Abstract
Background/Objectives
Low blood levels of 25-hydroxyvitamin D (25OHD) have been associated with cardiometabolic disease but results are inconsistent. The objective of the study was to investigate the association of 25OHD with metabolic syndrome in a population at increased risk for diabetes.
Subjects/Methods
Using baseline data from the placebo and lifestyle intervention arms of the Diabetes Prevention Program (DPP) (N=2000), multivariable logistic regression models were used to estimate the odds of prevalent metabolic syndrome and each of its individual components across 25OHD tertiles. Multivariable linear regression was used to estimate the adjusted mean difference of insulin secretion and sensitivity across the same 25OHD tertiles. In participants free of metabolic syndrome at baseline (N=546), incident metabolic syndrome in the first two years of follow-up was assessed using discrete-time proportional hazards regression to test its association with 25OHD concentration.
Results
After multivariate adjustment, participants in the highest tertile of 25OHD had lower odds of prevalent metabolic syndrome (odds ratio 0.62; 95%CI 0.45-0.84), smaller waist circumference, higher high-density lipoprotein, and lower fasting plasma glucose compared to participants in the lowest tertile of 25OHD. Higher plasma 25OHD concentration was associated with greater insulin sensitivity and lower insulin secretion. After multivariate adjustment, there was a non-significant lower risk of metabolic syndrome in the highest tertile of 25OHD (hazard ratio 0.79; 95% CI, 0.48-1.32) compared to the lowest tertile.
Conclusion
In a population at increased risk for diabetes, higher plasma 25OHD concentration was inversely associated with prevalent metabolic syndrome and non-significantly with incident metabolic syndrome.
Keywords: metabolic syndrome, vitamin D
INTRODUCTION
A growing body of evidence suggests that low 25-hydroxyvitamin D (25OHD) concentration is associated with cardiometabolic disease.1-3 The apparent link may be explained by vitamin D modifying cardiovascular disease risk factors, such as metabolic syndrome and each of its individual components.
Recent studies have reported on the association between vitamin D status and metabolic syndrome and its individual components. While some studies showed an inverse association between vitamin D and metabolic syndrome, other studies have failed to show this association. 4-13 The lack of concordance is likely secondary to lack of adjustment for important potential confounders such as adiposity, race/ethnicity, systemic inflammation and kidney function. It is well established that adiposity and insulin resistance play a pivotal role in the pathogenesis of metabolic syndrome. However, studies in overweight and obese populations have not consistently shown an inverse association between vitamin D concentration and metabolic syndrome.10, 13-15 Furthermore, while there are well-recognized differences in vitamin D metabolism among different race/ethnic groups,16 most of the literature on vitamin D and metabolic syndrome in non-white populations come from studies in Asians.3 Prior studies have not examined the association between vitamin D status and metabolic syndrome in specific multiethnic populations identified as being at increased risk for diabetes and cardiovascular disease.
The purpose of the present study was to examine the association between plasma 25OHD concentration and prevalent metabolic syndrome and its traditional and non-traditional components, and risk of developing metabolic syndrome in the Diabetes Prevention Program (DPP), which represents a large multiethnic sample of U.S. adults with pre-diabetes.
MATERIAL AND METHODS
Study Participants
The DPP was a randomized controlled clinical trial conducted from 1996 to 2001 at 27 sites in the U.S. that compared the effects of intensive lifestyle intervention, metformin, or placebo on the development of diabetes in adults at high risk for the disease. The eligibility criteria, design, and methods of the DPP have been described in detail elsewhere.17 Briefly, inclusion criteria included age ≥25 years, body mass index (body mass index) ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), fasting plasma glucose 5.3 to 6.9 mmol/L (95 to 125 mg/dL) (≤6.9 mmol/L for American Indian sites) and a 2-hour plasma glucose 7.8 to 11 mmol/L (140 to 199 mg/dL) after a 75-gram oral glucose tolerance test. The Institutional Review Board at each site approved the protocol and all participants gave written informed consent. The present study was approved by the Tufts University Institutional Review Board.
The present observational study was conducted among participants randomized to two arms, the intensive lifestyle (n=1,079) and placebo (standard lifestyle, n=1,082). The metformin arm of the DPP was excluded to minimize the cost associated with measurement of plasma 25OHD. One hundred and twenty-two participants were also excluded because of lack of consent for ancillary studies (n=120) or no available specimen for measurement of 25OHD (n=2) or other covariates (n=9). After exclusions, 2,000 participants had data available for the unadjusted cross-sectional analyses and 1,959 had complete data for all covariates used in the multivariate analyses. For the prospective analysis of incident metabolic syndrome, 578 participants were free of the condition at baseline and 546 had available data on 25OHD and metabolic syndrome during the first two years of the study (32 developed diabetes or were lost to follow-up).
Measurement of plasma 25OHD concentration
Plasma 25OHD concentration was measured in baseline samples stored since collection at −70°C. Stability of vitamin D metabolites during transport and long-term freezing has been documented previously.18 Plasma 25OHD was measured at the Metabolic Laboratory at Tufts Medical Center by liquid chromatography, tandem mass spectrometry (LC/MS/MS) (Waters Acquity UPLC with TQD triple quadrupole mass spectrometer), certified through the National Institute of Standards and Technology (NIST) vitamin D quality assurance program. In the most recent testing, correlation with the NIST external standard for total 25-hydroxyvitamin D was r2=0.994.
Metabolic Syndrome, traditional components
Metabolic syndrome was defined according to the modified criteria from the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP III)19 based on the presence of three or more of the following five criteria: 1) central obesity: waist circumference ≥102 cm (men), ≥88 cm (women); 2) triglycerides ≥150 mg/dl, 3) high density lipoprotein (HDL) <40 mg/dL (men), <50 mg/dL (women); 4) systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg or current antihypertensive drug treatment in a patient with a history of hypertension; and 5) fasting plasma glucose ≥100 mg/dL. We also repeated the analyses with the higher threshold of glucose (≥110 mg/dL) used in the original definition of metabolic syndrome.
