Abstract
Objective
Coronary heart disease is associated with monocytosis. Studies using animal models of monocytosis and atherosclerosis such as Apoe-/- mice have shown bone marrow (BM) hematopoietic stem and multi-potential progenitor cell (HSPC) expansion, associated with increased cell surface expression of the common β subunit of the GM-CSF/IL-3 receptor (CBS) on HSPCs. Apoe-/- mice also display increased GM-CSF-dependent monocyte production in the spleen. We investigated the role of the CBS in cholesterol-driven HSPC expansion, monocytosis and atherosclerosis.
Approach and Results
Ldlr-/- mice were transplanted with Apoe-/-Cbs-/- or Apoe-/- BM followed by Western-type diet (WTD) feeding. Compared to Apoe-/- BM transplanted controls, Apoe-/-Cbs-/- BM transplanted mice had reduced BM and splenic HSPC proliferation, fewer blood monocytes and neutrophils, and reduced macrophage content and area of early atherosclerotic lesions. More advanced lesions showed diminished macrophage and collagen content; however, lesion size was unchanged reflecting an increase in necrotic core area, associated with a marked decrease in Abcg1 expression and increased macrophage apoptosis. Compared with wild-type mice, WTD-fed Apoe-/- mice showed increased CBS expression on GM-CSF-producing innate response activator (IRA) B cells and expansion of this population. Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice showed a marked decrease in IRA B cells compared to Apoe-/- BM transplanted Ldlr-/- controls.
Conclusions
Increased levels of CBS on HSPCs and splenic IRA B cells leads to expansion of these populations in Apoe-/- BM transplanted Ldlr-/- mice, contributing to monocytosis and increased lesional macrophage content. However, in more advanced lesions, the CBS also has a role in atherosclerotic plaque stabilization.
Keywords: atherosclerosis, monocytosis, HSPC, IRA B cells, GM-CSF
Introduction
Leukocytosis has been associated with higher rates of ischemic vascular disease in numerous prospective and cross-sectional studies.1-3 The monocyte count, in particular, independently predicts risk for coronary artery disease following adjustment for conventional risk factors.4 Monocytosis and neutrophilia have also been observed in animal models of atherosclerosis including pigs and rabbits and appear to contribute to atherogenesis.5,6 Hypercholesterolemia-induced monocytosis has been documented in Apoe-/- mice in association with a marked increase in the Ly-6Chi monocyte subset, a subpopulation that more readily infiltrates lesions.7, 8 Hypercholesterolemia-induced neutrophilia has also been reported to contribute to early atherosclerotic lesion formation in Apoe-/- mice.9 Conversely, reduction of monocytes in the circulation through interruption of M-CSF or MCP-1 activity decreased atherosclerosis in Apoe-/- and Ldlr-/- mice.10-12
Recent studies have suggested that HSPC expansion underlies the monocytosis and neutrophilia observed in these models.13, 14 Mice deficient in the adenosine triphosphate-binding cassette (ABC) transporters A1 and G1 (ABCA1 and ABCG1), which promote cholesterol efflux from myeloid cells, develop monocytosis, neutrophilia, and expansion of HSPCs in the bone marrow (BM).15 A similar observation was made in Apoe-/- mice fed a Western-type diet (WTD), where monocytosis and neutrophilia were associated with HSPC expansion.16 Chimeric BM transplantation experiments revealed that the Apoe-/- HSPCs outcompeted WT HSPCs in giving rise to monocytes and neutrophils when transplanted into Ldlr-/- mice, and that the Abca1-/-Abcg1-/- HSPCs outcompeted wild-type (WT) HSPCs when transplanted into WT mice, suggesting a cell intrinsic proliferative advantage of Apoe-/- and Abca1-/-Abcg1-/- HSPCs compared to WT HSPCs.16 In both studies, the cell intrinsic proliferative advantage was associated with increased cell surface levels of the common β subunit of the GM-CSF/IL-3 receptor (CBS). Another study, using Apoe-/- mice as a model, identified increased numbers of GM-CSF producing cells in the spleen and underscored the importance of extramedullary expansion of HSPCs and myeloid progenitor cells in the spleen as drivers of monocytosis and atherogenesis.17 The same group later described innate response activator (IRA) B cells that protect the hosts from polymicrobial sepsis through LPS-induced production of GM-CSF specifically in the spleen.18 Interestingly, these IRA B cells develop from peritoneal B1a cells in a CBS-dependent manner, suggesting a feed-forward loop in GM-CSF production from IRA B cells under certain stress conditions.
