Abstract
Alterations in peripheral nervous system (PNS) insulin support may contribute to diabetic neuropathy (DN); yet, PNS insulin signaling is not fully defined. Here, we investigated in vivo insulin signaling in the PNS and compared the insulin-responsiveness to that of muscle, liver, and adipose. Nondiabetic mice were administered increasing doses of insulin to define a dose response relationship between insulin and Akt activation in the DRG and sciatic nerve. Resulting EC50 doses were used to characterize the PNS insulin signaling time course and make comparisons between insulin signaling in the PNS and other peripheral tissues (i.e., muscle, liver, adipose). The results demonstrate that the PNS is responsive to insulin and that differences in insulin signaling pathway activation exist between PNS compartments. At a therapeutically relevant dose, Akt was activated in the muscle, liver, and adipose at 30 minutes, correlating with the changes in blood glucose levels. Interestingly, the sciatic nerve showed a similar signaling profile as insulin-sensitive tissues, however there was not a comparable activation in the DRG or spinal cord. These results present new evidence regarding PNS insulin signaling pathways in vivo and provide a baseline for studies investigating the contribution of disrupted PNS insulin signaling to DN pathogenesis.
Keywords: diabetic neuropathy, neuronal Akt activation, neuronal insulin signaling, peripheral nervous system
Introduction
Approximately 366 million patients are currently diagnosed with diabetes worldwide, and projections indicate that approximately 552 million will be diagnosed by 2030 (Whiting et al., 2011). Conservative estimates suggest that 40–50% of diabetic patients suffer from peripheral neuropathy (Zochodne, 2007; CDC, 2011). Diabetic neuropathy (DN) presents with chronic pain or peripheral insensitivity and is a major contributing factor to lower limb amputation in the United States (Zochodne, 2007; CDC, 2011). Many mechanisms of DN pathogenesis have been investigated (Vincent et al., 2011), yet few therapeutic options are available (Apfel et al., 2000; Chalk et al., 2007; Habib and Brannagan, 2010).
An attractive mechanism of DN that is receiving additional attention is the loss of neuronal insulin signaling, either through insulinopenia or insulin resistance (Brussee et al., 2004; Toth et al., 2006b; Romanovsky et al., 2010; Grote et al., 2011; Singh et al., 2012). Mounting evidence has established insulin as an important neurotrophic factor in both the central and peripheral nervous system. Insulin induces neurite outgrowth (Recio-Pinto et al., 1986; Fernyhough et al., 1989), facilitates in vivo nerve regeneration (Xu et al., 2004; Toth et al., 2006a; Guo et al., 2011), and improves memory formation (Haj-ali et al., 2009; Craft et al., 2011).
Insulin signaling begins with activation of the insulin receptor tyrosine kinase that then phosphorylates tyrosine residues on docking proteins, such as insulin receptor substrate (IRS). Tyrosine phosphorylation of IRS allows downstream mediators to bind and propagate the signal. Insulin activates both the PI3K-Akt pathway as well as the MAPK pathway. Cellular actions of insulin in “insulin sensitive tissues” (i.e., muscle, liver, and adipose) include: increased glucose uptake, decreased gluconeogenesis, as well as increased glycogen, protein, and lipid synthesis (for review, see Le Roith and Zick, 2001).
Unfortunately, a lack of information regarding insulin signaling in vivo in the PNS has hindered our understanding of the role insulin signaling may play in DN. Varying results using insulin supplementation have been published with respect to the timing, dose, and delivery method needed for proper insulin signaling and functional changes (Xu et al., 2004; Toth et al., 2006a; Francis et al., 2009). Insulin signaling has been investigated in primary DRG culture, but translation of these results is difficult due to the severely altered microenvironment. Furthermore, culture models do not allow for the simultaneous comparison between tissues or provide information about the physiological effects of insulin signaling (Grote et al., 2011; Kim et al., 2011). The aim of the current study was to begin to establish PNS insulin signaling physiologic parameters in response to systemically delivered insulin. Delineating the PNS insulin signaling pathway should provide insight about insulin function in the PNS and how disruptions in those mechanisms may lead to DN.
Our results indicate that while PNS insulin signaling is dose dependent, high doses of intraperitoneal insulin were needed to activate signaling pathways in DRG neurons. In fact, only moderate insulin signaling activation was observed in the PNS as compared to the robust response of muscle, liver, and adipose following insulin administration. These studies provide new information related to PNS insulin signaling in comparison to other insulin-sensitive tissues and will be crucial in guiding future research aimed at delineating the role of insulin in DN pathogenesis.
Materials and Methods
Animals
All experiments were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. Male nondiabetic C57bl/6 mice aged 8 to 11 weeks were used for all studies. Mice were given access to food and water ad libitum and housed on a 12-hour light/dark cycle. Mice were fasted 3 hours prior to the start of all experiments and data collection. Tissues collected included the lumbar DRG, sciatic nerve, lumbar enlargement of spinal cord, liver, gastrocnemius muscle, and epididymal fat pad.
Antibodies and reagents
Humulin R insulin (Eli Lilly, Indianapolis, IN) was used for all experiments. Blood glucose levels were measured via tail clip with a glucose diagnostic assay (Sigma, St. Louis, MO). All antibodies for Western blot analysis were purchased from Cell Signaling (Danvers, MA) unless otherwise noted: total Akt, p(Ser473)Akt, p(Thr308)Akt, total GSK3β, p(Ser9)GSK3β, total p44/42 MAPK (ERK1/2), p(Thr202/Tyr204)p44/42 MAPK (ERK1/2), total mTor, p(Ser2448)mTor, total AS160 (Millipore, Billerica, MA), and p(Thr642)AS160 (Millipore). Secondary antibodies included: HRP-conjugated anti-mouse and anti-rabbit (Santa Cruz, Santa Cruz, CA).
