Introduction
The many uses of methylene blue
Methylene blue (methylthioninium chloride) is a ‘jack of all trades’ with a litany of clinical uses. In vivo, it is indicated for use as a therapy for drug-induced methemoglobinemia [1-3], can be used for the treatment of infections, pathologies, or poisoning, and as a dye for diagnostics. It is also commonly used as a dye in vitro – for example, as a component in staining of cells, tissues, DNA, parasites, and bacteria [4-6]. In the 1890s, Ehrlich demonstrated its use to target the malarial parasite, and more recently it has been reinvestigated for inclusion in antimalarial regimens in the wake of parasite drug resistance [7-9]. Further examples of its clinical use include treatment of ifosfamide-induced neurotoxicity (although treatment has been reported ineffective), an antidote for cyanide poisoning, visualization of fallopian tubes or ruptured membranes, a marker of tumors, and even as a potential therapy for septic shock and ischemic brain injury [5,8,10-18]. In phase II clinical trials, a modified version of methylene blue is reported to slow cognitive decline in mild–moderate Alzheimer’s disease patients compared to placebo, and phase III trials are planned, although these results remain unpublished [19]. The mechanism of action is unclear – possibly by preventing tau protein aggregation or increasing amyloid-β clearance by enhancing proteasome activities [19,20]. As a photodynamic therapy, methylene blue could be used to treat psoriasis, West Nile virus infection, AIDS-related Kaposi’s sarcoma, antibiotic-resistant bacterial strains and decontaminate blood before transfusion [5,21-25]. Methylene blue has also contributed to drug development, forming the structural chemical basis of other therapeutic drugs, including the antimalarial drug, chloroquine; the antihistamine, promethazine; and the antipsychotic, chlorpromazine [5]. Acknowledging the many uses of methylene blue, this article focuses on the pharmacodynamics of methylene blue in the context of methemoglobinemia treatment and the pathways surrounding this, due to known pharmacogenetic associations that relate to these pathways.
Methemoglobinemia
Methemoglobinemia is an increase in the methemoglobin (MetHb) content of red blood cells (RBCs) [26,27]. MetHb is formed when heme iron atoms within hemoglobin are oxidized and can no longer bind oxygen or carbon dioxide [26-30]. Normal levels of MetHb in circulating RBCs are around 1%. Methemoglobinemia occurs when these levels increase and can be due to inherited factors (hereditary) or induced by exogenous oxidizing agents such as therapeutic drugs (acquired) [26,27]. Cyanosis occurs at around 15% MetHb, and tissue hypoxia can occur as levels rise further–MetHb levels of 70% or higher can be fatal [27].
G6PD deficiency
Methylene blue is an effective treatment for reducing MetHb however, it is associated with adverse reactions in glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals (Table 1). Drug labels for methylene blue contraindicate or advise precaution for use in G6PD-deficient individuals due to a risk of hemolytic anemia and/or methemoglobinemia [1-3]. Three hundred and thirty million individuals are estimated to have a deficiency in the G6PD enzyme, with the highest prevalence found in Africa, the Middle East, and Asia [48]. G6PD-deficient individuals are more susceptible to RBC oxidative stress induced by exogenous agents such as therapeutic drugs because reduced nicotinamide adenine dinucleotide phosphate (NADPH) production cannot meet the demand required by regulatory mechanisms (described below in further detail) [46,49,50]. The variants within the G6PD gene that have been identified (currently > 180) are categorized into WHO classes (I–V) depending on the extent of enzyme deficiency and clinical manifestations they confer (see the PharmGKB G6PD very important pharmacogene summary http://www.pharmgkb.org/vip/PA28469) [50-54].
Table 1. Cases of adverse reactions reported after methylene blue treatment in G6PD-deficient individuals.
| Study details | Methemoglobinemia reported to be triggered by | Consequence of subsequent methylene blue treatment | G6PD deficiency? Test carried out? | References |
|---|---|---|---|---|
| Case study, male adult (Filipino) with metastatic renal cell carcinoma | Triapine | Developed jaundice, hemoglobinuria, hemolysis | G6PD-deficient, enzyme activity assay | Foltz et al. [31] and Dalal et al. [32] |
| Case study, male adult (Mexican-American) | Cleaning liquid containing aniline and toluene | No change in MetHb levels, hemolysis on day 2 which may have been triggered by aniline, toluene, methylene blue (or the subsequent vitamin Ca treatment) | G6PD deficiency A – variant, quantitative and qualitative enzyme activity assays | Rosen et al. [33] |
| Report of three premature neonates who were exposed to methylene blue prenatally (two male, one female) | Not applicable | Development of severe hemolysis resulting in hyperbilirubinemia, all required exchange transfusions | G6PD deficiency was confirmed in two of the three cases, enzyme activity assay | Gauthier [14] |
| Case study, 26-month-old boy | Nail removal fluid (containing nitroethane) | MetHb levels were only transiently reduced, and rose, but were resolved by RBC exchange | G6PD-deficient, enzyme activity, and 53% HbA, 41% HbS sickle trait | Golden et al. [34] |
| Case study, 23-year-old woman (India) | Aniline | Was also given vitamin Ca. No improvement. Developed hemolysis and required transfusion | G6PD-deficient, methemoglobin reduction method | Mullick et al. [35] |
| A 3-month-old who had undergone cardiac surgery | Could not define, but the authors discuss that it may be due to the effects of cardiopulmonary bypass on enzyme activity, glyceryl trinitrate treatment, or other cardiac or respiratory causes | Low dose of methylene blue was given over 10 min and MetHb levels reduced. Developed jaundice and mild hematuria attributed to hemolysis | Prior knowledge of a partial G6PD deficiency | Maddali et al. [36] |
| A case study, 40-year-old man | Fungicide containing copper-8-hydroxyquinolate. He was initially treated with vitamin Ca. Methemoglobinemia and hemolysis developed | Methylene blue was administered, along with continued treatment with vitamin Ca. Treatment was not effective, and hemolysis became more severe | Underlying G6PD deficiency, as well as inhibition of G6PD activity by copper. Activity assay | Yang et al. [37] |
| A case study, adult, in a trial of 80 patients (patients with known G6PD deficiency were not enrolled) | Rasburicase | MetHb levels decreased but hemolysis worsened | Previously unknown G6PD deficiency, test not reported | Vadhan-Raj et al. [38] |
| A case study, 12-year-old boy (Laotian) | Rasburicase | Treatment was not effective | G6PD-deficient, enzyme activity test | Bhat et al. [39] |
| A case study, a male patient (Jordanian) with chronic renal failure | Metoclopramide (impaired renal function and cytochrome b5 reductase deficiency also contributed) | Also given vitamin Ca. Treatment was not effective, his condition worsened, and he likely developed hemolysis. The patient died | Cytochrome b5 reductase- deficient, quantitative assay, and G6PD-deficient, enzyme activity assay | Karadsheh et al. [40] |
| A case study, 25-year-old man | Aniline | Symptoms diminished and MetHb levels reduced, however hemolytic anemia developed several days later | G6PD deficiency, assay | Liao et al. [41] |
| A case study, 23-year-old woman undergoing laparoscopy | Methylene blue (administered through the cervix to visualize the fallopian tubes) | Cyanosis was treated with vitamin Ca rather than with methylene blue | G6PD deficiency, enzyme levels | Bilgin et al. [13] |
| n = 409 children were genotyped, n = 88 were G6PD-deficient. Dose-finding study – treated with combination of chloroquine and different doses of methylene blue. Burkino Faso | Not applicable | In one child, hemoglobin dropped below 5 g/dl on day 5, and in seven children, hemoglobin value dropped by more than 3 g/dl | The child whose hemoglobin dropped below 5 g/dl was a G6PD-deficient hemizygous male. Three of the seven children with a greater than 3 g/dl drop in hemoglobin were G6PD-deficient. Genotyping method or variant not described | Meissner et al. [42] |
| Pooled analysis of four randomized control trials (n = 1005 children) (includes PMID: 16179085, 17026773 (above), 18286187, and unpublished data). A 21-month-old girl and a 28-month- old boy, both with malaria | Not applicable | n = 844 children were treated with methylene blue combined with other antimalarial drugs. In these two patients, hemoglobin levels fell to 5 g/dl or less (indicating severe anemia) | Female was heterozygous for G6PD A –, the male was hemizygous (likely that described above in Meissner et al. [42]). Specifics of genotyping were not provided | Muller et al. [43] |
G6PD, glucose-6-phosphate dehydrogenase; MetHb, methemoglobin; PMID, PubMed identification number.
