Abstract
The decarboxylated thyroid hormone derivative 3-iodothyronamine (T1AM) has been reported as having behavioral and physiological consequences distinct from those of thyroid hormones. Here, we investigate the effects of T1AM on EEG-defined sleep after acute administration to the preoptic region of adult male rats. Our laboratory recently demonstrated a decrease in EEG-defined sleep after administration of 3,3′,5-triiodo-L-thyronine (T3) to the same brain region. After injection of T1AM or vehicle solution, EEG, EMG, activity, and core body temperature were recorded for 24 h. Sleep parameters were determined from EEG and EMG data. Earlier investigations found contrasting systemic effects of T3 and T1AM, such as decreased heart rate and body temperature after intraperitoneal T1AM injection. However, nREM sleep was decreased in the present study after injections of 1 or 3 μg T1AM, but not after 0.3 or 10 μg, closely mimicking the previously reported effects of T3 administration to the preoptic region. The biphasic dose–response observed after either T1AM or T3 administration seems to indicate shared mechanisms and/or functions of sleep regulation in the preoptic region. Consistent with systemic administration of T1AM, however, microinjection of T1AM decreased body temperature. The current study is the first to show modulation of sleep by T1AM, and suggests that T1AM and T3 have both shared and independent effects in the adult mammalian brain.
Keywords: 3-Iodothyronamine, T1AM, Thyroid hormone, Sleep, Preoptic region, Hypothermia
Introduction
Several recent discoveries have shed light on the function of thyroid hormones in the adult brain. Sleep disturbances and other neurological maladies in patients suffering from thyroid dysfunction have been repeatedly reported in clinical studies (Demet et al., 2002; Watt et al., 2006; Whybrow and Bauer, 2005a, 2005b), but to date, results of behavioral and neurophysiological studies have been inconsistent (Browning et al., 1954; Carpenter and Timiras, 1982; Eastman and Rechtschaffen, 1979; Kales et al., 1967; Salin-Pascual et al., 1997). However, recent data from our lab has shown that acute administration of the thyroid hormone 3,3′,5-triiodo-L-thyronine (T3) to the preoptic region reduced EEG-defined sleep in adult male rats (Martin et al., 2013; Moffett et al., 2013). By showing that acute T3 administration alters sleep, these recent studies from our lab provide a new approach to clarify earlier conflicting reports.
Detection of the thyroid hormone derivative 3-iodothyronamine (T1AM) in extracts of rodent brains (Scanlan et al., 2004) has also furthered interest in thyroid hormone action in the adult central nervous system. Localization of thyroid hormones within specific neuronal populations of the adult mammalian brain has been extensively described, but currently only limited data is available for T1AM (Dratman and Crutchfield, 1978; Dratman et al., 1976, 1982; Gordon et al., 1999; Rozanov and Dratman, 1996; Sarkar and Ray, 1994). Initially it was suspected that T1AM was produced in the brain by aromatic amino acid decarboxylase (AADC) (Berry, 2004; Dratman, 1974; Piehl et al., 2008; Scanlan et al., 2004), which produces dopamine and several trace amines from tyrosine and its analogues. Local production of T3 from thyroxine (T4) (Campos-Barros et al., 1997), and further metabolism to the deaminated metabolites triiodothyroacetic acid (triac) and tetraiodothyroacetic acid (tetrac) (Rall et al., 1957), have been described as occurring in the brain. However, recent studies show iodothyronines are not substrates for AADC (Hoefig et al., 2012) and there is no extrathyroidal conversion of T4 to T1AM (Hackenmueller et al., 2012).
In contrast to T3, tetrac and triac, T1AM is reported as not binding to or interacting with thyroid hormone nuclear receptors (Koury et al., 2009; Scanlan et al., 2004). However, thyroid hormones are known to alter neuronal processes and excitability independent of binding to nuclear receptors (Chapell et al., 1998; Dratman and Gordon, 1996; Martin et al., 1996, 2004; Sarkar et al., 2006, 2011), and several mechanisms of T1AM action that might be potentially relevant to sleep regulation have been reported. T1AM activates the inhibitory metabotropic Trace Amine-Associated Receptor 1 (TAAR1) (Scanlan et al., 2004) and binds the α2A adrenergic receptor (Regard et al., 2007), both of which are expressed in the preoptic region (Bucheler et al., 2002; Lindemann et al., 2008; Modirrousta et al., 2004). Serotonin vesicular transporter (VMAT2) purified from rat synaptosomes and recombinant dopamine and norepinephrine plasma membrane transporters (DAT and NET, respectively) were also inhibited by T1AM (Snead et al., 2007).
Behavioral and physiological effects of T1AM have also been reported recently, and these effects of T1AM should be considered alongside those of thyroid hormones and norepinephrine. In contrast to the hyper-activity observed in rats made chronically hyperthyroid (Emlen et al., 1972), intraperitoneal (IP) injection of T1AM resulted in sudden behavioral changes including a decrease in activity (Dhillo et al., 2009; Scanlan et al., 2004). Additionally, T1AM caused a drop in body temperature of up to 10 °C for several hours after an IP injection (Scanlan et al., 2004). Injection of T1AM to the locus coeruleus (LC) resulted in an increase in firing of a subset of neurons (Dhillo et al., 2009; Gompf et al., 2010). In contrast, hippocampal cells showed a rapid decrease in excitability upon local administration of T4 in anesthetized rats (Caria et al., 2009). Administration of norepinephrine and norepinephrine agonists to the preoptic region rapidly decreased EEG-defined sleep (Mohan Kumar et al., 1986; Ramesh and Kumar, 1998; Ramesh et al., 1995). Within minutes of injection of norepinephrine to the lateral ventricle, rats showed increased activity, and the increase was augmented in rats made chronically hyperthyroid by IP injection of T4 (Emlen et al., 1972).
