Abstract
Lung maturation is regulated by interactions between mesenchymal and epithelial cells, and is delayed by androgens. Fibroblast–Type II cell communications are dependent on extracellular signal-regulated kinases (ERK) 1/2 activation by the ErbB receptor ligands epidermal growth factor (EGF), transforming growth factor (TGF)-α, and neuregulin (Nrg). In other tissues, dihydrotestosterone (DHT) has been shown to activate SRC by a novel nontranscriptional mechanism, which phosphorylates EGF receptors to potentiate EGF-induced ERK1/2 activation. This study sought to determine if DHT potentiates EGFR signaling by a nontranscriptional mechanism. Embryonic day (E)17 fetal lung cells were isolated from dams treated with or without DHT since E12. Cells were exposed to 30 ng/ml DHT for periods of 30 minutes to 3 days before being stimulated with 100 ng/ml EGF, TGF-α, or Nrg for up to 30 minutes. Lysates were immunoblotted for ErbB and SRC pathway signaling intermediates. DHT increased ERK1/2 activation by EGF, TGF-α, and Nrg in fibroblasts and Type II cells. Characterization in fibroblasts showed that potentiation of the EGF pathway was significant after 60 minutes of DHT exposure and persisted in the presence of the translational inhibitor cycloheximide. SRC and EGF receptor phosphorylation was increased by DHT, as was EGF-induced SHC1 phosphorylation and subsequent association with GRB2. Finally, SRC silencing, SRC inhibition with PP2, and overexpression of a dominant-negative SRC each prevented DHT from increasing EGF-induced ERK1/2 phosphorylation. These results suggest that DHT activates SRC to potentiate the signaling pathway leading from the EGF receptor to ERK activation in primary fetal lung fibroblasts.
Keywords: ErbB, androgens, fibroblasts, MAP kinases
Clinical Relevance
Fetal lung maturation is regulated by growth factor signaling through ERK1/ERK2 and is retarded by androgens. Androgens canonically regulate gene transcription and have been associated with decreased ERK activity in vivo. Signaling assays show that dihydrotestosterone stimulation potentiates EGF activation of ERK1/2. This potentiation is mediated by SRC kinase through a rapid nontranscriptional mechanism. Additional mechanisms must be invoked to reconcile this finding with the decreased ERK activation observed in vivo.
Premature male infants have long been known to be at greater risk for neonatal respiratory distress syndrome than their female counterparts (1–3). This observation has been attributed to developmental androgen expression, which delays lung maturation (4). Exposure of female fetuses to dihydrotestosterone (DHT) inhibits surfactant production, whereas antiandrogen treatment increases surfactant synthesis in male fetuses.
Lung maturation, as assessed by surfactant synthesis, is regulated by communications between fibroblasts and adjacent epithelial Type II cells (5–11). Among the mediators of this communication are neuregulin (Nrg), a peptide growth factor that is expressed by fibroblasts to stimulate epithelial ErbB-4 receptor tyrosine kinases (12). Fibroblasts, in turn, are regulated by peptide growth factors (13) that culminate in the activation of extracellular signal-regulated kinases (ERK) 1 and 2. Among the most potent examples are epidermal growth factor (EGF) and transforming growth factor (TGF)-α, which signal through ErbB-1 receptors (13, 14). In fetal lung fibroblasts, peak EGF receptor (EGFR) activity coincides with the onset of surfactant production in Type II cells, suggesting that its ligands activate fibroblast ERK1/2 to regulate fetal lung Type II cell maturation (14).
Androgens act on fetal lung fibroblast to delay the development of fibroblast–Type II cell communication (15, 16). The previously described peak in EGFR activity occurs later in the male fetuses relative to female fetuses (17), suggesting that androgens regulate EGF signaling. Moreover, delayed lung maturation in the male fetus and the response of the fetal lung to androgen are dependent on the presence of functional androgen receptors (15, 18).
The specific effects of androgens on EGF signaling appear to depend on the duration of exposure. Acute DHT treatment of cultured late-gestation primary fetal lung fibroblasts increases EGF binding and EGFR protein expression (19). In contrast, fibroblasts isolated from animals treated with DHT for 7 days have reduced EGF binding and EGF-induced receptor phosphorylation (20).