Here, we use three pathway diagrams to explain the pharmacogenetics underlying the mechanism of action of methylene blue in the treatment of methemoglobinemia. We introduce the essential role of G6PD in the production of NADPH via the pentose phosphate pathway (PPP) in RBCs (Fig. 1) and detail some of the mechanisms that are dependent on NADPH to regulate oxidative stress in RBCs (Fig. 2), including methylene blue treatment (Fig. 3). Many of the steps within these pathways involve cycling redox reactions – oxidation is the loss of electrons and reduction is the accompanied gain of electrons [29]. These pathways help to explain why individuals with genetic variants that confer G6PD deficiency may be more susceptible to methemoglobinemia triggered by exogenous agents and why methylene blue treatment is ineffective in these individuals, possibly even exacerbating RBC oxidative stress. Interactive versions of these pathways, with links to gene and drug pages can be found on the PharmGKB website at: http://www.pharmgkb.org/search/browse/pathways.action.
Fig. 1.
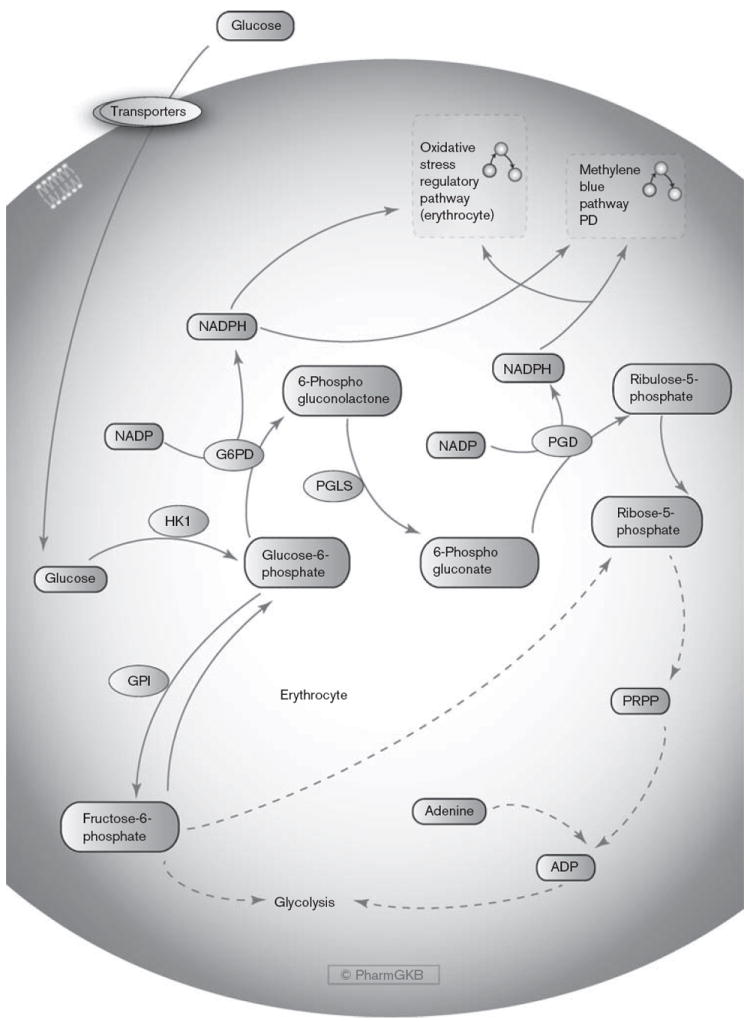
The pentose phosphate pathway in erythrocytes. A graphical representation of the genes involved in the generation of NADPH in red blood cells. The NADPH produced can be utilized in mechanisms that regulate oxidative stress and by methylene blue to reduce methemoglobin. An interactive version of this pathway with links to genes and the interconnecting pathways can be found on the PharmGKB website: http://www.pharmgkb.org/pathway/PA165971634. G6PD, glucose-6-phosphate dehydrogenase; GPI, glucose-6-phosphate isomerase; HK1, hexokinase 1; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PGD, 6-phosphogluconate dehydrogenase; PGLS, 6-phosphogluconolactonase; PRPP, phosphoribosylpyrophosphate.
Fig. 2.
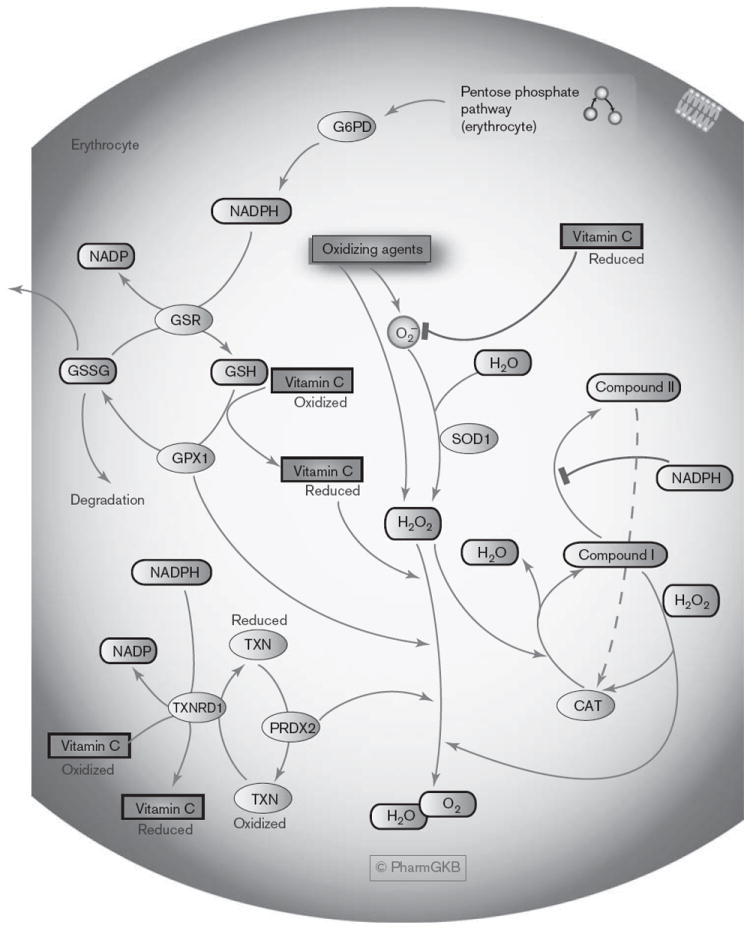
The oxidative stress regulatory pathway in erythrocytes. A graphical representation showing several of the regulatory mechanisms that prevent oxidative stress in red blood cells, many of which require NADPH from the pentose phosphate pathway. An interactive full color version of this pathway with links to genes and the interconnecting pathways can be found on the PharmGKB website: http://www.pharmgkb.org/pathway/PA165980399. CAT, catalase; G6PD, glucose-6-phosphate dehydrogenase; GPX1, glutathione peroxidase; GSH, reduced glutathione; GSR, glutathione reductase; GSSG, oxidized glutathione; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PRDX2, peroxiredoxin 2; SOD1, superoxide dismutase 1; TXN, thioredoxin; TXNRD1, thioredoxin reductase.
