Abstract
Osteoporosis can result from intestinal inflammation, as is seen with inflammatory bowel disease. Probiotics, microorganisms that provide a health benefit to the host when ingested in adequate amounts, can have anti-inflammatory properties and are currently being examined to treat inflammatory bowel disease. Here, we examined if treating healthy male mice with Lactobacillus reuteri ATCC PTA 6475 (a candidate probiotic with anti-TNFα activity) could affect intestinal TNFα levels and enhance bone density. Adult male mice were given L. reuteri 6475 orally by gavage for 3 ×/week for 4 weeks. Examination of jejunal and ileal RNA profiles indicates that L. reuteri suppressed basal TNFα mRNA levels in the jejunum and ileum in male mice, but surprisingly not in female mice. Next, we examined bone responses. Micro-computed tomography demonstrated that L. reuteri 6475 treatment increased male trabecular bone parameters (mineral density, bone volume fraction, trabecular number, and trabecular thickness) in the distal femur metaphyseal region as well as in the lumbar vertebrae. Cortical bone parameters were unaffected. Dynamic and static histomorphometry and serum remodeling parameters indicate that L. reuteri ingestion increases osteoblast serum markers and dynamic measures of bone formation in male mice. In contrast to male mice, L. reuteri had no effect on bone parameters in female mice. Taken together our studies indicate that femoral and vertebral bone formation increases in response to oral probiotic use, leading to increased trabecular bone volume in male mice.
By the year 2020, more than half of the United States population is expected to have low bone density or osteoporosis (Foundation, 2012). One in two women and one in four men over the age of 50 will experience an osteoporotic associated fracture in their lifetime. The health care costs associated with osteoporosis and related maladies (fractures) is expected to be over $25 billion dollars in the United States alone by the year 2025. Although several medications are available that have shown success in slowing bone loss, most have unwanted side effects that are beginning to emerge since they have been in widespread use for a long period of time. For example, the most widely used class of anti-resportive drugs, bisphosphonates, can result in joint and muscle pain and are associated with increasing reports of unusual fractures (Rizzoli et al., 2011; Abrahamsen and Einhorn, 2012). One possible cause of the unusual fractures is that bisphosphonate reduces bone remodeling that ultimately leads to weakened bone. Therefore, novel therapeutics for the treatment of osteoporosis, with reduced side effects, are desired.
While post-menopausal women make up the majority of osteoporosis patients, bone loss can be associated with a number of conditions that affect both men and women of all ages. In some cases, such as diabetes, inflammatory bowel disease (IBD) as well as menopause, inflammation within the bone is a key driver of the disruption of normal bone remodeling and initiation of bone loss (McCabe, 2007; Tilg et al., 2008; Harris et al., 2009; Pacifici, 2010). For example, increased levels of TNF are associated with osteoporosis, both in animal models of menopause and in post-menopausal women. Moreover, evidence is accumulating that resolution of this inflammation can be beneficial to bone which supports the investigation of anti-inflammatory agents in reducing osteoporosis (Kimble et al., 1997; Charatcharoenwitthaya et al., 2007). Thus, therapeutics that target bone inflammation may be beneficial in multiple conditions associated with osteoporosis.
The intestinal microbiota plays a critical role in human health and dysbiosis can result in disease, including IBD, diabetes, fatty liver disease, and cardiovascular disease (Wen et al., 2008; Wang et al., 2011; Henao-Mejia et al., 2012; Mathis and Benoist, 2012; Manichanh et al., 2012a). This is in part due to the key role that intestinal bacteria play in shaping the immune system (Chung et al., 2012; Maynard et al., 2012). Recent work has also linked the intestinal microbiota to bone health in animal models. Germ-free mice have increased bone density when compared to mice that contain a conventional microbiota in their intestinal tract. Moreover, antibiotic treatment of weaning mice results in increased bone density in these young mice (Cho et al., 2012). These studies indicate that the intestinal microbiota can have profound effects on bone health, however the mechanisms of how this occurs are largely unknown.
Probiotics are live microbial feed supplements that when administered in adequate amounts provide a health benefit to the host. One mechanism by which probiotics mediate their beneficial effects is by alteration of the host immune system. We are interested in one such strain, L. reuteri ATCC PTA 6475, due to its ability to suppress TNF production in the human monocytoid cell line THP-1 and in primary monocytes (Lin et al., 2008; Jones et al., 2011; Thomas et al., 2012). We therefore hypothesized that L. reuteri 6475 may have a beneficial effect on bone health by reducing TNF levels in the host and limiting bone resorption. To begin to explore the role of L. reuteri 6475 in bone health we treated healthy male and female mice and assessed the effects on numerous bone parameters. Our results demonstrate that male mice display significant improvement in bone health upon treatment with L. reuteri 6475.