Electrophysiological, c-Fos and lesioning studies have shown that the preoptic region contains subsets of sleep-active neurons that are implicated in the regulation of sleep, including the median preoptic nucleus (MnPN), medial preoptic area, and ventrolateral preoptic nucleus (Gong et al., 2000; Gvilia et al., 2006; McGinty and Sterman, 1968; Modirrousta et al., 2004; Sterman and Clemente, 1968; Suntsova et al., 2002). Innervation by the noradrenergic LC and expression of α2A adrenergic receptors in sleep-active neurons of the preoptic region suggests that this brain region might be sensitive to the noradrenergic receptor-mediated effects of T1AM; however, other mechanisms should not be ruled out. In this study, we explore the effect of administration of T1AM to the preoptic region of rats on EEG-defined sleep.
Materials and methods
Animals
Eight adult (8–12 weeks old) male Sprague-Dawley rats (Hilltop Lab Animals, Scottdale, PA) were housed individually and given food and water ad libitum. The temperature of the facility was maintained at 22.2–23.3 °C on a 12 hour light/12 hour dark cycle (lights on at 07:00). Rats were handled frequently prior to the start of the experiment to reduce the effects of stress and given at least 5 days post-surgical recovery. All procedures were approved by the Rutgers Institutional Animal Care and Use Committee (IACUC) in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Surgical procedure
Rats were anesthetized with 2.5% isoflurane using an EZ Anesthesia vaporizer apparatus (EZ Systems) at 2 L/min and placed in a stereotaxic device on a heating pad maintained at 37 °C. A Mini-Mitter transponder, which encodes temperature and activity data via radio communication with a receiver underneath the animal’s cage, was inserted into the peritoneal cavity via a small ventral incision. Next, an incision was made in the scalp, the skull was exposed, and a stainless-steel screw EEG electrode (Plastics One E363-20) was inserted in each quadrant of the skull. Two Teflon-coated wires with stripped ends were inserted into the neck musculature to serve as EMG electrodes. The electrode sockets were inserted into an electrode pedestal (Plastics One MS363). A hole was drilled into the skull +0.5 mm medial–lateral and −0.2 mm dorsal–ventral, with respect to bregma, using a 0.0125″ drill bit. Two stainless steel guide cannulae were inserted −7.1 mm dorsal/ventral with respect to the surface of the skull and 1 mm apart from each other. Electrodes and cannulae were secured with dental acrylic and a topical antibiotic ointment was applied to the periphery of the surgical site.
Experimental procedure
The current study was carried out over 4 weeks, during which 4 dosages of T1AM were administered, with animals individually receiving 1 randomly-selected dosage per week. The administrations of T1AM were at least one week apart. On the first day of each week of the study, animals were housed in individual chambers in an electrically-isolated, sound-attenuated facility for 24 h under the same light/dark schedule prior to the experimental procedure. On the second day of each week, rats were injected with vehicle solution (aCSF: 0.25 mM Na2HPO4, 0.5 mM NaH2PO4, 0.4 mM MgCl2, 0.65 mM CaCl2, 3 mM KCl, 128 mM NaCl, 25 mM NaHCO2, pH =7.4), supplemented with 2% v/v DMSO). A total of 0.5 μL was injected over 2 min, with 0.25 μL injected in each cannula. On the third day of each week of the study, rats were bilaterally injected with a total of 0.3, 1, 3, or 10 μg T1AM dissolved in vehicle. All injections were made approximately 2 h into the light phase of the cycle (09:00, or ZT 2), while the animal was quietly restrained.
After injection, animals were returned to their individual cages and connected to a multichannel amplifier (Grass Instruments Model 15) via a shielded cable leading through a multichannel commutator (Plastics One SL6C). EEG and EMG were digitized using a data acquisition unit (CED Micro 1401) and recorded for 24 h following each injection using Spike2 Software. Locomotor activity and core body temperature were measured continuously using a Mini-Mitter receiver placed under each chamber and recorded with VitalView software.
Data analysis
EEG and EMG data were analyzed offline by a trained researcher unaware of experimental conditions. Each 24-hour record was evaluated in 30-second epochs after a 1–30 Hz band pass filter and a notch filter at 20 Hz was applied, and a state of arousal (nREM, REM, or Awake) was assigned according to standard criteria (Martin et al., 1989). Temperature and activity data were registered in one-minute intervals. Data were included only after histological confirmation of cannula placement (Fig. 1) according to a rat brain atlas (Kruger et al., 1995). Although placement of cannula injection tips centered around the MnPN, injection sites are conservatively referred to as being in the “preoptic region”, due to the significant radius of diffusion from the cannulae (Lohman et al., 2005).
Fig. 1.