Fig. 3.
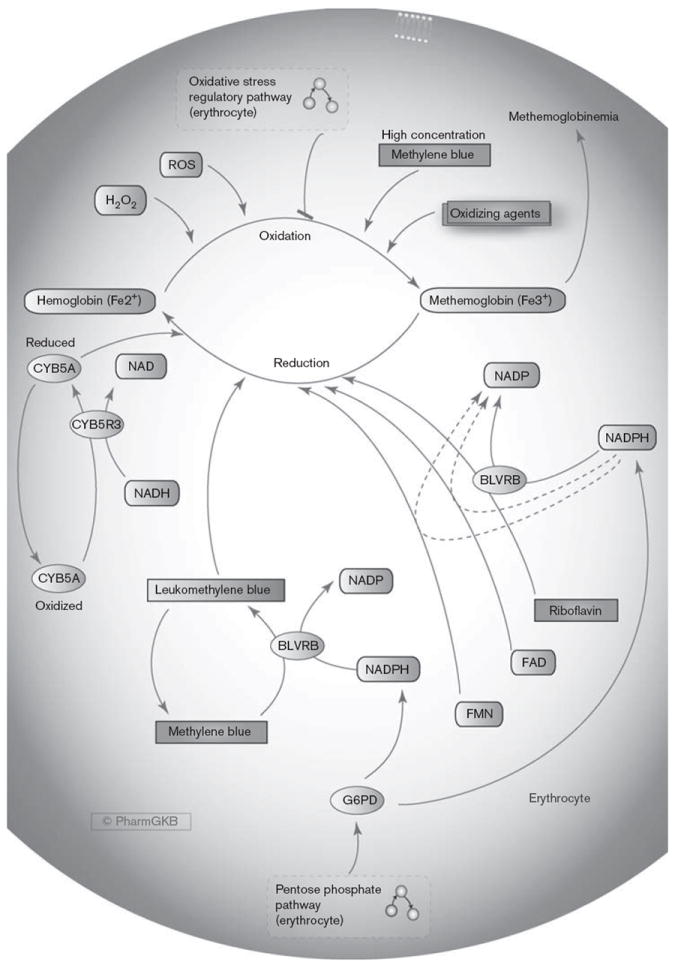
Methylene blue pathway, pharmacodynamics (PD). A graphical representation of some of the triggering agents that can cause methemoglobin production in red blood cells and the control mechanisms to prevent methemoglobinemia, including treatment with methylene blue which requires NADPH from the pentose phosphate pathway. An interactive full color version of this pathway with links to genes, drugs, and the interconnecting pathways can be found on the PharmGKB website:http://www.pharmgkb.org/pathway/PA165980834. BLVRB, biliverdin reductase B; CYB5A, cytochrome b5; CYB5R3, cytochrome b5 reductase; FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; G6PD, glucose-6-phosphate dehydrogenase; NAD, nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species.
Pathway descriptions
The pentose phosphate pathway and production of NADPH in RBCs (Fig. 1)
Glucose is converted to glucose-6-phosphate by hexokinase (HK1), and then enters either the glycolysis pathway via conversion to the isomer fructose-6-phosphate or the PPP (also known as hexose monophosphate shunt) via oxidation into 6-phosphogluconolactone [29,55-61]. Two steps within the PPP produce NADPH (Fig. 1); the conversion of glucose-6-phosphate to 6-phosphogluconolactone by G6PD and the conversion of 6-phosphogluconate to ribulose-5-phosphate by 6-phosphogluconate dehydrogenase (PGD, 6PGD) [55-58,62]. The end product of the pathway is ribose-5-phosphate, utilized for the production of nucleotides, polysaccharides, and coenzymes, and used in RBCs for phosphoribosylpyrophosphate (PRPP) production to generate ADP for use in the Embden–Meyerhof glycolysis pathway [57,63,64]. Within RBCs, NADPH is required for the regulation of oxidative stress and is utilized for methylene blue function [27,57,65]. The only source of NADPH in RBCs is the via the PPP, in which G6PDis the rate-limiting step [29,49,50,55-57]. As RBCs age, enzyme activities involved in glucose metabolism diminish, including that of G6PD, reducing energy production and the ability to protect cell membrane integrity and hemoglobin from oxidation [60,66].
The role of NADPH in neutralization of ROS in RBCs
Release of reactive oxygen species (ROS) such as superoxide (O2−) and/or peroxide (e.g. hydrogen peroxide, H2O2) can be triggered by exogenous oxidizing agents such as therapeutics or their metabolites [30,50]. For example, H2O2 is produced as a byproduct of the conversion of uric acid to allantoin by the drug rasburicase [67,68]. RBCs are constantly subjected to oxidative stress, from their role as an oxygen transporter to their exposure to xenobiotics in circulation [29,30]. NADPH is utilized as reducing power in many of the processes that protect RBCs from these oxidative stresses (Figs 2 and 3). Inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by H2O2 is thought to reroute glucose from glycolysis to the PPP to increase NADPH production [58,69].
The oxidative stress regulatory pathway (erythrocytes)
The oxidative stress regulatory pathway focuses on some of the mechanisms requiring NADPH from the PPP to neutralize ROS such as O2− and H2O2 (Fig. 2). O2− can be converted to H2O2 by superoxide dismutase (SOD1) [50,62]. Glutathione reductase (GSR) utilizes NADPH to convert oxidized glutathione (GSSG) to reduced glutathione (GSH) [55-57,70,71]. GSH is then oxidized back to GSSG by glutathione peroxidase (GPX1) in a cyclical reaction to neutralize 2H2O2 into 2H2O and O2 [50,55-57,70]. GSH also protects hemoglobin by preventing and reversing oxidation that causes the formation of disulfide crosslinks between globin chains, which distorts hemoglobin structure, potentially resulting in the precipitation of ‘Heinz bodies’ [29]. RBCs with normal G6PD enzyme activity have higher PPP activity and levels of GSH compared to G6PD-deficient RBCs, however, deficient cells can cope with low levels of available NADPH under normal conditions [49,50]. When oxidative stress occurs, G6PD ‘normal’ RBCs maintain GSH levels by enhancing PPP activity, whereas in G6PD-deficient cells the PPP remains at minimum capacity and GSH levels decrease [49]. Because the PPP in G6PD-deficient RBCs is already close to the maximum activity rate obtainable under normal conditions, these cells cannot cope with oxidative stresses and are more susceptible to lysis triggered by oxidative stress, which can lead to hemolytic anemia [49,50].