Insulin dosing
Insulin was delivered via intraperitoneal injection for all experiments and sterile PBS was used as a vehicle control. To establish an insulin dose curve, doses were increased from 0.01 U/kg to 10,000 U/kg. Accordingly, minimum and maximum doses given were approximately 0.00025 and 250 units, respectively (assuming a 25 gram mouse). A 30-min insulin stimulation timeframe was used for these studies based on previous observations of PNS insulin signaling. Blood glucose levels were measured immediately prior to insulin administration and directly following the 30-min insulin stimulation.
An additional experiment was run to investigate PNS insulin signaling at a “therapeutically relevant” insulin dose, defined as a dose sufficient to decrease blood glucose levels without causing signs of hypoglycemia. An insulin dose of 1.29 U/kg (3 times the dose curve glucose percent change EC50) was delivered via IP injection and mice were sacrificed 0.5, 2, 4, and 6 h thereafter. Glucose levels were measured before insulin administration and at every subsequent time point.
Mice were carefully monitored for clinical signs of hypoglycemia (lethargy, tremor, or coma) during all experiments. At all doses administered for the dose curve experiment, mice did not show clinical signs of hypoglycemia. 60 min after an insulin dose of 33.05 U/Kg mice become lethargic. Accordingly, no time points beyond 60 min were investigated at a dose of 33.05 U/Kg. Mice showed no clinical signs of hypoglycemia during studies using a 1.29 U/Kg insulin dose.
Western blot analysis
At sacrifice, tissues were harvested and snap frozen in liquid nitrogen. Samples were homogenized in cell extraction buffer (Invitrogen, Carlsbad, CA) containing 55.55 µl/ml protease inhibitor cocktail, 200 mM Na3VO4, and 200 mM NaF and incubated on ice for 60 min for protein extraction. Following centrifugation, the protein concentration of the supernatant was measured via Bradford assay (Bio-Rad, Hercules, CA) and samples were boiled with lane marker reducing sample buffer (Thermo Scientific, Waltham, MA). 30 µg of protein was loaded per sample. Samples were separated on a 4–15% gradient tris-glycine gel (Bio-Rad) and transferred to a nitrocellulose membrane. Following incubation with primary and secondary antibodies, resultant bands were visualized with film and analyzed with ImageJ (NIH). All Western blot data is reported as the band density of the phospho-protein normalized to the band density of the total protein.
Statistical analysis
All data are expressed as means ± standard error of the mean. Insulin dose curve data was analyzed with a non-linear fit curve. The resultant EC50’s and R squared values are reported. Dose curve data was also analyzed with a 1-way ANOVA and Dunnett’s post-hoc to compare all groups back to control. Experiments investigating signaling time courses and 1.29 U/Kg insulin doses were also analyzed with a 1-way ANOVA and Dunnett’s post-hoc. Outliers greater than or less than 2 standard deviations from the mean were not included in the analysis. All statistical tests were performed using GraphPad Prism software and a P value <0.05 was considered significant.
