia are summarized in Table 3.
Table 2.
Exposure information comparing cases and controls
| Exposure information | Cases (N = 50) % or mean ± SD | Controls (N = 100)% or mean ± SD |
|---|---|---|
| Number of exposures | 2.3 ± 1.6 | 2.2 ± 1.3 |
| Single drug overdose | 38 | 35 |
| Multiple drug overdose | 60 | 61 |
| Unknown drug exposure* | 2 | 5 |
| Proof of at least one exposure | 80 | 83 |
N, number of subjects; SD, standard deviation.
At least one of the drugs to which the patient was exposed was an unknown drug.
Table 3.
Most common drug exposures in study subjects and risk of hyperlactatemia by exposure class
| Exposure class | Number of total subjects | HL* (%) | OR | 95% CI |
|---|---|---|---|---|
| Ethanol (co-ingestion) | 40 | 53 | 1.1 | 0.54–2.2 |
| Acetaminophen | 39 | 62 | 1.8 | 0.86–3.8 |
| Benzodiazepines | 26 | 31 | 0.37 | 0.15–0.91 |
| Opioids | 26 | 58 | 1.4 | 0.6–3.3 |
| Sympathomimetics | 23 | 48 | 0.87 | 0.36–2.1 |
| Antipsychotics | 22 | 50 | 0.97 | 0.39–2.4 |
| Antidepressants | 17 | 47 | 0.85 | 0.31–2.3 |
| Anticonvulsants | 11 | 73 | 2.8 | 0.71–10.9 |
| Cardiovascular Medications | 8 | 38 | 0.57 | 0.13–2.5 |
| Salicylates | 8 | 75 | 3.1 | 0.6–15.8 |
| Total | 150 | 51 | – | – |
Exposure class listed in descending order of frequency of ingestion among all 150 subjects. Statistically significant OR listed in bold. CI, confidence interval; HL, hyperlactatemia; OR, odds ratio.
Hyperlactatemia defined as above the upper normal limit of venous lactate concentration, >2.5 mmol/L.
Lactate concentration and blood gas data
Mean serum lactate concentration (mmol/L) was 9.88 ± 6.7 for cases and 2.76 ± 2.9 for controls (p < 0.001). Raw lactate data comparing cases to controls are demonstrated in Fig. 1. Elements of the blood gas (pH, PCO2, HCO3) were available in 45 (90%) cases and 97 (97%) controls. Elements of the blood gas were highly associated with fatality (all p < 0.001 using t-test) and are each summarized in Table 4. Sensitivity analysis was performed by removing all 39 subjects with acetaminophen (APAP) co-ingestion from the analysis (because of prior data associating lactate with fatality in APAP overdose);19 in the remaining 111 subjects, mean serum lactate remained significantly associated with fatality (p < 0.001).
Fig. 1.
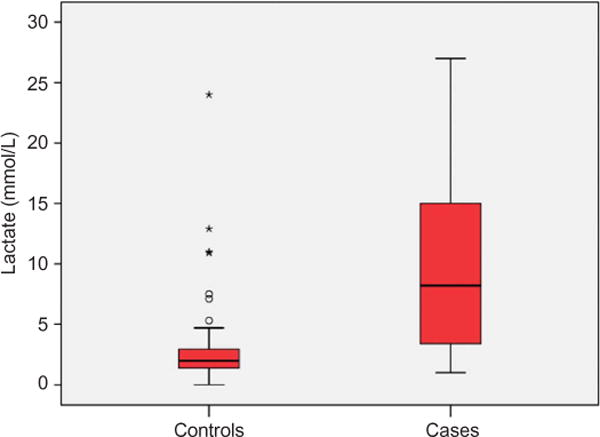
Lactate data comparing cases with controls. This figure demonstrates a box-plot of lactate concentration for cases (fatalities) and control (survivors) subjects. The box-plot represents the median, 25th and 75th quartiles, outliers, and extreme outliers by a line, a box, open circles (○) and asterisks (*), respectively.
Table 4.