The CBS (aka CD131) is the beta subunit shared by the receptors of IL-3, GM-CSF, and IL-5. In humans, CD131 is encoded by the gene Csf2rb.19 In mice, Csf2rb1 encodes IL3-βc, the ortholog of CD131, while Csf2rb2 encodes IL3 receptor class II β chain, a second protein that is homologous to CD131 but can only form a functional receptor with the IL-3 alpha subunit.20 Due to their partial functional redundancy and the fact that a Csf2rb1-/-Csf2rb2-/- mouse will be used in this study, CBS will be used to denote the protein product of Csf2rb1 and Csf2rb2 collectively, and Cbs-/- will denote Csf2rb1-/-Csf2rb2-/-. The CBS plays a central role in hematopoiesis reflecting the function of GM-CSF and IL-3 in myeloid lineage development.21, 22 IL-3 promotes HSPC survival and proliferation, and GM-CSF determines myeloid lineage specification and mediates the development of monocyte and dendritic cells (DCs),23, 24 two cell types that are intimately involved in atherosclerosis.
The hypothesis underlying this study was that increased expression of the CBS in HSPCs and possibly IRA-B cells or their progenitors would promote HSPC and IRA-B cell expansion, contributing to monocytosis and atherogenesis in Apoe-/- mice.
Methods
An expanded Methods section is available in the online data supplement.
Results
CBS deficiency decreased monocyte levels specifically in Apoe-/- BM transplanted mice
To investigate the role of CBS in monocytosis, we generated Apoe-/-Cbs-/- mice by crossbreeding Apoe-/- and Cbs-/- mice (C57BL/6J background). In preliminary studies, we found that CBS deficiency in whole body Apoe deficiency decreased plasma cholesterol levels (results not shown); therefore we transplanted Apoe-/- or Apoe-/-Cbs-/- BM into Ldlr-/- mice, which did not change plasma cholesterol levels. At 5 weeks after transplantation, BM reconstitution was >90% (results not shown). We observed a major effect of diet-induced hypercholesterolemia in the Ldlr-/- background on blood monocyte and neutrophil counts as reported.25 Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice had significantly fewer monocytes than Apoe-/- BM transplanted Ldlr-/- mice after 5 weeks of WTD feeding, and the difference was even more marked after 8 weeks of WTD feeding (Figure 1A). CBS deficiency also reduced neutrophil counts after WTD feeding (Figure 1B). The changes in monocytes and neutrophil levels were observed in the absence of any significant difference in the cholesterol levels (Figure 1C). We also studied the effect of CBS deficiency on monocyte levels in the presence of ApoE in the BM. When we transplanted Ldlr-/- mice with wild-type or Cbs-/- BM and fed them the WTD, no effect on blood monocytes was found (Supplemental Figure I). This was likely due to increased CBS on Apoe-/- but not wild-type HSPCs, as reported.16 Therefore, for subsequent studies, we used BM donors on the Apoe-/- background.
Figure 1. CBS deficiency decreases blood monocyte levels in the Apoe-/- background in the absence of any significant differences in cholesterol levels.
Ldlr-/- mice were transplanted with Apoe-/- or Apoe-/-Cbs-/- bone marrow (BM). At 5 weeks post-BM transplantation (presented as baseline), mice were fed the WTD. Blood monocytes (A), neutrophils (B), and plasma cholesterol levels (C) were monitored at baseline, 5 and 8 weeks of WTD feeding. N=6. *P<0.05, as determined by t-test.
CBS deficiency decreased HSPC expansion in BM and spleen in Apoe-/- BM transplanted mice
We compared the HSPC levels and their proliferative activity in the BM and the spleen after 9 weeks of WTD feeding (Figure 2 and Supplemental Figure II). Compared with Apoe-/- BM transplanted Ldlr-/- mice, the Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice had significantly lower numbers of Lin-Sca1+c-Kit+ cells (LSKs, referred to as HSPCs), common myeloid progenitor cells (CMPs) and granulocyte-monocyte progenitor cells (GMPs) in both BM and spleen (Figure 2A-B, and Supplemental Figure II). Apoe-/- mice are known to develop splenomegaly upon WTD feeding,16 and this was also decreased in the Apoe-/-Cbs-/- group (Supplemental Figure III). In BM of the Apoe-/-Cbs-/- group, the decreased HSPC, CMP, and GMP percentages were associated with a decrease only in the proliferation of HSPCs (Supplemental Figure IIA and IIB); whereas in the spleen, both HSPCs and GMPs showed decreased proliferative activity (Supplemental Figure IIC). These data suggest that CBS facilitates monocytosis in the hematopoietic Apoe-/- background by enhancing HSPC proliferation in the BM and HSPC and GMP production in the spleen.
Figure 2. CBS deficiency decreases HSPC expansion in BM and spleen in the Apoe-/- background.
