Abstract
With over 500,000 coronary artery bypass grafts (CABG) performed annually in the United States alone, there is a significant clinical need for a small diameter tissue engineered vascular graft. A principle goal in tissue engineering is to develop materials and growth conditions that encourage appropriate re-cellularization and extracellular matrix formation in vivo. A particular challenge in vascular tissue engineering results from the inability of adult cells to produce elastin, as its expression is developmentally limited. We investigated factors to stimulate elastogenesis in vitro, and found that heparin treatment of adult human vascular smooth muscle cells promoted the formation of elastic fibers. This effect was heparin-specific, and dependent on cell density and growth state. We then applied this information to a silk-based construct, and found that immobilized heparin showed essentially identical biological effects to that of soluble heparin. These findings indicate that heparinized vascular grafts may promote elastin formation and regulate restenosis, in addition to heparin’s well-established antithrombotic properties. Given the increase in elastin mRNA level and the increase in extracellular elastin present, our data suggests that there may be multiple levels of elastin regulation that are mediated by heparin treatment.
Keywords: elastin, heparin, silk, vascular tissue engineering
1. Introduction
The high incidence of heart disease results in more than 500,000 coronary artery bypass grafts (CABGs) performed each year in the United States (AHA, 2007). In these procedures, a donor vessel is typically harvested from the patient’s internal mammary artery or saphenous vein to use for transplant. Donor vessels are often unsuitable for transplant due to advanced age or illness. Vascular conduits made of Dacron or expanded polytetrafluoroethylene (ePTFE) are available for large diameter (>6 mm) grafts; however, these materials fail at smaller diameters due to thrombosis (Harris and Seikaly, 2002). This unmet need drives research into alternative tissue engineered vascular grafts for small diameter vessels.
A key challenge in vascular conduit engineering is the development of robust elastic laminae throughout the new matrix material upon repopulation with native cells and their deposited extracellular matrix (Mitchell and Niklason, 2003). Elastic laminae are critical for proper functioning of elastic arteries, which include the major coronary vessels. In addition to their mechanical properties, elastic laminae biochemically regulate SMC proliferation and migration (Karnik et al., 2003). Elastic fiber biology has been studied in vitro using fetal and neonatal mammalian cells, which has shed significant light on the assembly process. Current data indicate that initially, fibrillin-1 fibers are assembled and deposited extracellularly. Tropoelastin monomers are then deposited onto the fibrillin, in a process likely organized by fibulin-5 and other elastin associated proteins. Tropoelastin is then crosslinked by lysyl oxidase and related family members to form mature elastin fibers (Kielty, 2006). Though this process has been extensively studied, two challenges to inducing exogenous elastin generation are presented. First, properly organized elastin is not produced from adult smooth muscle cells, as its expression is developmentally limited and ceases after childhood (Davis, 1993; El-Hallous et al., 2007). As adult SMC in culture do not produce elastin, attempts to study differences in molecular organization, mechanical properties, or even to optimize elastin production between the adult-originating cells and fetal cells have been largely unsuccessful. (Kelleher et al., 2004; Swee et al., 1995) Thus, developing and characterizing a system to robustly promote elastin formation from adult tissues becomes a necessary first step. The lack of elastin deposition by adult SMCs significantly impacts the field of tissue engineering, as any biodegradable scaffold would ideally be populated with ECM-depositing cells. As elastin is a critical protein for mechanical properties of blood vessels, its deposition from SMCs is highly desirable. Though some elastin is formed in response to injury, this is generally not properly organized (Arribas et al., 2008). Second, the complex nature of the elastic fiber (due to its many accessory proteins) may be partly responsible for the inability of tropoelastin over-expression alone to form mechanically robust fibers (Katsuta et al., 2008).