Lactate and selected blood gas data comparison
| Laboratory test | Cases: mean (SD) |
Controls: mean (SD) |
p-Value |
|---|---|---|---|
| Lactate (mmol/L) | 9.88 (6.7) | 2.77 (2.9) | <0.001 |
| pH† | 7.14 (0.20) | 7.38 (0.09) | <0.001 |
| PCO2 (mmHg) | 29.6 (11) | 41.9 (10.1) | <0.001 |
| HCO3‡ (mmol/L) | 10.5 (5.4) | 23.7 (3.5) | <0.001 |
| Total | n = 45 | n = 97 | – |
p-Values calculated using the t-test for continuous variables.
Venous and arterial pH values used unchanged for cases; venous pH for al controls.
Calculated using formula: pH = 6.1 + log HCO3/0.0306 × PCO2.
Notes: HCO3, bicarbonate; mmHg, millimeters mercury; mmol/L, milli moles per liter; N, number; PCO2, partial pressure of carbon dioxide; SD standard deviation.
Lactate cutpoint
The ROC curve for ability of a serum lactate concentration to predict fatality is demonstrated in Fig. 2. The c-statistic for area under the ROC curve was 0.87 (95% CI, 0.81–0.94) and was statistically significant (p < 0.0001). The optimal serum lactate concentration integer cutpoints that maximized the sum of sensitivity and specificity were 3.0 mmol/L (84% sensitivity, 75% specificity) and 5.0 mmol/L (68% sensitivity, 93% specificity), respectively. A lactate cutpoint of 3.0 mmol/ L conferred 15.8-fold increased odds of fatality (OR 15.8; CI 6.5–38). Diagnostic test characteristics of selected serum lactate concentration cutpoints are summarized in Table 5.
Fig. 2.
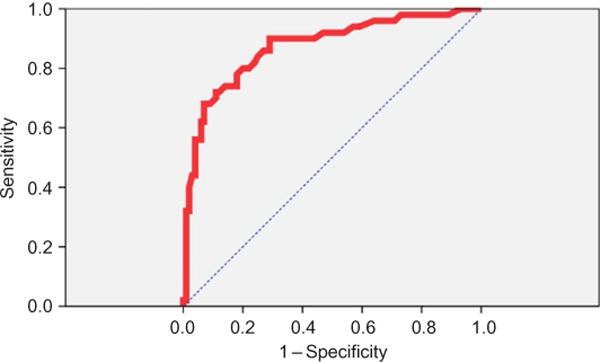
ROC curve for prediction of overdose fatality using initial serum lactate. This figure demonstrates the ROC curve of initial serum lactate concentration to predict mortality. The area under the curve of 0.87 was statistically significant. The cutpoints that maximized the sum of sensitivity and specificity were 3.0 and 5.0 mmol/L, respectively. ROC, receiver operating characteristics.
Table 5.
Diagnostic test characteristics of selected lactate cutpoints
| Lactate cutpoint | Sensitivity % (CI) |
Specificity % (CI) |
NPV % (CI) |
PPV % (CI) |
|---|---|---|---|---|
| HL* | 90 (82–98) | 66 (57–75) | 93 (87–99) | 57 (46–68) |
| >3.0 | 84 (74–94) | 75 (66–84) | 90 (84–97) | 63 (51–75) |
| >4.0 | 72 (60–85) | 88 (82–95) | 86 (79–93) | 75 (63–87) |
| >5.0 | 68 (55–81) | 93 (88–98) | 85 (78–92) | 83 (71–95) |
CI, confidence intervals; HL, hyperlactatemia; NPV, negative predictive value; PPV, positive predictive value.
Hyperlactatemia from assay defined per manufacturer as lactate concentration >2.5 mmol/L.
Discussion
In this derivation study, serum lactate concentration had excellent prognostic utility to predict fatality in drug-overdose emergencies. Using ROC analysis, initial venous lactate concentrations obtained in the ED had outstanding diagnostic test characteristics. By maximizing the sum of sensitivity and specificity, selection of optimal integer cutpoints for lactate concentration occurred at 3.0 and 5.0 mmol/L.