Methods
Mice
C57Bl/6 mice, 14 weeks of age were from breeding pairs housed in a specific pathogen free environment and given autoclaved food, bedding, and water. Cage changes were performed in a laminar flow hood. Experimental mice were transferred to a conventional mouse room and allowed to acclimate for 1 week before treating. Mice were given Teklad 2019 chow (Madison, WI) and water ad libitum and were maintained on a 12 h light/dark cycle. Food and water intake were monitored and did not differ between groups (data not shown). All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee.
Bacterial culture conditions
L. reuteri ATCC PTA 6475 was cultured under anaerobic conditions in deMan, Rogosa, Sharpe media (MRS, Difco) for 16–18 h at 37°C. On the following day, the overnight culture is sub-cultured into fresh MRS and grown until log phase (OD600 = 0.4). L. retueri (300 μl of 1 × 109 cfu/ml) were then introduced directly into the stomach with a 24-guage ball-tipped gavage needle. Control mice were given MRS broth (vehicle control). Mice were treated three times per week for 4 weeks.
Bone and intestine RNA analysis
Immediately following euthanasia tibias and intestine were cleaned of connective tissue and luminal contents (respectively), snap frozen in liquid nitrogen and stored at −80°C. Frozen tibias and intestine were crushed under liquid nitrogen conditions with a Bessman Tissue Pulverizer (Spectrum Laboratories, Rancho Dominguez, CA). RNA was isolated using TriReagent (Molecular Research Center, Cincinnati, OH), and integrity assessed by formaldehyde-agarose gel electrophoresis. cDNA was synthesized by reverse transcription using Superscript II Reverse Transcriptase Kit and oligodT (12–18) primers (Invitrogen, Carlsbad, CA) and amplified by real-time PCR with iQ SYBR Green Supermix (BioRad, Hercules, CA), and gene specific primers (synthesized by Integrated DNA Technologies, Coralville, IA). RNA levels of the housekeeping gene hypoxanthine guanine phosphoribosyltransferase (HPRT) do not fluctuate with treatment and were therefore used as an internal control. Primers for real-time PCR were previously described (Xue et al., 2006; Motyl et al., 2009). Real-time PCR was carried out for 40 cycles using the iCycler (Bio-Rad) and data were evaluated using the iCycler software. Each cycle consisted of 95°C for 15 sec, 60°C for 30 sec (except for osteocalcin which had an annealing temperature of 65°C), and 72°C for 30 sec. cDNA-free samples, a negative control, did not produce amplicons. Melting curve and gel analyses (sizing, isolation, and sequencing) were used to verify single amplicon products of the appropriate base pair size.
μCT bone imaging
Fixed femurs and vertebrae were scanned using a GE Explore Locus microcomputed tomography (μCT) system at a voxel resolution of 20 μm obtained from 720 views. Beam angle of increment was 0.5, and beam strength was set at 80 peak kV and 450 μA. Each run consisted of control and L. reuteri treated mouse bones, and a calibration phantom to standardize grayscale values and maintain consistency. On the basis of auto threshold and isosurface analyses of multiple bone samples, a fixed threshold (750) was used to separate bone from bone marrow. Distal femur bone analyses were performed in a region of trabecular bone defined at 1% of the total length (~0.14 mm) proximal to the growth plate extending 2 mm toward the diaphysis excluding the outer cortical bone. Trabecular bone mineral content, bone volume fraction, thickness, spacing, and number values were computed by a GE Healthcare MicroView software application for visualization and analysis of volumetric image data. Cortical measurements were performed in a 2 × 2 × 2 mm3 cube centered midway down the length of the femur using a threshold of 1,000 to separate bone from marrow. Trabecular bone was also analyzed within the entire lumbar (L3) vertebrae.
Vertebraedynamic measures
For dynamic histomorphometric measures of bone formation, mice were injected intraperitoneally with 200 μl of 10 mg/ml calcein (Sigma, St. Louis, MO) dissolved in sterile saline at 7 and 2 days prior to harvest. L3–L4 vertebrae were fixed in formalin at time of harvest then transferred to 70% ethanol 48 h later. Vertebrae were then embedded, sectioned and examined under UV light. Five images were taken and the distance between the calcein lines (bone formation rate, BFR) and their length along the bone surface was measured and used to calculate mineral apposition rate (MAR).
