Abstract
TGF-β signaling promotes metastasis by controlling the expression of downstream target genes. In this issue of Cancer Cell, Yuan et al. discover a novel TGF-β-induced lncRNA, lncRNA-ATB, that stimulates EMT through sequestering miR-200s and facilitates colonization by stabilizing IL-11 mRNA, thus promoting both early and late steps of cancer metastasis.
The transforming growth factor-β (TGF-β) pathway plays crucial roles during development and homeostasis and exerts strong anti-proliferative effects on normal and premalignant cells. However, advanced stage cancers often become insensitive to the tumor-suppressive actions of TGF-β. Instead, advanced cancers benefit from TGF-β’s profound metastasis-promoting effects, such as epithelial-to-mesenchymal transition (EMT) induction, angiogenesis promotion, altered extracellular matrix deposition, immune suppression, and increased metastatic colonization (Ikushima and Miyazono, 2010; Massague, 2008). These pro-metastatic responses to TGF-β are mediated by a variety of downstream effector proteins, including transcription factors (e.g. AP-1, ID1, SNAIL, SLUG, TWIST, and ZEB1/2), cytokines, growth factors and other ligands (e.g. ANGPTL4, PTHrP, IL-11, JAGGED1, PDGF-B, CTGF, and VEGF), matrix proteins and proteases (e.g. TNC, MMPs) (Ikushima and Miyazono, 2010; Massague, 2008), and a growing number of microRNAs (miRNAs) (Butz et al., 2012).
In recent years, long non-coding RNAs (lncRNAs), a new class of non-coding RNAs longer than 200 nucleotides, have been recognized to regulate a wide variety of physiological and pathological processes through diverse mechanisms. For example, lncRNAs ANRIL and HOTAIR promote tumor growth or metastasis by recruiting chromatin-remodeling complexes to alter gene transcription, while tumor-suppressing lncRNA GAS-5 and tumor-promoting lncRNA HULC act as decoys for glucocorticoid receptor and miR-372, respectively (Wapinski and Chang, 2011). Although the diversity and abundance of lncRNAs seem to rival that of mRNAs in any given cell type, there is little understanding of crucial lncRNAs functioning downstream of the TGF-β pathway. In this issue of Cancer Cell, Yuan et al. report a novel TGF-β induced lncRNA that amplifies the pro-metastatic effect of TGF-β via two independent mechanisms (Yuan et al., 2014).
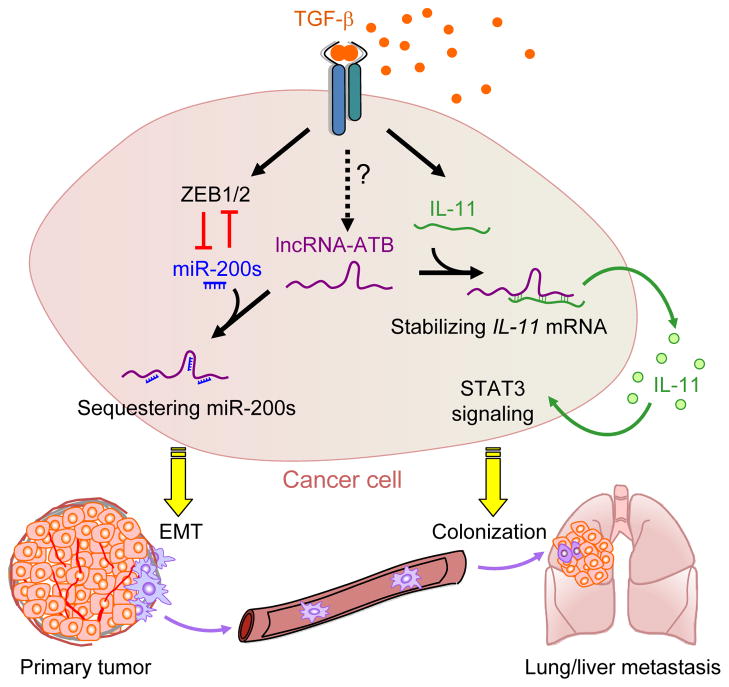
Aiming to identify TGF-β regulated lncRNAs involved in EMT, Yuan et al. profiled lncRNA expression in human hepatocellular carcinoma (HCC) cells after TGF-β treatment. The authors focused their attention on lncRNAs that might function as potential competing endogenous RNAs (ceRNAs) against the miR-200 family miRNAs, which are suppressed by TGF-β signaling and are potent inhibitors of EMT through targeting of two master EMT transcription factors ZEB1 and ZEB2 (Figure 1). One such lncRNA, aptly named lncRNA-Activated by TGF-β (lncRNA-ATB), stood out by virtue of containing three predicted miR-200 binding sites. Binding of lncRNA-ATB by miR-200s was