Metabolic Syndrome, Non-traditional (non-ATP III) components
Two measures of insulin secretion20 and two measures of insulin sensitivity21 were calculated as previously described in the DPP cohort.22 Glucose and insulin are expressed as mg/dL and μU/mL, respectively, unless otherwise specified. Insulin secretion was estimated as: insulinogenic index (IGI)), (insulin at 30 minutes – insulin at 0 minutes) ÷ (glucose at 30 minutes – glucose at 0 minutes); the corrected insulin response (CIR), calculated as follows: (100 × insulin at 30 minutes) ÷ (glucose at 30 minutes × [glucose at 30 minutes – 70 mg/dL]). Insulin sensitivity was estimated as: 1÷fasting insulin; the insulin-sensitivity index (ISI), which is the reciprocal of insulin resistance according to the homeostasis model assessment and is calculated by the following equation: 22.5 ÷ (fasting insulin × [fasting glucose ÷ 18.01]). Both measures of insulin secretion strongly correlate with each other, as do both measures of insulin sensitivity.22 The oral disposition index (DI), was calculated by two methods: DI1=IGI × (1/fasting insulin) and DI2= CIR × ISI.23
Assessment of Potential Confounders and Laboratory Assessment
At baseline, self-reported level of leisure physical activity was assessed with the Modifiable Activity Questionnaire.24 Usual daily nutrient intake was assessed with the use of a modified version of the Block food-frequency questionnaire.25 Data on vitamin D intake were not available. Standardized interviewer-administered questionnaires were used to obtain self-reported data on personal medical history, smoking, medications, alcohol use, and family medical history. Self-reported race/ethnicity was classified according to the 1990 U.S. Census questionnaire. Weight and height were measured using standard calibrated scale and stadiometer, respectively and body mass index was calculated (Kg/m2). Fasting blood was obtained and processed following standardized procedures. Measurement methods for glucose and C-reactive protein have been described previously.17 A yearly ultraviolet index for each site based on the National Weather Service data on the monthly means of ultraviolet index for each geographic location in 1997 was constructed.
Statistical Analysis
Descriptive statistics for 25OHD, outcomes of interest and each of the covariates were conducted. Variable distributions were assessed for potential violations of statistical assumptions and residual analyses were performed to identify violations and influential observations. Data transformations and non-parametric methods were performed when necessary. Characteristics of the study sample are presented by metabolic syndrome status and compared using two sample t-tests or Wilcoxon rank-sum tests for continuous variables where appropriate and chi-square tests for categorical variables. Multivariable logistic regression models were used to estimate the odds and 95% confidence interval of prevalent metabolic syndrome and each of its individual components by tertiles of 25OHD, after adjusting for potential confounders. Multivariable linear regression models were used to estimate the adjusted average differences in non-traditional metabolic syndrome components (insulin secretion and sensitivity) between 25OHD tertiles. In multivariable logistic and linear regression models, we adjusted for DPP clinical site location, month of blood draw, age, gender, race, ultraviolet radiation index at participant's recruitment location, smoking status, alcohol consumption, C-reactive protein, self-reported physical activity, total energy intake and BMI. We assessed the association between 25OHD concentration with the number of metabolic syndrome components using the Spearman correlation coefficient.
For ease of interpretation, we define differences in 25OHD concentration between participants categorized into three groups using tertiles (33.3rd and 66.7th percentiles) of baseline 25OHD concentration. Tests of linear trend across increasing groups were performed by modeling the median of each 25OHD tertile group as a continuous variable. The lowest tertile class is used as the referent group. The odds of metabolic syndrome and its individual components in each of the higher groups is compared with the referent by extrapolating the per unit change in estimated odds from the multivariate model. Similarly, the adjusted average difference of each continuous variable from the multivariate model is used to compare the highest two groups to the referent lowest group by extrapolating the per unit change from the multivariate model. Age, gender, race and month of blood draw were forced into all models. We accounted for clustering at sites using generalized estimating equation (GEE) models for correlated data.
For the prospective analysis, discrete-time proportional hazards models were used to assess the association between plasma 25OHD (by baseline defined tertile groups) and incident metabolic syndrome to account for interval-censored data. Participants who developed diabetes prior to an incident metabolic syndrome assessment were censored to account for competing risks. In multivariable models, we adjusted for similar potential confounders adjusted for in the cross-sectional. To account for additional unmeasured effects of intervention, we also adjusted for treatment assignment.
The predictor (25OHD) and other variables (physical activity and body weight) whose values were measured at multiple time points entered the prospective analyses as time-varying “lagged” covariates. At each successive annual visit when the outcome (metabolic syndrome) was assessed, the value of these variables was calculated as the mean of the current and previous non-missing value prior to that visit. For the time-varying variables, if either the current or most recent value was missing, we imputed values using the non-missing observation (current or most recent). If both current and most recent values were missing, then no value was imputed. Covariates measured only at baseline and year 1 visits (alcohol consumption, CRP, and total energy intake) were treated as time-invariant predictors by calculating the mean of the baseline and year 1 values. Tests of linear trend across increasing groups were performed by modeling the median of each 25OHD tertile group as a continuous variable and the risk of metabolic syndrome in each of the highest two groups was compared with the lowest group by extrapolating the per unit change in estimated hazard from the multivariable model.
We investigated the following possible effect modifiers on the associations of 25OHD with prevalent metabolic syndrome: baseline age, gender, race/ethnicity, and baseline body weight. We checked for the statistical significance of the interaction by using Wald chi-square tests. Tests for interactions were not conducted in the prospective analyses, due to the small number in each subgroup. All p-values are based on two-sided tests. Statistical analyses were performed using SAS version 9.2 (SAS, Cary, NC).
RESULTS
The mean age of the cohort was 51 years with 67% being women (table 1). The racial/ethnic distribution was diverse with 57% self-reported Whites and 20% self-reported African Americans. Participants with metabolic syndrome were younger, were more likely to be smokers, reported lower alcohol consumption, were less likely to be physically active, had higher CRP and were more likely to live in areas with high ultraviolet index.
Table 1.