Eight rats were histologically confirmed to have cannula tips within the preoptic region and centered in the median preoptic nucleus (MnPN). Each number points to the two cannula terminae. Other structures are indicated by the abbreviations ac (anterior commissure), Bst (bed nucleus of the stria terminalis), MP (medial preoptic area), PS (parastrial nucleus), LPO (lateral preoptic area), DB (diagonal band of Broca), MaPo (magnocellular preoptic nucleus).
Statistical analysis
Data were analyzed in 1-hour bins using a 2-way analysis of variance (ANOVA) on GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). Each 2-way ANOVA compared the effect of the vehicle injection to a dose of T3 as one factor and time after injection as a second factor. Temperature and activity data were normalized to account for placement of the Mini-Mitter in each animal. Body temperature was normalized in relation to the average maximal and minimal temperatures. Activity for each rat was normalized, with 100% corresponding to maximal observed activity and 0% corresponding to minimal observed activity for each experimental week.
Some rats detached their cables or lost their headsets on a particular day, so experimental data were compared to combined control data.
Results
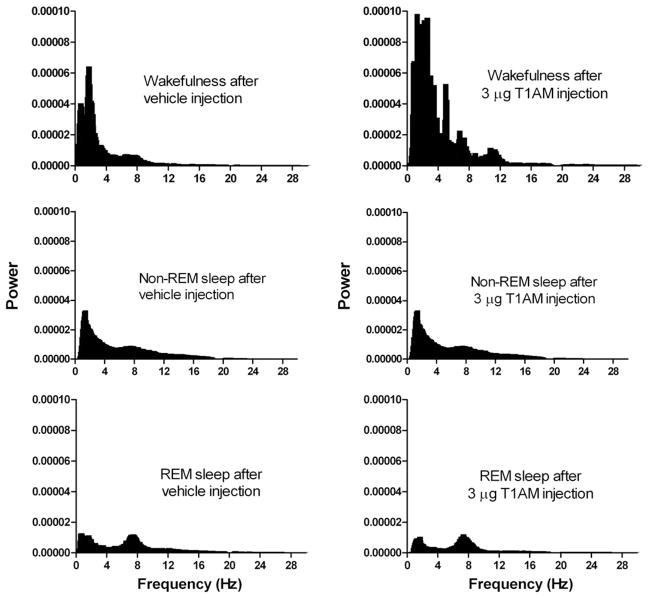
Acute microinjection of 3 μg T1AM to the preoptic region resulted in a change in the power spectrum corresponding to increased low-frequency power and theta in EEG-defined wakefulness, as shown in Fig. 2. The rats also showed significantly higher sleep fragmentation in the 10 h following injections of T1AM than after injection of the vehicle solution alone (Fig. 3). The bout length for vehicle-injected controls was 161.1 ± 2.8 s (mean ± SEM), while the bout length following experimental injections was 143.8 ± 2.8 s. A two-tailed T-test indicated a significant difference of P < 0.0001 between groups (T = 4.3, df = 5245).
Fig. 2.
Power spectra of consciousness state over a 4-hour period following representative vehicle and 3 μg T1AM injection to the preoptic region. Each sleep parameter was analyzed over multiple 30-second epochs. EEG signals were pre-processed with a notch filter at 19.5–20.5 Hz and low-pass filter set to remove any noise above the 30 Hz level.
Fig. 3.
Non-REM sleep bout length after vehicle injection and after T1AM injection. NREM sleep bout length was analyzed and found to be significantly different between vehicle and a combination of all T1AM treatments. NREM sleep bout length after control injection to the preoptic region was found to be longer than after T1AM injections.
Microinjections of T1AM to the preoptic region also resulted in a biphasic reduction of nREM sleep (see Fig. 4 and Table 1). A 2-way ANOVA showed a significant effect of T1AM injection at doses of 1 and 3 μg (P < 0.01, F(1522) = 6.82, ; P < 0.01, F(1487) = 6.75, , respectively), but not at 0.3 or 10 μg (P < 0.68, F(1546) = 0.17, ; P < 0.89, F(1498) = 0.02, , respectively). Figs. 4B and C show that this decrease in sleep was most apparent within the first 8 h of injection. However, no significant interaction between time and injection of T1AM was noted and no significant differences were noted in Bonferroni’s post-hoc test. Significant diurnal variation was observed in nREM sleep (P < 0.01 and ) for all dosages 0.3, 1, 3, and 10 μg T1AM, respectively.
Fig. 4.

Percentage of mean nREM sleep for each 1 hour period after administration of vehicle (solid black line) or T1AM (red dashed line) to the preoptic region of adult male rats. Black bar on x-axis denotes 12-hour dark phase of light cycle and error bars denote standard error of the mean (SEM). Doses of 1 and 3 μg T1AM, panels B and C, caused statistically significant changes in nREM sleep (two-way ANOVA, n = 5, n = 4, respectively). Diurnal variation in nREM sleep was noted, but there was no interaction between the effects of time and T1AM treatment.
Table 1.
Total time in consciousness state for 10-hour period following microinjection.1
| Control | 0.3 mg T1AM | 1 mg T1AM | 3 mg T1AM | 10 mg T1AM | |
|---|---|---|---|---|---|
| Waking | |||||
| Time (minutes) | 230.58 | 223.62 | 256.5* | 267.48* | 238.8 |
| Standard error | 3.6666 | 3.825 | 3.5058 | 3.3966 | 3.4626 |
| nREM | |||||
| Time (minutes) | 289.62 | 296.76 | 257.7* | 255.66* | 282.66 |
| Standard error | 2.7042 | 2.808 | 2.5152 | 2.5488 | 2.7972 |
| REM | |||||
| Time (minutes) | 76.38 | 77.28 | 82.62 | 80.88 | 77.4 |
| Standard error | 0.981 | 1.0104 | 1.0218 | 1.1562 | 0.8034 |
Asterisk denotes significance.