Another key system requiring NADPH is the conversion of oxidized thioredoxin (TXN) to a reduced form by thioredoxin reductase (TXNRD1), reduced thioredoxin is then utilized as an electron donor by the enzyme peroxiredoxin to neutralize H2O2 [70-72]. In RBCs, peroxiredoxin 2 (Prx 2, PRDX2) is the most abundant isoform, and also plays a protective role by binding to and stabilizing hemoglobin [72,73]. The importance of PRDX2 is demonstrated in knockout mice who display morphological RBC defects such as Heinz bodies, and upon exposure to H2O2 blood samples from knockout mice have enhanced MetHb formation compared to wildtype samples [73].
Both GSH and thioredoxin reductase can convert fully oxidized vitamin C (dehydroascorbic acid) to its reduced form (ascorbate, ascorbic acid), which can in turn donate electrons and hydrogens to O2−, H2O2, and oxygen free radicals [71,74-76]. Because humans cannot synthesize vitamin C, recycling it from an oxidized state is important in maintaining RBC and plasma levels of the antioxidant form [71,74,75].
The catalase (CAT) enzyme also neutralizes H2O2, involving reaction steps that form different states of the enzyme – NADPH is not required for the functional activity of catalase, rather the prevention of forming an inactive state of the enzyme [77]. The first reaction between resting catalase (ferricatalase) and H2O2 forms compound I and H2O, subsequent reaction with a further H2O2 molecule returns catalase to resting state and releases H2O and O2 [77,78]. Reduction of compound I can also form the inactive compound II state, that slowly spontaneously reverts back to catalase [77,78]. Formation of compound II can be prevented by the production of NADPH by G6PD from glucose-6-phosphate [77,79,80]. Evidence suggests that NADPH may also function to reduce compound I back to catalase under conditions that prolong this state of catalase, a strong oxidant (e.g. low H2O2) [77].
Methylene blue pathway, PD
The methylene blue pathway focuses on the mechanisms involved in oxidation of hemoglobin to MetHb and protection from this process, or reduction of MetHb back to hemoglobin (Fig. 3). Hemoglobin in RBCs has interchangeable structural forms that enable the uptake of oxygen and release of carbon dioxide in the lungs and the release of oxygen and uptake of carbon dioxide in the tissues (reviewed extensively in [81]). Deoxyhemoglobin is the tense conformation – as oxygen binds to one heme group, the structure relaxes and affinity for oxygen increases, enabling oxygen molecules to rapidly bind the remaining three heme groups (oxyhemoglobin) [28,81]. MetHb is formed through the oxidation of one or more heme iron atoms within deoxyhemoglobin from a ferrous to the ferric state (Fe2+ to Fe3+) by compounds such as H2O2 and O2− [26-30].
To prevent the formation of MetHb, RBCs have several different mechanisms that work either by reducing ROS in the cell, to prevent MetHb formation (including mechanisms described in the oxidative stress regulatory pathway; Fig. 2), or by reverting the ferric iron back to a ferrous state by reduction [26,27,29]. The main RBC enzyme that reduces MetHb in vivo is the soluble form of cytochrome b5 reductase (CYB5R3, also known as NADH-dependent MetHb reductase or diaphorase-1), utilizing the electron donor NADH to reduce cytochrome b5 (CYB5A) which can then in turn reduce MetHb [26-28,30,82]. More than 40 genetic variants within the CYB5R3 gene have been associated with recessive congenital methemoglobinemia [82]. Extracellular NADH in the presence of lactose dehydrogenase can enhance the rate of MetHb reduction in human erythrocytes [83].
A second enzyme, flavin reductase (NADPH) [biliverdin reductase B (BLVRB), NADPH-MetHb reductase, NADPH-MetHb-diaphorase] undertakes around 5% of this activity in RBCs under normal conditions, requiring NADPH and an electron acceptor cofactor [26,27,30,84]. Riboflavin, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) can all act as electron acceptors to then reduce MetHb [85-87].
Pharmacodynamics of methylene blue
Another such cofactor for BLVRB that greatly accelerates the reduction of MetHb is methylene blue [26,27,30]. Methylene blue is reduced to leukomethylene blue by BLVRB, accepting electrons from NADPH [27]. Leukomethylene blue acts as an electron donor to reduce MetHb to hemoglobin, converting back to methylene blue in a cyclical redox reaction [27,30]. Conversely, because methylene blue is an oxidizing agent, at high concentrations it can cause methemoglobinemia by oxidizing hemoglobin [27,30]. The efficacy of methylene blue treatment can be affected by numerous factors, for example an aniline intermediate may block RBC uptake of methylene blue [88].
Pharmacogenetics – G6PD deficiency
Early in-vitro studies demonstrate that the rate of MetHb reduction in G6PD-deficient RBCs incubated with methylene blue and glucose is severely reduced compared to normal RBCs [89]. However, this can be increased by incubating G6PD-deficient and ‘normal’ RBCs together. However, this can be increased by incubating G6PD deficient and ‘normal’ RBCs together-possibly by the diffusion of leukomethylene blue into G6PD-deficient RBCs [89]. Due to dependency on NADPH, methylene blue treatment is often ineffective at ameliorating methemoglobinemia in G6PD-deficient patients [33-35,37,39,40], and may exacerbate the condition and/or induce hemolysis in individuals with G6PD deficiency (Table 1) [14,26,31-33,35-38,40,41]. In fact, failure of methylene blue to reduce MetHb was developed as a diagnostic test for G6PD deficiency [90,91].
Is methylene blue safe in G6PD-deficient individuals?
A recent evidence-based review concluded that methylene blue treatment should be avoided in patients with G6PD deficiency due to a risk of hemolysis [47], backed by other reviews [46,62,92], the Italian G6PD Deficiency Association [93], and drug labels [1-3]. Alternative treatments for methemoglobinemia include vitamin C, an electron donor that can reduce ROS and therefore inhibit the production of MetHb (Fig. 2) [13,26,33,94]. However, methylene blue is a more potent and rapid reducer of MetHb than other options, such as riboflavin [95], vitamin C treatment is not always effective [35,37,40], and several NADPH-dependent mechanisms are required for the recycling of oxidized vitamin C [71,74,75].
Several studies show no association between risk of methylene blue-induced hemolysis and G6PD deficiency. No cases of severe hemolysis were observed in 24 children with G6PD deficiency in a study to treat uncomplicated malarial infection with methylene blue and chloroquine, a drug combination also found to be safe in 74 healthy G6PD-deficient men [96,97]. Both studies were carried out in an area of West Africa where G6PD class III variants are common [96-98].
It has therefore been debated whether methylene blue is safe to use in G6PD-deficient individuals or not. The answer may depend on the type of deficiency, as WHO class III G6PD variants are considered to confer less severe deficiency compared to class I or II [50,53,54,96,97,99]. However, one argument against using methylene blue in this patient population is that testing for G6PD deficiency is still based on enzyme activity in the majority of cases around the world, rather than genotyping or characterization of the underlying variant, and so methylene blue should be administered with extreme caution to those with known G6PD deficiency [48,99 (Author reply)]. Of the case studies identified in this extensive literature review (Table 1), only two publications describe the characterization of the G6PD electrophoretic variant (A –), and genotyping of the specific underlying genetic variants is not always reported (see G6PD A – haplotypes http://www.pharmgkb.org/gene/PA28469?tabType=tabHaplotypes) [33,43]. Another argument is that despite previous perceptions, G6PD A– should not clinically be regarded as ‘mild’ as patients are still at risk of life-threatening acute hemolytic anemia when challenged with a potent agent [100], and the distinction between WHO class II and III G6PD that variants is no longer clinically useful [101]. Illustrating this, a recent combined analysis of four randomized controlled trials of methylene blue treatment observed significant but small reductions in hemoglobin levels in hemi/homozygous children with class III G6PD A– compared to wildtype or heterozygous children (Table 1) [43]. This effect was described as being of limited clinical consequence (with only two children of 844 displaying severe anemia: one heterozygous and one hemizygous), though monitoring of adverse hematological events in G6PD-deficient patients was advised [43].