Baseline characteristics of the Diabetes Prevention Program population by metabolic syndrome status
| Characteristic | Overall cohort | Metabolic syndrome absent | Metabolic syndrome present | P-value1 |
|---|---|---|---|---|
| Number of participants | 2000 | 578 | 1422 | |
| Age, mean (SD), years | 51.0 (10.8) | 51.8 (11.5) | 50.7 (10.6) | 0.0392 |
| Gender, No. (%) women | 1337 (66.9) | 379 (65.6) | 958 (67.4) | 0.4385 |
| Race, No. (%) | ||||
| White | 1142 (57.1) | 313 (54.2) | 829 (58.3) | 0.0091 |
| African-American | 405 (20.3) | 142 (24.6) | 263 (18.5) | |
| Other (Hispanic, Asian, American Indian) | 453 (22.7) | 123 (21.3) | 330 (23.2) | |
| Weight, mean (SD), kg | 94.5 (20.5) | 86.2 (18.6) | 97.9 (20.3) | <0.0001 |
| Body mass index, mean (SD), kg/m2 | 34.0 (6.7) | 31.3 (6.1) | 35.1 (6.7) | <0.0001 |
| Waist circumference, mean (SD), cm | 105.1 (14.7) | 97.6 (13.3) | 108.1 (14.1) | <0.0001 |
| Annual UV index, mean (SD), 90J/m2/hour 2 | 4.6 (1.4) | 4.5 (1.3) | 4.7 (1.4) | 0.0135 |
| Hypertension, No. (%) 3 | 975 (48.8) | 131 (22.7) | 844 (59.4) | <0.0001 |
| Physical Activity, mean (SD), MET-hours 4 | 15.9 (25.6) | 17.4 (22.2) | 15.2 (26.9) | 0.0004 |
| Smoking status, No. (%) | ||||
| Never | 1145 (57.3) | 333 (57.6) | 812 (57.1) | 0.0085 |
| Past | 715 (35.8) | 220 (38.1) | 495 (34.8) | |
| Current | 140 (7.0) | 25 (4.3) | 115 (8.1) | |
| Alcohol consumption, mean (SD), g/day | 2.2 (5.6) | 2.6 (6.1) | 2.1 (5.4) | 0.0050 |
| Total energy intake, mean (SD), kcal/d | 2091.0 (1027.8) | 1978.7 (946.5) | 2136.6 (1056.0) | 0.0009 |
| Calcium intake, mean (SD), mg/day | 1102.2 (724.3) | 1086.2 (681.4) | 1108.7 (741.2) | 0.7559 |
| Systolic blood pressure, mean (SD), mmHg | 124.0 (14.6) | 118.5 (13.3) | 126.3 (14.5) | <0.0001 |
| Diastolic blood pressure, mean (SD), mmHg | 78.4 (9.1) | 75.2 (7.8) | 79.7 (9.3) | <0.0001 |
| Fasting plasma glucose, mean (SD), mg/dL | 106.8 (8.1) | 104.3 (8.4) | 107.8 (7.7) | <0.0001 |
| Fasting insulin, mean (SD), μU/mL | 26.4 (15.1) | 20.2 (10.7) | 28.9 (15.9) | <0.0001 |
| Insulin Sensitivity | ||||
| Insulin sensitivity, mean (SD), (μU/mL)−1 | 0.05 (0.03) | 0.06 (0.04) | 0.05 (0.03) | <0.0001 |
| Insulin sensitivity index (ISI), mean (SD), (μnU/mL)*( mg/dL)]−1 | 0.20 (0.14) | 0.25 (0.16) | 0.18 (0.12) | <0.0001 |
| Insulin Secretion | ||||
| Insulinogenic Index (IGI), mean (SD), (μU/mL)/(mg/dL) | 1.22 (0.88) | 1.12 (0.87) | 1.26 (0.89) | <0.0001 |
| Corrected insulin response (CIR), mean (SD), [(μU/mL)/(mg/dL)2] | 0.62 (0.40) | 0.57 (0.41) | 0.64 (0.40) | <0.0001 |
| Disposition Indices (DI) | ||||
| DI 1: IGI * Insulin sensitivity, mean (SD) | 0.05 (0.04) | 0.06 (0.05) | 0.05 (0.04) | <0.0001 |
| DI 2: CIR * ISI, mean (SD) | 0.10 (0.07) | 0.12 (0.08) | 0.10 (0.07) | <0.0001 |
| Total Cholesterol, mean (SD), mg/dL | 204.5 (36.2) | 204.5 (35.8) | 204.5 (36.4) | 0.9882 |
| Triglycerides, mean (SD), mg/dL | 165.6 (95.4) | 113.0 (48.8) | 186.8 (101.3) | <0.0001 |
| HDL cholesterol, mean (SD), mg/dL | 45.5 (12.1) | 54.0 (12.2) | 42.1 (10.2) | <0.0001 |
| LDL cholesterol, mean (SD), mg/dL | 125.6 (32.8) | 127.4 (32.7) | 124.9 (32.9) | 0.1250 |
| C-reactive protein, mean (SD), mg/L | 5.8 (6.9) | 5.0 (7.3) | 6.1 (6.7) | <0.0001 |
| 25-hydroxyvitamin D, mean (SD), ng/mL | 21.6 (9.7) | 23.2 (10.3) | 20.9 (9.4) | <0.0001 |
Values are means (standard deviation) for continuous variables or n (%) for categorical variables. To convert plasma 25OHD concentration from ng/mL to nmol/L multiply by 2.459; to convert triglycerides from mg/dL to mmol/L multiply by 0.0113; to convert glucose from mg/dL to mmol/L multiply by 0.0555; to convert cholesterol from mg/dL to mmol/L multiply by 0.0259.
P values for differences between metabolic syndrome status (present vs. absent) for continuous variables are based on t-tests. For categorical variables, the p value is based on chi-square tests.
Average ultraviolet index at participants’ clinical site.
Hypertension defined as blood pressure ≥ 130/85 mmHg or the use of antihypertensive medication.
MET denotes metabolic equivalent. MET-hours represent the average amount of 588 time engaged in specified physical activities multiplied by the MET value of each activity.