Results
Insulin induced Akt activation is dose and time dependent in the PNS
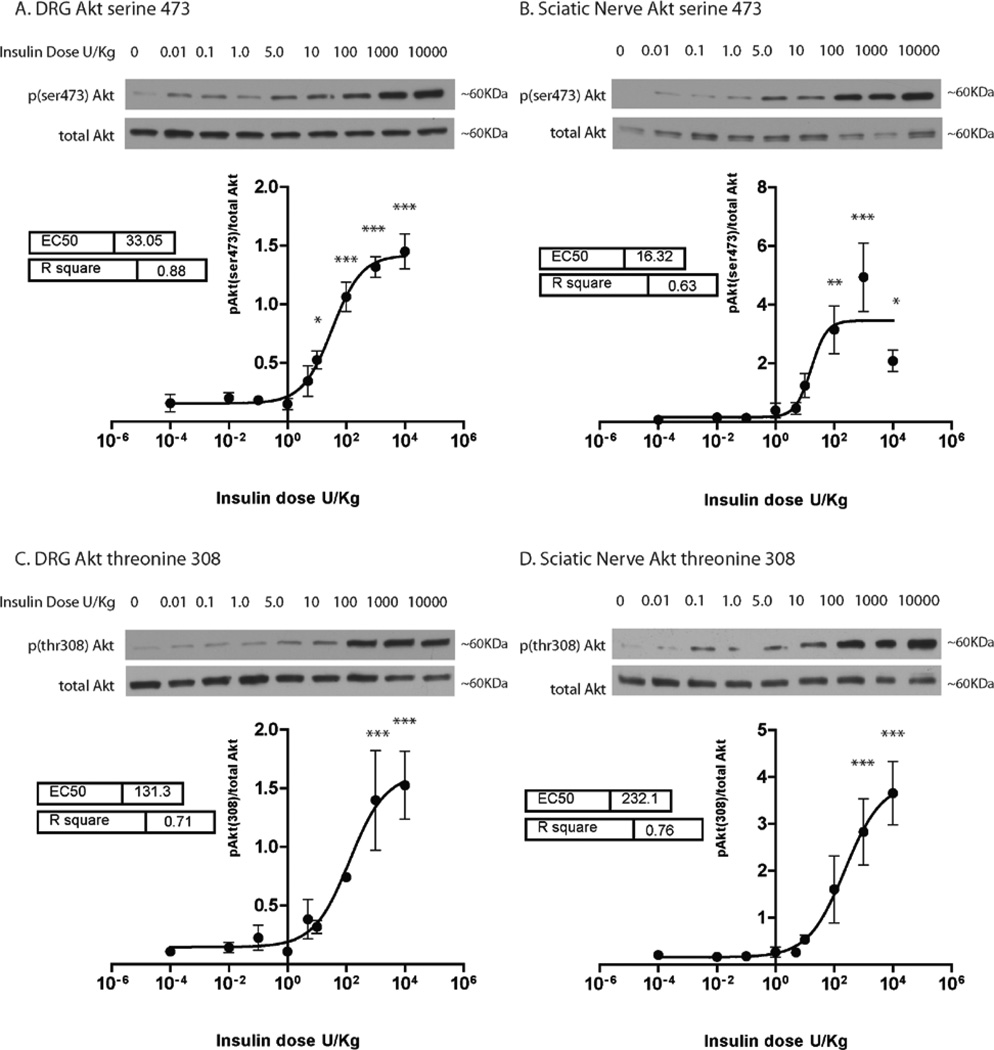
Akt is a major downstream mediator of insulin signaling and Akt phosphorylation (ser473 via mTorc2 and thr308 via PDK1) can be used to quantify insulin signal transduction. Here, insulin was delivered via IP injection in increasing doses and PNS insulin signaling was quantified with Western blots of activated Akt (p(Ser473)Akt/total Akt and p(Thr308)Akt/total Akt) in the DRG and sciatic nerve. Analysis of dose response curves indicated that insulin induced Akt ser473 and thr308 phosphorylation is dose dependent in both the DRG and sciatic nerve (Fig. 1). At the maximum dose of 10,000 U/kg the fold change for Akt ser473 phosphorylation as compared to baseline in the DRG and sciatic nerve was 9.2 and 30.5, respectively. Analysis of Akt thr308 phosphorylation indicates the fold change at 10,000 U/Kg as compared to baseline was 14.0 in the DRG and 18.1 in the sciatic nerve.
Figure 1. Insulin-induced Akt activation is dose dependent in the DRG and sciatic nerve.
Mice were administered insulin via IP injections at doses of 0.01, 0.1, 1.0, 5.0, 10.0, 100.0, 1,000.0 and 10,000 U/kg. Sterile PBS was used as a vehicle control. 30 min after insulin injection, Akt phosphorylation at sites serine 473 (A and B) and threonine 308 (C and D) were analyzed in the DRG and sciatic nerve and normalized to total Akt levels. Data was fit with a sigmoidal dose response curve and analyzed with a 1-way ANOVA and Dunnett’s post hoc. Results indicate that Akt activation in the DRG (A and C) and sciatic nerve (B and D) increased in a dose dependent manner with insulin. n=4–5 mice per dose. *=p<0.05, **=p<0.01, ***=p<0.001.
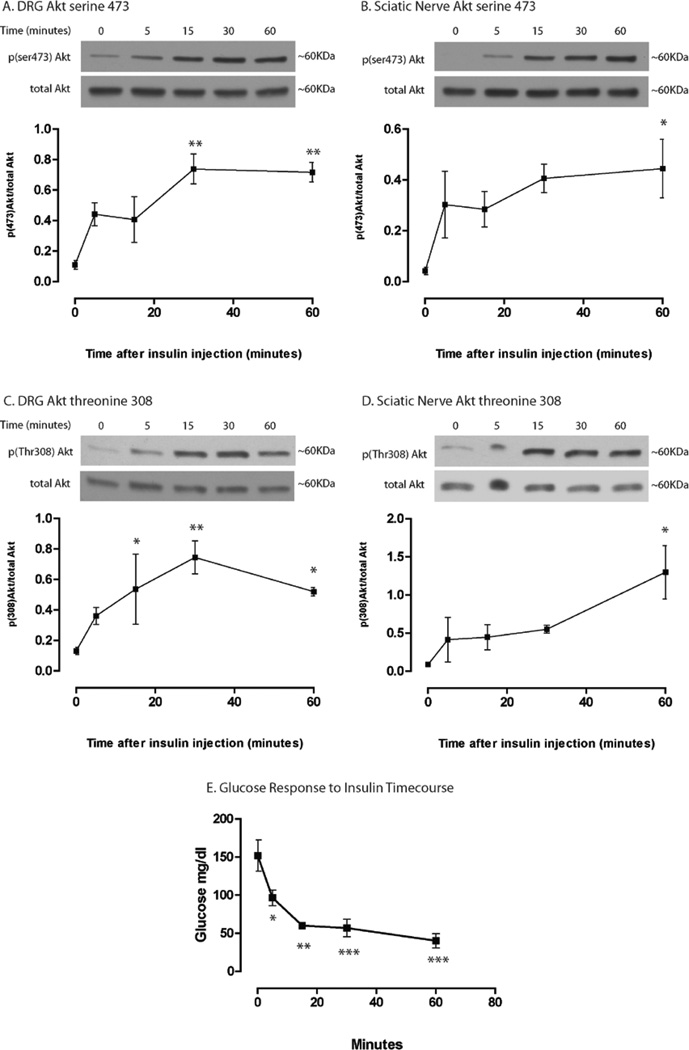
In addition to dose-sensitivity, insulin signaling in the PNS is time sensitive. To establish the PNS insulin signaling time course profile, mice were given IP injections of insulin at 33.05 U/Kg (DRG Akt ser473 phosphorylation EC50) and the DRG and sciatic nerve were harvested at 0, 5, 15, 30, and 60 min after insulin administration. At this dose, Akt activation was evident 5 min after insulin injection and appeared to be maximal at approximately 30 min in the DRG at both activation sites, ser473 (6.5-fold change, Fig. 2.2A) and thr308 (5.7-fold change, Fig. 2C). In the sciatic nerve, Akt activation appeared to continue increasing out to the 60-min time point at phosphorylation sites, ser473 (14.0-fold change, Fig. 2B) and thr308 (15.1-fold change, Fig. 2D). For comparison, blood glucose levels were also collected at these time points (Fig. 2E). Glucose levels decreased throughout the study; however, the rate of decrease was much slower from 15 to 60 min after an initial steep decline from 0 to 15 min. Experiments were not carried out past 60 min at this dose to avoid hypoglycemia.