There have been several recent reports demonstrating direct biochemical interactions between heparin, tropoelastin and fibrillin (Cain et al., 2005; Tu and Weiss, 2008). Heparin is a glycosaminoglycan (GAG) composed of alternating glucosamine and uronic acid sugars, which are extensively sulfated to confer a high degree of negative charge (Mishra-Gorur K, 1999). Native heparin ranges from 8,000–20,000 daltons (approximately 30–70 sugar residues) in mass, whereas clinical grade low molecular weight heparin (LMW-heparin; trade names Enoxaparin, Lovenox) is about 3,000 daltons (approximately 10 sugar residues). Heparan sulfate (HS) is a close biochemical relative of heparin, however HS covalently attached to one of many protein cores to form a heparin sulfate proteoglycan (HSPG). Additionally, HS consists of a lower percentage of iduronic acid residues, and is less sulfated than heparin, resulting in a more positive overall charge. Physiologically, heparin is stored in mast cells and is also secreted by endothelial cells, thereby forming part of the blood vessel extracellular matrix (Lever and Page, 2002). Heparin has both anticoagulant properties and antiproliferative properties, though these properties are independent as previously demonstrated (Castellot et al., 1984). These properties, in addition to our finding that heparin induces elastogenesis, make heparin addition an attractive biochemical modification for silk vascular grafts.
Silk derived from Bombyx mori silkworm cocoons is a useful biomaterial for vascular tissue engineering. Following initial processing to remove immunogenic sericin proteins, the liquid silk can be molded into vessels of any diameter, with varied porosity (Lovett et al., 2008). Silk is also non-immunogenic, mechanically strong, biodegradable with controlled kinetics, and can be chemically modified (Altman GH, 2003).
We hypothesized, given the known anti-proliferative and anticoagulant activities and the elastogenic potential of heparin described herein, that heparin contained in silk films would increase organized elastic fibers from SMCs cultured on these films. This would provide a foundation for a silk-based heparin delivery system for vascular graft applications. Indeed, here we present evidence of regulated smooth muscle cell response to heparinized silk constructs, and also describe parameters for elastin expression from adult human SMCs in culture.
2. Results
2.1. Heparin treatment of cultured hAoSMC induces organized elastin fibers
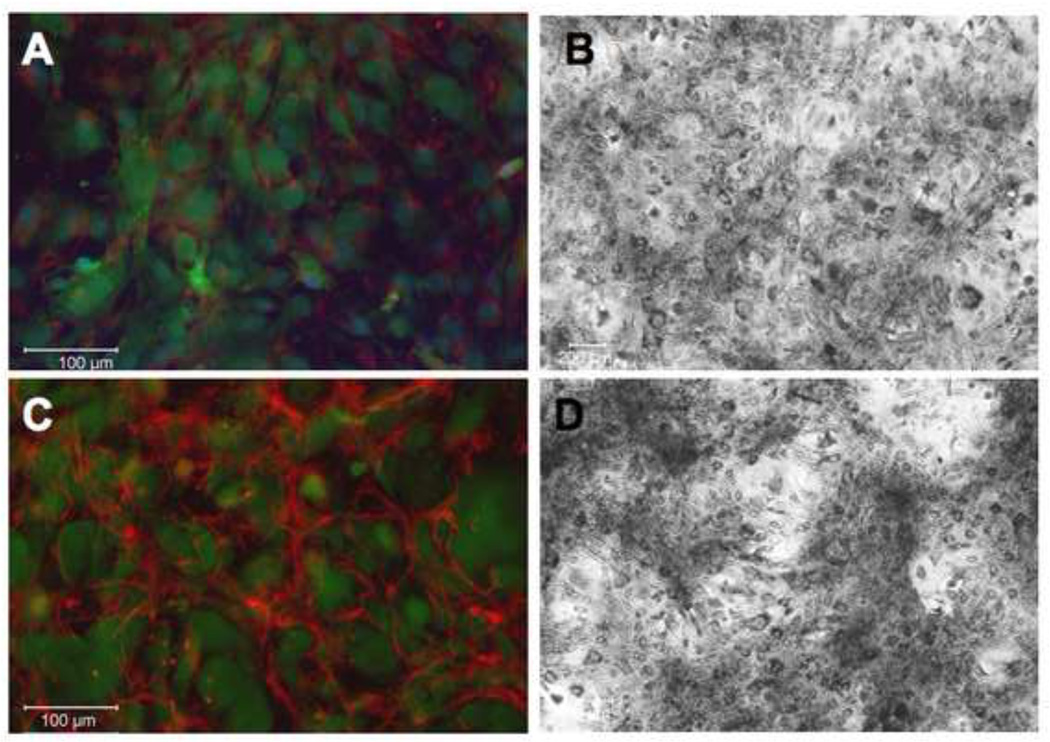
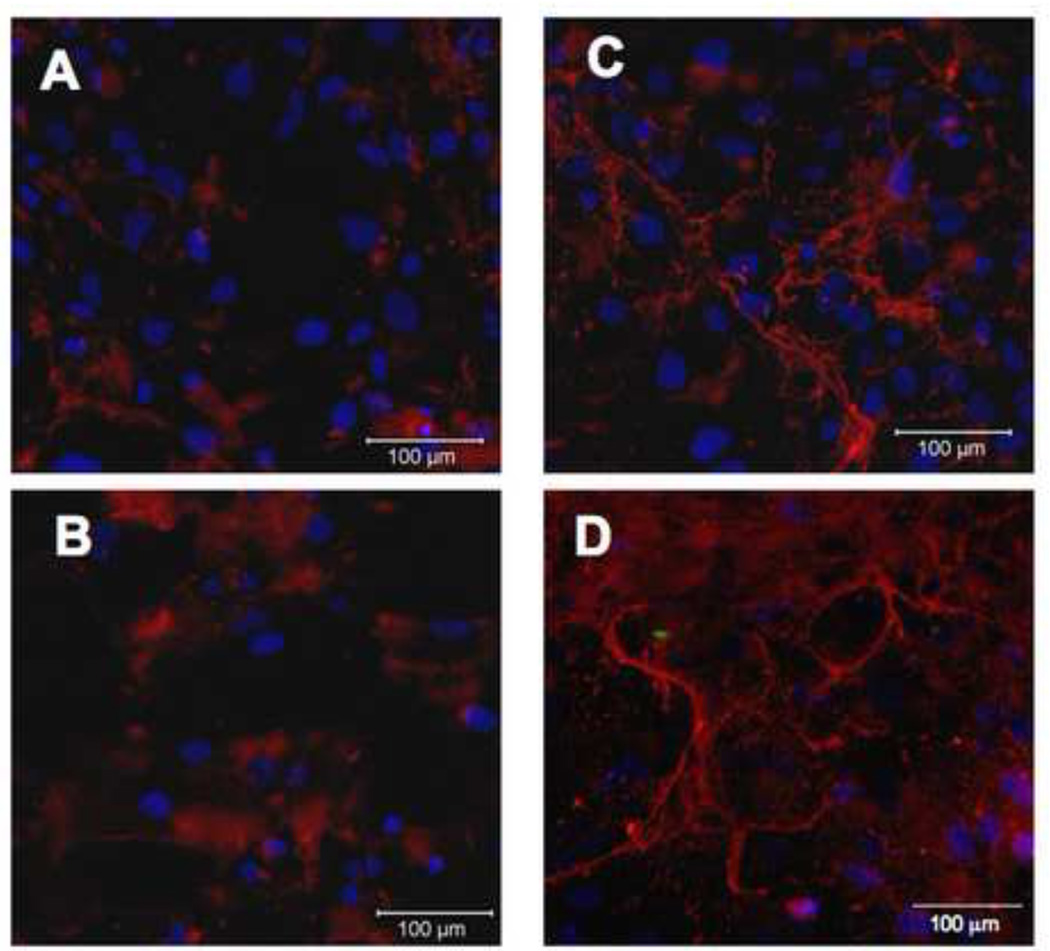
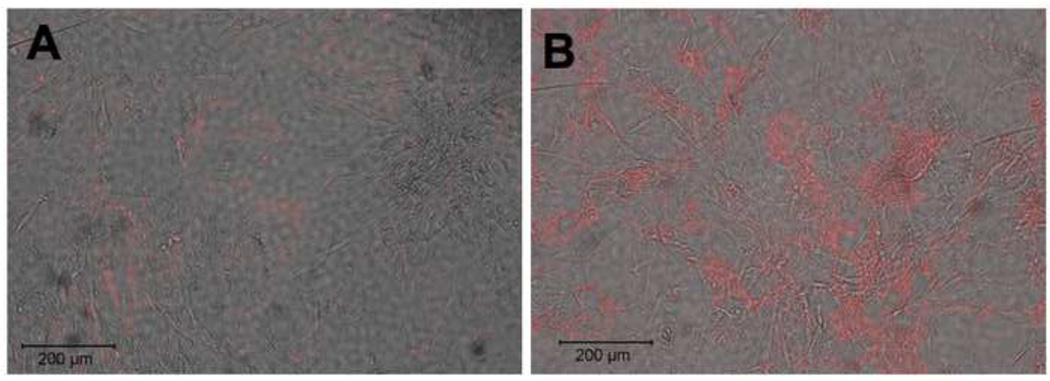
As heparin has been shown to bind tropoelastin monomers in vitro, we hypothesized that heparin may affect the regulation of elastic fiber formation or organization in vivo (Tu and Weiss, 2008, 2010). To address this hypothesis, we treated adult human aortic smooth muscle cells (hAoSMC) with 300 µg/ml heparin for 7 days. To determine if heparin increased extracellular elastin protein level and organization, we stained these samples using immunofluorescence on non-permeabilized cells (Figure 1). Little elastin is observed in untreated samples (1A, 1B), however an increase in organized elastin fibers was found upon heparin treatment (Figure 1C). The presented images use the Abcam antibody (see Methods), however nearly identical staining patterns were observed for all three antibodies tested. To confirm the identity of the fibers, we also stained these samples using a van Gieson-based elastin stain kit and observed an increase in extracellular elastin when treated with heparin (Figure 1D) relative to untreated control (Figure 1B).
Figure 1. Heparin treatment of primary hAoSMC shows organized elastin fibers.
Adult human aortic SMCs were grown in the absence (A,B) or presence (C,D) of 300 µg/ml heparin for 7 days, then fixed and processed for immunofluorescence microscopy. Non-permeabilized samples were labeled with polyclonal anti-elastin antibody (red), CellTracker Green for cell membrane, and DAPI (blue) for nuclear stain (A,C); or with van Gieson stain for elastin (B,D). Significant extracellular elastin fibers are shown in the heparin treated samples.
2.2. Elastogenesis depends on initial cell density and heparin concentration
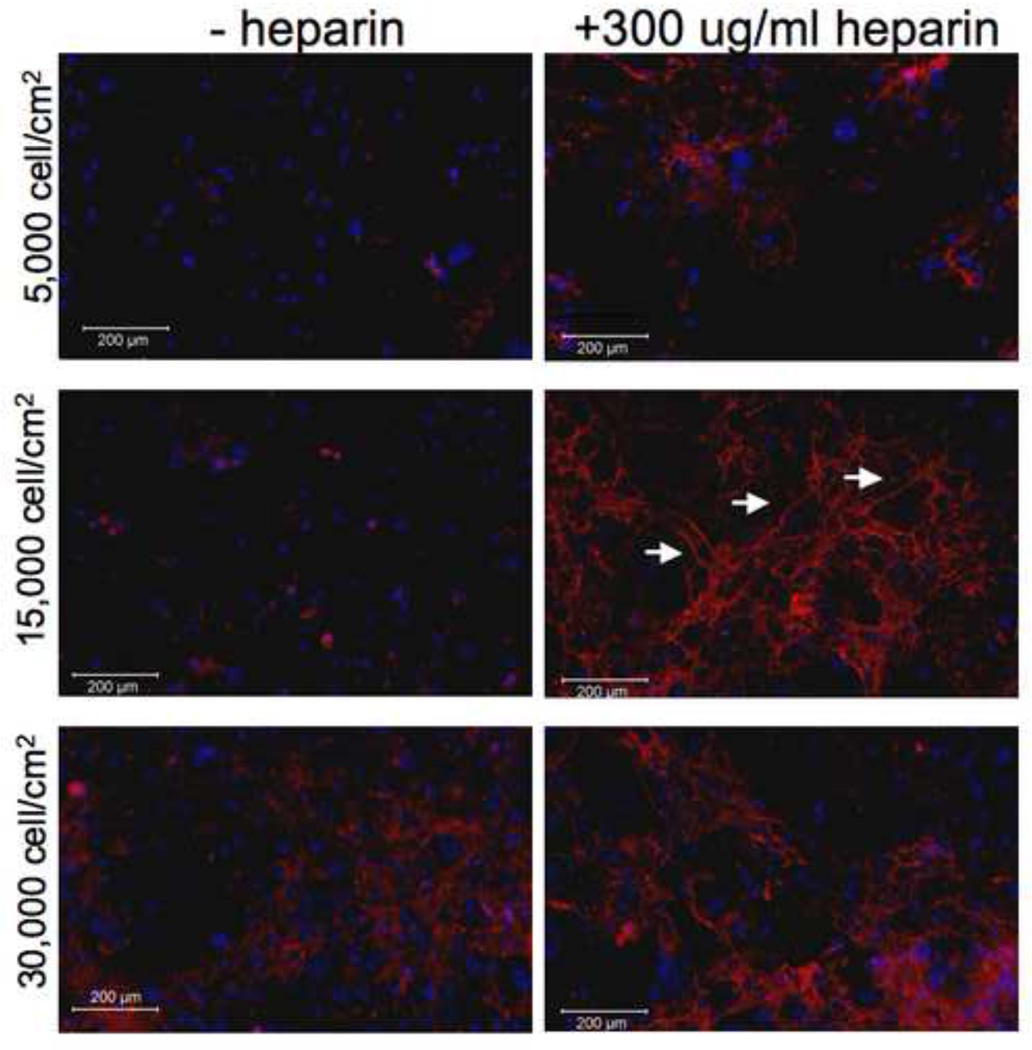
In these experiments, initial cell seeding density had a significant impact on elastogenesis. When cells were seeded at an initial density of 15,000 cells/cm2, elastic fibers became visible by 3 days in culture, and became more numerous and interconnected by day 7 (Figure 2). However, cells seeded at the lower initial density of 5,000 cells/cm2 developed far fewer and less extensive elastic fibers after 7 days in culture. Interestingly, cells seeded at the highest density tested, 30,000 cells/cm2, also developed fewer and less extensive elastic fibers than those at 15,000 cells/cm2. Despite the fact that each of the different seeding densities became confluent by 3 days in culture, the cell-cell interaction at the time of initial heparin treatment may determine extent of elastogenesis. Thus, all subsequent experiments were carried out at a seeding density of 15,000 cells/cm2.