Ldlr-/- mice were transplanted with Apoe-/- or Apoe-/-Cbs-/- BM and fed WTD for 9 weeks. The percentages of HSPCs, CMPs, and GMPs were assessed in the BM (A) and spleen (B) using flow cytometry. N=6-8. *P<0.05, **P<0.01, ***P<0.001, as determined by t-test.
Role of IRA B cells in elevated GM-CSF production in spleens of Apoe-/- mice
Given the significant reduction of cell proliferation in the spleens of the Apoe-/- Cbs-/- group, we investigated the underlying mechanisms. In Apoe-/- mice fed WTD, increased numbers of cells that produce GM-CSF and IL-3 accompanied the increase in monocyte production in the spleen.17 It has also been reported that peritoneal B1a cells develop into IRA B cells specifically in the spleen where they produce GM-CSF and IL-3 to protect the host against polymicrobial sepsis.18 Thus, we examined the possibility that IRA B cells are involved in the enhanced GM-CSF/IL-3 production in the spleen of WTD fed Apoe-/- mice. Indeed, compared with WT mice, Apoe-/- mice showed elevated numbers of IRA B cells and enhanced IRA B cell proliferation in the spleen (Figure 3A and 3B, Supplemental Figure IVA), but not the BM (Supplemental Figure IVB, C and D), suggesting that IRA B cells are involved in the increased GM-CSF and IL-3 production that give rise to myeloid expansion in the spleen.
Figure 3. CBS and apoE modulate splenic IRA B cell content and proliferation during atherogenesis.
A-D. WT or Apoe-/- mice were fed WTD for 5 weeks and splenic IRA B cell content (A), proliferation (B), and CBS surface expression (C) were assessed by flow cytometry. CBS surface expression was also assessed on peritoneal B1a cells (D). N=5. Ldlr-/- mice were transplanted with Apoe-/- or Apoe-/-Cbs-/- BM and fed WTD for 8 weeks. Splenic IRA B cell content (E) and proliferation (F) were assessed using flow cytometry. MFI denotes mean fluorescent intensity. N=7. *P<0.05, **P<0.01, as determined by t-test.
CBS plays a central role in IRA B cell expansion in Apoe-/- mice
Previous studies demonstrated that B1a cells failed to give rise to IRA B cells in the presence of an antibody against CBS,18 suggesting that CBS is required for the development of IRA B cells. Our laboratory has previously reported that Apoe-/- mice had increased cell surface level of CBS in HSPCs due to defective cholesterol efflux and lipid raft formation.16 These observations led us to hypothesize that the enhanced IRA B cell formation in spleen could be due to elevated CBS expression in B1a and/or IRA B cells. Indeed, Apoe-/- mice have increased CBS surface levels on both IRA B cells (Figure 3C and Supplemental Figure IVE) and peritoneal B1a cells (Figure 3D and Supplemental Figure IVF) compared with WT mice. Given that the number of B1a cells did not differ significantly between the two groups (Supplemental Figure IVG), these findings suggest that IRA B cell expansion is likely due to enhanced development of IRA B cells from B1a cells and increased IRA B cell proliferation as a result of the increased CBS expression on these two cell types. Conversely, lack of CBS decreased the number of IRA B cells in the spleen (Figure 3E) and their proliferative activity (Figure 3F) in Ldlr-/- mice transplanted with Apoe-/- or Apoe-/-Cbs-/- BM, suggesting a central role for CBS in elevating the number of IRA B cells in Apoe-/- mice.
Decreased macrophage content of atherosclerotic lesions of Apoe-/-Cbs-/- mice; increased complexity of advanced lesions
We next assessed the extent of atherosclerotic plaque burden in Apoe-/-and Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice after 4 weeks or 9 weeks of WTD feeding. After 4 weeks of WTD feeding, Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice showed a 19% reduction in atherosclerotic plaque size (P<0.05) compared with Apoe-/- BM transplanted Ldlr-/- mice (Figure 4A and 4C). This reduced lesion size was associated with a 24% reduction in macrophage-containing area as assessed by staining with an F4/80 antibody (Figure 4B and 4D), while the percentages of macrophages per lesion size were similar in both groups (results not shown). Consistent with these findings, the lesions in these two groups were largely cellular with little necrotic core formation (Figure 4A). These findings support our hypothesis that reduced monocyte counts arising through CBS deficiency in Apoe-/- hematopoietic tissue leads to decreased macrophage accumulation in early atherogenesis.
Figure 4. CBS deficiency reduces lesion size and macrophage-positive area in early atherosclerosis in BM Apoe deficiency.