As it is possible that leukomethylene blue diffuses into G6PD-deficient RBCs [89], heterozygous females may be less at risk for methylene blue-induced hemolysis, though to our knowledge this mechanism has yet to be examined.
Other genetic factors may contribute to risk – for example, individuals with cytochrome b5 reductase, catalase, or GSH synthetase deficiency may be at a higher risk of drug-induced methemoglobinemia and hemolytic anemia [40,102-107]. Newborns are particularly susceptible to methemoglobinemia due to low cytochrome b5 reductase levels, reduced catalase and glutathione peroxidase activity [27,28,108]. Similar to G6PD, hexokinase is able to enhance the rate of the PPP under conditions of oxidative stress (e.g. treatment with methylene blue), as demonstrated by a lack of enhanced PPP rate in cells when hexokinase is inhibited [109], and patients with hexokinase deficiency exhibit nonspherocytic hemolytic anemia [110]. BLVRB deficiency, a rare or potentially asymptomatic condition as indicated by a lack of literature, was described in an individual who had sufficient G6PD activity but an abnormal methylene blue screen test [111]. Due to role of BLVRB in the pharmacodynamics of methylene blue, methylene blue treatment may be unsuccessful in BLVRB-deficient patients, as reflected in European Union drug labeling, which contraindicates the use of the drug in these individuals [2,3,112,113].
Disease context may also play a role – patients with methemoglobinemia are already displaying clinical signs of oxidative stress within their RBCs and therefore may be at a higher risk for hemolysis. As an oxidizing agent, the dosage of methylene blue administered should also be considered [27,30]. Other factors, for example, baseline hemoglobin levels in patients with malarial that correlate with parasitemia levels, may also affect risk [43].
Does methylene blue cause hemolysis in G6PD-deficient individuals, or is it the agent that initiated methemoglobinemia?
Several of the same agents listed that can trigger acquired methemoglobinemia are also known to cause hemolytic anemia in G6PD-deficient individuals, for example, primaquine, acetanilide, and toluidine [27,46]. It is therefore difficult to pinpoint whether methylene blue is the cause of hemolysis in G6PD-deficient individuals being treated for acquired methemoglobinemia, rather than the precursor agent, though methylene blue is listed both as an agent that can cause hemolytic anemia in G6PD-deficient individuals and as an agent that can cause acquired methemoglobinemia [27,46]. In one study from our literature review, the development of methemoglobinemia in a G6PD-deficient patient was associated with methylene blue (Table 1) [13].
Are G6PD-deficient individuals also more susceptible to methemoglobinemia risk?
G6PD-deficient individuals may be more susceptible to acquired methemoglobinemia, though this direct association is unclear in the published literature. The development of methemoglobinemia has been linked to G6PD deficiency [114]. Rasburicase (contraindicated in G6PD-deficient patients) has been associated with both methemoglobinemia and hemolytic anemia in several reported cases of patients with G6PD deficiency [38,39,56,115-118]. Deficiency in G6PD may contribute to exacerbation of acquired methemoglobinemia in several ways via a decrease in available NADPH in RBCs, as described above, though the NADH-dependent cytochrome b5 reductase pathway dominates reduction of MetHb [26,27].
Conclusion
Methylene blue can be used for the treatment of methemoglobinemia, a condition triggered by oxidative stress in RBCs. However, the mechanism of action of methylene blue is dependent on the intracellular capacity for NADP/NADPH recycling, and as the PPP is the only source of NADPH in RBCs, methylene blue treatment to reduce MetHb relies on G6PD enzyme activity. G6PD deficiency is found in around 5% of the world’s population, with more than 180 genetic variants described that confer varying degrees of enzyme deficiency [48,52]. Methylene blue is therefore an unsuitable treatment option for methemoglobinemia in G6PD-deficient individuals, and has been associated with hemolysis likely caused by the exacerbation of oxidative stress in RBCs deficient in the G6PD enzyme.
Acknowledgments
This work is supported by the NIH/NIGMS (R24 GM61374) and the Spanish Ministry of Science and Innovation (BIO2010-17039). The authors thank Caroline F. Thorn and Julia Barbarino for critical reading of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.General Injectables & Vaccines Inc. [31 July 2012];Methylene blue injection, solution drug label. Available at: http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=04cea07f-f3f0-4c45-9f56-67dc8ebe7ddf.
- 2.European Medicines Agency. [7 February 2013];Methylthioninium chloride Proveblue page. 2012 Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002108/human_med_001444.jsp&mid =WC0b01ac058001d124.
- 3. [7 February 2013];PharmGKB methylene blue drug labels. Available at: http://www.pharmgkb.org/drug/PA450457?tabType=tabDrugLabels.
- 4.Horobin RW. How Romanowsky stains work and why they remain valuable – including a proposed universal Romanowsky staining mechanism and a rational troubleshooting scheme. Biotech Histochem. 2011;86:36–51. doi: 10.3109/10520295.2010.515491. [DOI] [PubMed] [Google Scholar]
- 5.Wainwright M. The use of dyes in modern biomedicine. Biotech Histochem. 2003;78:147–155. doi: 10.1080/10520290310001602404. [DOI] [PubMed] [Google Scholar]
- 6.Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you – methylene bluey…. Neurobiol Aging. 2011;32:2325.e7–2325.e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Krafts K, Hempelmann E, Skorska-Stania A. From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitol Res. 2012;111:1–6. doi: 10.1007/s00436-012-2886-x. [DOI] [PubMed] [Google Scholar]
- 8.Schirmer RH, Coulibaly B, Stich A, Scheiwein M, Merkle H, Eubel J, et al. Methylene blue as an antimalarial agent. Redox Rep. 2003;8:272–275. doi: 10.1179/135100003225002899. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright M, Amaral L. The phenothiazinium chromophore and the evolution of antimalarial drugs. Trop Med Int Health. 2005;10:501–511. doi: 10.1111/j.1365-3156.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 10.Sweiss KI, Beri R, Shord SS. Encephalopathy after high-dose Ifosfamide: a retrospective cohort study and review of the literature. Drug Saf. 2008;31:989–996. doi: 10.2165/00002018-200831110-00003. [DOI] [PubMed] [Google Scholar]
- 11.Tajino T, Kikuchi S, Yamada H, Takeda A, Konno S. Ifosfamide encephalopathy associated with chemotherapy for musculoskeletal sarcomas: incidence, severity, and risk factors. J Orthop Sci. 2010;15:104–111. doi: 10.1007/s00776-009-1431-y. [DOI] [PubMed] [Google Scholar]
- 12.Alici-Evcimen Y, Breitbart WS. Ifosfamide neuropsychiatric toxicity in patients with cancer. Psychooncology. 2007;16:956–960. doi: 10.1002/pon.1161. [DOI] [PubMed] [Google Scholar]
- 13.Bilgin H, Ozcan B, Bilgin T. Methemoglobinemia induced by methylene blue pertubation during laparoscopy. Acta Anaesthesiol Scand. 1998;42:594–595. doi: 10.1111/j.1399-6576.1998.tb05173.x. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier TW. Methylene blue-induced hyperbilirubinemia in neonatal glucose-6-phosphate dehydrogenase (G6PD) deficiency. J Matern Fetal Med. 2000;9:252–254. doi: 10.1002/1520-6661(200007/08)9:4<252::AID-MFM14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 16.Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010;30:702–715. doi: 10.1592/phco.30.7.702. [DOI] [PubMed] [Google Scholar]