Androgens regulate ErbB signaling through transcriptional or nontranscriptional mechanisms (21). Canonically, androgen ligand/receptor complexes translocate to the nucleus, where they regulate gene transcription. The resulting changes in cell behavior require new protein synthesis and are manifested over hours or days. Alternatively, androgen/receptor complexes can exert rapid changes in cell signaling that are independent of protein translation. Perhaps the best characterized androgen signaling pathway is mediated by c-src, a 60-kD nonreceptor protooncogenic tyrosine kinase that is activated by multiple signaling processes, including that of EGF (22). The c-src protein SRC is anchored to the plasma membrane by N-terminal myristylation, and its C-terminal tyrosine kinase is autoinhibited by association with its N-terminal SH3 domain (23). DHT-bound androgen receptor binds to the SRC SH3 domain, thereby releasing the kinase domain. SRC can then modulate ERK1/2 by phosphorylating SHC1 proteins to initiate the Ras-Raf-MEK-ERK pathway (24) or by phosphorylating EGFRs and increasing their ligand-induced activity (25). The experiments described herein suggest that DHT potentiates ERK1/2 signaling within 1 hour, leading us to hypothesize that androgens regulate ERK activation by a nontranscriptional mechanism.
Materials and Methods
In Utero DHT Exposure, Cell Isolation, and Culture
Pregnant Swiss Webster mice received subcutaneous DHT (1 mg/d) or vehicle beginning on Day 12 of gestation (26–28) under a protocol approved by the Tufts Medical Center IACUC. Dams were killed by CO2 inhalation at embryonic day (E)17. The fetal lungs were isolated and dissociated with 0.1% trypsin, and the fibroblasts and Type II cells were isolated by differential adherence (29, 30). Cells were maintained in Dulbecco’s modified Eagle medium with 10% charcoal-stripped FBS at 37°C in 5% CO2 and received DHT (30 ng/ml) during culture. Fetal lung fibroblasts were similarly isolated from untreated mice at E17 and cultured in the absence of DHT.
Signaling Analyses
At 80% confluence, cells were starved overnight in serum-free Dulbecco’s modified Eagle medium. For acute exposures, cells were first treated with DHT (30 ng/ml) for up to 24 hours. Cells were stimulated with 100 ng/ml EGF, TGF-α, or Nrg for up to 30 minutes and lysed in RIPA buffer (150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 0.1% SDS, 50 mM Tris [pH 8.0], 2 mM EDTA, 1 mM NaVO4, 10 mM NaF) with Complete protease inhibitor (Roche, Indianapolis, IN).
ERK1/2 pathway activation was estimated by ERK1/2 phosphorylation, SHC1 phosphorylation, and SHC1 association with GRB2. SRC activation was assessed by phosphorylation of Tyr424 or Tyr535. Lysate protein contents were quantified by micro BCA (Pierce, Rockford, IL), equalized, resolved by 10% SDS-PAGE, and subjected to chemiluminescence immunoblotting (Pierce). Phosphoprotein antibodies against murine SRC Tyr424 (44660G; Life Technologies, Carlsbad, CA and #6943; Cell Signaling Technologies [CST] Beverly, MA), SRC Tyr527 (CST #2105), EGFR Tyr1148 (CST #4404), EGFR Tyr845 (CST #2231), and ERK1/2 Thr202/Tyr204 (CST #4377) were used. Phosphoprotein content was quantitated densitometrically and normalized against total ERK1/2 (CST #4695), EGFR (ab2430; Abcam, Cambridge, MA), or SRC (Invitrogen) within the same samples but generally on separate blots. To assess SHC1 phosphorylation and GRB2 association, lysates were precipitated overnight at 4°C with 1 μg SHC1 antibody (610081; BD Transduction Laboratories, San Diego, CA) and 10 μl immobilized anti-rabbit IgG (EY Laboratories, San Mateo, CA) per 100 μg total protein. Immunoprecipitates were immunoblotted for GRB2 (BD Transduction Laboratories) and phosphotyrosine (CST #9411).
To inhibit protein synthesis, cells were treated with cycloheximide (1 μg/ml) for 2 hours before DHT stimulation (31). Inhibition was confirmed by metabolic labeling with 0.1 mCi/ml 35S-methionine and autoradiography. To abrogate SRC function, cells were treated with the small molecule inhibitor PP2 or its control reagent PP3 (Calbiochem, San Diego, CA) (10 μM) for 2 hours before stimulation. Src was silenced using small interfering RNA (siRNA) S74383 from Invitrogen. Cells were transfected with 50 nM siRNA using Oligofectamine (Life Technologies). Viability was assessed by annexin V and propidium iodide staining and by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (both from Life Technologies). Adenoviruses encoding dominant-negative Src were provided by William H. Walker (University of Pittsburgh, Pittsburgh, PA) (32).
Immunoblot quantitations were compared by one- and two-way ANOVA or paired t test assuming equal variance.