In addition to the decrease in nREM sleep, there was a complementary increase in wakefulness (see Fig. 5). A significant effect of T1AM was observed after injection of 1 and 3 μg (P < 0.04, F(1546) = 4.41, ; P < 0.02, F(1463) = 5.47, , respectively), but not at 0.3 or 10 μg T1AM (P < 0.96, F(1546) = 0.00, ; P < 0.81, F(1498) = 0.06, , respectively). The change in wakefulness was most apparent for the first 8 h post-injection (Figs. 5B, C). Again, significant diurnal variation in waking behavior was observed for all groups (P < 0.01 and ) for all dosages 0.3, 1, 3, and 10 μg T1AM, respectively, but no significant interaction between T1AM injection and time was noted and no significant differences were noted in Bonferroni’s post-hoc test.
Fig. 5.
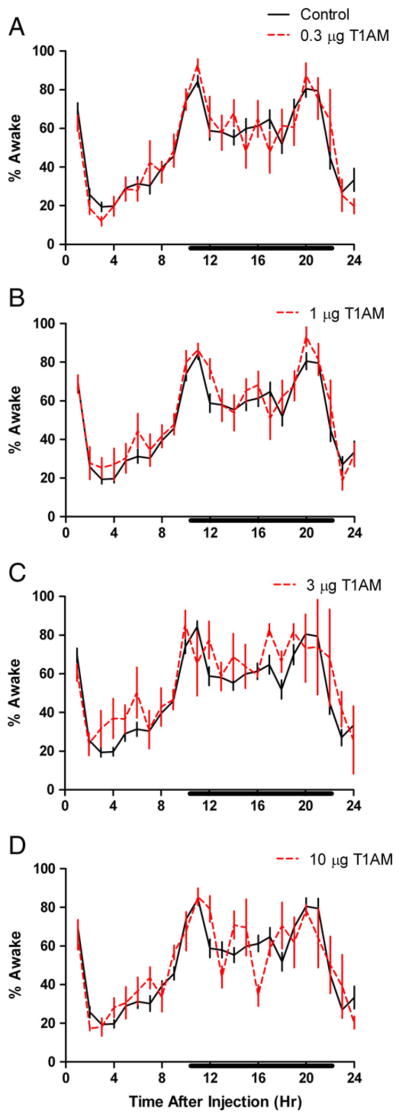
Percentage of mean time awake for each 1 hour period after administration of vehicle (solid black line) or T1AM (red dashed line) to the preoptic region of adult male rats. Black bar on x-axis denotes 12-hour dark phase of light cycle and error bars denote standard error of the mean (SEM). Doses of 1 and 3 μg T1AM, panels B and C, caused statistically significant changes in time awake (two-way ANOVA, n = 5, n = 4, respectively). Diurnal variation in time awake was noted, but there was no interaction between the effects of time and T1AM treatment.
No significant change in REM sleep was observed (Fig. 6). For each group, there was a significant diurnal variation in REM sleep (P < 0.01 and ) for all dosages, 0.3, 1, 3, and 10 μg T1AM, respectively, but there was no interaction between effects of injection of T1AM and time. We performed Bonferroni post-hoc tests on the data, which did not reveal significant differences at any individual time point.
Fig. 6.

Percentage of mean REM sleep for each 1 hour period after administration of vehicle (solid black line) or T1AM (red dashed line) to the preoptic region of adult male rats. Black bar on x-axis denotes 12-hour dark phase of light cycle and error bars denote standard error of the mean (SEM). No significant effect of T1AM on REM sleep was observed. Diurnal variation in REM sleep was noted, but there was no interaction between the effects of time and T1AM treatment.
After injection of 1 or 10 μg T1AM (Figs. 7B and D), there was a significant change in normalized body temperature (P < 0.01, F(1720) = 7.92, ; P < 0.01, F(1696) = 15.49, , respectively). The most apparent decrease in body temperature was observed 8 h after injection of 10 μg T1AM, but it did not reach significance (Fig. 7D). A significant change in body temperature over time was observed after injection of 1 or 3 μg T1AM (P < 0.03, F(23,720) = 1.67, ; P < 0.04, F(23, 696) = 1.60, , respectively). However, there was no significant interaction of the effects of injection of T1AM and time.
Fig. 7.

Mean normalized core body temperature for each 1 hour period after administration of vehicle (solid black line) or T1AM (red dashed line) to the preoptic region of adult male rats. Black bar on x-axis denotes 12-hour dark phase of light cycle and error bars denote the standard error of the mean (SEM). Significant change in body temperature was observed after injection of 1 or 10 μg T1AM (B and D) (two-way ANOVA, n = 6, n = 7, respectively). Diurnal variation in body temperature was noted only after doses of 1 or 3 μg T1AM (two-way ANOVA, n = 7, n = 6, respectively) but no interaction between the effects of time and T1AM treatment was found.