- 17.Donati A, Adrario E, Pelaia P, Preiser JC. Methylene blue as the future protecting agent for ischemic brain injury? Crit Care Med. 2010;38:2265–2266. doi: 10.1097/CCM.0b013e3181f7d8f7. [DOI] [PubMed] [Google Scholar]
- 18.Moldenhauer Brooks M. Methylene blue as an antidote for cyanide and carbon monoxide poisoning. Sci Mon. 1936;43:585–586. [Google Scholar]
- 19.Gravitz L. Drugs: a tangled web of targets. Nature. 2011;475:S9–S11. doi: 10.1038/475S9a. [DOI] [PubMed] [Google Scholar]
- 20.Medina DX, Caccamo A, Oddo S. Methylene blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salah M, Samy N, Fadel M. Methylene blue mediated photodynamic therapy for resistant plaque psoriasis. J Drugs Dermatol. 2009;8:42–49. [PubMed] [Google Scholar]
- 22.Papin JF, Floyd RA, Dittmer DP. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antiviral Res. 2005;68:84–87. doi: 10.1016/j.antiviral.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Tardivo JP, Del Giglio A, Paschoal LH, Baptista MS. New photodynamic therapy protocol to treat AIDS-related Kaposi’s sarcoma. Photomed Laser Surg. 2006;24:528–531. doi: 10.1089/pho.2006.24.528. [DOI] [PubMed] [Google Scholar]
- 24.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wainwright M. Photodynamic medicine and infection control. J Antimicrob Chemother. 2012;67:787–788. doi: 10.1093/jac/dkr562. [DOI] [PubMed] [Google Scholar]
- 26.Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. 2011;104:757–761. doi: 10.1097/SMJ.0b013e318232139f. [DOI] [PubMed] [Google Scholar]
- 27.Curry S. Methemoglobinemia. Ann Emerg Med. 1982;11:214–221. doi: 10.1016/s0196-0644(82)80502-7. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol. 1993;42:7–12. doi: 10.1002/ajh.2830420104. [DOI] [PubMed] [Google Scholar]
- 29.Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23:169–188. doi: 10.2165/00139709-200423030-00004. [DOI] [PubMed] [Google Scholar]
- 30.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 31.Foltz LM, Dalal BI, Wadsworth LD, Broady R, Chi K, Eisenhauer E, et al. Recognition and management of methemoglobinemia and hemolysis in a G6PD-deficient patient on experimental anticancer drug Triapine. Am J Hematol. 2006;81:210–211. doi: 10.1002/ajh.20547. [DOI] [PubMed] [Google Scholar]
- 32.Dalal BI, Kollmannsberger C. Drug-induced haemolysis and methaemoglobinaemia in glucose 6-phosphate dehydrogenase deficiency. Br J Haematol. 2005;129:291. doi: 10.1111/j.1365-2141.2005.05404.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosen PJ, Johnson C, McGehee WG, Beutler E. Failure of methylene blue treatment in toxic methemoglobinemia. Association with glucose-6-phosphate dehydrogenase deficiency. Ann Intern Med. 1971;75:83–86. doi: 10.7326/0003-4819-75-1-83. [DOI] [PubMed] [Google Scholar]
- 34.Golden PJ, Weinstein R. Treatment of high-risk, refractory acquired methemoglobinemia with automated red blood cell exchange. J Clin Apher. 1998;13:28–31. doi: 10.1002/(sici)1098-1101(1998)13:1<28::aid-jca6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Mullick P, Kumar A, Dayal M, Babbar S. Aniline-induced methaemoglobinaemia in a glucose-6-phosphate dehydrogenase enzyme deficient patient. Anaesth Intensive Care. 2007;35:286–288. doi: 10.1177/0310057X0703500222. [DOI] [PubMed] [Google Scholar]
- 36.Maddali MM, Fahr J. Postoperative methemoglobinemia with associated G-6-P-D deficiency in infant cardiac surgery – enigmas in diagnosis and management. Paediatr Anaesth. 2005;15:334–337. doi: 10.1111/j.1460-9592.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- 37.Yang CC, Wu ML, Deng JF. Prolonged hemolysis and methemoglobinemia following organic copper fungicide ingestion. Vet Hum Toxicol. 2004;46:321–323. [PubMed] [Google Scholar]
- 38.Vadhan-Raj S, Fayad LE, Fanale MA, Pro B, Rodriguez A, Hagemeister FB, et al. A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome. Ann Oncol. 2012;23:1640–1645. doi: 10.1093/annonc/mdr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhat P, Sisler I, Collier AB., 3rd Exchange transfusion as treatment for rasburicase induced methemoglobinemia in a glucose-6-phosphate dehydrogenase deficient patient. Pediatr Blood Cancer. 2008;51:568. doi: 10.1002/pbc.21582. [DOI] [PubMed] [Google Scholar]
- 40.Karadsheh NS, Shaker Q, Ratroat B. Metoclopramide-induced methemoglobinemia in a patient with co-existing deficiency of glucose-6- phosphate dehydrogenase and NADH-cytochrome b5 reductase: failure of methylene blue treatment. Haematologica. 2001;86:659–660. [PubMed] [Google Scholar]
- 41.Liao YP, Hung DZ, Yang DY. Hemolytic anemia after methylene blue therapy for aniline-induced methemoglobinemia. Vet Hum Toxicol. 2002;44:19–21. [PubMed] [Google Scholar]
- 42.Meissner PE, Mandi G, Coulibaly B, Witte S, Tapsoba T, Mansmann U, et al. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006;5:84. doi: 10.1186/1475-2875-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller OMF, Marks B, Meissner P, Coulibaly B, Kuhnert R, Buchner H, et al. Haemolysis risk in methylene blue treatment of G6PD-sufficient and G6PD-deficient West-African children with uncomplicated falciparum malaria: a synopsis of four RCTs. Pharmacoepidemiol Drug Saf. 2012;22:376–385. doi: 10.1002/pds.3370. [DOI] [PubMed] [Google Scholar]
- 44.Campbell GD, Jr, Steinberg MH, Bower JD. Letter: ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann Intern Med. 1975;82:810. doi: 10.7326/0003-4819-82-6-810_1. [DOI] [PubMed] [Google Scholar]
- 45.Mehta JB, Singhal SB, Mehta BC. Ascorbic-acid-induced haemolysis in G-6-PD deficiency. Lancet. 1990;336:944. doi: 10.1016/0140-6736(90)92317-b. [DOI] [PubMed] [Google Scholar]
- 46.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 47.Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, et al. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 2010;33:713–726. doi: 10.2165/11536520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42:267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Gaetani GD, Parker JC, Kirkman HN. Intracellular restraint: a new basis for the limitation in response to oxidative stress in human erythrocytes containing low-activity variants of glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci USA. 1974;71:3584–3587. doi: 10.1073/pnas.71.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bull World Health Organ. 1989;67:601–611. [PMC free article] [PubMed] [Google Scholar]
- 51.McDonagh EM, Thorn CF, Bautista JM, Youngster I, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for G6PD. Pharmacogenet Genomics. 2012;22:219–228. doi: 10.1097/FPC.0b013e32834eb313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the “old” and update of the new mutations. Blood Cells Mol Dis. 2012;48:154–165. doi: 10.1016/j.bcmd.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 53.The World Health Organization Scientific Group on Standardization of Procedures for the Study of Glucose-6-Phosphate Dehydrogenase. Nomenclature of glucose-6-phosphate dehydrogenase in man. Am J Hum Genet. 1967;19:757–761. [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida A, Beutler E, Motulsky AG. Human glucose-6-phosphate dehydrogenase variants. Bull World Health Organ. 1971;45:243–253. [PMC free article] [PubMed] [Google Scholar]