Figure 2. Insulin-induced Akt activation time course in DRG and sciatic nerve.
Mice were administered 33.05 U/Kg insulin and Akt activation in the DRG (A and C) and sciatic nerve (B and D) was analyzed via Western blot at 5, 15, 30, and 60 min post insulin injection. Akt activation appears to be maximal around 30 min in the DRG and 60 min in the sciatic nerve. Glucose levels were significantly decreased 5 min after insulin injection. Results were analyzed with a 1-way ANOVA and Dunnett’s post hoc. N=3 mice per time point. *=p<0.05, **=p<0.01, ***=p<0.001.
Downstream insulin signaling pathway activation in the PNS
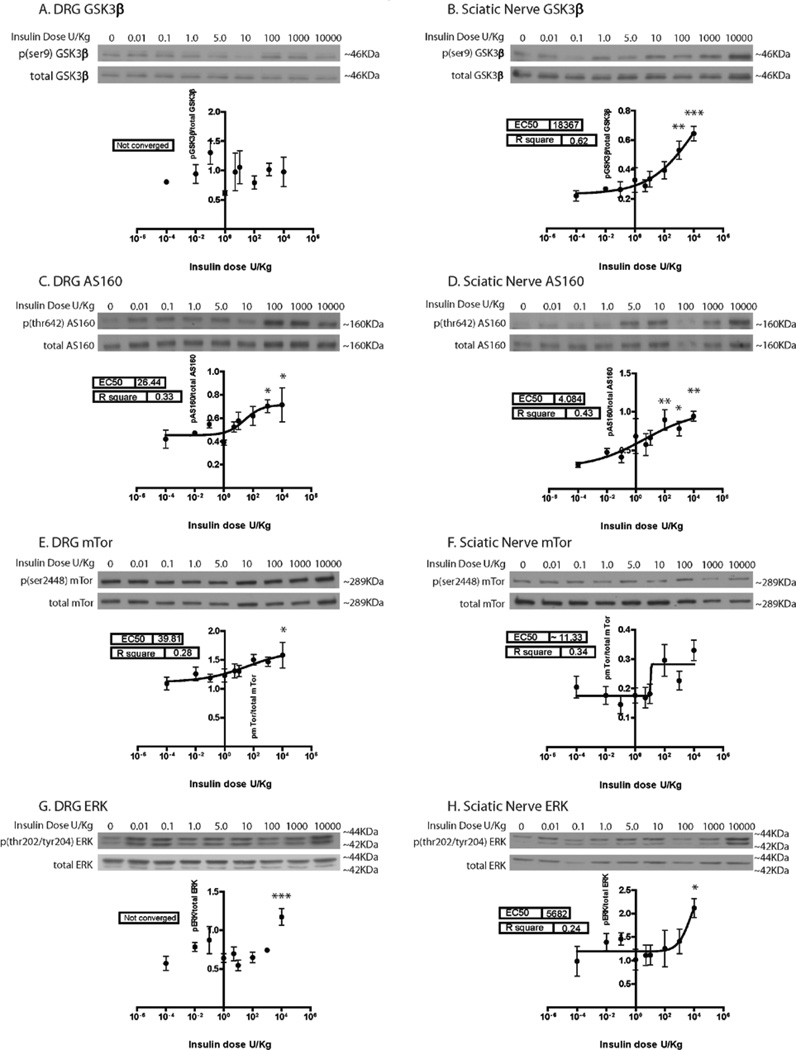
Downstream mediators of insulin-induced Akt activation in muscle, liver, and adipose tissue were investigated in the PNS, including GSK3β inhibition (glycogen synthesis), activation of mTor (protein synthesis), and activation of AS160 (glucose uptake). In addition, ERK activation was tested to evaluate Akt independent pathways (results are summarized in Table 1). Increasing phospho to total protein ratios represent increasing GSK3β inactivation, but increasing activation for AS160, mTor, and ERK. All proteins investigated responded to insulin in a dose-dependent manner in the sciatic nerve (Fig. 3). However, in the DRG, GSK3β (Fig. 3A) and ERK (Fig. 3G) signaling did not show a dose dependent relationship with insulin. In the sciatic nerve, it appeared that although both GSK3β (Fig. 3B) and ERK (Fig. 3H) signaling was dose dependent, a plateau level was not reached. Dunnett’s post hoc shows that at a dose of 10,000 U/Kg, GSK3β in the DRG (Fig. 3A) and mTor in the sciatic nerve (Fig. 3F) were the only points that did not show significant change as compared to baseline.
Table 1. Summary of insulin dose curve insulin signaling pathway activation in the DRG and sciatic nerve.
Analyzed results of in vivo insulin signaling in the DRG and sciatic nerve in response to an insulin dose curve indicate that both sensory neuron cell bodies and the peripheral nerve are insulin responsive. Interestingly, differences in downstream signaling may exist between the DRG and sciatic nerve. Data was fit with a sigmoidal dose response curve and analyzed with a 1-way ANOVA and Dunnett’s post hoc. “not converged” indicates that the sigmoidal dose response curve equation was unable to fit the data.
| Protein of Interest | Dose Curve EC50 | Dose Curve R2value | 10000U/kg Insulin-induced fold change as compared to baseline |
|||
|---|---|---|---|---|---|---|
| DRG | Sciatic Nerve | DRG | Sciatic Nerve | DRG | Sciatic Nerve | |
| Akt (t308) | 33.05 | 16.3 | 0.71 | 0.76 | 13.9*** | 18.1*** |
| Akt(s473) | 131.3 | 232.1 | 0.88 | 0.63 | 9.2*** | 30.5* |
| GSK3β (s9) | not converged | 18367.0 | not converted | 0.62 | 1.2 | 2.9*** |
| AS160(t642) | 26.4 | 4.1 | 0.33 | 0.43 | 1.7* | 3.0** |
| mTor (s2448) | 39.8 | ~11.3 | 0.28 | 0.34 | 1.4* | 1.6 |
| ERK (Thr202/Tyr204) | not converted | 5682.0 | not converted | 0.24 | 2.1*** | 1.8* |
=p<0.05,
=p<0.01,
=p<0.001.