confirmed by RNA immunoprecipitation (RIP) of lncRNA-ATB, luciferase assays, and anti-AGO2 RIP. LncRNA-ATB is non-polyadenylated, localizes primarily in the cytoplasm, and has three close homologs in the human genome. Notably, lncRNA-ATB is also up-regulated by TGF-β in MCF7 breast cancer cell line and SMAD4-deficient SW480 colorectal cancer cell line, implying that lncRNA-ATB may be activated through the SMAD-independent, non-canonical TGF-β pathway. LncRNA-ATB increases ZEB1 and ZEB2 mRNA and protein levels through competitively binding and sequestering miR-200s, thereby inducing EMT (Figure 1). Remarkably, depletion of lncRNA-ATB is sufficient to abolish TGF-β-induced EMT in HCC cells, even though TGF-β is known to strongly induce many other EMT drivers, such as SNAIL, SLUG, and TWIST. These findings suggest that lncRNA-ATB may represent an essential node in the EMT regulatory network.
Figure 1. LncRNA-ATB Acts Downstream of TGF-β to Promote Different Steps of Cancer Metastasis.
TGF-β signaling promotes metastasis by altering the expression of a variety of downstream genes, including many protein-coding mRNAs, miRNAs, and in the current study, a long non-coding RNA lncRNA-ATB. TGF-β signaling induces lncRNA-ATB, which reinforces the pro-metastatic TGF-β response via two distinct mechanisms. LncRNA-ATB competitively binds to miR-200s and sequesters them away from their mRNA targets ZEB1 and ZEB2, which encode two key EMT promoting transcription factors that repress the expression of E-cadherin and the miR-200s themselves, thus promoting EMT. LncRNA-ATB also binds to and stabilizes IL-11 mRNA, thereby increasing autocrine IL-11-STAT3 signaling to enhance the survival and metastatic colonization of disseminated tumor cells in the lung and liver. While ZEB1/2 and IL-11 are known to be activated by Smad-dependent pathways downstream of TGF-β receptor activation, lncRNA-ATB is activated by a Smad-independent non-canonical pathway that remains to be identified.
While mutations in miR-200 binding sites or miR-200 overexpression abolished lncRNA-ATB’s function in stimulating EMT, they only partially eliminated the pro-metastatic effect of lncRNA-ATB, suggesting that other mechanisms are at play. The authors first tested the role of lncRNA-ATB in different steps of the metastatic cascade. While lncRNA-ATB overexpression increases tumor dissemination in a miR-200-dependent manner, lncRNA-ATB promotes liver and lung colonization of HCC cells independent of miR-200s. Genome-wide RIP revealed IL-11 mRNA as one of the top transcripts bound by lncRNA-ATB. IL-11 is a TGF-β target gene that has been shown to promote bone metastasis of breast cancer (Kang et al., 2003) and liver colonization of colorectal cancer by activating the pro-survival GP130/STAT3 signaling pathway (Calon et al., 2012). Yuan et al. showed that lncRNA-ATB binds to and stabilizes IL-11 mRNAs, stimulates autocrine IL-11 production, thus triggering STAT3 signaling in tumor cells to promote colonization (Figure 1).