Hyperlactatemia in the setting of overdose may occur from many mechanisms, all of which suggest major metabolic insult and systemic compromise. Mechanisms to account for hyperlactatemia from specific drug overdoses are myriad and include the following: hypoperfusion because of vasoconstriction (e.g., ergots)23 or hypotension (e.g., beta blockers);24 muscle activity because of seizures (e.g., cocaine)25 or myoclonus (e.g., serotonin syndrome);26 altered metabolism of lactate because of increased production (e.g., propylene glycol)27 or decreased clearance (e.g., metformin);28 duration of unconsciousness;5 mitochondrial DNA changes (e.g., nucleoside inhibitors);29 and failure of cellular respiration because of poisoning of glycolysis (e.g., arsenic),30 the Kreb’s cycle (e.g., monofluoroacetate),31 electron transport (e.g., carbon monoxide),32 or uncoupling of oxidative phosphorylation (e.g., salicylism).33
The association of hyperlactatemia with mortality has been extensively studied in medical and surgical patients.6,7,10–11,34 It also correlates with mortality in ED patients with sepsis.7 The Surviving Sepsis Campaign uses a serum lactate concentration cutpoint as one trigger to begin early goal-directed therapy regardless of blood pressure,35 as hyperlactatemia correlates with mortality when studied in patients with severe sepsis even while excluding organ failure and shock.8 Organ failure and mortality during trauma also show a positive correlation to admission lactate concentrations.10 Similarly, hyperlactatemia is associated with assessing the severity of various poisonings such as with cyanide and acetaminophen-induced fulminant hepatic failure.36,37 Hyperlactatemia is now being studied in the pre-hospital setting and has shown promising results in identifying patients early in their presentation who are at increased risk for morbidity and mortality.38
Our data build upon the above prior evidence to include patients with acute drug overdose. We utilized a validated technique of adjudication22 to categorize overdoses resulting in PRF, and then correlated these results with observed hyperlactatemia. Cases were selected from the PCC database, rather than from the same two hospitals used to select the controls, because the study would not have otherwise been feasible given the rarity of PRF. Selection of controls from a 1-year period, rather than from the 7-year time period of case ascertainment, yielded enough patients to adequately power the statistical analysis; thus, evaluation of a longer time period was not necessary. We cannot exclude the possibility that changes in overdose epidemiology or differential use of lactate measurement between these two time periods may have biased our results; however, these possibilities are unlikely to have biased away from the null and are thus unlikely to have impacted the results meaningfully.
The implications of these results may be to help risk stratify patients early in the course of the ED presentation. Identification of those at risk for drug-overdose fatality may help prevent in-hospital adverse events and aid ICU triage. Risk stratification by serum lactate concentration may also help lead to expedite interventions such as life-saving antidotal therapy, when indicated. Prospective validation in the ED evaluation of drug overdoses is necessary to confirm these derivation data. Strategies that incorporate lactate concentration into ICU triage from the ED in drug-overdose emergencies may be warranted.
Interestingly, benzodiazepines were the only drugs that demonstrated a significantly decreased risk of hyperlactatemia. When benzodiazepines were co-ingested in overdose, there was 63% lower odds of hyperlactatemia (p < 0.05). This finding is novel but can be explained plausibly by CNS depression, muscle relaxation, and possible suppression of seizure activity, all of which contribute to lowering the serum lactate concentration.
Limitations
Our study has several limitations including those common to all retrospective case–control studies, namely the inability to calculate incidence or prevalence of hyperlactatemia or fatality. A large subset of subjects were excluded because of absence of ED serum lactate, which may have biased the lactate cutpoint data; however, this would probably bias toward the null hypothesis as clinicians are typically more likely to draw lactate in more severely ill patients. Another consideration is the study setting, as the study was performed in an urban tertiary referral center, and results might not be applicable to all centers; however, our subject population was highly diverse and likely represents a general ED population. The presence of a bedside medical toxicology consultation may have improved outcomes for controls as opposed to cases; however, this would have biased toward the null as sicker control subjects with theoretically high lactates would presumably have better prognosis. Cases were poison center referrals at the discretion of the ED physician, which may have biased our case population. Additionally, because of limited patients for subgroup analysis we could not identify drug combinations that were particularly prone to produce hyperlactatemia or fatality as an additive effect; however, in future larger studies we plan to examine particularly toxic drug combinations (type, dose, etc.). And finally, time to obtaining serum lactate concentration, as well as whether the source was arterial or venous, may also limit interpretation of our data. However, our data represent real-world scenario, which adds credence to the overall concept of generalizability.