Serum measurements
Blood was collected at the time of harvest, allowed to clot at room temperature for 5 min, then centrifuged at 4,000 rpm for 10 min. Serum was removed, frozen in liquid nitrogen and stored at −80°C. Serum went through no more than two freeze/thaw cycles. Serum TRAP5b and Osteocalcin were measured using a Mouse TRAP and OC assay kits (SB-TR103, Immunodiagnostic Systems Inc., Fountain Hills, AZ and BT-470, Biomedical Technologies Inc., Stoughton, MA, respectively) according to the manufacturer’s protocol.
Statistical analysis
All measurements are presented as the mean ± SE or SD as noted. Student’s t-test (two tail assuming equal variance) using Microsoft Excel (Microsoft, Redmond, WA) was used to determine significance where noted.
Results
Inflammatory bowel disease causes bone loss, suggesting the possibility that even low levels of intestinal inflammation can affect bone health. The probiotic L. reuteri is known to have anti-inflammatory, specifically anti-TNFα, properties. Therefore we examined if adult male mice, moved from pathogen free facilities to standard animal facilities, would obtain gut and bone health benefits from L. reuteri treatment. Mice were given either MRS broth (vehicle) or L. reuteri 6475 orally (by gavage) 3× a week for 4 weeks. Mice were harvested at the end of the experiment and general parameters examined. Interestingly, body weight and fat pad weight displayed a trend toward decreasing in L. reuteri 6475 treated mice, with visceral fat showing a significant reduction by nearly 50% (Table 1). Liver weight was also decreased in treated mice while muscle, spleen, and thymus weights did not differ between groups.
TABLE 1.
General mouse parameters
| Control | +L. reuteri | |
|---|---|---|
| Weight (g) | 29.6 ± 0.5 | 28.8 ± 0.6 |
| Femoral fat (mg) | 302 ± 37 | 246 ± 18 |
| Visceral fat (mg) | 124 ± 20 | 63 ± 8* |
| Liver (g) | 1.41 ± 0.04 | 1.28 ± 0.03* |
| Kidney (mg) | 341 ± 27 | 323 ± 23 |
| Tibialis (mg) | 92 ± 8 | 87 ± 7 |
| Heart (mg) | 174 ± 26 | 152 ± 7 |
| Spleen (mg) | 85 ± 4 | 84 ± 4 |
| Thymus (mg) | 35 ± 2 | 35 ± 2 |
Values are averages ± SE. n ≥ 11;
P < 0.05 compared to corresponding gender controlmice.
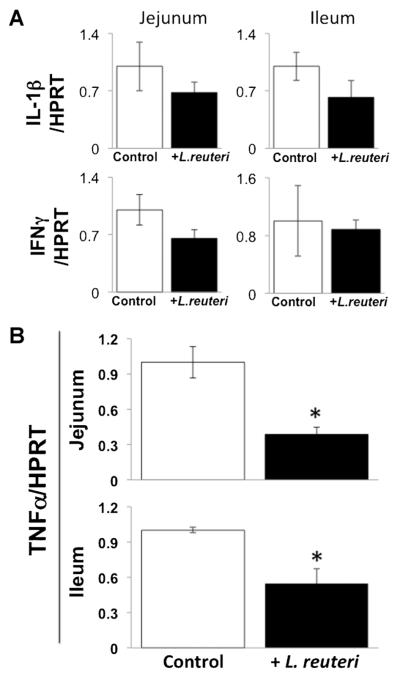
To assess the effect of L. reuteri treatment on basal/general intestinal inflammation, we examined cytokine expression in the proximal (jejunum) and distal (ileum) regions of the small intestine. While expression of several cytokines did not change (IL-6, MCP-1; data not shown), IL-1β and IFNγ expression in the jejunum and ileum displayed a trend toward decreasing in L. reuteri 6475 treated mice (Fig. 1A). More importantly, TNFα expression levels in both jejunal and ileal regions of the small intestine were significantly suppressed in mice given oral L. reuteri treatment (Fig. 1B).
Fig. 1.