LncRNA-ATB is up-regulated in HCC samples compared to paired noncancerous hepatic tissues and significantly correlates with liver cirrhosis, vascular invasion, and reduced recurrence-free and overall survival of HCC patients. LncRNA-ATB levels are also significantly higher in portal vein tumor thrombus (PVTT), the main route for intrahepatic metastasis of HCC cells, compared to primary tumor tissues. Furthermore, increased lncRNA-ATB levels significantly correlate with increased ZEB1/2 and IL-11 mRNA levels and decreased CDH1 (encoding for E-cadherin). Collectively, these data highlight strong clinical relevance and prognostic value for lncRNA-ATB and suggest its potential as a promising biomarker and therapeutic target.
This exciting study revealed a novel TGF-β-induced lncRNA that promotes both early and late steps of HCC metastasis by enhancing the pro-metastatic effects of TGF-β signaling in EMT and colonization. These findings also raised important questions that warrant future explorations. First, as lncRNA-ATB is responsive to TGF-β induction even in SMAD4-deficient cells, this indicates that non-canonical SMAD-independent pathways downstream of TGF-β (Moustakas and Heldin, 2005) are involved and that lncRNA-ATB might be able to mediate the pro-metastatic function of TGF-β in the context of Smad deficiency. Further studies are needed to connect TGF-β signaling to lncRNA-ATB activation and investigate the potential regulation of lncRNA-ATB by other oncogenic signaling pathways that are active in the tumor microenvironment. On the other hand, among >20,000 lncRNAs in the human genome, other lncRNAs (including those identified but not explored further in the current study) are also likely to be involved in mediating tumor-suppressive or tumor-promoting effects of TGF-β. The field remains wide open to identify these TGF-β-responsive lncRNAs and elucidate their mechanisms of action.
It is also worth noting that the pro-metastatic effects of lncRNA-ATB rely on miR-200s and IL-11, whose expressions are previously known to be regulated by TGF-β through transcriptional mechanisms independent of lncRNA-ATB. Thus, lncRNA-ATB functions by enhancing the existing network of pro-metastatic TGF-β signaling (Figure 1). It will be of great interest to put this new link into the tempo-spatial context of TGF-β signaling dynamics during tumor progression. How are the levels of lncRNA-ATB maintained in disseminated tumor cells during colonization after the cells depart from the TGF-β-rich primary tumor microenvironment? Is lncRNA-ATB expression maintained by a bistable control mechanism just like the miR-200/ZEB double-negative feedback loop, or perhaps lncRNA-ATB has a long half-life? Is lncRNA-ATB also involved in mediating the paracrine signaling effect of TGF-β in stromal cells during metastasis, as was previously described for the production of IL-11 from cancer-associated fibroblasts (Calon et al., 2012)?
Despite these questions, the strong clinical significance of lncRNA-ATB in HCC suggests its potential utility as a therapeutic target. Soluble antisense oligonucleotides against lncRNA-ATB or other agents that block lncRNA-ATB’s interactions with target miRNAs and mRNAs may be developed to specifically block the pro-metastatic branch of TGF-β signaling (Wahlestedt, 2013). To avoid potential detrimental side effects, it will be essential to understand the normal physiological function of lncRNA-ATB as well as its three closely related homologs. In this regard, it will also be important to characterize the role of other miRNAs and mRNAs that bind to lncRNA-ATB as they may also mediate lncRNA-ATB’s function during development, homeostatsis, and cancer progression. Despite these challenges, the discovery of lncRNA-ATB represents an exciting step forward toward harnessing lncRNAs, formerly among the “dark matters” of the genome, for therapeutic intervention against cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Butz H, Racz K, Hunyady L, Patocs A. Crosstalk between TGF-beta signaling and the microRNA machinery. Trends in pharmacological sciences. 2012;33:382–393. doi: 10.1016/j.tips.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nature reviews Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. Journal of cell science. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nature reviews Drug discovery. 2013;12:433–446. doi: 10.1038/nrd4018. [DOI] [PubMed] [Google Scholar]
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends in cell biology. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Yuan J-H, Yang F, Wang F, Ma J-Z, Guo Y-J, Tao Q-F, Liu F, Pan W, Wang T-T, Zhou C-C, et al. A long non-coding RNA activated by TGF-b promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer cell. 2014;XX:XX–XX. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]