Conclusions
In this derivation study, serum lactate concentration had excellent prognostic utility to predict drug-overdose fatality. Prospective validation in the ED evaluation of patients with drug overdose is warranted. Identification of those at risk for fatality may prevent adverse events and aid ICU triage.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network. 2005 May; http://druginfo.samhsa.gov/. Accessed on 2 June 2008. [PubMed]
- 2.Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2007 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila) 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- 3.Paulozzi L, Crosby A, Ryan G. Increases in age-group-specific injury mortality – United States, 1999–2004. MMWR. 2007;56:1281–1284. [PubMed] [Google Scholar]
- 4.McCaig LF, Burt CW. Poisoning-related visits to emergency departments in the United States, 1993–1996. J Toxicol Clin Toxicol. 1999;37:817–826. doi: 10.1081/clt-100102460. [DOI] [PubMed] [Google Scholar]
- 5.Schuster HP, Kapp S, Prellwitz W, Schuster CJ, Weilemann LS. Significance of hyperlactatemia in acute hypnotic drug poisoning. J Mol Med. 1981;59:599–605. doi: 10.1007/BF02593849. [DOI] [PubMed] [Google Scholar]
- 6.Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005;20:255–271. doi: 10.1177/0885066605278644. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure or shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 9.Jones AE, Puskarich MA. Is lactate the “Holy Grail” of biomarkers for sepsis prognosis? Crit Care Med. 2009;37:1812–1813. doi: 10.1097/CCM.0b013e3181a09487. [DOI] [PubMed] [Google Scholar]
- 10.Mankis P, Jankowski S, Zhang H, Kahn RJ, Vincent JL. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med. 1995;13:619–622. doi: 10.1016/0735-6757(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 11.Callaway DW, Shapiro NI, Donnino MW, Baker C, Rosen CL. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma. 2009;66:1040–1044. doi: 10.1097/TA.0b013e3181895e9e. [DOI] [PubMed] [Google Scholar]
- 12.Valente S, Lazzeri C, Vecchio S, Giglioli C, Margheri M, Bernardo P, Comeglio M, Chiocchini S, Gensini GF. Predictors of in-hospital mortality after percutaneous coronary intervention for cardiogenic shock. Int J Cardiol. 2007;114:176–182. doi: 10.1016/j.ijcard.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009;13:R90. doi: 10.1186/cc7918. Epub 2009 June 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson TB, Thompson TM, Lu JJ. The approach to the patient with an unknown overdose. Emerg Med Clin North Am. 2007;25:249–281. doi: 10.1016/j.emc.2007.02.004. abstract vii. [DOI] [PubMed] [Google Scholar]
- 15.Burns MJ, Schwartzstein RM. In: General approach to drug poisoning in adults. Basow DS, editor. UpToDate; Waltham, MA: 2010. [Google Scholar]
- 16.Wolf SJ, Heard K, Sloan EP, Jagoda AS, American College of Emergency Physicians Clinical policy: critical issues in the management of patients presenting to the emergency department with acetaminophen overdose. Ann Emerg Med. 2007;50:292–313. doi: 10.1016/j.annemergmed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Manini AF, Hoffman RS, McMartin KE, Nelson LS. Relationship between serum glycolate and falsely elevated lactate in severe ethylene glycol poisoning. J Anal Toxicol. 2009;33:174–176. doi: 10.1093/jat/33.3.174. [DOI] [PubMed] [Google Scholar]
- 18.Dell’aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54:818–823. doi: 10.1016/j.annemergmed.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- 20.Miller KD, Cameron M, Wood LV, Dalakas MC, Ko-vacs LA. Lactic acidosis and hepatic steatosis associated with use of stavudine: report of four cases. Ann Int Med. 2000;133:192–195. doi: 10.7326/0003-4819-133-3-200008010-00010. [DOI] [PubMed] [Google Scholar]