Ldlr-/- mice were transplanted with Apoe-/- or Apoe-/-Cbs-/- BM and fed the WTD for 4 weeks. Hearts were isolated and fixed, and paraffin sections of the aortic root were made. A. Representative H&E stained sections are shown. B. Sections were stained for macrophages using an F4/80 antibody. Representative F4/80-stained sections are shown. C. Quantification of atherosclerotic lesion area by morphometric analysis on H&E stained sections. D. Quantification of macrophage-positive area on F4/80 stained sections. Each datapoint represents a single mouse. N=12-16. *P<0.05, as determined by a Mann-Whitney test.
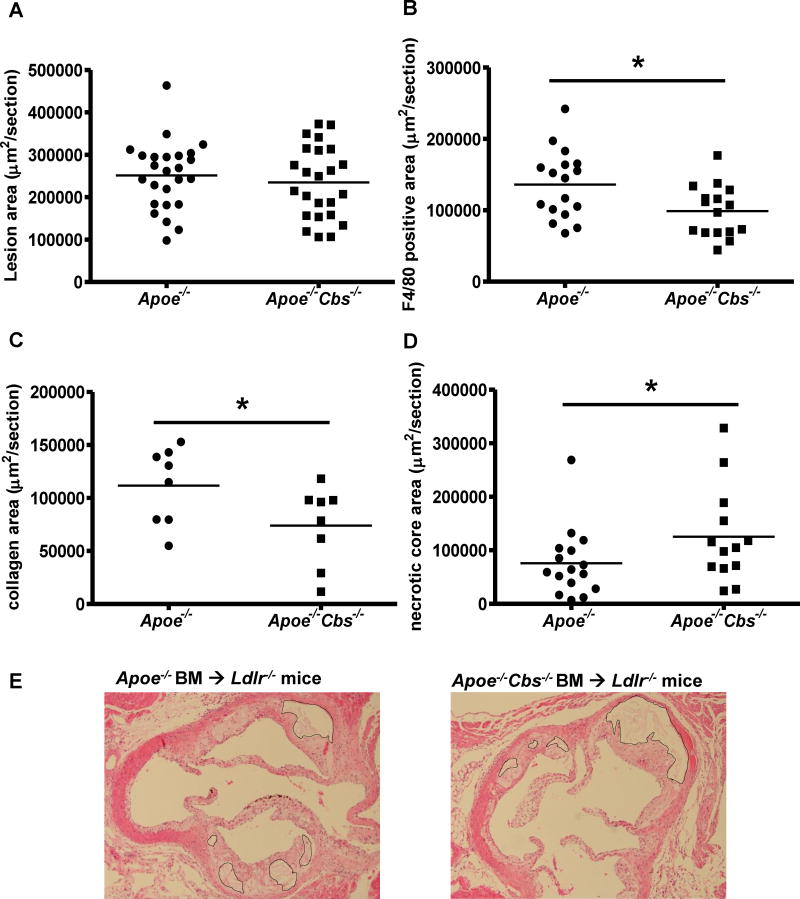
After 9 weeks of WTD feeding, CBS deficiency did not reduce the overall atherosclerotic plaque size comparing Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice and Apoe-/- BM transplanted mice (Figure 5A). However, there was a significant 36% reduction of macrophage content in the Apoe-/-Cbs-/- BM transplanted mice (Figure 5B and Supplemental Figure VA), as well as a ∼30% reduction in collagen content (Figure 5C and Supplemental Figure VB). Upon closer characterization of the lesions, we identified a significant increase of necrotic core area in the Apoe-/-Cbs-/- group (Figure 5D and 5E), suggesting that the potential athero-protective effect of reduced monocyte and macrophage content of lesions might be counteracted by increased necrotic core formation.
Figure 5. CBS deficiency reduces macrophage-positive area and increases the size of necrotic cores without affecting atherosclerotic lesion size in advanced lesions in BM Apoe deficiency.
Ldlr-/- mice were transplanted with Apoe-/- or Apoe-/-Cbs-/- BM and fed the WTD for 9 weeks. Hearts were isolated and fixed, and paraffin sections of the aortic root were made. A. Sections were stained with H&E and atherosclerotic lesion area was quantified by morphometric analysis. N=24-25. B. Sections were stained for F4/80 and macrophage-positive area was quantified. N=14-16. C. Sections were stained for Masson-Trichrome and collagen-positive area was quantified. N=8. D. Necrotic core area was quantified on H&E stained sections. N=13-16. E. Representative H&E sections containing necrotic cores are shown. Each datapoint represents a single mouse. *P<0.05, as determined by a Mann-Whitney test.