Association between plasma 25OHD concentration and prevalent metabolic syndrome
The overall prevalence of metabolic syndrome in the cohort was 71%. This number is higher compared to the previously reported prevalence of metabolic syndrome in the entire DPP cohort,26 because the previous publication used the higher glucose criterion of 110 mg/dL. When participants were categorized into tertiles of 25OHD, the prevalence of metabolic syndrome was 76%, 72% and 66% in the lower, middle and higher vitamin D tertiles, respectively (Table 2). There was a 50% lower odds of metabolic syndrome (OR 0.50; 95% CI 0.38 to 0.65) in the highest tertile of 25OHD concentration (median [interquartile range] 25OHD 30.6 [27.5-34.9] ng/mL) compared to the lowest tertile (12.1 [9.7-14.3] ng/mL) after adjusting for location, month of blood draw, age, gender and race (Table 2). Further adjustments for average yearly ultraviolet radiation index at participant's study site, smoking status, alcohol consumption, C-reactive protein, self-reported physical activity, and total energy intake, did not change the association (OR 0.49; 95% CI 0.36 to 0.67). Further adjustment for BMI attenuated the association but it remained significant (OR 0.62; 95% CI 0.45 to 0.84). After repeating the analyses using the higher cutoff for glucose (110 mg/dL), results were unchanged (data not shown).
Table 2.
Adjusted odds ratios (OR) of prevalent metabolic syndrome and its components by tertiles of plasma 25-hydroxyvitamin D concentration in the lifestyle and placebo arms of the Diabetes Prevention Program population.
| Metabolic syndrome | Metabolic syndrome components | |||||
|---|---|---|---|---|---|---|
| Large waist circumference | High blood pressure | High triglycerides | High fasting plasma glucose | Low HDL cholesterol | ||
| 25OHD concentration* | Prevalence (n/N) | Prevalence (n/N) | Prevalence (n/N) | Prevalence (n/N) | Prevalence (n/N) | Prevalence (n/N) |
| 1st tertile | 75.7 (504/666) | 86.8 (577/665) | 48.8 (325/666) | 40.2 (267/664) | 85.6 (570/666) | 62.8 (417/664) |
| 2nd tertile | 71.5 (477/667) | 79.5 (530/667) | 51.1 (341/667) | 49.3 (328/666) | 81.0 (540/667) | 57.7 (384/666) |
| 3rd tertile | 66.1 (441/667) | 72.7 (485/667) | 46.3 (309/667) | 49.3 (328/666) | 77.4 (516/667) | 50.2 (334/666) |
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| 1st tertile | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref |
| 2nd tertile | 0.82 (0.73, 0.91) | 0.73 (0.65, 0.81) | 0.90 (0.80, 1.01) | 1.12 (1.00, 1.26) | 0.76 (0.67, 0.86) | 0.83 (0.75, 0.93) |
| 3rd tertile | 0.63 (0.49, 0.81)2 | 0.49 (0.38, 0.63)2 | 0.78 (0.60, 1.03) | 1.30 (1.00, 1.68) | 0.54 (0.40, 0.71)2 | 0.66 (0.52, 0.84)2 |
| 1st tertile | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref |
| 2nd tertile | 0.73 (0.65, 0.83) | 0.67 (0.58, 0.78) | 0.95 (0.84, 1.07) | 0.94 (0.85, 1.04) | 0.76 (0.67, 0.87) | 0.77 (0.69, 0.85) |
| 3rd tertile | 0.50 (0.38, 0.65)2 | 0.41 (0.29, 0.57)2 | 0.89 (0.68, 1.17) | 0.87 (0.69, 1.10) | 0.54 (0.40, 0.74)2 | 0.55 (0.43, 0.70)2 |
| 1st tertile | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref |
| 2nd tertile | 0.73 (0.64, 0.84) | 0.66 (0.57, 0.78) | 0.95 (0.84, 1.08) | 0.94 (0.85, 1.03) | 0.76 (0.67, 0.88) | 0.77 (0.69, 0.85) |
| 3rd tertile | 0.49 (0.36, 0.67)2 | 0.40 (0.27, 0.57)2 | 0.89 (0.67, 1.19) | 0.86 (0.69, 1.07) | 0.54 (0.40, 0.74)2 | 0.55 (0.44, 0.69)2 |
| 1st tertile | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref | 1.00 ref |
| 2nd tertile | 0.81 (0.71, 0.93) | 0.77 (0.64, 0.93) | 1.01 (0.88, 1.16) | 0.94 (0.85, 1.04) | 0.80 (0.70, 0.93) | 0.82 (0.74, 0.91) |
| 3rd tertile | 0.62 (0.45, 0.84)2 | 0.56 (0.36, 0.85)2 | 1.03 (0.76, 1.41) | 0.86 (0.68, 1.08) | 0.61 (0.44, 0.85)2 | 0.63 (0.50, 0.80)2 |
Results are presented for tertiles of plasma 25-hydroxyvitamin D (median [interquartile range] concentration, ng/mL, 1st tertile 12.1 [9.7,14.3]; 2nd tertile 20.3 [18.3, 22.7]; 3rd tertile 30.6 [27.5, 34.9]; odds ratio and 95% confidence interval (CI) of metabolic syndrome and its components in each of the two highest tertiles of plasma 25-hydroxyvitamin D concentration was compared with the lowest tertile by extrapolating the per unit change in estimated odds from the multivariate model; large waist circumference is defined as waist circumference ≥ 102 cm (male) and ≥ 88 cm (female); high blood pressure is defined as systolic blood pressure ≥ 130 mm Hg and/or diastolic blood pressure > 85 mm Hg or current antihypertensive drug treatment in a patient with a history of hypertension; high triglycerides is defined as triglycerides ≥ 150 mg/dL; high fasting plasma glucose is defined as fasting plasma glucose ≥ 100 mg/dL; low HDL is defined as HDL < 40 mg/dL in men and < 50 mg/dL in women; to convert plasma 25OHD concentration from ng/mL to nmol/L multiply by 2.459; to convert triglycerides from mg/dL to mmol/L multiply by 0.0113; to convert glucose from mg/dL to mmol/L multiply by 0.0555; to convert HDL- cholesterol from mg/dL to mmol/L multiply by 0.0259.
p-value for trend was less than 0.01.