- 55.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 56.Browning LA, Kruse JA. Hemolysis and methemoglobinemia secondary to rasburicase administration. Ann Pharmacother. 2005;39:1932–1935. doi: 10.1345/aph.1G272. [DOI] [PubMed] [Google Scholar]
- 57.Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011;17:395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant CM. Metabolic reconfiguration is a regulated response to oxidative stress. J Biol. 2008;7:1. doi: 10.1186/jbiol63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinoshita A, Nakayama Y, Kitayama T, Tomita M. Simulation study of methemoglobin reduction in erythrocytes. Differential contributions of two pathways to tolerance to oxidative stress. FEBS J. 2007;274:1449–1458. doi: 10.1111/j.1742-4658.2007.05685.x. [DOI] [PubMed] [Google Scholar]
- 60.Bernstein RE. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruzzo A, Andreoni F, Magnani M. Structure of the human hexokinase type I gene and nucleotide sequence of the 5′ flanking region. Biochem J. 1998;331(Pt 2):607–613. doi: 10.1042/bj3310607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manganelli G, Fico A, Martini G, Filosa S. Discussion on pharmacogenetic interaction in G6PD deficiency and methods to identify potential hemolytic drugs. Cardiovasc Hematol Disord Drug Targets. 2010;10:143–150. doi: 10.2174/187152910791292547. [DOI] [PubMed] [Google Scholar]
- 63.Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenwalt TJ. The Ernest Witebsky memorial lecture. Red but not dead: not a hapless sac of hemoglobin. Immunol Invest. 1995;24:3–21. doi: 10.3109/08820139509062760. [DOI] [PubMed] [Google Scholar]
- 65.Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brewer GJ, Powell RD. Hexokinase activity as a function of age of the human erythrocyte. Nature. 1963;199:704–705. doi: 10.1038/199704a0. [DOI] [PubMed] [Google Scholar]
- 67.Navolanic PM, Pui CH, Larson RA, Bishop MR, Pearce TE, Cairo MS, et al. Elitek-rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a Meeting Report, Dallas, Texas, January 2002. Leukemia. 2003;17:499–514. doi: 10.1038/sj.leu.2402847. [DOI] [PubMed] [Google Scholar]
- 68.Oldfield V, Perry CM. Rasburicase: a review of its use in the management of anticancer therapy-induced hyperuricemia. Drugs. 2006;66:529–545. doi: 10.2165/00003495-200666040-00008. [DOI] [PubMed] [Google Scholar]
- 69.Ralser M, Wamelink MM, Kowald A, Gerisch B, Heeren G, Struys EA, et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J Biol. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotypephenotype association. Blood Rev. 2007;21:267–283. doi: 10.1016/j.blre.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- 72.Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 73.Han YH, Kim SU, Kwon TH, Lee DS, Ha HL, Park DS, et al. Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem Biophys Res Commun. 2012;426:427–432. doi: 10.1016/j.bbrc.2012.08.113. [DOI] [PubMed] [Google Scholar]
- 74.Mendiratta S, Qu ZC, May JM. Enzyme-dependent ascorbate recycling in human erythrocytes: role of thioredoxin reductase. Free Radic Biol Med. 1998;25:221–228. doi: 10.1016/s0891-5849(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 75.May JM. Ascorbate function and metabolism in the human erythrocyte. Front Biosci. 1998;3:d1–d10. doi: 10.2741/a262. [DOI] [PubMed] [Google Scholar]
- 76.May JM, Qu Z, Morrow JD. Mechanisms of ascorbic acid recycling in human erythrocytes. Biochim Biophys Acta. 2001;1528:159–166. doi: 10.1016/s0304-4165(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 77.Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Alfonso-Prieto M, Vidossich P, Rovira C. The reaction mechanisms of heme catalases: an atomistic view by ab initio molecular dynamics. Arch Biochem Biophys. 2012;525:121–130. doi: 10.1016/j.abb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Kirkman HN, Galiano S, Gaetani GF. The function of catalasebound NADPH. J Biol Chem. 1987;262:660–666. [PubMed] [Google Scholar]
- 80.Gaetani GF, Rolfo M, Arena S, Mangerini R, Meloni GF, Ferraris AM. Active involvement of catalase during hemolytic crises of favism. Blood. 1996;88:1084–1088. [PubMed] [Google Scholar]
- 81.Hsia CC. Respiratory function of hemoglobin. N Engl J Med. 1998;338:239–247. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 82.Percy MJ, Lappin TR. Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. Br J Haematol. 2008;141:298–308. doi: 10.1111/j.1365-2141.2008.07017.x. [DOI] [PubMed] [Google Scholar]
- 83.Kennett EC, Ogawa E, Agar NS, Godwin IR, Bubb WA, Kuchel PW. Investigation of methaemoglobin reduction by extracellular NADH in mammalian erythrocytes. Int J Biochem Cell Biol. 2005;37:1438–1445. doi: 10.1016/j.biocel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Shalloe F, Elliott G, Ennis O, Mantle TJ. Evidence that biliverdin-IX beta reductase and flavin reductase are identical. Biochem J. 1996;316(Pt 2):385–387. doi: 10.1042/bj3160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yubisui T, Matsuki T, Tanishima K, Takeshita M, Yoneyama Y. NADPH-flavin reductase in human erythrocytes and the reduction of methemoglobin through flavin by the enzyme. Biochem Biophys Res Commun. 1977;76:174–182. doi: 10.1016/0006-291x(77)91683-7. [DOI] [PubMed] [Google Scholar]
- 86.Yubisui T, Takeshita M, Yoneyama Y. Reduction of methemoglobin through flavin at the physiological concentration by NADPH-flavin reductase of human erythrocytes. J Biochem. 1980;87:1715–1720. doi: 10.1093/oxfordjournals.jbchem.a132915. [DOI] [PubMed] [Google Scholar]
- 87.Matsuki T, Yubisui T, Tomoda A, Yoneyama Y, Takeshita M, Hirano M, et al. Acceleration of methaemoglobin reduction by riboflavin in human erythrocytes. Br J Haematol. 1978;39:523–528. doi: 10.1111/j.1365-2141.1978.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 88.Clifton J, 2nd, Leikin JB. Methylene blue. Am J Ther. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Beutler E, Baluda MC. Methemoglobin reduction. Studies of the interaction between cell populations and of the role of methylene blue. Blood. 1963;22:323–333. [PubMed] [Google Scholar]
- 90.Brewer GJ, Tarlov AR, Alving AS. Methaemoglobin reduction test: a new, simple, in vitro test for identifying primaquine-sensitivity. Bull World Health Organ. 1960;22:633–640. [PMC free article] [PubMed] [Google Scholar]
- 91.Brewer GJ, Tarlov AR, Alving AS. The methemoglobin reduction test for primaquine-type sensitivity of erythrocytes. A simplified procedure for detecting a specific hypersusceptibility to drug hemolysis. JAMA. 1962;180:386–388. doi: 10.1001/jama.1962.03050180032008. [DOI] [PubMed] [Google Scholar]
- 92.Elyassi AR, Rowshan HH. Perioperative management of the glucose-6- phosphate dehydrogenase deficient patient: a review of literature. Anesth Prog. 2009;56:86–91. doi: 10.2344/0003-3006-56.3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.G6PD deficiency Favism Association. [7 February 2013];The Association is affiliated with UNIAMO, Italian Federation for Rare Diseases, which is a member of EURORDIS, European Organization for Rare Diseases. Available at: http://www.g6pd.org.