In an effort to understand the mechanisms that might explain the lack of reduction of overall size of advanced lesions, we considered a number of potential hypotheses. First, since the CBS transduces signals mediated by IL-5, and IL-5 has been implicated in the production of potentially athero-protective natural antibodies, such as the IgM E06/T15,26 we measured titers of these antibodies in plasma. The titers of antibodies were not reduced in Apoe-/-Cbs-/- BM compared to Apoe-/- BM transplanted Ldlr-/- mice (Supplemental Figure VI), indicating that this mechanism could not explain the lack of decrease in lesion area in the Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice. GM-CSF is known to play a role in the development of DCs that activate Tregs,27, 28 an immunosuppressive T cell population that has been reported to attenuate atherosclerosis, likely through suppressing both T cell- and macrophage-mediated inflammation and lesional MCP-1 expression.29, 30 Therefore, we quantified the level of DCs and Tregs and found slightly decreased numbers of both cell types in spleens of Apoe-/-Cbs-/- BM compared with Apoe-/- BM transplanted Ldlr-/- mice (Supplemental Figure VIIA and VIIB), presumably due to the decreased GM-CSF production as a result of reduced IRA B cells. Lymph node populations were unchanged (Supplemental Figure VIIC and VIID), as expected.31 In an attempt to assess a possible causative role of altered Tregs in lesions, we carried out gene expression studies using laser capture microdissection (LCM) analysis of the cellular area of lesions. There was a trend to a reduction of Foxp3 mRNA level in lesions from Apoe-/-Cbs-/- BM compared to Apoe-/- BM transplanted Ldlr-/- mice (Supplemental Figure VIIIA), with the wide variation probably due to the scarcity of Tregs in lesions. However, this possible decrease in Foxp3 expression was not associated with an expected parallel decrease of Tgf-β and increase in Mcp-1 expression.30 On the contrary, Tgf-β level was significantly increased and Mcp-1 levels decreased in lesions from Apoe-/-Cbs-/- BM compared to Apoe-/- BM transplanted Ldlr-/- mice (Supplemental Figure VIIIB and VIIIC). These effects are inconsistent with a major effect of lesional Treg content on lesion development in this model.
It has been shown that GM-CSF promotes Abcg1 expression by targeting Pparγ,32 and ABCG1 deficiency has been linked to increased macrophage apoptosis and increased necrotic core formation in lesions.33, 34 Indeed, while Apoe-/- BM transplanted Ldlr-/- mice had readily detectable Abcg1 expression, Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice demonstrated an absence of detectable Abcg1 mRNA in the lesions (Figure 6A), and this was associated with a decrease in Pparγ espression (Figure 6B), a ∼70% increase in the pro-apoptotic protein caspase 3 (Figure 6C and 6E), and a 3-fold increase in the number of apoptotic cells as determined by TUNEL staining (Figure 6D and Supplemental Figure IX).
Figure 6. CBS deficiency in BM Apoe deficiency decreases Abcg1 and Pparγ mRNA expression and increases apoptosis in atherosclerotic lesions.
Ldlr-/- mice transplanted with BM of specified genotype were fed WTD for 9 weeks, and frozen sections of aortic roots were prepared. RNA was extracted from laser-captured macrophages from atherosclerotic lesions, and Abcg1 (A) and Pparγ (B) mRNA were measured. C. Sections were stained for the pro-apoptotic protein caspase 3. D. TUNEL staining was performed to identify apoptotic cells. E. Representative pictures of caspase 3 staining. Arrows indicate caspase 3 positive cells. N=9-10. *P<0.05, **P<0.01, as determined by t-test. F. Under hypercholesterolemic conditions in hematopoietic apoE deficiency, increased CBS expression in HSPCs increases the production of myeloid cells. In spleen, CBS increases GM-CSF and IL-3 production by facilitating IRA B cell development, and potentially enhances extramedullary myeloid proliferation and monocytosis.
Discussion
Previous studies have shown increased levels of the CBS associated with increased myeloid proliferative responses to GM-CSF and IL-3 in BM HSPCs of hypercholesterolemic mice with defective cellular cholesterol efflux pathways and also in the widely used Apoe-/- mouse model.25, 35 However, the in vivo significance of increased cell surface CBS levels and signaling in monocytosis and atherosclerosis remained uncertain. Our study provides direct evidence that the CBS plays a key role in mediating the monocytosis that develops in hypercholesterolemic Apoe-/- mice. In the BM and spleen, the CBS is required for the hypercholesterolemia-driven HSPC and myeloid expansion; in the spleen, the CBS also likely increases GM-CSF and IL-3 production by facilitating IRA B cell formation. IRA B cells account for ∼70% of the GM-CSF production in the spleen;36 suggesting IRA B cells could contribute to enhanced myeloid proliferation and monocytosis in Apoe-/- BM transplanted Ldlr-/- mice (schematic Figure 6F). Moreover, CBS deficiency ameliorated plaque burden, including macrophage content during early atherogenesis. Unexpectedly, in more advanced atherosclerotic lesions, CBS deficiency decreased macrophage content but increased necrotic core areas, resulting in no net change of plaque area. This suggests that GM-CSF signaling via the CBS promotes atherosclerotic plaque stabilization in more advanced lesions.