Association between plasma 25OHD concentration and individual metabolic syndrome components
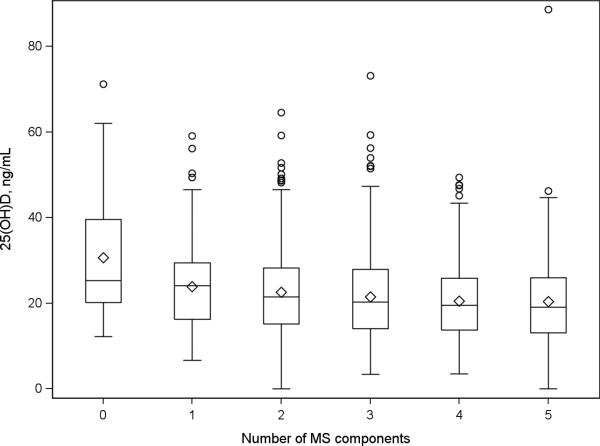
There was a small but statistically significant inverse association between 25OHD concentration and the number of metabolic syndrome components (Spearman correlation coefficient, r=−0.11, p<0.0001) (Figure 1). There was a significant inverse linear trend in the multivariate adjusted odds of metabolic syndrome across increasing tertiles of 25OHD for three components of metabolic syndrome, larger waist circumference, higher fasting plasma glucose and lower HDL cholesterol (Table 2). There was no statistically significant difference in the prevalence of high triglyceride concentration or high blood pressure according to 25OHD tertiles; however the odds ratios were in the same direction as other components.
Figure 1.
Cross-sectional association between 25-hydroxyvitamin D (25OHD) and the number of metabolic syndrome components.
Association between plasma 25OHD concentration and insulin sensitivity and insulin secretion
Insulin sensitivity significantly increased across tertiles of 25OHD (adjusted average difference 0.143; 95% CI 0.081 to 0.212) (Table 4). Conversely, insulinogenic index decreased across 25OHD tertiles (adjusted average difference −0.098; 95%CI −0.158 to −0.025). The disposition indices increased across 25OHD tertiles, although only the DI1 was statistically significantly different (adjusted average difference 0.348; 95% CI 0.089 to 0.607).
Table 4.
Adjusted average difference of non-traditional components of metabolic syndrome factors by tertiles of continuous plasma 25-hydroxyvitamin D concentration in the lifestyle and placebo arms of the Diabetes Prevention Program population.
| No. of participants | Tertile of 25- hydroxyvitamin D concentration | P value | |||
|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 (Highest) | |||
| Number of participants | 2000 | N = 666 | N = 667 | N = 667 | --- |
| Number with MS | 2000 | N = 504 | N = 477 | N = 441 | --- |
| Plasma 25-hydroxyvitamin D concentration, median (interquartile range), ng/mL | 2000 | 12.1 (9.7, 14.3) | 20.3 (18.3, 22.7) | 30.6 (27.5, 34.9) | --- |
| Adjusted average difference (95% confidence interval) | |||||
| 1Insulin sensitivity, μU/mL−1 | 1957 | 0.00 (reference) | 0.061 (0.035, 0.089) | 0.143 (0.081, 0.212) | < 0.001 |
| 1Insulin sensitivity index, [(μU/mL)*( mg/dL)]-1 | 1957 | 0.00 (reference) | 0.070 (0.041, 0.101) | 0.164 (0.095, 0.241) | < 0.001 |
| 1Insulinogenic index, IGI (μU/mL)/(mg/dL) | 1893 | 0.00 (reference) | −0.045 (−0.074, −0.011) | −0.098 (−0.158, −0.025) | 0.0111 |
| 1Corrected insulin response, CIR (μU/mL)/(mg/dL)2 | 1926 | 0.00 (reference) | −0.039 (−0.065, −0.009) | −0.086 (−0.141, −0.020) | 0.013 |
| Disposition Index 1 (LN(IGI) * LN(Insulin sensitivity)) | 1893 | 0.00 (reference) | 0.154 (0.039, 0.268) | 0.348 (0.089, 0.607) | 0.0083 |
| Disposition Index 2 (LN(CIR) * LN(Insulin sensitivity index)) | 1925 | 0.00 (reference) | 0.016 (−0.042, 0.074) | 0.037 (−0.095, 0.168) | 0.5855 |
Results are presented for tertiles of plasma 25-hydroxyvitamin D concentration; differences in each of the two highest tertiles was compared with the lowest tertile by calculating the difference in predicted values from the multivariate model; all models are adjusted for recruitment site and month of blood draw, age (years), gender (male or female), race (black, white, or other), ultraviolet radiation index at participant's study site (mean annual in 1997, 90J/m2/hour), smoking status at baseline (never, past, or currently smoking), alcohol consumption (g/day), C-reactive protein (mg/L), self-reported physical activity (MET-hours per week), total energy intake (kcal/day), and body mass index; to convert plasma 25-hydroxyvitamin D concentration from ng/mL to nmol/L multiply by 2.459.
Differences between tertiles for log transformed dependent variables are presented in the original scale.
Subgroup Analyses
The inverse associations between 25OHD concentration and metabolic syndrome were generally consistent across all subgroups (Table 5) and did not differ by age, gender or race. The association between 25OHD and metabolic syndrome appeared to be stronger among non-obese versus obese. However, the study was not powered to assess the significance of the association across subgroups and the tests for interaction were not statistically significant for any of these factors (Table 5, p for interactions >0.05).
Table 5.