As shown in Fig. 8, doses of 0.3 and 3 μg T1AM caused an increase in normalized activity detected by the Mini-Mitter (P < 0.01, F(1743) =8.18, ; P < 0.01, F(1743) = 6.98, ). A trend towards a decrease in activity was observed in the first 5 h following injection of 10 μg T1AM, but this did not reach statistical significance (P < 0.08, F(1719) = 3.10, ). For all animals, statistically significant diurnal variation in activity was noted (P < 0.01 and ) for all dosages, 0.3, 1, 3, and 10 μg T1AM respectively, but there was no significant interaction between injection of T1AM and time.
Fig. 8.

Normalized activity counts for each 1 hour period after administration of vehicle (solid black line) or T1AM (red dashed line) to the preoptic region of adult male rats. Black bar on x-axis denotes 12-hour dark phase of light cycle and error bars denote standard errors of the mean (SEM). Significant change in locomotor activity was observed after injection of 0.3 or 3 μg T1AM (A and C) (two-way ANOVA, n = 7, n = 7, respectively). Significant diurnal variation in body temperature was noted after each dose of T1AM (two-way ANOVA) but no significant interaction between the effects of time and T1AM treatment was found.
Discussion
Previous reports of single-dose T1AM action suggested that the effects were generally opposite to those of thyroid hormones. Specifically, T1AM decreased body temperature, heart rate, and activity (Scanlan et al., 2004). Our lab has previously shown that administration of T3 to the preoptic region reduced EEG-defined sleep (Martin et al., 2013; Moffett et al., 2013). According to these and other data, the preoptic region would be predicted to be sensitive to the effects of T1AM, and T1AM would increase sleep in adult male rats. Although the concentration of T1AM should have been greatest at the MnPN, and it would be reasonable to assert the effects described here were due to changes in MnPN activity, we cannot exclude action of T1AM at other nearby sites and consequently consider the actions of T1AM more inclusively as occurring within the preoptic region. In contrast to our prediction, T1AM decreased EEG-defined sleep when administered to the preoptic region. Interestingly, reduced sleep was observed after injection of 1 or 3 μg T1AM, but not 0.3 or 10 μg, consistent with dose–response profile observed after T3 microinjection to the same brain region (Martin et al., 2013; Moffett et al., 2013). The inverted U-shaped magnitude of effect of T1AM on sleep behavior is also mirrored by T3 effects on neuronal protein phosphorylation (Sarkar et al., 2006, 2011). It is unlikely that local production of T1AM from T3 is the source of the similar activity of these agents because recent work has shown that T1AM is not produced from extrathyroidal T4 whereas T3 is, implying that extrathyroidal T3 is also not a biosynthetic precursor to T1AM (Hackenmueller et al., 2012). Instead, our results suggest that T1AM and T3 have some shared targets and/or actions that result in changes in sleep behavior. The results of this study are part of a growing body of evidence showing that thyroid hormones and their derivatives have functions in the developed central nervous system and potentially modify behavior.
Although IP injection of 50 mg/kg T1AM caused a decrease in activity in mice (Dhillo et al., 2009; Scanlan et al., 2004), the administration of 0.3 and 3 μg T1AM to the preoptic region resulted in an increase in activity in the current study. Mice made chronically hyperthyroid by IP injection of T4 and rats acutely microinjected with T3 or norepinephrine to the preoptic region all showed an increase in activity (Emlen et al., 1972). It is, of course, possible that T1AM has different actions in different brain regions. Effects on multiple brain sites after IP injection might result in distinct behavioral outputs. In addition, the dose used in Scanlan et al. (2004) was quite high, and the behavioral inhibition described in that study might therefore be attributed to an “inverted U” type of dose–response curve, such as seen after amphetamine administration (Glick and Muller, 1971).
Consistent with the results of Scanlan et al. (2004), T1AM decreased body temperature in the current study. Again, the quantity of T1AM administered by Scanlan et al.’s (2004) study, 50 mg/kg body weight administered IP, was significantly greater than the quantities injected in the present study (although injected by a different route), which might account for the less dramatic change in body temperature seen in the current work. Norepinephrine has also been shown to decrease body temperature when administered to the preoptic region, and there is evidence that this is mediated through α2 adrenergic receptors (Day et al., 1979) Since it has been proposed that T1AM can have some of its actions through noradrenergic mechanisms (Regard et al., 2007), it is possible that the decrease in body temperature observed in the current study was due to T1AM action on noradrenergic systems of the preoptic region. Furthermore, TAAR1 knockout mice exhibited hypothermia due to IP injection of T1AM and other TAAR1 agonists, suggesting that the change in body temperature observed in the current study is not due to effects mediated by the TAAR1 receptor (Panas et al., 2010).