BM derived HSPCs are known to traffic through blood to extramedullary sites including the spleen where they proliferate and give rise to resident myeloid cells during both steady-state conditions and inflammation.37-39 This phenomenon also occurs in animal models of atherosclerosis.15, 17 Abca1-/-Abcg1-/- mice showed infiltration of HSPCs in the lung, spleen and liver as a result of increased signaling through the IL-23/IL-17/G-CSF axis,40 and Apoe-/- mice developed splenomegaly with WTD feeding, reflecting enhanced HSPC proliferation and myelopoiesis in the spleen.17 Recent studies have highlighted the significance of the spleen as a site of extramedullary myeloproliferation and a source of monocytes in accelerated atherosclerosis following myocardial infarction.39, 41 Our study shows a critical role of the CBS in facilitating extramedullary monocyte production through the development and expansion of IRA B cells, which likely contribute to myeloid proliferation in the spleen by producing GM-CSF and IL-3. Moreover, a decrease in IRA B cells was associated with reduced monocytosis and lesional macrophage burden, suggesting that these cells may contribute to atherogenesis in the Apoe-/- mouse model.
Very recently, Hilgendorf et al.36 also showed an increase in splenic IRA B cells in mouse atherosclerosis models. Based on an elegant chimeric BM transplantation approach that resulted in a specific depletion of GM-CSF producing B cells, these authors suggested that GM-CSF producing IRA B cells accelerate atherogenesis by increasing IL12p40 producing CD11b+ DCs in the spleen, leading to activation of CD4+ T-cells and production of IFNγ, contributing to macrophage inflammation in lesions. As we observed a decrease in splenic DCs in Apoe-/-Cbs-/- BM transplanted Ldlr-/- mice, this mechanism may have also played a role in our study. However, Hilgendorf et al.36 did not observe any effects on monocyte counts. While this may seem discrepant with our data, Hilgendorf et al.36 used Apoe+/+ BM for transplantation whereas our studies employed Apoe-/- BM. IRA B cells are 4-fold expanded in Apoe-/- mice compared to Ldlr-/- mice.36 When we used Apoe+/+Cbs-/- BM for transplantation into Ldlr-/- mice we also observed no effect on monocyte counts (Supplemental Figure I). We conclude that the marked expansion of IRA B cells probably does contribute to the prominent monocytosis that develops in WTD fed Apoe-/- BM transplanted Ldlr-/- mice, but not in WTD fed Ldlr-/- mice where IRA B cell expansion36 and monocytosis16 are more subtle. The increase in CBS on HSPCs and IRA B cells may be brought out by defective cellular cholesterol efflux caused by BM Apoe deficiency. Overall, the increased blood monocytes in hypercholesterolemic mice with Apoe-/- hematopoietic tissue is likely caused both by increased CBS on HSPCs16 and on IRA B cells, driving expansion of both populations.
Studies in the past decade have firmly established a central role of monocyte-derived macrophages in the development of atherosclerotic lesions.42 Evidence has also emerged to paint a more complex picture of the pathogenesis with differing characteristics between early and advanced atherosclerotic lesions.43, 44 Strikingly, in our model, the reduction of monocyte levels had differential effects on early lesions and advanced lesions. In early lesions, the reduction of monocytes resulted in decreased macrophage content and decreased lesion size, consistent with the largely cellular nature of early lesions. Our findings are consistent with several earlier studies involving GM-CSF injection or deficiency in atherogenesis. With one exception,45 most of these studies have shown a pro-atherogenic role of GM-CSF,46-48 thought to be related to proliferation of CD11c+ dendritic-like cells in lesions.46, 47 Many cells that stain for CD11c also stain for macrophage markers,49 consistent with our findings of decreased lesional macrophages.
In advanced atherosclerotic lesions, CBS deficiency did not decrease lesion size despite a sustained reduction of blood monocyte counts. This was explained by increased necrotic core formation due at least in part to the lack of ABCG1 expression in the lesions. Prior studies have shown that GM-CSF induces macrophage Abcg1 expression mediated by PPARγ.50 It is known that ABCG1 is critical in sustaining macrophage survival likely by promoting cholesterol and oxysterol efflux, and lack of ABCG1 results in elevated cholesterol and oxysterol levels and enhanced apoptosis.51 Studies have established that macrophage death may reduce the volume of early atherosclerotic plaques. However, advanced lesions are characterized by defective efferocytosis in which debris from the apoptotic macrophages is not adequately removed, aggravating the inflammatory process in lesions.52 A recent study found that local macrophage proliferation, rather than monocyte recruitment, plays a major role in maintaining the macrophage content of established atherosclerotic lesions.53 However, a role for GM-CSF as a proliferative stimulus was specifically excluded in that report, indicating that such a mechanism was not likely involved in our study.