Subgroup analyses for odds of metabolic syndrome by tertiles of continuous plasma 25-hydroxyvitamin D concentration in the lifestyle and placebo arms of the Diabetes Prevention Program cohort by subgroups
| No. of participants | Tertile of 25-hydroxyvitamin D concentration | P value | P value for interaction | |||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3(Highest) | ||||
| Number of participants | 2000 | N = 666 | N = 667 | N = 667 | --- | --- |
| Number with MS | 2000 | N = 504 | N = 477 | N = 441 | --- | --- |
| Plasma 25-hydroxyvitamin D concentration, median (interquartile range), ng/mL | 2000 | 12.1 (9.7, 14.3) | 20.3 (18.3, 22.7) | 30.6 (27.5, 34.9) | --- | --- |
| Odds ratio (95% confidence interval) | ||||||
| Age (median, y) | 0.1190 | |||||
| < 50 | 932 | 1.00 (reference) | 0.83 (0.71, 0.96) | 0.65 (0.46, 0.92) | 0.0154 | |
| Participants (number with Metabolic Syndrome) | 359 (272) | 280 (208) | 293 (199) | |||
| >= 50 | 1027 | 1.00 (reference) | 0.79 (0.65, 0.96) | 0.59 (0.38, 0.90) | 0.0153 | |
| Participants (number with Metabolic Syndrome) | 289 (219) | 375 (260) | 363 (235) | |||
| Gender | 0.5047 | |||||
| Female | 1308 | 1.00 (reference) | 0.84 (0.71, 0.98) | 0.67 (0.47, 0.97) | 0.0317 | |
| Participants (number with Metabolic Syndrome) | 486 (369) | 412 (295) | 410 (274) | |||
| Male | 651 | 1.00 (reference) | 0.75 (0.58, 0.99) | 0.53 (0.29, 0.97) | 0.0393 | |
| Participants (number with Metabolic Syndrome) | 162 (122) | 243 (173) | 246 (160) | |||
| Race | 0.7822 | |||||
| White | 1128 | 1.00 (reference) | 0.86 (0.72, 1.02) | 0.71 (0.48, 1.05) | 0.0841 | |
| Participants (number with Metabolic Syndrome) | 242 (194) | 407 (305) | 479 (318) | |||
| Non-White | 831 | 1.00 (reference) | 0.80 (0.65, 0.99) | 0.61 (0.38, 0.98) | 0.0393 | |
| Participants (number with Metabolic Syndrome) | 406 (297) | 248 (163) | 177 (116) | |||
| Body mass index (kg/m2) | 0.0648 | |||||
| < 30 | 637 | 1.00 (reference) | 0.76 (0.58, 1.00) | 0.54 (0.29, 1.00) | 0.0503 | |
| Participants (number with Metabolic Syndrome) | 138 (79) | 221 (119) | 278 (136) | |||
| >= 30 | 1322 | 1.00 (reference) | 0.85 (0.71, 1.01) | 0.69 (0.46, 1.02) | 0.0596 | |
| Participants (number with Metabolic Syndrome) | 510 (412) | 434 (349) | 378 (298) | |||
Results are presented for tertiles of plasma 25-hydroxyvitamin D concentration at the baseline visit of the Diabetes Prevention Program cohort; odds ratio of metabolic syndrome in each of the two highest tertiles was compared with the lowest tertile by extrapolating the per unit change in estimated odds from the multivariate model; all models are adjusted for recruitment site and month of blood draw; to convert plasma 25-hydroxyvitamin D concentration from ng/mL to nmol/L multiply by 2.459. Subgroup models adjusted for recruitment location, month of blood draw, age (years), gender (male or female; except for analysis by gender), race (black, white, or other; except for analysis by race), ultraviolet radiation index at participant's study site (mean annual in 1997, 90J/m2/hour), smoking status at baseline (never, past, or currently smoking), alcohol consumption (g/day), C-reactive protein (mg/L), self-reported physical activity (MET-hours per week), total energy intake (kcal/day), and body mass index.
Association between plasma 25OHD concentration and incident metabolic syndrome
In the prospective analysis, after multivariate adjustment including treatment arm, participants in the highest tertile of 25OHD (median 25OHD 31 ng/mL) had a non-significant lower risk for developing metabolic syndrome (HR 0.79; 95% CI, 0.48 to 1.32) compared to participants in the lowest tertile (median 25OHD 12.3 ng/mL) (Table 3). After adjusting further for change in body weight, the direction of the association remained the same (HR 0.83; 95%CI 0.50, 1.39).
Table 3.
Risk of incident metabolic syndrome during the first 2 years of follow-up by tertiles of continuous plasma 25-hydroxyvitamin D concentration in the lifestyle and placebo arms of the Diabetes Prevention Program.
| No. of person years | No. of participants | Tertile of 25-hydroxyvitamin D concentration | P value | |||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 (Highest) | ||||
| Number of participants at baseline (events) | 162 (53) | 190 (68) | 226 (60) | |||
| Plasma 25-hydroxyvitamin D concentration at baseline, median (interquartile range), ng/mL | --- | 578 | 12.3 (10.0, 14.7) | 20.4 (18.4, 23.1) | 31.0 (27.4, 36.8) | --- |
| Hazard ratio (95% confidence interval) | ||||||
| Unadjusted Model | 949 | 546 | 1.00 (reference) | 0.82 (0.70, 0.98) | 0.65 (0.44, 0.95) | 0.0261 |
| Model adjusted for age, gender | 949 | 546 | 1.00 (reference) | 0.85 (0.71, 1.00) | 0.68 (0.46, 1.01) | 0.0542 |
| Multivariate model 1 | 945 | 544 | 1.00 (reference) | 0.81 (0.65, 1.01) | 0.62 (0.38, 1.01) | 0.0562 |
| Multivariate model and baseline weight 2 | 945 | 544 | 1.00 (reference) | 0.87 (0.70, 1.09) | 0.73 (0.44, 1.21) | 0.2207 |
| Multivariate model and treatment arm 3 | 945 | 544 | 1.00 (reference) | 0.90 (0.72, 1.13) | 0.79 (0.48, 1.32) | 0.3749 |
| Multivariate model and change in weight 4 | 945 | 544 | 1.00 (reference) | 0.92 (0.73, 1.16) | 0.83 (0.50, 1.39) | 0.4831 |
Results are presented for baseline tertiles of plasma 25-hydroxyvitamin D concentration; hazard ratio of diabetes in each of the two highest tertiles was compared with the lowest tertile by extrapolating the per unit change in estimated hazard from the multivariate model; variables measured at multiple time points throughout the study (25-hydroxyvitamin D and physical activity) entered the analyses as time-varying “lagged” covariates, as the mean of the previous and current visit at which diabetes status was assessed; to convert plasma 25- hydroxyvitamin D concentration from ng/mL to nmol/L multiply by 2.459
Adjusted for recruitment location, age (years), gender (male or female), month of blood draw, race (black, white, or other), smoking status at baseline (never, past, or currently smoking), alcohol consumption (average of values self-reported at baseline and 1-year follow-up visit, g/day), C-reactive protein (average of values at baseline, 1-year follow-up visits, mg/L), and self-reported physical activity (MET-hours per week) and total energy intake (average of values self-reported at baseline and 1-year follow-up visit, kcal/day) plus ultraviolet radiation index at participant's recruitment location (mean annual in 1997, 90J/m2/hour).