The preoptic region has thermoregulatory functions and a relationship between body temperature and sleep behavior has been observed (McGinty and Szymusiak, 1990). Physiological and behavioral changes, such as shivering or vasodilation, due to local temperature changes, glutamate administration, or electrical stimulation have suggested a role of the preoptic region in thermal homeostasis (Satinoff, 1964; Zhang et al., 1995). Furthermore, local warming of the preoptic region has been reported to induce sleep in cats (Roberts and Robinson, 1969). In addition, elevated ambient temperature increased the number of sleep-active c-Fos immunoreactive neurons in the MnPN (Gong et al., 2000). Indeed, the MnPN is thought to integrate information about thermal homeostasis and temperature-dependent changes in neuronal firing rates have been observed within the MnPN (Travis and Johnson, 1993). In this current study, however, no change in percent of time in nREM sleep was observed after injection of 10 μg T1AM, but the most apparent change in body temperature was observed following that dose. Other evidence indicates dissociation between thermal and sleep regulation in the preoptic region. No temperature-dependent changes in firing rates were reported after single-unit recordings of sleep-active neurons in the MnPN (Suntsova et al., 2002). Conversely, triazolam increased sleep without significantly changing body temperature when administered to the preoptic region (Mendelson and Martin, 1992; Mendelson et al., 1989). The current findings indicate that the preoptic region mechanisms for lowering of body temperature do not necessarily increase nREM sleep.
Several decades ago, Dratman (1974) posited that thyroid hormones might have an interaction with noradrenergic systems and speculated as to the existence of a decarboxylated thyroid hormone derivative. Approximately 30 years later, Scanlan et al. (2004) discovered T1AM, a decarboxylated thyroid hormone derivative, in rodent brain extracts. Further confirming Dratman’s predictions, T1AM has been shown to activate α2A adrenergic receptors (Regard et al., 2007). It is not yet clear whether T1AM has any interaction(s) with the GABAA receptor or other nongenomic modes of thyroid hormone action. Our results make it seem likely, however, that thyroid hormones and T1AM share common pathways or mechanisms in the adult brain to produce similar behavioral consequences. Like thyroid hormones, several potential mechanisms of T1AM action in adult brain have been proposed, so the behavioral and physiological effects described here might be due to one or more independent or interacting mechanisms within the preoptic region. Though other groups have reported changes in CNS-derived behavior due to T1AM, the present study is the first to document the effects of T1AM on EEG-defined sleep.
Acknowledgments
This work was supported by the National Science Foundation grant IOS 0724962.
References
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Browning TB, Atkins RW, Weiner H. Cerebral metabolic disturbances in hypothyroidism; clinical and electroencephalographic studies of the psychosis of myxedema and hypothyroidism. AMA Arch Intern Med. 1954;93:938–950. doi: 10.1001/archinte.1954.00240300132014. [DOI] [PubMed] [Google Scholar]
- Bucheler MM, Hadamek K, Hein L. Two alpha(2)-adrenergic receptor subtypes, alpha(2A) and alpha(2C), inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Musa A, Flechner A, Hessenius C, Gaio U, Meinhold H, Baumgartner A. Evidence for circadian variations of thyroid hormone concentrations and type II 5′-iodothyronine deiodinase activity in the rat central nervous system. J Neurochem. 1997;68:795–803. doi: 10.1046/j.1471-4159.1997.68020795.x. [DOI] [PubMed] [Google Scholar]
- Caria MA, Dratman MB, Kow LM, Mameli O, Pavlides C. Thyroid hormone action: nongenomic modulation of neuronal excitability in the hippocampus. J Neuroendocrinol. 2009;21:98–107. doi: 10.1111/j.1365-2826.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Timiras PS. Sleep organization in hypo- and hyperthyroid rats. Neuroendocrinology. 1982;34:438–443. doi: 10.1159/000123342. [DOI] [PubMed] [Google Scholar]
- Chapell R, Martin J, Machu TK, Leidenheimer NJ. Direct channel-gating and modulatory effects of triiodothyronine on recombinant GABA(A) receptors. Eur J Pharmacol. 1998;349:115–121. doi: 10.1016/s0014-2999(98)00182-4. [DOI] [PubMed] [Google Scholar]
- Day TA, Willoughby JO, Geffen LB. Thermoregulatory effects of preoptic area injections of noradrenaline in restrained and unrestrained rats. Brain Res. 1979;174:175–179. doi: 10.1016/0006-8993(79)90814-x. [DOI] [PubMed] [Google Scholar]
- Demet MM, Ozmen B, Deveci A, Boyvada S, Adiguzel H, Aydemir O. Depression and anxiety in hyperthyroidism. Arch Med Res. 2002;33:552–556. doi: 10.1016/s0188-4409(02)00410-1. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Bewick GA, White NE, Gardiner JV, Thompson EL, Bataveljic A, Murphy KG, Roy D, Patel NA, Scutt JN, Armstrong A, Ghatei MA, Bloom SR. The thyroid hormone derivative 3-iodothyronamine increases food intake in rodents. Diabetes Obes Metab. 2009;11:251–260. doi: 10.1111/j.1463-1326.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- Dratman MB. On the mechanism of action of thyroxine, an amino acid analog of tyrosine. J Theor Biol. 1974;46:225–270. doi: 10.1016/0022-5193(74)90151-9. [DOI] [PubMed] [Google Scholar]