Figure 2. Initial cell density is critical for heparin-induced elastic fiber formation.
SMCs were plated at 5,000, 15,000 and 30,000 cells/cm2 on day 0. Media was changed on day 1, with or without 300 µg/ml heparin. Cells were grown for 7 days, fixed and processed by immunofluorescence using an anti-elastin primary antibody and Cy3-labeled secondary antibody. Elastin fibers (white arrows) are more prevalent in the 15,000 cells/cm2 condition, while minimal in cultures plated more and less densely, despite complete confluence of all cultures at the time of fixation.
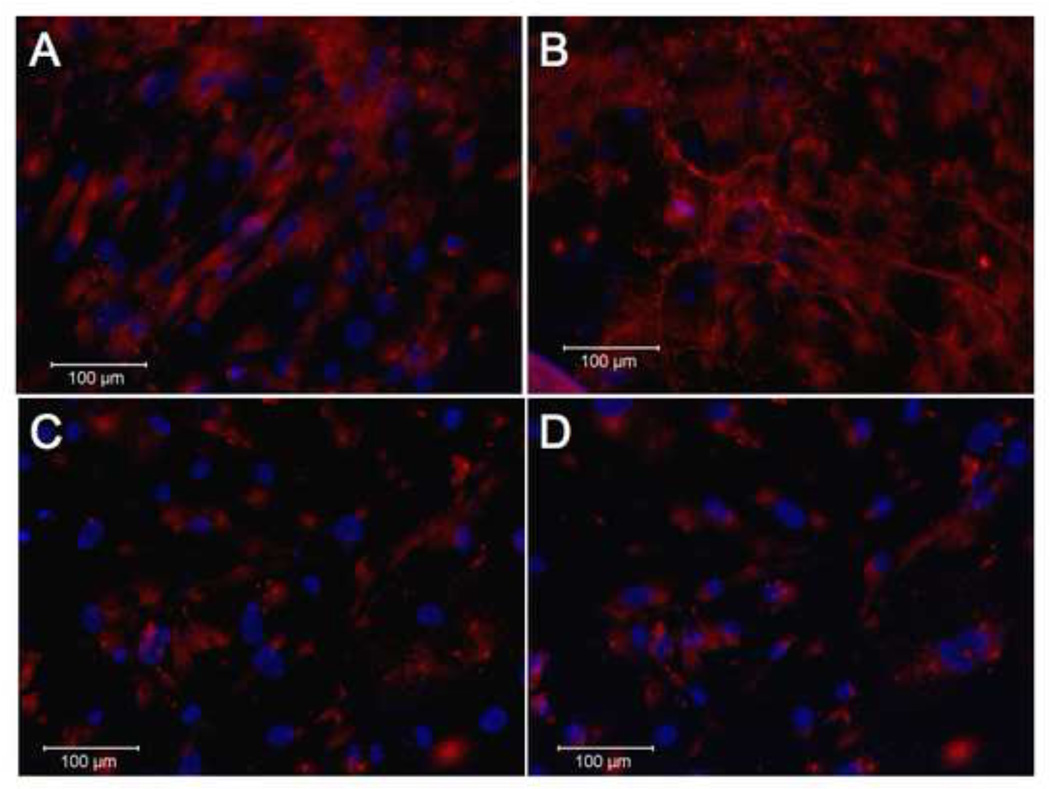
Smooth muscle cells undergo significant changes in phenotype as a result of growth conditions, including cell culture density and serum concentration – both of which can lead to growth arrest. Heparin has also been shown to induce and maintain growth arrest in smooth muscle cells, and there is some evidence indicating an increase in elastin deposition when embryonic chicken SMCs are in a proliferative phase (Castellot JJ Jr, 1981; Herman IM, 1987; Wachi et al., 1995). Thus, we hypothesized that the elastogenic properties of heparin as shown in Figure 1 may be dependent on growth state. When SMCs were grown in media with 10% serum, they responded to heparin by developing elastin fibers (Figure 3). However, cells cultured in 0.4% serum, which induces growth arrest, did not produce elastin fibers in response to heparin treatment, despite remaining healthy and viable.
Figure 3. Growth arrested cells do not respond to heparin by producing elastic fibers.
SMCs were grown in either growth media (A, B; DMEM+10% serum) or growth-arrest media (C, D; DMEM+0.4% serum), with (B, D) or without (A,C) heparin, for 7 days, then fixed and stained for elastin as in previous experiments. Elastin is indicated in red, nuclei in blue (DAPI).
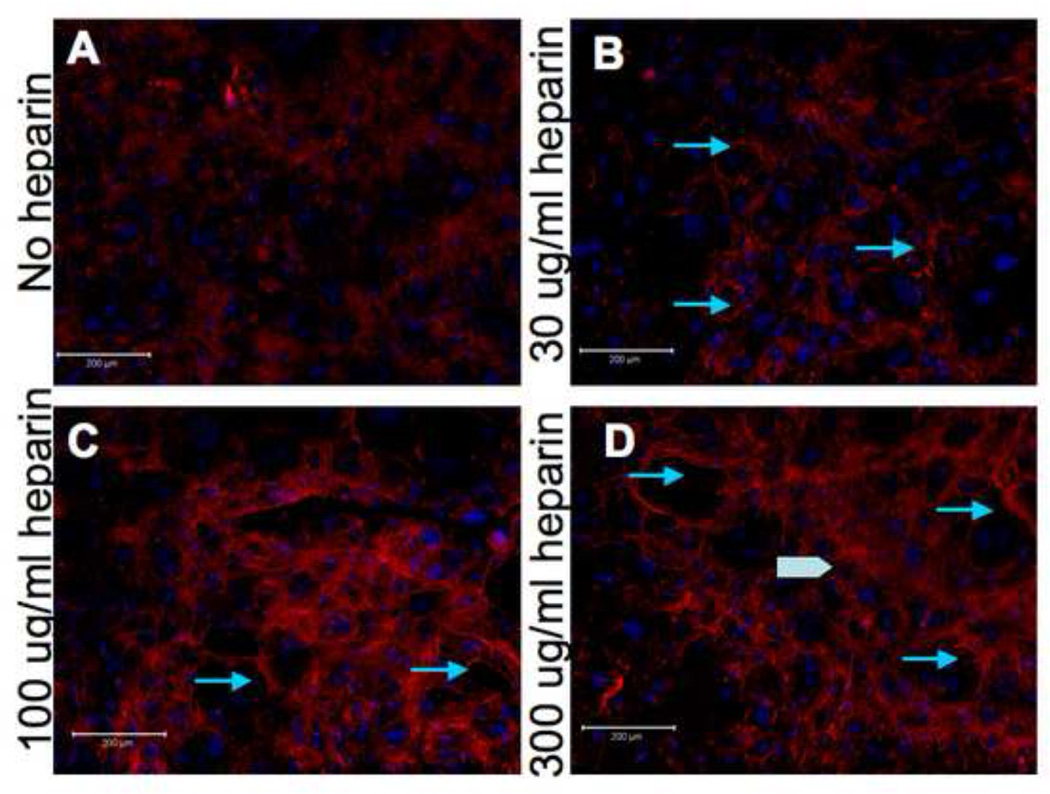
We next wanted to characterize other conditions that might affect the activity of heparin, to determine optimal treatment parameters. To assess if the effects of heparin were dose-dependent, we treated cultured SMCs with 30, 100 or 300 µg/ml of heparin for 5 days. There is a significant increase in elastic fibers in the 100 µg/ml of heparin treatment as compared to 30 µg/ml, and a smaller but still notable difference between 100 µg and 300 µg/ml treatments. Thus, we found that elastic fibers were more extensive and interconnected at higher doses of heparin, though they did appear at all treatment doses (Figure 4).