Adjusted for everything in in footnote (1) plus baseline body weight (kg)
Adjusted for everything in in footnote (2) plus treatment arm (intensive lifestyle or placebo)
Adjusted for everything in in footnote (3) plus change in body weight from previous non-missing visit
DISCUSSION
In a large multiethnic population at increased risk for diabetes, plasma 25OHD concentration was inversely associated with prevalence of metabolic syndrome and risk of developing metabolic syndrome although the latter association lost statistical significance after multivariate adjustment. The prevalence of larger waist circumference, lower HDL-cholesterol and higher fasting plasma glucose was lower with increasing 25OHD levels. Although the difference in mean 25OHD between those with and those without metabolic syndrome did not appear to be large, increasing 25OHD concentration was associated with lower prevalence of metabolic syndrome, especially when comparing those in the highest tertile (mean 30.6 ng/mL) vs. the lowest quartile (12.1 ng/mL) of 25OHD concentration. Insulin sensitivity was greater and insulin secretion was lower with increasing levels of 25OHD.
The biological mechanisms by which vitamin D may influence cardiometabolic risk factors have not been completely elucidated. There is a growing body of evidence suggesting that vitamin D plays a role in insulin resistance, which is generally regarded as the central mechanism for metabolic syndrome.27 On the other hand, insulin secretion is central to development of hyperglycemia. Vitamin D may enhance insulin sensitivity in several ways, including increasing the expression of insulin receptors,28 activating transcription factors important in glucose homeostasis29 or indirectly via regulating calcium, which is essential for insulin-mediated intracellular processes. In vivo and in vitro studies have also shown an effect of vitamin D on insulin secretion.30-32 The effect on beta cell function is likely mediated by binding of the active form, 1,25(OH)2D, to vitamin D receptor, which is expressed in beta cells33 or by the activation of vitamin D which may occur within the beta cell by the 25-OHD-1α-hydroxylase (CYP27B1), which is expressed in beta cells.34 Vitamin D can also affect beta-cell function indirectly via calcium regulation, which in turn affects insulin secretion, a calcium-dependent process.35
Our results from the cross-sectional analysis are consistent with, and build on, the results of other studies.3, 7, 9-11, 36-39 Based on data from the third National Health and Nutrition Examination Survey (NHANES III),9 25OHD concentration was inversely associated with metabolic syndrome but not after adjustment for BMI. In contrast, our results remained significant after adjustment for BMI suggesting that the relationship between vitamin D and metabolic syndrome is independent of obesity. More recently, Reis et al showed an inverse association between vitamin D and metabolic syndrome in the NHANES; however, the study was limited by the inability to account for the season in which blood samples were obtained.36 The same authors had previously failed to show this association between vitamin D and metabolic syndrome in the Rancho-Bernardo study, which included US residents from southern California, which may – at least in part – be attributed to generally higher vitamin D levels.12 The mean level of vitamin D in the current study was 21.6 ng/mL, which is about 50% lower than the mean levels among participants from the Rancho Bernardo study. It is possible that there is a threshold or range for the association between vitamin D and metabolic syndrome.
Results from other prospective observational studies on 25OHD and incident metabolic syndrome are inconsistent. Forouhi et al. found that higher baseline 25OHD was associated with lower metabolic syndrome risk after 10 years of follow-up; however the association lost significant after multivariate adjustment, similarly to our results.40 On the other hand, Gagnon et al. found an inverse association between vitamin D and metabolic syndrome, where the incidence of metabolic syndrome was higher in the lowest vitamin D quintile (25OHD < 18ng/mL) compared to the highest quintile (25OHD ≥ 34 ng/mL), (OR 1.41; 95%CI 1.02-1.95).41 Our results showed an inverse association, which was non-statistically significant possibly due to inadequate statistical power and also the fact that the DPP study included an intervention known to improve many of the components of metabolic syndrome.
There are well-recognized differences in vitamin D metabolism among different race/ethnic groups; 16 In our study, the observed cross-sectional association did not differ by race, as a proxy for altered vitamin D homeostasis in persons with dark skin,42 suggesting that in persons at high risk for diabetes, vitamin D may be important in modulating cardiometabolic risk independent of race/ethnicity. However, it is important to note that our study was not powered to test for differences in ethnic groups.
The complementary changes in insulin sensitivity and insulin secretion are in line with some observational studies that have reported an association between vitamin D status and insulin sensitivity.40, 43-45 However, previous studies assessing the association between 25OHD and beta cell function have yielded inconsistent results.38, 46 This is likely secondary to use of different measures of beta-cell function and lack of concurrent adjustment for insulin resistance. In the present study, disposition index, a measure of insulin secretion that accounts for the prevailing insulin sensitivity, and a validated predictor of diabetes risk, increased across 25OHD tertiles indicating improved beta cell function among participants with higher 25OHD concentration. These results are consistent with our previous findings in the DPP cohort, where higher 25OHD concentration was associated with a lower rate of progression to type 2 diabetes.47
Our study has a number of strengths. Primarily, we used data from a large multiethnic sample reflecting the diversity of the U.S. population with pre-diabetes. Our analyses took into account many potential covariates that might confound the observed association and we used validated measurements of the exposure and outcome variables and covariates; nevertheless, residual confounding remains a potential limitation. Additionally, in the cross-sectional analysis, the potential of reverse causation cannot be ruled out. And since the study is observational, we refrain from making any from statements about optimal 25OHD concentration. In the prospective analysis, the lack of significant inverse association between 25OHD and metabolic syndrome could be attributed to the lack of power. Finally, the use of a single 25OHD measurement may not capture overall vitamin D status due to geographical and seasonal variation;48 however, our analyses adjusted for recruitment location and month of blood collection and in addition we have used the mean of repeated measures of 25OHD for the prospective analysis.
In conclusion, higher plasma 25OHD concentration was associated with a lower prevalence of metabolic syndrome among persons at increased high risk of diabetes and a lower, but non-statistically significant, risk of incident metabolic syndrome. A causal relationship needs to be established in randomized trials of vitamin D supplementation in high-risk populations.