Figure 3. Insulin dose curve downstream mediator activation.
In addition to Akt, the in vivo signaling of several other proteins in the insulin pathway was investigated in the DRG and sciatic nerve in response to an IP insulin dose curve. Data was fit with a sigmoidal dose response curve and analyzed with a 1-way ANOVA and Dunnet’s post hoc. The sigmoidal dose response curve was unable to fit the GSK3β (A) and ERK (F) results in the DRG (not converged). All other proteins appear to have a dose dependent relationship with insulin. The overall ANOVA p-value was significant for all proteins investigated except mTor and GSK3β in the DRG and ERK in the SN. Results of Dunnett’s post hoc are denoted with *. n=4–5 mice per dose. *=p<0.05, **=p<0.01, ***=p<0.001.
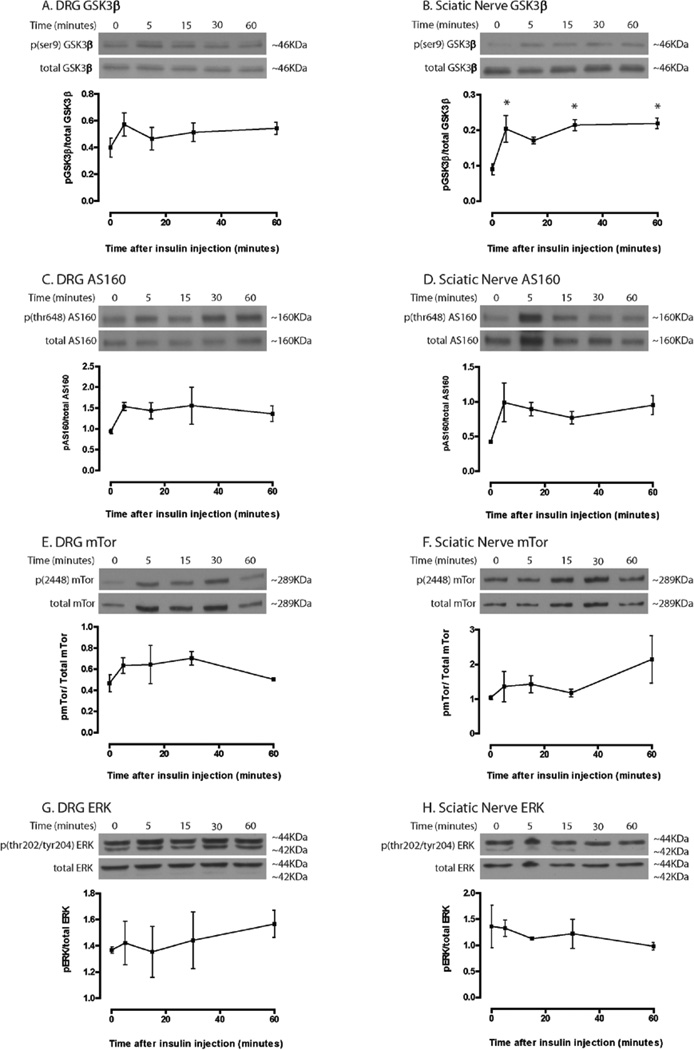
The time course of downstream mediator activation was also investigated at an insulin dose of 33.05 U/Kg. Only GSK3β phosphorylation in the sciatic nerve showed significant changes under these experimental conditions (Fig. 4), suggesting that either a longer timeframe or higher insulin doses are needed to effectively study downstream insulin signaling in the PNS.
Figure 4. Time course of insulin signaling downstream mediator activation.
In contrast to Akt results, few significant changes were observed in downstream insulin signaling throughout the time course at a dose of 33.05 U/Kg. Only GSK3β in the sciatic nerve showed significant differences from baseline when analyzed with a 1-way ANOVA and Dunnett’s post hoc (Fig. 4B). n=3 mice per time point. *=p<0.05, **=p<0.01, ***=p<0.001.