L. reuteri treatment reduces TNFα expression in proximal and distal regions of the small intestine. Adult male mice were treated for 4 weeks with L. reuteri or broth (vehicle control). Total RNA was isolated from sections of jejunum and ileum and analyzed for cytokine mRNA expression. A: Interleukin 1-β (IL-1β) and interferon (IFN) γ mRNA levels expressed relative to HPRT (a non-modulated control gene). B: TNFα mRNA levels. Values are average ± standard error, n ≥ 8 per group, *P < 0.05 to control by Student’s t-test.
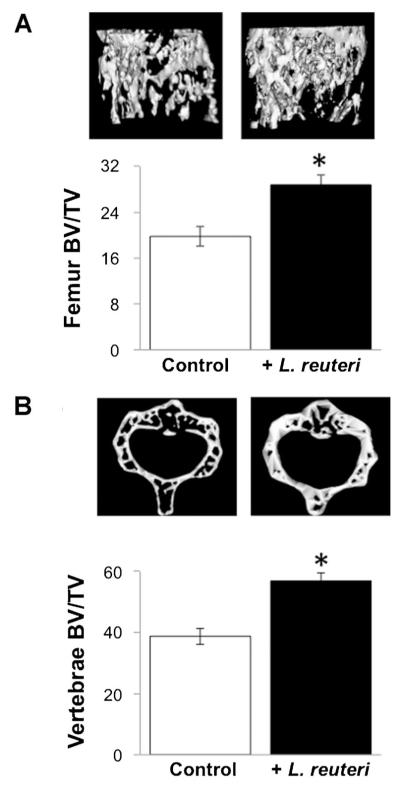
Next, we examined if L. reuteri treatment could benefit bone health. We examined two bones, femur, and vertebrae, to assess if there were effects and if they were site/bone specific. Microcomputed tomography analysis of the distal femur metaphyseal trabecular region identified a significant increase in bone volume fraction (Fig. 2A), bone mineral density and bone mineral content (Table 2). Consistent with these measures, trabecular number and thickness were increased in L. reuteri treated mice, while trabecular spacing was decreased. Analysis of the cortical regions of the femur (diaphyseal region) did not demonstrate any significant differences between the groups, although total cortical area trended toward an increase (Table 2). Similar to femoral trabecular bone changes, vertebral trabecular bone volume increased in L. reuteri treated mice (Fig. 2B), indicating that positive effects on bone density are broad based. Vertebral trabecular number and thickness were increased consistent with trabecular spacing being decreased (Table 3).
Fig. 2.
Femoral and vertebral trabecular bone volume is increased in L. reuteri treated male mice. A: Representative micro-computed tomography isosurface images (top) and bone volume fraction (BV/TV) quantitative data (bottom) obtained from the distal femur trabecular bone of control and L. reuteri treated mice. B: Representative micro-computed tomography isosurface images (top) and %BV/TV (bottom) obtained from the trabecular region of the L3 vertebrae from control and L. retueri treated mice. Mice were treated for 4 weeks. Values are averages ± standard error, n = 10 per group, *P < 0.05 to control as determined by Student’s t-test.
TABLE 2.
Femur bone parameters
| Control | +L. reuteri | |
|---|---|---|
| Trabecular | ||
| BV/TV | 19.8 ± 1.7 | 28.8 ± 1.8* |
| Tb. N. (1/mm) | 4.97 ± 0.25 | 5.87 ± 0.20* |
| Tb. Th. (mm) | 39 ± 2 | 49 ± 2* |
| Tb. Sp. (mm) | 0.17 ± 0.01 | 0.12 ± 0.01* |
| BMC (mg) | 0.50 ± 0.02 | 0.58 ± 0.02* |
| BMD (mg/cc) | 166 ± 6 | 193 ± 7* |
| Cortical | ||
| Tt. Ar. (mm2) | 2.13 ± 0.08 | 2.32 ± 0.06 |
| Ct. Ar. (mm2) | 1.21 ± 0.04 | 1.28 ± 0.03 |
| Ma. Ar. (mm2) | 0.92 ± 0.05 | 1.04 ± 0.05 |
| Ct. Ar./Tt. Ar. | 0.57 ± 0.01 | 0.55 ± 0.01 |
| Ct. Th (mm) | 0.17 ± 0.01 | 0.17 ± 0.01 |
BV/TV, bone volume; Tb. N, trabecular number; Tb. Th, trabecular thickness; Tb. Sp trabecular spacing; BMC, bone mineral content; BMD, bone mineral density; Tft. Ar, total cortical area, Ct. Ar, cortical area; Ma. Ar, marrow area; Ct. Ar/Tt.Ar, cortical area fraction; Ct. Th, cortical thickness. Data are averages ± standard error. N = 10 per group,
P ≤ 0.05 using Student’s t-test.