- Dratman MB, Crutchfield FL. Synaptosomal [125 I] triiodothyronine after intravenous [125 I] thyroxine. Am J Physiol. 1978;235:E638–E647. doi: 10.1152/ajpendo.1978.235.6.E638. [DOI] [PubMed] [Google Scholar]
- Dratman MB, Gordon JT. Thyroid hormones as neurotransmitters. Thyroid. 1996;6:639–647. doi: 10.1089/thy.1996.6.639. [DOI] [PubMed] [Google Scholar]
- Dratman MB, Crutchfield FL, Axelrod J, Colburn RW, Thoa N. Localization of triiodothyronine in nerve ending fractions of rat brain. Proc Natl Acad Sci U S A. 1976;73:941–944. doi: 10.1073/pnas.73.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman MB, Futaesaku Y, Crutchfield FL, Berman N, Payne B, Stumpf WE, Sar M. Iodine125-labeled triiodothyronine in rat brain: Evidence for localization in discrete neural systems. Science. 1982;215:309–312. doi: 10.1126/science.7053582. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Rechtschaffen A. Effect of thyroxine on sleep in the rat. Sleep. 1979;2:215–232. [PubMed] [Google Scholar]
- Emlen W, Segal DS, Mandell AJ. Thyroid state: effects on pre- and postsynaptic central noradrenergic mechanisms. Science. 1972;175:79–82. doi: 10.1126/science.175.4017.79. [DOI] [PubMed] [Google Scholar]
- Glick SD, Muller RU. Paradoxical effects of low doses of d-amphetamine in rats. Psychopharmacologia. 1971;22:396–402. doi: 10.1007/BF00406877. [DOI] [PubMed] [Google Scholar]
- Gompf HS, Greenberg JH, Aston-Jones G, Ianculescu AG, Scanlan TS, Dratman MB. 3-Monoiodothyronamine: the rationale for its action as an endogenous adrenergic-blocking neuromodulator. Brain Res. 2010;1351:130–140. doi: 10.1016/j.brainres.2010.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3′,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience. 1999;93:943–954. doi: 10.1016/s0306-4522(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenmueller SA, Marchini M, Saba A, Zucchi R, Scanlan TS. Biosynthesis of 3-iodothyronamine (T1AM) is dependent on the sodium-iodide symporter and thyroperoxidase but does not involve extrathyroidal metabolism of T4. Endocrinology. 2012;153:5659–5667. doi: 10.1210/en.2012-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefig CS, Renko K, Piehl S, Scanlan TS, Bertoldi M, Opladen T, Hoffmann GF, Klein J, Blankenstein O, Schweizer U, Kohrle J. Does the aromatic L-amino acid decarboxylase contribute to thyronamine biosynthesis? Mol Cell Endocrinol. 2012;349:195–201. doi: 10.1016/j.mce.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Kales A, Heuser G, Jacobson A, Kales JD, Hanley J, Zweizig JR, Paulson MJ. All night sleep studies in hypothyroid patients, before and after treatment. J Clin Endocrinol Metab. 1967;27:1593–1599. doi: 10.1210/jcem-27-11-1593. [DOI] [PubMed] [Google Scholar]
- Koury EJ, Pawlyk AC, Berrodin TJ, Smolenski CL, Nagpal S, Deecher DC. Characterization of ligands for thyroid receptor subtypes and their interactions with co-regulators. Steroids. 2009;74:270–276. doi: 10.1016/j.steroids.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Kruger L, Saporta S, Swanson LW. Photographic Atlas of the Rat Brain: The Cell and Fiber Architecture Illustrated in Three Planes With Stereotaxic Coordinates. Cambridge University Press; Cambridge; New York: 1995. [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Lohman RJ, Liu L, Morris M, O’Brien TJ. Validation of a method for localised microinjection of drugs into thalamic subregions in rats for epilepsy pharmacological studies. J Neurosci Methods. 2005;146:191–197. doi: 10.1016/j.jneumeth.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Martin JV, Cook JM, Hagen TJ, Mendelson WB. Inhibition of sleep and benzodiazepine receptor binding by a beta-carboline derivative. Pharmacol Biochem Behav. 1989;34:37–42. doi: 10.1016/0091-3057(89)90349-3. [DOI] [PubMed] [Google Scholar]
- Martin JV, Williams DB, Fitzgerald RM, Im HK, VonVoigtlander PF. Thyroid hormonal modulation of the binding and activity of the GABA A receptor complex of brain. Neuroscience. 1996;73:705–713. doi: 10.1016/0306-4522(96)00052-8. [DOI] [PubMed] [Google Scholar]
- Martin JV, Padron JM, Newman MA, Chapell R, Leidenheimer NJ, Burke LA. Inhibition of the activity of the native gamma-aminobutyric acid(A) receptor by metabolites of thyroid hormones: correlations with molecular modeling studies. Brain Res. 2004;1004:98–107. doi: 10.1016/j.brainres.2003.12.043. [DOI] [PubMed] [Google Scholar]
- Martin JV, Giannopoulos PF, Moffett SX, James TD. Effects of acute microinjections of thyroid hormone to the preoptic region of euthyroid adult male rats on sleep and motor activity. Brain Res. 2013 doi: 10.1016/j.brainres.2013.01.032. http://dx.doi.org/10.1016/j.brainres.2013.01.032 (in press) [DOI] [PubMed]