Differences in insulin signaling exist between PNS and “classically” insulin sensitive tissues
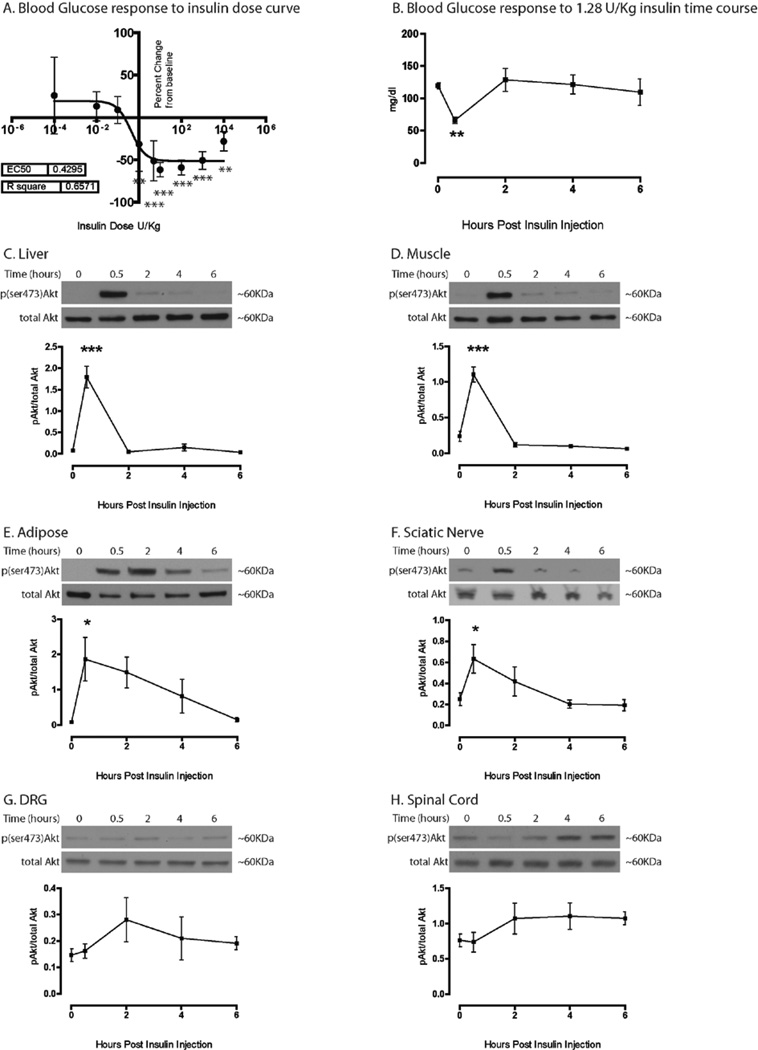
An interesting observation from the dose curve experiments was the large difference between the EC50 for glucose percent change (0.43 U/kg, Fig. 5A) and the EC50 for Akt activation in the DRG (33.05 U/kg, Fig. 1A), approximately a 77-fold difference. Furthermore, it is recognized that 33.05 U/Kg is a supraphysiological insulin dose and potentially lethal in humans (Megarbane et al., 2007). To further explore the difference in glucose percent change and PNS insulin signaling at a more physiological insulin dose; Akt activation was tested over a 6 h period in the PNS as well as in muscle, liver, and adipose at a “therapeutically relevant” dose. An insulin dose 3 times the EC50 for glucose percent change, 1.29 U/kg, was used for these experiments. Time points chosen were based on a previous publication investigating the delivery of insulin to neural structures via intranasal or subcutaneous injections (Francis et al., 2009). As expected, significant increases in Akt activation were seen in the liver (21.8-fold change, Fig. 5C), muscle (4.6-fold change, Fig. 5D), and adipose (21.5-fold change, Fig. 5E) at 30 min, correlating with the change in glucose levels (Fig. 5B). However, of the neural tissues investigated, only the sciatic nerve demonstrated a significant increase in Akt activation (2.5-fold change) at 30 min (Fig. 5F). No significant changes in Akt activation were observed in the DRG or spinal cord at all-time points investigated (Fig. 5G and 5H, respectively). These results differ from previous results presented using higher insulin doses; indicating the dose and timeframe of insulin signaling in the PNS are codependent.
Figure 5. “Therapeutic” insulin dose Akt activation time course in liver, muscle, adipose, sciatic nerve, DRG, and spinal cord.
The EC50 for glucose percent change was 0.43 U/Kg in insulin dose curve studies (A). A 6-h time course study using 1.29 U/Kg insulin showed a significant decrease in glucose levels 30 min after insulin injection, with a return to baseline by 2 h (B). Significant Akt activation was observed at 30 min post insulin in the liver (C), muscle (D), adipose (E), and sciatic nerve (F). No significant changes were seen in the DRG (G) or lumbar spinal cord (H). Data was analyzed with a 1-way ANOVA and Dunnett’s post hoc. n=6 at 0 time point, n=6 at 30 min time point, n=4 at 2 h time point, n=5 at 4 h time point, n=3 at 6 h time point. *=p<0.05, **=p<0.01, ***=p<0.001.
Discussion
Due to the growing incidence of diabetes, diabetic complications are becoming alarmingly prevalent. DN occurs with an elevated incidence as compared to other complications and is associated with dramatic decreases in patient quality of life (CDC, 2011). The goal of this study was to establish the profile of insulin signaling in the PNS in vivo to better understand how disruptions in PNS insulin signaling may impact sensory neuron function. Our results indicate that the PNS is clearly insulin responsive and that differences in insulin signaling may exist between somal and axonal compartments. Furthermore, it also appears that PNS insulin signaling is comparably less sensitive than muscle, liver, and adipose at a therapeutically relevant dose. Importantly, these results will further guide researchers when completing in vivo studies evaluating the impact of altered insulin signaling on DN pathogenesis.
One consistent theme throughout the presented data was an increased insulin response in the sciatic nerve as compared to the DRG. For several of the investigated proteins, a higher fold change in activation (inhibition for GSK3β) was observed in the sciatic nerve. These results closely mimic previous observations of insulin signaling in the PNS using intrathecal injections (Grote, unpublished observations). Moreover, at a “therapeutically relevant” insulin dose, only the sciatic nerve showed a significant increase in Akt activation. It is plausible that the postmitotic DRG neurons are buffered from large swings in insulin levels and rely more on basal insulin for support, whereas the Schwann cells of the peripheral nerve readily react to changing insulin levels via Akt activation to modulate proliferation (Fex Svenningsen and Kanje, 1996), differentiation (Ogata et al., 2004) and myelination (Liang et al., 2007). In fact, it has been recently discovered that Schwann cells express insulin receptors (Shetter et al., 2011) and that insulin receptor signaling regulates glycoprotein P0 expression (Shettar and Muttagi, 2012). Furthermore, Schwann cell dysfunction has been implicated in DN (Eckersley, 2002). Thus, we propose that reductions in insulin signaling associated with diabetes impact sensory neuron cell bodies, but also strongly affect axons and Schwann cells in the peripheral nerve, and perhaps diminish the regenerative/repair capacity of distal axons. It is plausible to suggest that in DN reduced PNS insulin signaling may be more of a propagating event than an inciting event, such that nerves cannot recover from hyperglycemia injury due to the lack of insulin support.