TABLE 3.
Vertebral bone parameters
| Control | +L. reuteri | |
|---|---|---|
| Trabecular | ||
| BV/TV | 38.8 ± 2.7 | 56.9 ± 2.7* |
| Tb. N (1/mm) | 9.03 ± 0.31 | 9.36 ± 0.29* |
| Tb. Th (mm) | 42.8 ± 1.7 | 61.3 ± 3.3* |
| Tb. Sp (mm) | 68.3 ± 5.2 | 46.3 ± 2.3* |
| BMC (mg) | 0.49 ± 0.02 | 0.61 ± 0.02* |
| BMD (mg/cc) | 216 ± 8 | 270 ± 7* |
BV/TV, bone volume fraction; BMD, bone mineral density; BMC, bone mineral content; Tb. Th., trabecular thickness; Tb. N., trabecular number; Tb. Sp., trabecular spacing. Data are averages ± standard error, n = 8 per group,
P < 0.05 to control as determined by Student’s t-test.
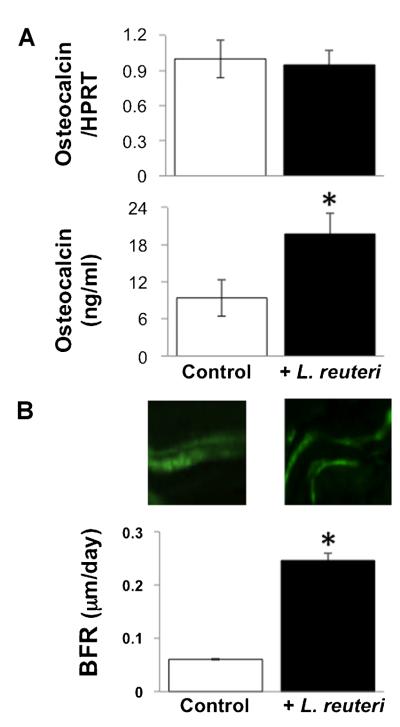
To determine the mechanism accounting for the bone density changes, decreased catabolic, and/or increased anabolic bone processes, we measured markers of osteoclast and osteoblast activity. Serum protein and bone RNA levels of TRAP5, a specific marker of osteoclast activity, were not altered in L. reuteri treated mice (Fig. 3). Finally, we examined markers of osteoblast activity and determined that while bone osteocalcin mRNA levels were unchanged, serum osteocalcin levels were significantly increased in L. reuteri treated mice (Fig. 4A). To follow up on this observation, we examined a dynamic measure of bone formation by pulsing mice with calcein and measuring bone formation rate. Our findings demonstrated that L. reuteri ingestion causes a dramatic and significant increase in bone formation rate (Fig. 4B).
Fig. 3.
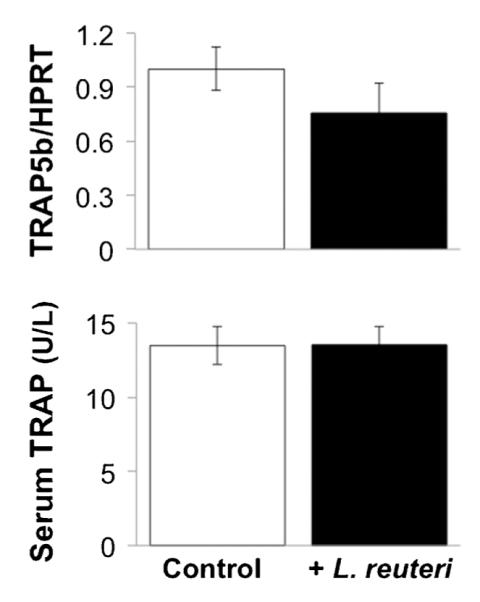
Osteoclast activity measures are not altered by L. reuteri treatment. Bar graphs displaying levels of TRAP5 tibial RNA and serum active protein levels. Mice were treated for 4 weeks. Values are averages ± standard error, n = 8 per group.
Fig. 4.