- 21.Baud FJ, Borron SW, Mégarbane B, Trout H, Lapostolle F, Vicaut E, Debray M, Bismuth C. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30:2044–2050. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S, WHI Morbidity and Mortality Committee Outcomes ascertainment and adjudication in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn FE, Johnson MN, Gillis RA, Visner MS, Schaer GL. Effect of cocaine on the coronary circulation and systemic hemodynamics in dogs. J Am Coll Cardiol. 1990;16:1481–1491. doi: 10.1016/0735-1097(90)90396-7. [DOI] [PubMed] [Google Scholar]
- 24.Love JN, Howell JM, Litovitz TL, Klein-Schwartz W. Acute beta blocker overdose: factors associated with the development of cardiovascular morbidity. J Toxicol Clin Toxicol. 2000;38:275–281. doi: 10.1081/clt-100100932. [DOI] [PubMed] [Google Scholar]
- 25.Hulme J, Sherwood N. Severe lactic acidosis following alcohol related generalized seizures. Anaesthesia. 2004;59:1228–1230. doi: 10.1111/j.1365-2044.2004.03962.x. [DOI] [PubMed] [Google Scholar]
- 26.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 27.Jorens PG, Demey HE, Schepens PJ, Coucke V, Verpooten GA, Couttenye MM, Van Hoof V. Unusual D-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol. 2004;42:163–169. doi: 10.1081/clt-120030942. [DOI] [PubMed] [Google Scholar]
- 28.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 29.Cote HCF, Brumme ZL, Kevin JP, Craib J, Alexander CS, Wynhoven B, Ting L, Wong H, Harris M, Harrigan PR, O’Shaughnessy MV, Montaner JSG. Changes in mitochondrial DNA as a matter of nucleoside toxicity in HIV-infected patients. N Engl J Med. 2002;346:811–820. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- 30.Pal S, Chatterjee AK. Protective effect of methionine supplementation on arsenic-induced alteration of glucose homeostasis. Food Chem Toxicol. 2004;42:737–742. doi: 10.1016/j.fct.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Chi CH, Chen KW, Chan SH, Wu MH, Huang JJ. Clinical presentation and prognostic factors in sodium monofluoroacetate intoxication. J Toxicol Clin Toxicol. 1996;34:707–712. doi: 10.3109/15563659609013833. [DOI] [PubMed] [Google Scholar]
- 32.Benaissa ML, Mégarbane B, Borron SW, Baud FJ. Is elevated plasma lactate a useful marker in the evaluation of pure carbon monoxide poisoning? Intensive Care Med. 2003;29:1372–1375. doi: 10.1007/s00134-003-1866-0. [DOI] [PubMed] [Google Scholar]
- 33.Stolbach AI, Hoffman RS, Nelson LS. Mechanical ventilation was associated with acidemia in a case series of salicylate-poisoned patients. Acad Emerg Med. 2008;15:866–869. doi: 10.1111/j.1553-2712.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, Bennett ED. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27:74–83. doi: 10.1007/s001340051352. [DOI] [PubMed] [Google Scholar]
- 35.Rivers EP, Coba V, Visbal A, Whitmill M, Amponsah D. Management of sepsis with early resuscitation. Clin Chest Med. 2008;29:689–704. doi: 10.1016/j.ccm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Baud FJ, Borron SW, Megarbane B, Trout H, Lapostolle F, Vicaut E, Debray M, Bismuth C. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30:2044–2050. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt LE, Larsen FS. Prognostic implications of hyperlactemia, multiple organ failure and systemic inflammatory response syndrome in patients with acetaminophen-induced acute liver disease. Crit Care Med. 2006;34:337–343. doi: 10.1097/01.ccm.0000194724.70031.b6. [DOI] [PubMed] [Google Scholar]
- 38.Jansen TC, van Bommel J, Mulder PG. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care. 2008;12:R160. doi: 10.1186/cc7159. [DOI] [PMC free article] [PubMed] [Google Scholar]