One important consideration when interpreting the data presented here is that these studies utilized a single IP injection of Humulin R insulin, and that difference in delivery (insulin pumps) or insulin formulations may give differing results. Furthermore, it should be noted that cross talk between insulin and the IGF1 receptor does occur (Fernyhough et al., 1993). Insulin at high concentrations can signal through the IGF1 receptor and activate many of the same intracellular pathways (namely Akt). Additionally, PNS insulin signaling is both dose and time dependent. Thus, using a different time frame (experiment was done at 30 min) would shift the curve and generate different EC50s.
While these studies have provided a new understanding of insulin signaling in the PNS, further research is needed to fully understand the physiologic role of insulin in the PNS. However, based on our findings, we can begin to extrapolate from the downstream mediators investigated. ERK, GSK3β, AS160, and mTor were chosen for investigation based on their known roles in the cellular actions of insulin in insulin-responsive tissues and their potential role in DN. ERK is a mediator of neurite outgrowth (Perron and Bixby, 1999), GSK3β has roles in neuronal plasticity and myelination (Peineau et al., 2008; Makoukji et al., 2012), AS160 is involved with glucose uptake (Kramer et al., 2006), and mTor can regulate neurite outgrowth via protein synthesis (Abe et al., 2010). Thus, disruptions in insulin regulation of these mediators may have detrimental effects on sensory neuron function and contribute to DN. The results of two downstream mediators were of particular interest, GSK3β and AS160. Inhibition of GSK3β was dose dependent in the sciatic nerve but not the DRG, suggesting that insulin may have divergent downstream signaling within different compartments of the PNS. Interestingly, inhibition of GSK3β has been shown to play a pivotal role in peripheral nerve remyelination (Makoukji et al., 2012) and this may occur via the PI3K-Akt pathway (Ogata et al., 2004). Reductions in insulin-induced GSK3β inhibition in the peripheral nerve may contribute to pathological changes in DN and warrant further investigation.
AS160 is predominantly thought to be involved in glucose uptake via translocation of Glut4 to the plasma membrane (Kramer et al., 2006). It is currently believed that DRG neurons and Schwann cells do not take up glucose in an insulin dependent manner (Patel et al., 1994; Magnani et al., 1998). Furthermore, similar to the CNS, the main glucose transporters present in the PNS include Glut1 and Glut3 (Muona et al., 1992; Magnani et al., 1996; Choeiri et al., 2002). The insulin-responsive transporter Glut4, is not strongly expressed in the PNS, but is expressed in discrete areas of the CNS (Leloup et al., 1996). Thus, a dose dependent increase in AS160 activation was surprising. These results suggest that either high dose insulin may trigger glucose uptake in the PNS or that AS160 may have a previously undiscovered function in these tissues. Further experiments are needed to fully elucidate these possibilities.
A growing trend in the literature indicates that direct administration of insulin to the nervous system has a greater neurotrophic potency as compared to systemic insulin administration (Brussee et al., 2004; Francis et al., 2009); an idea first proposed by Kan et al. (2012). The results presented here may explain some of this observed effect. The quick utilization of insulin by muscle, liver and adipose, may be acting as a “sink” and could reduce available insulin to the PNS and blunt a neurotrophic response. Furthermore, it appears that only the peripheral nerve is responsive to low doses of systemic insulin, whereas both the neuronal cell body and peripheral nerve are responsive to intrathecal insulin (Grote, unpublished observation). Insulin stimulation of both PNS compartments may produce a greater neurotrophic effect compared to peripheral nerve signaling only. These results suggest that while current insulin formulations and delivery methods are very adequate for reducing elevated glucose levels, they may not reach the nervous system appropriately, and thus fail to deliver the needed neurotrophic support. Perhaps the development and integration of better insulin delivery to the nervous system either through different routes (i.e., intranasal) or through insulin peptide modification is warranted.
In conclusion, these studies have established that the PNS is insulin responsive in vivo and that the peripheral nerve and DRG may have different insulin signaling profiles. This information provides a new basis for future experiments designed to explore the role of insulin in the PNS and how disruptions in PNS insulin signaling may be contributing to DN pathogenesis.