In summary, our study highlights the important role of increased levels of the CBS in mediating cholesterol-driven HSPC expansion in the BM, as well as extramedullary myeloid expansion via IRA-B cells in the spleen. These processes contribute to monocytosis and plaque macrophage burden. However, the CBS also appears to play a role in cell survival, at least in part through effects on ABCG1 expression, and thus decreases necrotic core formation and increases collagen content in advanced atherosclerotic lesions. These observations may have significant implications for the development of new anti-inflammatory therapies for auto-immune diseases such as rheumatoid arthritis or multiple sclerosis, based on interruption of GM-CSF signaling.54
Supplementary Material
Significance.
Monocytosis has been associated with an increased cardiovascular risk in humans and increased atherosclerosis in mice. The CBS is increased on HSPCs of several mouse models exhibiting monocytosis and accelerated atherosclerosis.
We investigated the role of the CBS in monocytosis and atherosclerosis in hypercholesterolemic mice containing Apoe-/- BM. CBS deficiency decreased monocytosis, BM and splenic HSPC proliferation, and splenic IRA B cells, which are the main source of splenic GM-CSF, resulting in decreased macrophage accumulation and lesion size in early atherosclerosis. In advanced atherosclerosis, CBS deficiency decreased macrophage accumulation, but did not affect lesion size, associated with increased apoptosis, decreased collagen, and increased necrotic cores, suggesting decreased plaque stability. This reflected a decrease in macrophage ABCG1 expression which is known to be increased by GM-CSF. These findings could have important implications for therapies aimed at disrupting the GM-CSF pathway, which are currently being developed for various autoimmune disorders in humans.
Acknowledgments
Sources of funding: This work has been supported by NIH grant HL107653 (to A.R.T.), AHA Predoctoral Fellowship 10PRE3020048 (to M. Wang), and Netherlands Organization of Sciences VENI-grant 916.11.072 (to M. Westerterp).
Nonstandard abbreviations and acronyms
- ABC
adenosine triphosphate-binding cassette
- A.U
arbitrary units
- BM
bone marrow
- CBS
the common β subunit of GM-CSF/IL-3/IL-5 receptor
- CMP
common myeloid progenitor
- DC
Dendritic Cell
- GM-CSF
Granulocyte Macrophage-Colony Stimulating Factor
- GMP
granulocyte macrophage progenitor
- HSPC
hematopoietic stem and multi-potential progenitor cell
- IRA
innate response activator
- LCM
Laser Capture Microdissection
- LSK
Lin-Sca1+c-Kit+ cell
- Treg
regulatory T cell
- WTD
Western Type Diet
Footnotes
Disclosures: A.R.T. is a consultant to Pfizer, Merck, Amgen and CSL.
References
- 1.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality. Arterioscler Thromb Vasc Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 2.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in african-american and white men and women: Atherosclerosis risk in communities study. Am J Epidemiol. 2001;154:758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 3.Sweetnam PM, Thomas HF, Yarnell JWG, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: The caerphilly and speedwell studies. Am J Epidemiol. 1997;145:416–421. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 4.Olivares R, Ducimetière P, Claude J. Monocyte count: A risk factor for coronary heart disease? Am J Epidemiol. 1993;137:49–53. doi: 10.1093/oxfordjournals.aje.a116601. [DOI] [PubMed] [Google Scholar]
- 5.Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol. 1989;135:369–377. [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman DL, Mogelesky TC, Liptak BF, Gerrity RG. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991;11:985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- 7.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drechsler M, Megens RTA, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis / clinical perspective. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 10.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein e. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in ldl receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 13.Tall AR, Yvan-Charvet L, Westerterp M, Murphy AJ. Cholesterol efflux: A novel regulator of myelopoiesis and atherogenesis. Arterioscler Thromb Vasc Biol. 2012;32:2547–2552. doi: 10.1161/ATVBAHA.112.300134. [DOI] [PubMed] [Google Scholar]
- 14.Soehnlein O, Swirski FK. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol Metab. 2012 doi: 10.1016/j.tem.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates ly-6c(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch PJ, Chudnovskiy A, Robbins CS, et al. Innate response activator b cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagley C, Woodcock J, Hercus T, Shannon M, Lopez A. Interaction of gm-csf and il-3 with the common beta-chain of their receptors. J Leukoc Biol. 1995;57:739–746. doi: 10.1002/jlb.57.5.739. [DOI] [PubMed] [Google Scholar]