Figure 4. Effect of heparin on elastogenesis is dose-dependent.
15,000 hAoSMC/cm2 were plated and grown in medium containing 10% serum with the indicated concentrations of heparin. After 5 days, the cells were fixed and processed for immunofluorescence using an anti-elastin antibody as described in Materials and Methods. More extensive elastin fibers are seen with increasing heparin treatment (arrows). Some potential sheets of extracellular elastin are visible in the highest heparin concentration samples (arrowhead).
Given the dramatic and dose-dependent response to heparin treatment, we wondered if the effect was a specific property of heparin, or would be produced from treatment with other glycosaminoglycans. Chondroitin sulfate is a negatively charged, sulfated glycosaminoglycan composed of N-acetylgalactosamine and glucuronic acid, and is closely related to heparin chemically. Thus, we treated the SMCs with 300 µg/ml of chondroitin sulfate and assessed elastin formation as above. We observed no increase in elastic fiber formation above the level found in untreated cells (Figure 5).
Figure 5. Elastogenic response is specific to heparin but independent of size.
15,000 hAoSMC/cm2 were plated and grown in medium containing 10% serum alone (A), or medium containing 10% serum plus 300 µg/ml chondroitin sulfate (B), 300 µg/ml heparin (C), or 300 µg/ml LMW-heparin (D). After 5 days, cells were fixed and processed for immunofluorescence microscopy as described in Materials and Methods. Few elastic fibers are seen with chondroitin treatment, similar to the untreated control, whereas numerous fibers are seen in heparin treated samples.
We also wanted to determine if the effect of heparin was dependent on size, as heparin also is commonly used as low molecular weight (LMW) heparin (6–8 disaccharide repeats). LMW-heparin is clinically prescribed as an antithrombotic, but is far less anticoagulant than full-length heparin, which would make LMW heparin a good option for vascular graft treatment if there were risk of bleeding. Indeed, we saw that LMW heparin does induce a robust elastogenic response, as shown in Figure 5D.
2.3. Heparin upregulates elastin mRNA levels
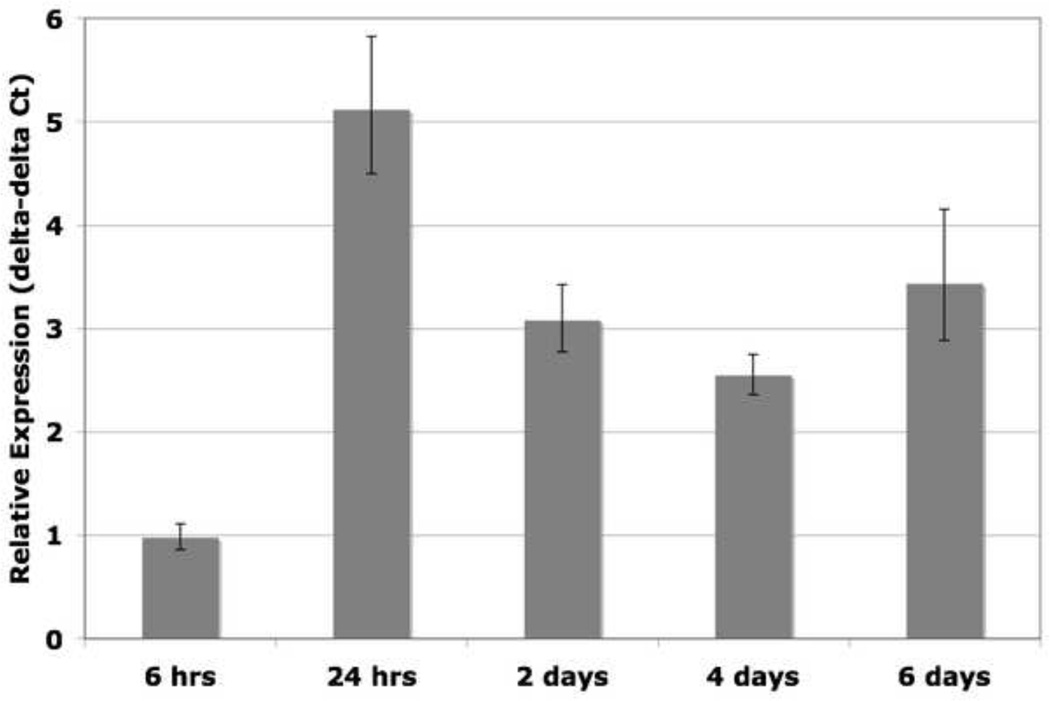
Next, we asked whether heparin addition stimulates transcription of elastin mRNA, as this could be a mechanism for increased elastin deposition. As shown in Figure 6, quantitative PCR shows an increase in elastin mRNA upon heparin treatment beginning at approximately 24 hours post-heparin treatment, and level remains elevated for up to six days. It has been reported that heparin treatment can increase elastin mRNA in neonatal rat lung fibroblast cultures, and as we have measured a similar response in adult human smooth muscle cells, this may be a contributing factor to the mechanism of elastogenesis in this system (McGowan et al., 1993).
Figure 6. Heparin stimulates elastin mRNA.
15,000 hAoSMCs/cm2 were treated with or without heparin, and samples were harvested at 6 hrs, 24 hrs, 2 days, 4 days, and 6 days. RNA was extracted, reverse-transcribed, and qPCR was used to measure relative amounts of elastin mRNA expression. Each sample was normalized to GAPDH expression, then to elastin expression in the untreated sample at the corresponding time point. Results are given as delta-delta Ct values, and are the average of 3 independent experiments. Standard deviation indicated by error bars. Elastin mRNA levels rise, beginning at 24 hrs post-heparin treatment and remain elevated for the duration of the experiment.
2.4. Heparin and elastic fibers colocalize in vitro
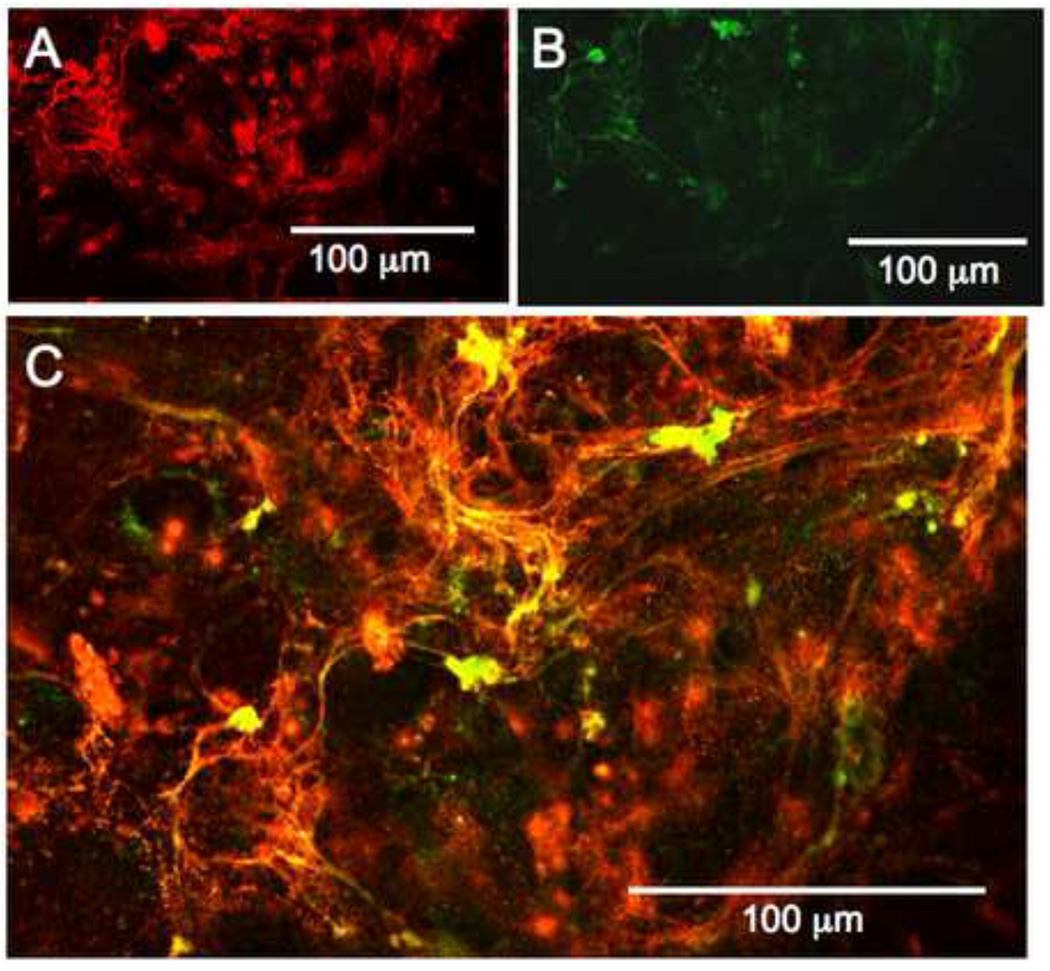
Given the initial findings presented above, and published reports indicating a heparin-elastin interaction in vitro, we asked if the added heparin might be responsible for fiber organization by interacting with elastin in the extracellular compartment (Tu and Weiss, 2008). To determine colocalization of the two components, fluorescein-labeled heparin (DTAF-heparin) was prepared. When DTAF-heparin was added to SMC cultures, it was elastogenic, showing it had retained biological activity, consistent with previous published data (Stearns, 1997). The elastic fibers induced by the DTAF-heparin were visualized using immunofluorescence and confocal microscopy. DTAF-heparin and elastin colocalized in nearly all areas, supporting the hypothesis that heparin may bind elastin, or some component of elastic fibers, in culture (Figure 7).