- 94.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 95.Dotsch J, Demirakca S, Kratz M, Repp R, Knerr I, Rascher W. Comparison of methylene blue, riboflavin, and N-acetylcysteine for the reduction of nitric oxide-induced methemoglobinemia. Crit Care Med. 2000;28:958–961. doi: 10.1097/00003246-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Meissner PE, Mandi G, Witte S, Coulibaly B, Mansmann U, Rengelshausen J, et al. Safety of the methylene blue plus chloroquine combination in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso [ISRCTN27290841] Malar J. 2005;4:45. doi: 10.1186/1475-2875-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mandi G, Witte S, Meissner P, Coulibaly B, Mansmann U, Rengelshausen J, et al. Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Trop Med Int Health. 2005;10:32–38. doi: 10.1111/j.1365-3156.2004.01356.x. [DOI] [PubMed] [Google Scholar]
- 98.Meissner PE, Coulibaly B, Mandi G, Mansmann U, Witte S, Schie W, et al. Diagnosis of red cell G6PD deficiency in rural Burkina Faso: comparison of a rapid fluorescent enzyme test on filter paper with polymerase chain reaction based genotyping. Br J Haematol. 2005;131:395–399. doi: 10.1111/j.1365-2141.2005.05778.x. [DOI] [PubMed] [Google Scholar]
- 99.Muller O, Meissner P, Mansmann U. Glucose-6-phosphate dehydrogenase deficiency and safety of methylene blue. Drug Saf. 2012;35:85. doi: 10.2165/11597790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Pamba ARN, Carter N, Duparc S, Premji Z, Tiono AB, Luzzatto L. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120:4123–4133. doi: 10.1182/blood-2012-03-416032. [DOI] [PubMed] [Google Scholar]
- 101.Luzzatto L, Poggi V. Glucose-6-phosphate dehydrogenase deficiency. In: Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE, editors. Nathan and Oski’s hematology of infancy and childhood. 7. Philadelphia, PA: Saunders, Elsevier; 2009. [Google Scholar]
- 102.Sannes LJ, Hultquist DE. Effects of hemolysate concentration, ionic strength and cytochrome b5 concentration on the rate of methemoglobin reduction in hemolysates of human erythrocytes. Biochim Biophys Acta. 1978;544:547–554. doi: 10.1016/0304-4165(78)90329-x. [DOI] [PubMed] [Google Scholar]
- 103.Ganer A, Knobel B, Fryd CH, Rachmilewitz EA. Dapsone-induced methemoglobinemia and hemolysis in the presence of familial hemoglobinopathy Hasharon and familial methemoglobin reductase deficiency. Isr J Med Sci. 1981;17:703–704. [PubMed] [Google Scholar]
- 104.Ogata M. Acatalasemia. Hum Genet. 1991;86:331–340. doi: 10.1007/BF00201829. [DOI] [PubMed] [Google Scholar]
- 105.Daly JS, Hultquist DE, Rucknagel DL. Phenazopyridine induced methaemoglobinaemia associated with decreased activity of erythrocyte cytochrome b5 reductase. J Med Genet. 1983;20:307–309. doi: 10.1136/jmg.20.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen RJ, Sachs JR, Wicker DJ, Conrad ME. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N Engl J Med. 1968;279:1127–1131. doi: 10.1056/NEJM196811212792102. [DOI] [PubMed] [Google Scholar]
- 107.Corrons JL, Alvarez R, Pujades A, Zarza R, Oliva E, Lasheras G, et al. Hereditary non-spherocytic haemolytic anaemia due to red blood cell glutathione synthetase deficiency in four unrelated patients from Spain: clinical and molecular studies. Br J Haematol. 2001;112:475–482. doi: 10.1046/j.1365-2141.2001.02526.x. [DOI] [PubMed] [Google Scholar]
- 108.Lo SC, Agar NS. NADH-methemoglobin reductase activity in the erythrocytes of newborn and adult mammals. Experientia. 1986;42:1264–1265. doi: 10.1007/BF01946415. [DOI] [PubMed] [Google Scholar]
- 109.Magnani M, Rossi L, Bianchi M, Serafini G, Stocchi V. Role of hexokinase in the regulation of erythrocyte hexose monophosphate pathway under oxidative stress. Biochem Biophys Res Commun. 1988;155:423–428. doi: 10.1016/s0006-291x(88)81103-3. [DOI] [PubMed] [Google Scholar]
- 110.McMullin MF. The molecular basis of disorders of red cell enzymes. J Clin Pathol. 1999;52:241–244. doi: 10.1136/jcp.52.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sass MD, Caruso CJ, Farhangi M. TPNH-methemoglobin reductase deficiency: a new red-cell enzyme defect. J Lab Clin Med. 1967;70:760–767. [PubMed] [Google Scholar]
- 112.Edwards RJ, Ujma J. Extreme methaemoglobinaemia secondary to recreational use of amyl nitrite. J Accid Emerg Med. 1995;12:138–142. doi: 10.1136/emj.12.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saha SA, Kordouni MR, Siddiqui M, Arora RR. Methemoglobinemia-induced cardio-respiratory failure secondary to topical anesthesia. Am J Ther. 2006;13:545–549. doi: 10.1097/01.mjt.0000208876.69103.c7. [DOI] [PubMed] [Google Scholar]
- 114.Moretti S, Jouvet P, Schleiermacher G, Hubert P, Doz F, Zucker JM, Cloup M. Pulse oximetry and methemoglobinemia. Arch Pediatr. 1996;3:258–260. doi: 10.1016/0929-693x(96)81305-4. [DOI] [PubMed] [Google Scholar]
- 115.Ng JS, Edwards EM, Egelund TA. Methemoglobinemia induced by rasburicase in a pediatric patient: a case report and literature review. J Oncol Pharm Pract. 2011;18:425–431. doi: 10.1177/1078155211429385. [DOI] [PubMed] [Google Scholar]
- 116.Sonbol MB, Yadav H, Vaidya R, Rana V, Witzig TE. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2012;88:152–154. doi: 10.1002/ajh.23182. [DOI] [PubMed] [Google Scholar]
- 117.Borinstein SC, Xu M, Hawkins DS. Methemoglobinemia and hemolytic anemia caused by rasburicase administration in a newly diagnosed child with Burkitt lymphoma/leukemia. Pediatr Blood Cancer. 2008;50:189. doi: 10.1002/pbc.21193. [DOI] [PubMed] [Google Scholar]
- 118.Bosly A, Sonet A, Pinkerton CR, McCowage G, Bron D, Sanz MA, Van den Berg H. Rasburicase (recombinant urate oxidase) for the management of hyperuricemia in patients with cancer: report of an international compassionate use study. Cancer. 2003;98:1048–1054. doi: 10.1002/cncr.11612. [DOI] [PubMed] [Google Scholar]