Osteoblast markers and bone formation rate are increased in male mice treated with L. reuteri. A: Levels of osteocalcin tibia RNA and serum protein are shown. Mice were treated with vehicle (control) or L. reuteri for 4 weeks. RNA levels were expressed relative to HPRT, a non-modulated house-keeping gene. B: Top: Representative fluorescence microscopy photographs depicting two pulses of calcein incorporation. The space between the two bands represents the mineral apposition rate (MAR). Bottom: Bar graphs of bone formation rate (BFR). Mice were treated for 4 weeks. Values are averages ± standard error, n ≥ 8 per group, *P < 0.05 to control by Student’s t-test.
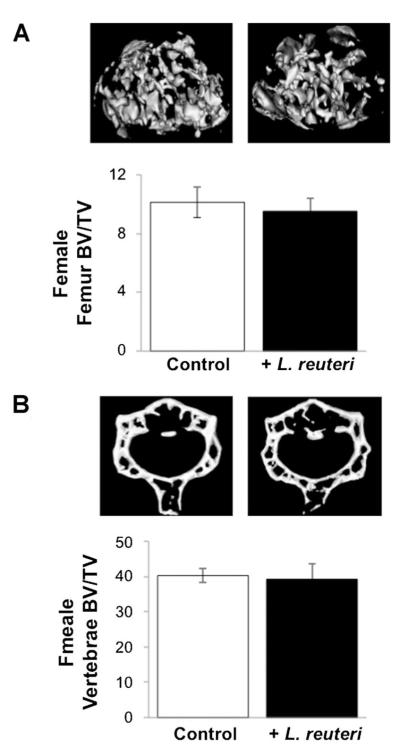
Given that the simple ingestion of L. reuteri could lead to a significant increase in bone density in male mice, it was logical to ask if similar effects could occur in adult female mice. An identical experiment was run (with part of the study overlapping with the male mouse study) with healthy female mice being treated for 4 weeks with L. reuteri. Analysis of general mouse parameters, such as body, organ, and fat pad weights, were not altered in female mice ingesting L. reuteri (data not shown). Even more surprising was that L. reuteri treatment had no effect on female mouse femur and vertebral trabecular bone density (Fig. 5A,B).
Fig. 5.
Female adult mouse femoral and vertebral trabecular bone volume is not altered by L. reuteri treatment. A: Representative micro-computed tomography isosurface images (top) and bone volume fraction (BV/TV) quantitative data (bottom) obtained from the distal femur trabecular bone of control and L. reuteri treated adult female mice. B: Representative micro-computed tomography isosurface images (top) and %BV/TV (bottom) obtained from the trabecular region of the L3 vertebrae from control and L. retueri treated adult female mice. Mice were treated for 4 weeks. Values are averages ± standard error, n = 10 per group, *P < 0.05 to control as determined by Student’s t-test.
Discussion
We have shown that L. reuteri 6475 can improve bone health in a gender specific manner. The choice of this particular bacterial strain was driven mainly by its ability to reduce TNF production from human monocytes in vitro. Given the key role of proinflammatory cytokines (including TNF) in some models of osteoporosis (diabetes (Graves and Kayal, 2008; Coe et al., 2011), ovariectomy (Li et al., 2011), and inflammatory bowel disease (Uno et al., 2006; Paganelli et al., 2007)), we surmised that supplementation of L. reuteri 6475 to animals experiencing inflammation driven bone loss would be beneficial. However, we were surprised to discover that treatment of “healthy” male mice with L. reuteri caused a significant increase in trabecular bone volume fraction, bone mineral density and bone mineral content in both vertebral and femoral locations. It should be noted that in these studies the mice were maintained under specific pathogen free (SPF) conditions and then moved to standard mouse housing at the time of treatment. Why would this protocol allow us to see an L. reuteri induced anabolic effect? At least two possibilities exist. First, ultra clean conditions may alter intestinal microbiota in such a way that bone formation is suppressed by a reduced interaction between the immune system and specific gut microbes; this in turn enhances the response to treatment with a beneficial/anabolic bacteria, L. reuteri. Second, upon being moved from SPF conditions to normal housing the mice may become infected with intestinal bacteria that now elicit a pro-inflammatory response, causing a reduction in bone health that is benefited by L. reuteri treatment. The association between intestinal inflammation and bone loss, as seen in inflammatory bowel disease (IBD) clinically and in animal models, further supports the role for gut health in the regulation of bone health (Sylvester et al., 2007; Harris et al., 2009; Agrawal et al., 2011). Even mild cases of intestinal inflammation can cause bone loss in male mice in the absence of any overt nutritional deficiencies or weight loss (manuscript submitted).