Figure 7. Fluorescently labeled heparin localizes to elastic fibers in vitro.
hAoSMC cultured for 7 days with 300 µg/ml DTAF-heparin. Confocal sections were taken, and different wavelength images of the same z-sections were reported. (A) shows the distribution of labeled heparin. As shown in (B), the DTAF-heparin retains biological activity, as it promotes elastic fiber formation. As seen in (C), the heparin and nascent elastin fibers colocalize extensively.
2.5. Silk-heparin constructs induce biologically appropriate cell behavior
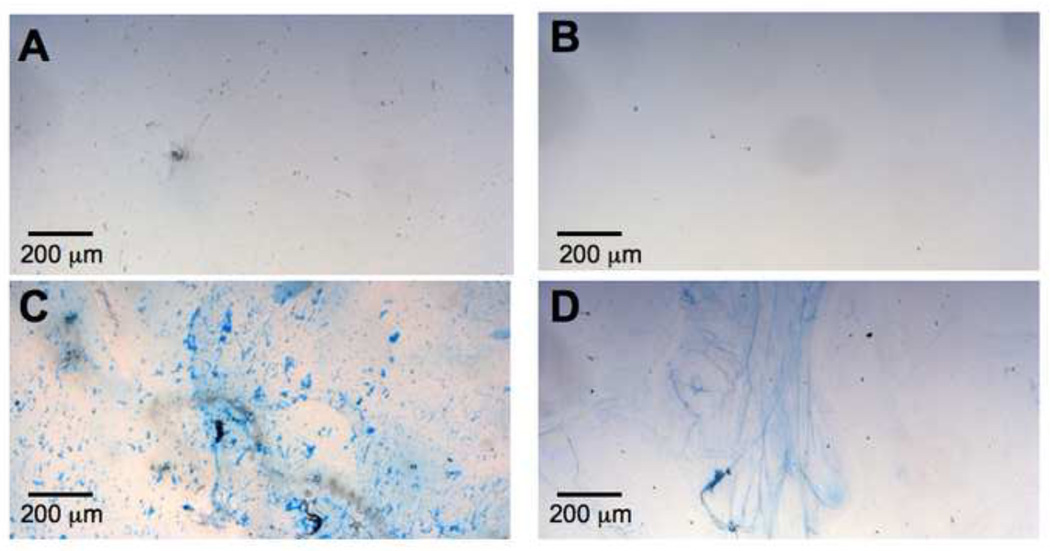
Given the increased elastogenesis induced by soluble heparin, we asked whether substrate-associated heparin would promote similar cell responses. Initially, silk films with or without admixed heparin were prepared and characterized with Alcian Blue. Heparin was found distributed throughout the silk films by the staining, both in the cast films and in the layer-by-layer (LbL) deposition (Figure 8; see methods). The slight speckled appearance of the caste films is likely due to microphase separation during drying, whereas the LbL films are spread out more when subjected to a stream of nitrogen.
Figure 8. Alcian blue reveals heparin incorporation into silk films.
In two types of silk film processing, heparin is incorporated by admixing silk and heparin solution. Caste films have higher total amount of silk (A,C), while layer-by-layer films contain less silk in thinner layers(B,D). In each situation, the heparin is fairly evenly distributed throughout the film, as shown by the dark blue staining in ‘with heparin’ samples (C, D). Some pale blue background staining arises from nonspecific affinity of silk for alcian blue in ‘silk only’ samples (A, B).
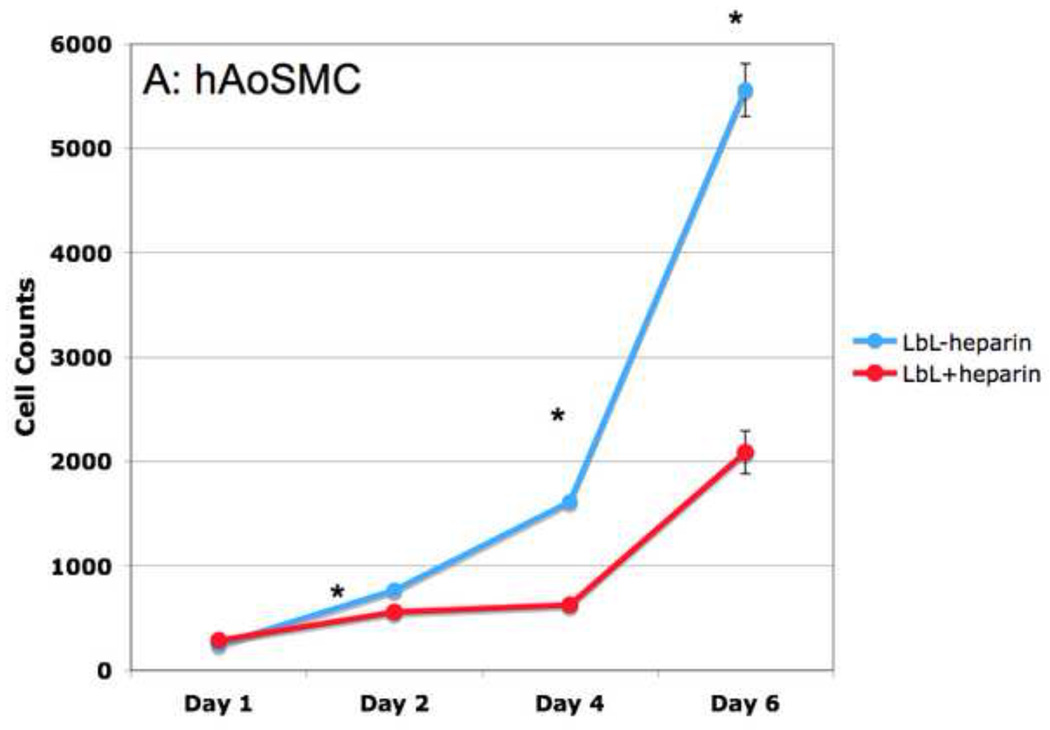
To demonstrate that the admixed heparin was still biologically active, we first assessed the well-documented antiproliferative effects of heparin on smooth muscle cells. Proliferation assays were performed with SMCs seeded onto silk films alone, or silk films mixed with heparin, at the indicated timepoints. The silk films containing heparin inhibited SMC proliferation, whereas silk films alone did not cause a decrease in SMC doubling time (Figure 9A). Neither films appeared to negatively effect the health of the cells. These experiments were carried out on both LbL and caste silk films with nearly identical results.