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13:480–487. doi: 10.1016/0166-2236(90)90081-k. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV. Characterization of the hypnotic effects of triazolam microinjections into the medial preoptic area. Life Sci. 1992;50:1117–1128. doi: 10.1016/0024-3205(92)90349-t. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV, Perlis M, Wagner R. Enhancement of sleep by microinjection of triazolam into the medial preoptic area. Neuropsychopharmacology. 1989;2:61–66. doi: 10.1016/0893-133x(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. Gabaergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129:803–810. doi: 10.1016/j.neuroscience.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Moffett SX, Giannopoulos PF, James TD, Martin JV. Effects of acute microinjections of thyroid hormone to the preoptic region of hypothyroid adult male rats on sleep, motor activity and body temperature. Brain Res. 2013 doi: 10.1016/j.brainres.2013.04.017. http://dx.doi.org/10.1016/j.brainres.2013.04.017 (in press) [DOI] [PubMed]
- Mohan Kumar V, Datta S, Chhina GS, Singh B. Alpha adrenergic system in medial preoptic area involved in sleep–wakefulness in rats. Brain Res Bull. 1986;16:463–468. doi: 10.1016/0361-9230(86)90174-7. [DOI] [PubMed] [Google Scholar]
- Panas HN, Lynch LJ, Vallender EJ, Xie Z, Chen GL, Lynn SK, Scanlan TS, Miller GM. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res. 2010;88:1962–1969. doi: 10.1002/jnr.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl S, Heberer T, Balizs G, Scanlan TS, Smits R, Koksch B, Kohrle J. Thyronamines are isozyme-specific substrates of deiodinases. Endocrinology. 2008;149:3037–3045. doi: 10.1210/en.2007-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JE, Rawson RW, Tata JR. Metabolism of L-thyroxine and L-3:5:3′-triiodothyronine by brain tissue preparations. Endocrinology. 1957;60:83–98. doi: 10.1210/endo-60-1-83. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Kumar VM. The role of alpha-2 receptors in the medial preoptic area in the regulation of sleep–wakefulness and body temperature. Neuroscience. 1998;85:807–817. doi: 10.1016/s0306-4522(97)00663-5. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Kumar VM, John J, Mallick H. Medial preoptic alpha-2 adrenoceptors in the regulation of sleep–wakefulness. Physiol Behav. 1995;57:171–175. doi: 10.1016/0031-9384(94)00297-i. [DOI] [PubMed] [Google Scholar]
- Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WW, Robinson TC. Relaxation and sleep induced by warming of preoptic region and anterior hypothalamus in cats. Exp Neurol. 1969;25:282. doi: 10.1016/0014-4886(69)90051-x. [DOI] [PubMed] [Google Scholar]
- Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience. 1996;74:897–915. doi: 10.1016/0306-4522(96)00186-8. [DOI] [PubMed] [Google Scholar]
- Salin-Pascual RJ, Franco M, Garcia-Ferrero R, Vazquez J, Suarez J, Sanchez L, Jimenez-Anguiano A. Differences in sleep variables, blood adenosine, and body temperature between hypothyroid and euthyroid rats before and after REM sleep deprivation. Sleep. 1997;20:957–962. doi: 10.1093/sleep/20.11.957. [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Ray AK. Synaptosomal T 3 content in cerebral cortex of adult rat in different thyroidal states. Neuropsychopharmacology. 1994;11:151–155. doi: 10.1038/sj.npp.1380101. [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Durga ND, Morris JJ, Martin JV. In vitro thyroid hormone rapidly modulates protein phosphorylation in cerebrocortical synaptosomes from adult rat brain. Neuroscience. 2006;137:125–132. doi: 10.1016/j.neuroscience.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Morris JJ, Martin JV. Non-genomic effect of L-triiodothyronine on calmodulin-dependent synaptosomal protein phosphorylation in adult rat cerebral cortex. Indian J Exp Biol. 2011;49:169–176. [PubMed] [Google Scholar]
- Satinoff E. Behavioral thermoregulation in response to local cooling of rat brain. Am J Physiol. 1964;206:1389. doi: 10.1152/ajplegacy.1964.206.6.1389. [DOI] [PubMed] [Google Scholar]
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- Snead AN, Santos MS, Seal RP, Miyakawa M, Edwards RH, Scanlan TS. Thyronamines inhibit plasma membrane and vesicular monoamine transport. ACS Chem Biol. 2007;2:390–398. doi: 10.1021/cb700057b. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Clemente CD. Basal forebrain structures and sleep. Acta Neurol Latinoam. 1968;14:228–244. [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep–waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol-Lond. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KA, Johnson AK. In-vitro sensitivity of median preoptic neurons to angiotensin-II, osmotic-pressure, and temperature. Am J Physiol. 1993;264:R1200–R1205. doi: 10.1152/ajpregu.1993.264.6.R1200. [DOI] [PubMed] [Google Scholar]
- Watt T, Groenvold M, Rasmussen AK, Bonnema SJ, Hegedus L, Bjorner JB, Feldt-Rasmussen U. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154:501–510. doi: 10.1530/eje.1.02124. [DOI] [PubMed] [Google Scholar]
- Whybrow PC, Bauer M. Behavioral and psychiatric aspects of hypothyroidism. In: Braverman LE, Utiger RD, editors. The Thyroid: A Fundamental and Clinical Text. Lippincott Williams and Wilkins; Philadephia: 2005. pp. 842–863. [Google Scholar]
- Whybrow PC, Bauer M. Behavioral and psychiatric aspects of thyrotoxicosis. In: Braverman LE, Utiger RD, editors. The Thyroid: A Fundamental and Clinical Text. 9. Lippincott Williams and Wilkins; Philadephia: 2005. pp. 644–650. [Google Scholar]
- Zhang YH, Yanasefujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area—which contribute more to the control of shivering in rats. J Physiol-Lond. 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]