Recent studies support a link between altered gut microbiota (dysbiosis) and IBD (Manichanh et al., 2012b) as well as other diseases (Lozupone et al., 2012). In the current studies, we demonstrate that ingestion of a probiotic decreases intestinal inflammation and enhances bone density in male mice, suggesting that bone health could be driven by microbes harbored in the gut. In support of this idea recent work has shown the intestinal microbiota can have a significant impact on bone health (Sjogren et al., 2012), although the precise mechanisms by how bone is affected by these bacteria are not understood.
How could bacterial supplementation impact bone health? Interestingly, L. reuteri 6475 reduced pro-inflammatory cytokine expression in the jejunum and ileum, indicating that the anti-inflammatory effects observed in vitro also take place in the intestinal tract. Although it is tantalizing to speculate that L. reuteri 6475 is generating a system-wide reduction in pro-inflammatory cytokine levels and this is responsible for increased bone volume fraction in males, we did not find that L. reuteri 6475 supplementation had any effect on cytokine RNA levels directly in the bone at the 4-week time point (data not shown). Alternatively, previous work with prebiotics (non-digestable sugars that enhance the growth of certain members of the microbiota) has shown beneficial effects on bone health that are speculated to be regulated by increased calcium and other mineral uptake (Abrams et al., 2005; Legette et al., 2012 #336). Thus L. reuteri 6475 could impact bone health by increasing calcium uptake through mechanisms that could include increasing calcium solubility/uptake and/or reducing intestinal epithelial cell inflammation to directly enhance transport. Finally, a novel mechanism may be employed by the bacterium, such as the production or transformation of estrogen like compounds that act on the gut epithelium or circulate through the blood stream to directly affect bone cells.
Surprisingly we found that the effect was gender specific, with females showing no response to L. reuteri 6475 supplementation with regard to bone health, general mouse parameters, or intestinal cytokine expression. Interestingly, we have observed that female mice are also more resistant to bacterial induced colitis and its associated bone loss, when compared to male mice (Irwin et al., in preparation). This further supports a gender dependent difference/signaling pathway that could be based in the intestine and/or immune system. It is know that estrogen can affect gut (Alzamora et al., 2011) and immune system function (Pacifici, 2012). Thus, it is possible that L. reuteri impacts estrogen and/or progesterone sensitive pathways in male mice that are fully active in adult females and thus insensitive to the bacterium.
Currently we are working to identify if L. reuteri 6475 increases bone health under conditions associated with bone loss and determining if gender specific differences are observed. In addition, we are interested in exploring if the beneficial effects of L. reuteri 6475 can be extended to cortical bone by treating mice for longer periods of time. While bone formation and resorption are typically coupled, our studies suggest that L. reuteri has a predominant anabolic effect on bone in our “healthy” male condition; this effect was characterized by both static and dynamic measures. It is possible that osteoclast activity is altered at an earlier time point and by 4 weeks of treatment we are only able to detect changes in mature functioning osteoblasts. As most of the available treatments for osteoporosis function by inhibiting of bone resorption and have unwanted side effects, it is exciting that L. reuteri 6475 appears to impact bone health at least in part by promoting bone formation. Thus one can envision L. reuteri 6475 being utilized either alone or in combination with existing therapies to treat osteoporosis.
Acknowledgments
The authors thank Jing Zhang and Darin Quach for their discussions and critical review of the manuscript, the Investigative Histology Laboratory in the Department of Physiology, Division of Human Pathology and the Biomedical Imaging Center at Michigan State University for their assistance with histology and imaging. These studies were supported by funding from the National Institute of Health (R21RAT005472 to L.R.M. and R.A.B.) and a Strategic Partnership Grant from Michigan State University.
Contract grant sponsor: National Institute of Health;
Contract grant number: R21RAT005472.
Contract grant sponsor: Michigan State University.
Footnotes
The authors have no conflict of interest to disclose.