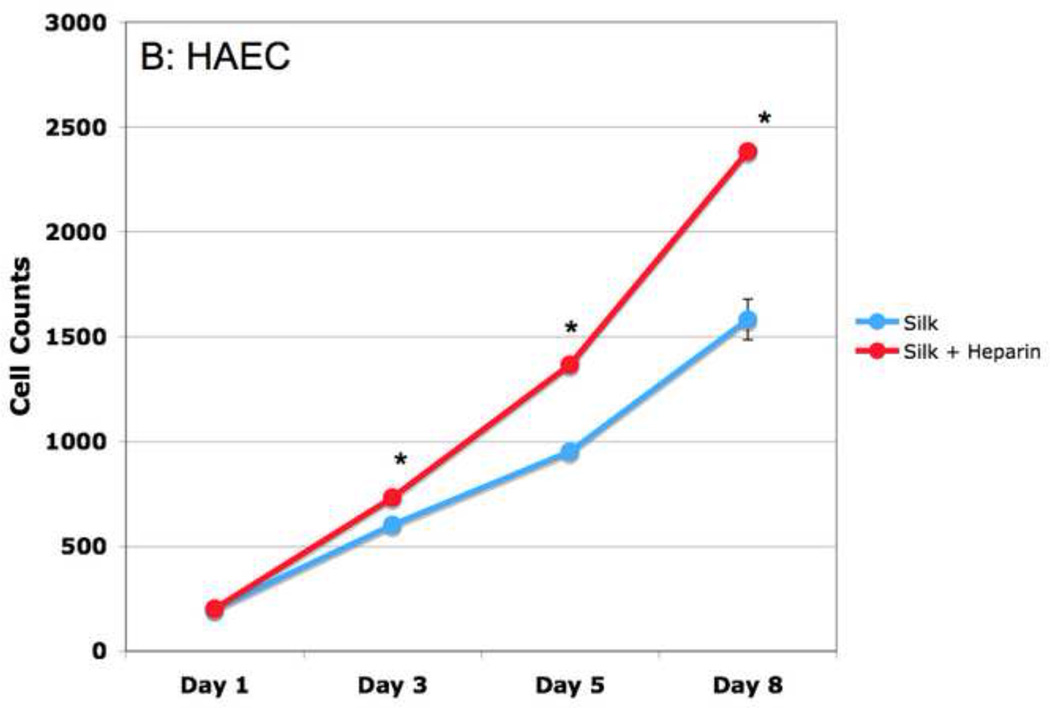
Figure 9. Heparin films inhibit hAoSMC proliferation, but promote HAEC proliferation.
Adult human aortic smooth muscle (hAoSMC, A) or endothelial (HAEC, B) cells were seeded onto silk films with or without heparin incorporated. At indicated timepoints, cells were trypsinized and counted. Data are the average of three experiments, with standard error of the mean (SEM) indicated by error bars. Stars indicate significance of P< 0.01.
Another important factor in developing potentially useful biomaterials for vascular grafts, one of our long term goals, is the ability to promote the development of a healthy endothelial monolayer. To examine the effect of heparinized silk films on endothelial cell proliferation, primary human aortic endothelial cells (HAECs) seeded onto heparinized silk films were tracked. The proliferation rate of the endothelial cells not only was not inhibited, but increased with heparin treatment. This is consistent with reported effects of heparin on endothelial cell growth (Figure 9B) (Maciag et al., 1984).
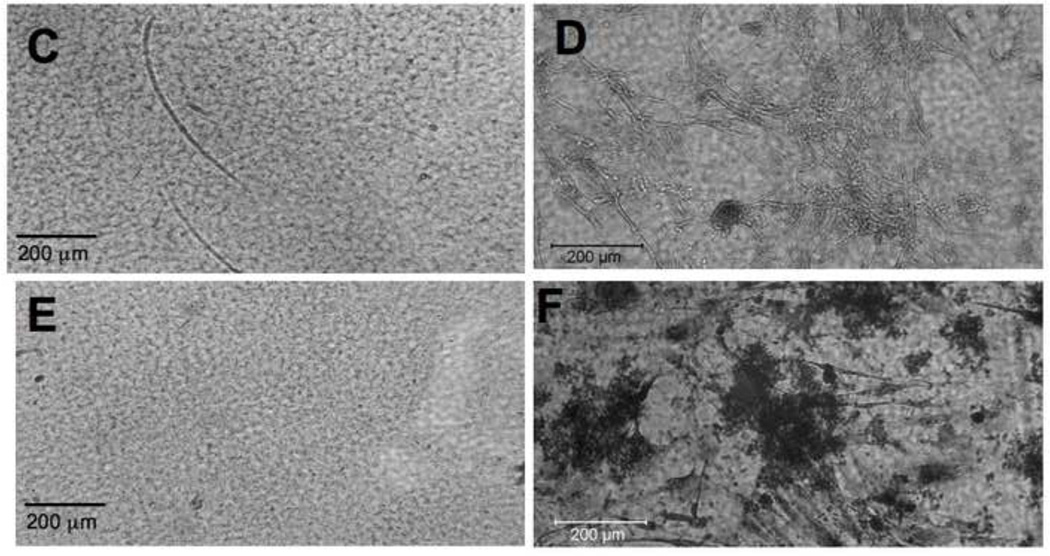
To determine if heparinized silk films promoted elastogenesis from SMCs, as is the case with soluble heparin (Figure 1), we next plated SMCs onto silk and heparinized silk films, then stained the films using immunofluorescence (after 9 days) and van Gieson stain (after 18 days). There is a clear increase in the amount of elastin formed from SMCs grown on heparinized silk films when compared to the silk-only films (Figure 10).
Figure 10. SMCs produce elastin on heparinized silk films.
hAoSMC were plated onto silk films. Samples were allowed to grow for 9 days on silk films (A) or silk+heparin films (B) and were then fixed and stained by immunofluorescence for elastin (in red with phase contrast overlay). Longer timepoint samples, grown for 18 days on silk films (D) and silk+heparin films (F) were stained by van Gieson stain. Nonspecific response to van Gieson stain was ruled out by silk (C), or silk+heparin (E), films stained without cells.
3. Discussion
In this manuscript, we report several novel observations: 1) we demonstrate for the first time that elastogenesis in adult human SMC is strongly stimulated by heparin; 2) structure-function analysis indicates that induction of elastogenesis in these adult cells is specific for heparin compared to other glycosaminoglycans, and can be elicited by low molecular weight heparin species; 3) heparinized silk films promote elastogenesis in adult human SMC while reducing proliferation of SMCs and concomitantly permitting normal proliferation of endothelial cells, all of which are critical to long-term vessel function using biomaterials considered for vascular repairs; 4) heparin and elastin co-localize extracellularly; and 5) heparin increases elastin mRNA expression in adult human SMCs. These new findings—and in particular the use of adult human SMC in all of these studies—strongly support the use of heparinized silk as a potential biomaterial for vascular repair.
In exploring factors that promote maximal elastic fiber expression, initial cell seeding density was important in determining the extent of elastogenesis, even at later timepoints well after the cells had reached confluence. This is particularly interesting in light of the density- and growth factor-dependent phenotype switching characteristic of SMCs. Cell cultures seeded at low density and/or under rich serum conditions typically retain the proliferative phenotype, where smooth muscle actin is diffuse, morphology is flat and spread out, and protein production is generally high. This phenotype is also observed in vivo in response to injury, when cells begin to proliferate for wound repair. However, SMCs switch to the contractile phenotype - characterized by strong smooth muscle actin staining and low proliferation rates - under quiescent conditions such as high cell density or low serum (Owens, 2004). As shown in the study, slightly subconfluent, proliferating cultures responded most effectively to heparin-induced elastogenesis. This is in contrast with other published reports indicating that only confluent cultures produce elastin, however these studies used neonatal fibroblasts, which may explain the different behavior (Mecham et al., 1981).
As we are most interested in examining the organization of elastic fibers at a cellular level, immunofluorescence was used to examine the distribution of elastic fibers. We find this to be the most relevant analytical tool, as total amounts of tropoelastin are not necessarily equivalent to the amount of properly organized elastic fibers (Mecham et al., 1981).
An initial increase in elastin mRNA level was correlated with heparin treatment, which is supported by reports with other cell types (Wachi et al., 1995). As the threshold for elastin expression in the adult is unknown, it could be possible that the slight increase in elastin mRNA is sufficient for protein expression. Additionally, it has been reported that tropoelastin expression can cause a positive-feedback loop, which could increase levels beyond our initial mRNA findings (Coquerel et al., 2009; Wachi et al., 1995). There also may be feedback to associated elastic fibers proteins, which may regulate elastin as well.