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel SC, Schwartz S, Adornato BT, Freeman R, Biton V, Rendell M, Vinik A, Giuliani M, Stevens JC, Barbano R, Dyck PJ. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284:2215–2221. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824–1830. doi: 10.2337/diabetes.53.7.1824. [DOI] [PubMed] [Google Scholar]
- CDC. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Centers for Disease Control and Prevention, Atlanta, GA: US Department of Health and Human Services; 2011. [Google Scholar]
- Chalk C, Benstead TJ, Moore F. Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database Syst Rev. 2007;4:CD004572. doi: 10.1002/14651858.CD004572.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience. 2002;111:19–34. doi: 10.1016/s0306-4522(01)00619-4. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2011;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Mill JF, Roberts JL, Ishii DN. Stabilization of tubulin mRNAs by insulin and insulin-like growth factor I during neurite formation. Brain Res Mol Brain Res. 1989;6:109–120. doi: 10.1016/0169-328x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain research. 1993;607:117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- Fex Svenningsen A, Kanje M. Insulin and the insulin-like growth factors I and II are mitogenic to cultured rat sciatic nerve segments and stimulate [3H]thymidine incorporation through their respective receptors. Glia. 1996;18:68–72. doi: 10.1002/(SICI)1098-1136(199609)18:1<68::AID-GLIA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH, 2nd, Toth C. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes. 2009;58:934–945. doi: 10.2337/db08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grote CW, Morris JK, Ryals JM, Geiger PC, Wright DE. Insulin receptor substrate 2 expression and involvement in neuronal insulin resistance in diabetic neuropathy. Exp Diabetes Res. 2011;2011:212571. doi: 10.1155/2011/212571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Kan M, Martinez JA, Zochodne DW. Local insulin and the rapid regrowth of diabetic epidermal axons. Neurobiol Dis. 2011;43:414–421. doi: 10.1016/j.nbd.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Habib AA, Brannagan TH., 3rd Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep. 2010;10:92–100. doi: 10.1007/s11910-010-0093-7. [DOI] [PubMed] [Google Scholar]
- Haj-ali V, Mohaddes G, Babri SH. Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci. 2009;123:1309–1314. doi: 10.1037/a0017722. [DOI] [PubMed] [Google Scholar]
- Kan M, Guo G, Singh B, Singh V, Zochodne DW. Glucagon-like peptide 1, insulin, sensory neurons, and diabetic neuropathy. J Neuropathol Exp Neurol. 2012;71:494–510. doi: 10.1097/NEN.0b013e3182580673. [DOI] [PubMed] [Google Scholar]
- Kim B, McLean LL, Philip SS, Feldman EL. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011;152:3638–3647. doi: 10.1210/en.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Zick Y. Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care. 2001;24:588–597. doi: 10.2337/diacare.24.3.588. [DOI] [PubMed] [Google Scholar]
- Leloup C, Arluison M, Kassis N, Lepetit N, Cartier N, Ferre P, Penicaud L. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res Mol Brain Res. 1996;38:45–53. doi: 10.1016/0169-328x(95)00306-d. [DOI] [PubMed] [Google Scholar]
- Liang G, Cline GW, Macica CM. IGF-1 stimulates de novo fatty acid biosynthesis by Schwann cells during myelination. Glia. 2007;55:632–641. doi: 10.1002/glia.20496. [DOI] [PubMed] [Google Scholar]
- Magnani P, Cherian PV, Gould GW, Greene DA, Sima AA, Brosius FC., 3rd Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metabolism. 1996;45:1466–1473. doi: 10.1016/s0026-0495(96)90174-2. [DOI] [PubMed] [Google Scholar]
- Magnani P, Thomas TP, Tennekoon G, DeVries GH, Greene DA, Brosius FC., 3rd Regulation of glucose transport in cultured Schwann cells. J Peripher Nerv Syst. 1998;3:28–36. [PubMed] [Google Scholar]
- Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G, Fledrich R, Fonte C, Branchu J, Goulard M, de Waele C, Charbonnier F, Sereda MW, Baulieu EE, Schumacher M, Bernard S, Massaad C. Lithium enhances remyelination of peripheral nerves. Proc Natl Acad Sci U S A. 2012;109:3973–3978. doi: 10.1073/pnas.1121367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megarbane B, Deye N, Bloch V, Sonneville R, Collet C, Launay JM, Baud FJ. Intentional overdose with insulin: prognostic factors and toxicokinetic/toxicodynamic profiles. Crit Care. 2007;11:R115. doi: 10.1186/cc6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muona P, Sollberg S, Peltonen J, Uitto J. Glucose transporters of rat peripheral nerve. Differential expression of GLUT1 gene by Schwann cells and perineural cells in vivo and in vitro. Diabetes. 1992;41:1587–1596. doi: 10.2337/diab.41.12.1587. [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NJ, Llewelyn JG, Wright DW, Thomas PK. Glucose and leucine uptake by rat dorsal root ganglia is not insulin sensitive. J Neurol Sci. 1994;121:159–162. doi: 10.1016/0022-510x(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153:S428–S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron JC, Bixby JL. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol Cell Neurosci. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E, Rechler MM, Ishii DN. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986;6:1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky D, Wang J, Al-Chaer ED, Stimers JR, Dobretsov M. Comparison of metabolic and neuropathy profiles of rats with streptozotocin-induced overt and moderate insulinopenia. Neuroscience. 2010;170:337–347. doi: 10.1016/j.neuroscience.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettar A, Muttagi G. Developmental regulation of insulin receptor gene in sciatic nerves and role of insulin on glycoprotein P0 in the Schwann cells. Peptides. 2012;36:46–53. doi: 10.1016/j.peptides.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Shetter AR, Muttagi G, Sagar CB. Expression and localization of insulin receptors in dissociated primary cultures of rat Schwann cells. Cell Biol Int. 2011;35:299–304. doi: 10.1042/CBI20100523. [DOI] [PubMed] [Google Scholar]
- Singh B, Xu Y, McLaughlin T, Singh V, Martinez JA, Krishnan A, Zochodne DW. Resistance to trophic neurite outgrowth of sensory neurons exposed to insulin. J Neurochem. 2012;121:263–276. doi: 10.1111/j.1471-4159.2012.07681.x. [DOI] [PubMed] [Google Scholar]
- Toth C, Brussee V, Martinez JA, McDonald D, Cunningham FA, Zochodne DW. Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience. 2006a;139:429–449. doi: 10.1016/j.neuroscience.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia. 2006b;49:1081–1088. doi: 10.1007/s00125-006-0169-8. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. Insulin as an in vivo growth factor. Exp Neurol. 2004;188:43–51. doi: 10.1016/j.expneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve. 2007;36:144–166. doi: 10.1002/mus.20785. [DOI] [PubMed] [Google Scholar]