Literature Cited
- Abrahamsen B, Einhorn TA. Beyond a reasonable doubt? Bisphosphonates and atypical femur fractures. Bone. 2012;50:1196–1200. doi: 10.1016/j.bone.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82:471–476. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- Agrawal M, Arora S, Li J, Rahmani R, Sun L, Steinlauf AF, Mechanick JI, Zaidi M. Bone, inflammation, and inflammatory bowel disease. Curr Osteoporos Rep. 2011;9:251–257. doi: 10.1007/s11914-011-0077-9. [DOI] [PubMed] [Google Scholar]
- Alzamora R, O’Mahony F, Harvey BJ. Estrogen inhibits chloride secretion caused by cholera and Escherichia coli enterotoxins in female rat distal colon. Steroids. 2011;76:867–876. doi: 10.1016/j.steroids.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Byrne FR, Morony S, Warmington K, Geng Z, Brown HL, Flores SA, Fiorino M, Yin SL, Hill D, Porkess V, Duryea D, Pretorius JK, Adamu S, Manoukian R, Danilenko DM, Sarosi I, Lacey DL, Kostenuik PJ, Senaldi G. CD4+CD45RBHi T cell transfer induced colitis in mice is accompanied by osteopenia which is treatable with recombinant human osteoprotegerin. Gut. 2005;54:78–86. doi: 10.1136/gut.2003.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res. 2007;22:724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe LM, Irwin R, Lippner D, McCabe LR. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. Journal of cellular physiology. 2011;226:477–483. doi: 10.1002/jcp.22357. [DOI] [PubMed] [Google Scholar]
- Foundation NO National Osteoporosis Foundation. 2012 [Google Scholar]
- Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227–1239. doi: 10.2741/2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1020–G1029. doi: 10.1152/ajpgi.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis. doi: 10.1097/MIB.0b013e318289e17b. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Whitehead K, Saulnier D, Thomas CM, Versalovic J, Britton RA. Cyclopropane fatty acid synthase mutants of probiotic human-derived Lactobacillus reuteri are defective in TNF inhibition. Gut Microbes. 2011;2:69–79. doi: 10.4161/gmic.2.2.15282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- Legette LL, Lee W, Martin BR, Story JA, Campbell JK, Weaver CM. Prebiotics enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J Food Sci. 2012;77:H88–H94. doi: 10.1111/j.1750-3841.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, Zayzafoon M, Weitzmann MN, Pacifici R. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Thibodeaux CH, Peña JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis. 2008;14:1068–1083. doi: 10.1002/ibd.20448. [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012a;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012b;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. The influence of the microbiota on type-1 diabetes: On the threshold of a leap forward in our understanding. Immunol Rev. 2012;245:239–249. doi: 10.1111/j.1600-065X.2011.01084.x. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102:1343–1357. doi: 10.1002/jcb.21573. [DOI] [PubMed] [Google Scholar]
- Motyl KJ, Botolin S, Irwin R, Appledorn DM, Kadakia T, Amalfitano A, Schwartz RC, McCabe LR. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218:575–583. doi: 10.1002/jcp.21626. [DOI] [PubMed] [Google Scholar]
- Pacifici R. The immune system and bone. Arch Biochem Biophys. 2010;503:41–53. doi: 10.1016/j.abb.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R. Role of T cells in ovariectomy induced bone loss–revisited. J Bone Miner Res. 2012;27:231–239. doi: 10.1002/jbmr.1500. [DOI] [PubMed] [Google Scholar]
- Paganelli M, Albanese C, Borrelli O, Civitelli F, Canitano N, Viola F, Passariello R, Cucchiara S. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:416–423. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Reginster JY, Boonen S, Breart G, Diez-Perez A, Felsenberg D, Kaufman JM, Kanis JA, Cooper C. Adverse reactions and drug–drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. 2011;89:91–104. doi: 10.1007/s00223-011-9499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, Hawker G, Griffiths AM. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR, Kaser A, Pines A, Dotan I. Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut. 2008;57:684–694. doi: 10.1136/gut.2006.117382. [DOI] [PubMed] [Google Scholar]
- Tomita T, Kanai T, Nemoto Y, Fujii T, Nozaki K, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Totsuka T, Watanabe M. Colitogenic CD4(+) effector-memory T cells actively recirculate in chronic colitic mice. Inflamm Bowel Dis. 2008;14:1630–1640. doi: 10.1002/ibd.20636. [DOI] [PubMed] [Google Scholar]
- Uno JK, Kolek OI, Hines ER, Xu H, Timmermann BN, Kiela PR, Ghishan FK. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology. 2006;131:497–509. doi: 10.1053/j.gastro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Lai KT, Huang JF, Gu Y, Karlsson L, Fourie A. Anti-inflammatory activity in vitro and in vivo of the protein farnesyltransferase inhibitor tipifarnib. J Pharmacol Exp Ther. 2006;317:53–60. doi: 10.1124/jpet.105.095976. [DOI] [PubMed] [Google Scholar]