Heparin and elastin extensively co-localized in the extracellular compartment, which could support the hypothesis that the role of heparin in elastogenesis is at least partially organizational. The reported biochemical interactions between heparin and elastin support a model in which electrochemical interactions between negatively charged side chains of heparin and positively charged tropoelastin allow organization of tropoelastin monomers in preparation for lysyl oxidase-mediated crosslinking, as supported by additional biochemical data from Wu et al. (Wu et al., 1999). This hypothesis is also supported by the work of Tu and Weiss, who show that heparin addition to purified tropoelastin decreased the critical concentration for tropoelastin coacervation in vitro (Tu and Weiss, 2010). Additionally, the Ramumurthi group has shown that copper ions, hyaluronic acid, and TGFβ can induce cross-linked elastin from adult rat smooth muscle cells (Kothapalli and Ramamurthi, 2009a, b). While useful from a bioengineering standpoint, the physiological relevance of these findings remains unknown.
As the number of coronary artery bypass grafts performed each year continues to rise, the need for a small-diameter tissue engineered vascular grafts increases. While Dacron and ePTFE have shown success when used for grafts of greater than 6 mm diameter, these materials fail at smaller diameters due to thrombosis. It is also likely that these failures are influenced by mechanical compliance mismatches between the surrounding native tissue and the implanted material. Silk is a suitable biomaterial to address these concerns, as it is non-immunogenic, can be chemically modified, is biologically remodeled and can be mechanically tuned to address compliance needs. In addition, biomolecules or growth factors can be covalently attached to silk, or can be entrapped with retention of bioactivity (Altman GH, 2003; Lovett M, 2007). As silk is easily molded into vessel-sized tubes, the films discusses here can be readily translated into a three dimensional construct, which is an area of ongoing study.
Another challenge for a long-term biodegradable small diameter vascular grafts, which we address here, is the elaboration of a robust elastic lamellar network. As the most promising tissue engineered vascular grafts would not require pre-cellularization, but could be repopulated by native cells following implantation, we have focused on adult human SMCs for our studies. Though these cells are the most physiologically relevant, they do not typically produce significant amounts of either tropoelastin or mature, cross-linked elastin fibers. Elastin production has in fact been reported to sharply decline after birth, ceasing almost entirely by early adulthood (Swee et al., 1995). Therefore, discovering and incorporating factors which stimulate elastin re-expression from adult cells is essential for proper production of extracellular matrix upon graft repopulation.
In conclusion, we have shown that heparin alone promotes elaborate elastic fiber assemblies in adult human smooth muscle cell cultures (Davis, 1993). Furthermore, a silk-based heparin-releasing biomaterial system preserves the biological function of heparin, and also promotes elastogenesis from SMCs. Thus, our findings support further study into the mechanisms and possible limitations of heparin-induced elastogenesis in adult SMC, as well as the formulation of new heparin-containing silk biomaterial constructs for small diameter vascular tissue engineering.
4. Experimental Procedures
4.1. Cell culture
Primary human aortic smooth muscle cells (hAoSMC) from a 79-year old female patient were obtained as a kind gift from Michael Mendelsohn at the Molecular Cardiology Research Institute (MCRI) at Tufts University School of Medicine. This cell line was transformed using E6/E7 and used for the majority of the experiments, with nearly identical results to the untransformed primary cells. Cells were grown in Dulbecco’s Modified Essential Media (DMEM) supplemented with 10% bovine growth serum, 100U/ml penicillin, 1000U/ml streptomyocin, and 1% L-glutamine at 37°C in a 95% air/5% CO2 atmosphere. Media was changed every 2–3 days and cells were passaged every week. Primary cells were used between P3 and P8; transformed cells, between P3 and P15. Heparin sodium salt from porcine intestinal mucosa (MW ≈ 15,000 Da) was purchased from SigmaAlrich (St. Louis, MO) and used without further purification.
4.2. Preparation of silk solution
Silk fibroin aqueous stock solutions were prepared as previous described (Li et al., 2006) (Li et al., 2006). Briefly, Bombyx mori cocoons were boiled for 40 minutes in an aqueous solution of 0.02 M Na2CO3, and then rinsed thoroughly with distilled water to extract the glue-like sericin proteins. The extracted silk fibroin was then dissolved in 9.3 M LiBr solution at 60°C for 4 h, yielding a 20% (w/v) solution. This solution was dialyzed against distilled water using a Slide-a-Lyzer dialysis cassette (MWCO 3,500, Pierce) at room temperature for 48 h to remove the salt. The dialysate was centrifuged 2 times, each at 4°C for 20 min, to remove impurities and the aggregates that formed during dialysis. The final concentration of silk fibroin aqueous solution was approximately 8% (w/v). This concentration was determined by weighing the residual solid of a known volume of solution after drying at 60°C for 24 h.
4.3. Caste silk film preparation
Silk solutions were prepared by diluting the stock silk solution with deionized (DI) water to a concentration of 1% (w/v). Mixed heparin and silk solutions were prepared by dissolving heparin in deionized (DI) water first, and then adding this to the silk solution to obtain a final concentration of heparin of 0.3 mg/ml. Films were prepared by depositing silk alone or the silk + heparin solution described above onto glass coverslips and allowing them to dry 24–48 hours. Films were then rendered water-insoluble by the water annealing technique described previously (Lawrence et al., 2009). Briefly, films were suspended above 250 ml DI water in the bottom of a vacuum chamber, which was then sealed and depressurized to remove all air. Films were incubated under vacuum for at least 3 hours.
4.4. Layer-by-layer films
Layer-by-layer (LbL) films were produced similarly to previously used methods (Wang, 2007). Tissue culture plates, with or without a 9 mm-diameter glass coverslip in the bottom, were incubated in a 1% w/v silk + 25 mg/ml heparin solution for 5 minutes at room temperature. Excess solution was removed, and the adsorbed material was dried under a stream of nitrogen gas for 2 minutes. The process was repeated for 2, 5 or 10 layers as indicated. A 1% w/v silk-only solution was used as a negative control.
4.5. Proliferation Assays
Prior to cell plating, cast silk films or layered films (as described above) were sprayed with 70% ethanol and treated with ultraviolet light for 30 minutes to sterilize them. Twenty µg/ml fibronectin in PBS was then added to each well and incubated overnight at 4°C to normalize cell adhesion. Cells were collected at indicated time points by trypsinization, and counted using a Coulter counter. Triplicate counts of triplicate wells were averaged, and data are presented as the average, with standard error of the mean (SEM) indicated by error bars.
4.6. Immunofluorescence Microscopy
Cells were seeded onto 9 mm coverslips or silk films in 24 well plates at 1.5 × 104 cells/cm2 unless otherwise indicated. At indicated time points, cells were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) pH 7.4 for 30 minutes, then blocked in 3% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. Primary antibodies against elastin (Abcam, Cambridge, MA; rabbit polyclonal, 1:200), and (Elastin Products Company, rabbit polyclonal anti-human tropoelastin and anti-human α-elastin, 1:100 each) were diluted in BSA as indicated, followed by incubation for 2 hours at room temperature or 24 hours at 4°C. Cy3-labelled anti-rabbit (Jackson Laboratories, West Grove, PA) was diluted 1:400 in 3% BSA and incubated with sample for 1 hour at room temperature protected from light. Samples were washed in PBS and mounted using Vectashield (Burlingham, CA) mounting media with DAPI. Imaging fields were acquired by selecting random areas under the DAPI filter. At least four images of each condition were captured per experiment and compared visually. Confocal images (Figure 7) were taken of the same z-section using a Leica microscope. Attempts to use total fluorescence quantification were thwarted by relatively high amounts of cell-surface associated elastin, and therefore did not adequately describe prominent fibers.
4.7. Quantitative PCR
For RNA isolation, cells were plated at 15,000 cells/cm2 in 6-well plates and treated with 300 µg/ml of heparin for 6 hours, 24 hours, 2 days, 4 days, and 6 days. At each time point, samples were harvested, stored at −80°C until RNA was isolated following instructions in the Qiagen RNeasy kit. RNA was quantified using a Nanodrop, then reverse transcribed into cDNA using the Ambion RETROscript kit. Primers for elastin and GAPDH were purchased as Assay-on-Demand kits from Applied Biosciences (elastin: Hs00355783_m1; GAPDH: Hs03929097_g1). Taqman 2× Master Mix was also from Applied Biosciences. All samples were run in triplicate with ‘no template’ controls, using a Stratagene M×3000P machine. Data were analyzed using the delta-delta Ct method, and are presented as the average of three independent experiments.