Acknowledgements
We thank David Kent, MD MSc. Associate Professor of Medicine, Director of the Clinical and Translational Science MS/PhD Program at the Sackler School for his valuable input into interpretation of the results. We also acknowledge the commitment and dedication of the DPP participants. A portion of the work was presented at the 2012 Endocrine Society meeting as an oral presentation, on June 23rd, 2012, in Houston, TX.
Source of funding: By research grants R01DK79003, U34DK091958 and U01DK098245 (to AGP) from the National Institute of Diabetes and Digestive and Kidney Disease, the Office Of The Director - National Institutes of Health, and the National Institutes of Health Office of Dietary Supplements; UL1RR025752 (to Tufts University) from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health. The US Department of Agriculture Agreement 58-1950-9001 (to BDH); the Marilyn Fishman Grant for Diabetes Research (to JM) from the Endocrine Fellows Foundation; U01DK48489 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health to the DPP clinical centers and the Coordinating Center for the design and conduct of the DPP study. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Research on Women's Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention, the Indian Health Service, and the American Diabetes Association. Lipha (Merck-Sante) provided medication. LifeScan Inc., Merck-Medco Managed Care, Inc., and Merck and Co. donated materials, equipment, or medicines for concomitant conditions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the above listed institutions.
Footnotes
Potential Financial Conflicts of Interest: None disclosed.
References
- 1.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32(7):1278–83. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beydoun MA, Boueiz A, Shroff MR, Beydoun HA, Wang Y, Zonderman AB. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in adults in the United States. J Clin Endocrinol Metab. 2010;95(8):3814–27. doi: 10.1210/jc.2010-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayaniyil S, Vieth R, Harris SB, Retnakaran R, Knight JA, Gerstein HC, et al. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab. 2011;96(1):168–75. doi: 10.1210/jc.2010-1439. [DOI] [PubMed] [Google Scholar]
- 6.Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr. 2011;94(1):225–33. doi: 10.3945/ajcn.111.013516. [DOI] [PubMed] [Google Scholar]
- 7.Lee DM, Rutter MK, O'Neill TW, Boonen S, Vanderschueren D, Bouillon R, et al. Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur J Endocrinol. 2009;161(6):947–54. doi: 10.1530/EJE-09-0496. [DOI] [PubMed] [Google Scholar]
- 8.He JL, Scragg RK. Vitamin D, parathyroid hormone, and blood pressure in the National Health and Nutrition Examination Surveys. Am J Hypertens. 2011;24(8):911–7. doi: 10.1038/ajh.2011.73. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28(5):1228–30. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 10.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vazquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26(5):573–80. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57(2):298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 12.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30(6):1549–55. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 13.Hjelmesaeth J, Hofso D, Aasheim ET, Jenssen T, Moan J, Hager H, et al. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study. Cardiovasc Diabetol. 2009;8:7. doi: 10.1186/1475-2840-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutrition journal. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rueda S, Fernandez-Fernandez C, Romero F, Martinez de Osaba J, Vidal J. Vitamin D, PTH, and the metabolic syndrome in severely obese subjects. Obes Surg. 2008;18(2):151–4. doi: 10.1007/s11695-007-9352-3. [DOI] [PubMed] [Google Scholar]
- 16.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470–3. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, et al. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35(12):2313–6. [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286–92. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151(2):190–8. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 22.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–14. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32(2):335–41. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(Suppl):S5–S9. [PubMed] [Google Scholar]
- 25.Mayer-Davis EJ, Sparks KC, Hirst K, Costacou T, Lovejoy JC, Regensteiner JG, et al. Dietary intake in the diabetes prevention program cohort: baseline and 1-year post randomization. Ann Epidemiol. 2004;14(10):763–72. doi: 10.1016/j.annepidem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Annals of internal medicine. 2005;142(8):611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 28.Maestro B, Molero S, Bajo S, Davila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct. 2002;20(3):227–32. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 29.Dunlop TW, Vaisanen S, Frank C, Molnar F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005;349(2):248–60. doi: 10.1016/j.jmb.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 30.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest. 1984;73(3):759–66. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka Y, Seino Y, Ishida M, Yamaoka K, Yabuuchi H, Ishida H, et al. Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol (Copenh) 1984;105(4):528–33. doi: 10.1530/acta.0.1050528. [DOI] [PubMed] [Google Scholar]
- 32.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119(1):84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267(3 Pt 1):E356–60. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 34.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90(1-5):121–5. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 35.Sergeev IN, Rhoten WB. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136(7):2852–61. doi: 10.1210/endo.136.7.7789310. [DOI] [PubMed] [Google Scholar]
- 36.Reis JP, von Muhlen D, Miller ER., 3rd. Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol. 2008;159(1):41–8. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 37.Kim MK, Il Kang M, Won Oh K, Kwon HS, Lee JH, Lee WC, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 2010;73(3):330–8. doi: 10.1111/j.1365-2265.2010.03798.x. [DOI] [PubMed] [Google Scholar]
- 38.Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–81. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chacko SA, Song Y, Manson JE, Van Horn L, Eaton C, Martin LW, et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am J Clin Nutr. 2011;94(1):209–17. doi: 10.3945/ajcn.110.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57(10):2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab. 2012;97(6):1953–61. doi: 10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 42.Harris SS. Does vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans? Am J Clin Nutr. 2011;93(5):1175S–8S. doi: 10.3945/ajcn.110.003491. [DOI] [PubMed] [Google Scholar]
- 43.Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, et al. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139(2):329–34. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 45.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33(6):1373–5. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulseth HL, Gjelstad IM, Tierney AC, Lovegrove JA, Defoort C, Blaak EE, et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care. 2010;33(4):923–5. doi: 10.2337/dc09-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pittas AG, Nelson J, Mitri J, Hillmann W, Garganta C, Nathan DM, et al. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care. 2012;35(3):565–73. doi: 10.2337/dc11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoben AB, Kestenbaum B, Levin G, Hoofnagle AN, Psaty BM, Siscovick DS, et al. Seasonal variation in 25-hydroxyvitamin d concentrations in the cardiovascular health study. Am J Epidemiol. 2011;174(12):1363–72. doi: 10.1093/aje/kwr258. [DOI] [PMC free article] [PubMed] [Google Scholar]