- 20.Itoh N, Yonehara S, Schreurs J, Gorman DM, Maruyama K, Ishii A, Yahara I, Arai K, Miyajima A. Cloning of an interleukin-3 receptor gene: A member of a distinct receptor gene family. Science. 1990;247:324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- 21.Bagley CJ, Woodcock JM, Stomski FC, Lopez AF. The structural and functional basis of cytokine receptor activation: Lessons from the common beta subunit of the granulocyte-macrophage colony-stimulating factor, interleukin-3 (il-3), and il-5 receptors. Blood. 1997;89:1471–1482. [PubMed] [Google Scholar]
- 22.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. The granulocyte-macrophage colony-stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Testa U, Fossati C, Samoggia P, Masciulli R, Mariani G, Hassan H, Sposi N, Guerriero R, Rosato V, Gabbianelli M, Pelosi E, Valtieri M, Peschle C. Expression of growth factor receptors in unilineage differentiation culture of purified hematopoietic progenitors. Blood. 1996;88:3391–3406. [PubMed] [Google Scholar]
- 24.Murphy JM, Young IG. Il-3, il-5, and gm-csf signaling: Crystal structure of the human beta-common receptor. Vitamin Hormon. 2006;74:1–30. doi: 10.1016/S0083-6729(06)74001-8. [DOI] [PubMed] [Google Scholar]
- 25.Murphy A, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem A, Kuivenhoven J, Yvan-Charvet L, Tall A. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder C, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss L, Corr M, Witztum J. Il-5 links adaptive and natural immunity specific for epitopes of oxidized ldl and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. Gm-csf-induced, bone-marrow-derived dendritic cells can expand natural tregs and induce adaptive tregs by different mechanisms. J Leukoc Biol. 2011;89:235–249. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. Gm-csf-induced cd11c+cd8a—dendritic cells facilitate foxp3+ and il-10+ regulatory t cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, Bogunovic M, Gautier EL, Miller J, Leboeuf M, Lu G, Aloman C, Brown BD, Pollard JW, Xiong H, Randolph GJ, Chipuk JE, Frenette PS, Merad M. Gm-csf controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker AD, Malur A, Barna BP, Ghosh S, Kavuru MS, Malur AG, Thomassen MJ. Targeted ppar{gamma} deficiency in alveolar macrophages disrupts surfactant catabolism. J Lipid Res. 2010;51:1325–1331. doi: 10.1194/jlr.M001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR. Abca1 and abcg1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarling EJ, Bojanic DD, Tangirala RK, Wang X, Lovgren-Sandblom A, Lusis AJ, Bjorkhem I, Edwards PA. Impaired development of atherosclerosis in abcg1-/- apoe-/- mice: Identification of specific oxysterols that both accumulate in abcg1-/- apoe-/- tissues and induce apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:1174–1180. doi: 10.1161/ATVBAHA.110.205617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yvan-Charvet L, Pagler T, Gautier E, Avagyan S, Siry R, Han S, Welch C, Wang N, Randolph G, Snoeck H, Tall A. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator b cells aggravate atherosclerosis by stimulating th1 adaptive immunity. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 39.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerterp M, Gourion-Arsiquaud S, Murphy A, Shih A, Cremers S, Levine R, Tall A, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell stem cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randolph GJ. Proliferating macrophages prevail in atherosclerosis. Nat Med. 2013;19:1094–1095. doi: 10.1038/nm.3316. [DOI] [PubMed] [Google Scholar]
- 45.Ditiatkovski M, Toh BH, Bobik A. Gm-csf deficiency reduces macrophage ppar-gamma expression and aggravates atherosclerosis in apoe-deficient mice. Arterioscl Throm Vas. 2006;26:2337–2344. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- 46.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. Gm-csf regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaposhnik Z, Wang X, Weinstein M, Bennett B, Lusis A. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- 49.Hume DA. Macrophages as apc and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 50.Malur A, Baker AD, McCoy AJ, Wells G, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Restoration of ppargamma reverses lipid accumulation in alveolar macrophages of gm-csf knockout mice. Am J Physiol. 2011;300:L73–80. doi: 10.1152/ajplung.00128.2010. [DOI] [PubMed] [Google Scholar]
- 51.Terasaka N, Wang N, Yvan-Charvet L, Tall A. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via abcg1. Proc Natl Acad Sci USA. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Nieuwenhuijze A, Koenders M, Roeleveld D, Sleeman MA, van den Berg W, Wicks IP. Gm-csf as a therapeutic target in inflammatory diseases. Mol Immunol. 2013;56:675–682. doi: 10.1016/j.molimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.