4.8. DTAF-heparin and DTAF-chondroitin sulfate preparation
DTAF-heparin and -chondroitin were purified according to the method of Letourneur et al. with minor modifications (Castellot et al., 1984). Briefly, 50 mg of 5([4,6 dichlorotriazin-2-yl] amino) fluorescein (DTAF, Sigma Aldrich, St. Louis MO) was dissolved in 7.5 ml of 0.1M borate buffer pH 9 (Pierce Biotechnology), and added to 150 mg of heparin/chondroitin in 7.5 ml borate buffer. Reaction was allowed to proceed overnight at room temperature protected from light. Free DTAF was separated from DTAF-heparin/chondroitin by extensive washing through a 5000 MWCO Amicon filter. DTAF-heparin/chondroitin was treated with 5% v/v trichloroacetic acid (TCA) for 30 minutes on ice to remove contaminating proteins. Supernatant was added to 3 volumes ice cold 100% ethanol and heparin was precipitated on ice for 30 minutes. Resulting pellet was washed once in 100% ethanol and dried to powder. When used for cell culture, powder was resuspended in DMEM; for silk incorporation purified water was used.
4.9. Alcian Blue staining
Samples were washed in PBS twice, washed with 0.1N HCl for 30 minutes, then stained for 1–24 hours with 1% Alcian Blue in 0.1N HCl at room temperature. They were then washed with 0.1N HCl at least twice to remove excess dye. Samples were then imaged for spatial distribution.
4.10. Elastin staining
Cells were fixed with 4%PFA for 30 minutes then washed twice in PBS and twice in water. An elastin stain kit (Sigma-Aldrich) was used according to package instructions. Briefly, samples were then stained in a hematoxylin/FeCl2 / Weigert’s iodine solution (“working elastin stain”) for 10 minutes, then washed in distilled water. Samples were differentiated in FeCl2 solution, rinsed in 95% ethanol, and counterstained in van Gieson. All steps were performed at room temperature.
Acknowledgments
We thank the NIH (EB002520) Tissue Engineering Resource Center for support of the project, as well as NIH grant HL046773 (JJC) and the American Heart Association Predoctoral Fellowship (0815680D) to CB.
Abbreviations
- hAoSMC
human aortic smooth muscle cell
- DTAF
dichlorotriazinylaminofluorescein
- HAEC
human aortic endothelial cell
- LbL
layer-by-layer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AHA. Cardiovascular Procedures. Factsheet. 2007 [Google Scholar]
- Altman GH, D F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Arribas SM, Briones AM, Bellingham C, Gonzalez MC, Salaices M, Liu K, Wang Y, Hinek A. Heightened aberrant deposition of hard-wearing elastin in conduit arteries of prehypertensive SHR is associated with increased stiffness and inward remodeling. Am J Physiol Heart Circ Physiol. 2008;295:H2299–H2307. doi: 10.1152/ajpheart.00155.2008. [DOI] [PubMed] [Google Scholar]
- Cain SA, Baldock C, Gallagher J, Morgan A, Bax DV, Weiss AS, Shuttleworth CA, Kielty CM. Fibrillin-1 interactions with heparin. Implications for microfibril and elastic fiber assembly. J Biol Chem. 2005;280:30526–30537. doi: 10.1074/jbc.M501390200. [DOI] [PubMed] [Google Scholar]
- Castellot JJ, Jr, A M, Rosenberg R, Karnovsky MJ. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biology. 1981;90:372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot JJ, Jr, Beeler DL, Rosenberg RD, Karnovsky MJ. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. J Cell Physiol. 1984;120:315–320. doi: 10.1002/jcp.1041200309. [DOI] [PubMed] [Google Scholar]
- Coquerel B, Poyer F, Torossian F, Dulong V, Bellon G, Dubus I, Reber A, Vannier JP. Elastin-derived peptides: matrikines critical for glioblastoma cell aggressiveness in a 3-D system. Glia. 2009;57:1716–1726. doi: 10.1002/glia.20884. [DOI] [PubMed] [Google Scholar]
- Davis EC. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry. 1993;100:17–26. doi: 10.1007/BF00268874. [DOI] [PubMed] [Google Scholar]
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Batge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J Biol Chem. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- Harris JR, Seikaly H. Evaluation of polytetrafluoroethylene micrografts in microvascular surgery. J Otolaryngol. 2002;31:89–92. doi: 10.2310/7070.2002.18928. [DOI] [PubMed] [Google Scholar]
- Herman IM, C JJ. Regulation of vascular smooth muscle cell growth by endothelial-synthesized extracellular matrix. Arteriosclerosis. 1987;7:463–469. doi: 10.1161/01.atv.7.5.463. [DOI] [PubMed] [Google Scholar]
- Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- Katsuta Y, Ogura Y, Iriyama S, Goetinck PF, Klement JF, Uitto J, Amano S. Fibulin-5 accelerates elastic fibre assembly in human skin fibroblasts. Exp Dermatol. 2008;17:837–842. doi: 10.1111/j.1600-0625.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- Kothapalli CR, Ramamurthi A. Biomimetic regeneration of elastin matrices using hyaluronan and copper ion cues. Tissue Eng Part A. 2009a;15:103–113. doi: 10.1089/ten.tea.2007.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothapalli CR, Ramamurthi A. Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells. J Tissue Eng Regen Med. 2009b;3:655–661. doi: 10.1002/term.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30:1299–1308. doi: 10.1016/j.biomaterials.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Lovett M, C C, Daheron L, Messmer B, Vunjak-Novakovic G, Kaplan DL. Silk fibroin microtubes for blood vessel engineering. Biomaterials. 2007;35:5271–5279. doi: 10.1016/j.biomaterials.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett ML, Cannizzaro CM, Vunjak-Novakovic G, Kaplan DL. Gel spinning of silk tubes for tissue engineering. Biomaterials. 2008;29:4650–4657. doi: 10.1016/j.biomaterials.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T, Mehlman T, Friesel R, Schreiber AB. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984;225:932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- McGowan SE, Liu R, Harvey CS. Effects of heparin and other glycosaminoglycans on elastin production by cultured neonatal rat lung fibroblasts. Arch Biochem Biophys. 1993;302:322–331. doi: 10.1006/abbi.1993.1218. [DOI] [PubMed] [Google Scholar]
- Mecham RP, Lange G, Madaras J, Starcher B. Elastin synthesis by ligamentum nuchae fibroblasts: effects of culture conditions and extracellular matrix on elastin production. J Cell Biol. 1981;90:332–338. doi: 10.1083/jcb.90.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra-Gorur K, C JJ. Heparin rapidly and selectively regulates tyrosine phosphorylation in vascular smooth muscle cells. J Cell Physiology. 1999;178:205–215. doi: 10.1002/(SICI)1097-4652(199902)178:2<205::AID-JCP10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Mitchell SL, Niklason LE. Requirements for growing tissue-engineered vascular grafts. Cardiovasc Pathol. 2003;12:59–64. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- Owens G, Kumar M, Wamhoff B. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiology Review. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Swee MH, Parks WC, Pierce RA. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J Biol Chem. 1995;270:14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- Tu Y, Weiss AS. Glycosaminoglycan-mediated coacervation of tropoelastin abolishes the critical concentration, accelerates coacervate formation, and facilitates spherule fusion: implications for tropoelastin microassembly. Biomacromolecules. 2008;9:1739–1744. doi: 10.1021/bm7013153. [DOI] [PubMed] [Google Scholar]
- Tu Y, Weiss AS. Transient tropoelastin nanoparticles are early-stage intermediates in the coacervation of human tropoelastin whose aggregation is facilitated by heparan sulfate and heparin decasaccharides. Matrix Biol. 2010;29:152–159. doi: 10.1016/j.matbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Wachi H, Seyama Y, Tajima S. Modulation of elastin expression by heparin is dependent on the growth condition of vascular smooth muscle cells: up-regulation of elastin expression by heparin in the proliferating cells is mediated by the inhibition of protein kinase C activity. J Biochem. 1995;118:582–586. doi: 10.1093/oxfordjournals.jbchem.a124949. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Castellot J, Herman I, Iafrati M, Kaplan DL. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials. 2007 doi: 10.1016/j.biomaterials.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Vrhovski B, Weiss AS. Glycosaminoglycans mediate the coacervation of human tropoelastin through dominant charge interactions involving lysine side chains. J Biol Chem. 1999;274:21719–21724. doi: 10.1074/jbc.274.31.21719. [DOI] [PubMed] [Google Scholar]