Results
Intrauterine DHT Increases ERK1/2 Activation by ErbB Ligands in Isolated Fibroblasts and Epithelial Cells
Lung maturation is regulated by androgens, the ErbB-4 ligand Nrg, and the ErbB-1 ligands EGF and TGF-α (12). Because ErbB ligands canonically activate ERK MAP kinases, we hypothesized that ERK activation is regulated by androgens. To assess this, pregnant mice were treated with subcutaneous timed-release pellets containing DHT (1 mg/d) or vehicle beginning on Day 12 of gestation as previously described (26–28). Fetal lungs were harvested at Day 17 and dissociated into fibroblasts and Type II cells. Cells from DHT-exposed fetuses continued to receive DHT (30 ng/ml) during culture and were maintained for approximately 3 days until reaching 80% confluence. The cells were then starved in serum-free medium overnight before stimulation with no growth factor (labeled as 0 m) or with up to 100 ng/ml of EGF, TGF-α, or Nrg for 5 or 30 minutes.
EGF is a strong inducer of ERK1/2 phosphorylation in primary fetal lung fibroblasts (Figure 1A). Densitometric quantitation (see Figure E1A in the online supplement) indicates that EGF significantly increased ERK phosphorylation (by ANOVA, P = 0.011 for ERK1 and P = 0.00002 for ERK2; n = 5). TGF-α (Figure 1B) also significantly induced ERK1 and ERK2 (P = 0.015 for ERK1 and P = 0.00027 for ERK2; n = 4) (Figure E1B). Nrg did not significantly increase fibroblast ERK1 or ERK2 phosphorylation (n = 4) (Figures 1C and E1C). There was some experimental variation, but peak ERK1/2 phosphorylation occurred within 30 minutes of growth factor stimulation.
Figure 1.
Dihydrotestosterone (DHT) increases ErbB-induced extracellular signal-regulated (ERK) activation in primary canalicular stage Type II cells and fibroblasts. Fibroblasts and Type II cells continuously exposed to DHT since embryonic day (E)12 were isolated from E17 lungs, cultured in the presence of DHT until 80% confluent, and then stimulated with epidermal growth factor (EGF), transforming growth factor (TGF)-α, or neuregulin (Nrg) at 100 ng/ml for the indicated times. The resulting phosphorylation of ERK1 and ERK2 was detected by immunoblotting and normalized against total ERK1 and ERK 2 within the same samples. Images shown are paired phosphoERK1/2 and total ERK1/2 immunoblots of the same samples but not necessarily of the same membrane. EGF induced fibroblast ERK1/2 phosphorylation regardless of the presence or absence of DHT (A). However, DHT significantly increased EGF responsiveness (densitometric quantitation and statistical comparisons are provided as Figure E1). (B) TGF-α also activated fibroblast ERK1 and ERK2. This activation was significantly increased by DHT. (C) Nrg had nonsignificant effects on fibroblast ERK1/2 induction in the absence of DHT. In its presence, Nrg responsiveness increased and became significant. DHT had similar effects on primary fetal Type II cells. In the absence of DHT, EGF significantly activated ERK1 but not ERK2 (D). DHT treatment increased EGF-induced phosphorylation of ERK1 and ERK2 so that in its presence both were induced by EGF. (E) TGF-α conversely phosphorylated ERK2 but not ERK1. DHT treatment also increased TGF-α–induced phosphorylation of ERK1 and ERK2. (F) Nrg had insignificant effects on ERK1 and ERK2 phosphorylation in Type II cells. DHT treatment increased responsiveness to Nrg so that in its presence both ERKs were activated.
DHT increased the strength of ERK activation by all three ErbB ligands. Relative to untreated controls, fibroblasts exposed to DHT before EGF stimulation had a 1.8-fold greater peak ERK1 phosphorylation (P = 0.012; n = 5) and a 1.7-fold higher peak ERK2 phosphorylation (P = 0.035) (Figures 1A and E1A). Similarly, DHT increased peak TGF-α–induced phosphorylation of ERK1 by 2.0-fold (P = 0.015; n = 4) and ERK2 by 2.4-fold (P = 0.00027) (Figures 1B and E1B). DHT pretreatment also doubled the Nrg-induced phosphorylation of ERK1 and ERK2 (P = 0.0001 for both); in fact, Nrg significantly induced ERK1 phosphorylation only in the presence of DHT (P = 0.009; n = 4) (Figures 1C and E1C). Nrg induction of ERK2 approached significance in the presence of DHT (P = 0.053; n = 4).
When these experiments were repeated using cultured primary fetal lung Type II cells in the absence of DHT, ERK1 but not ERK2 was significantly activated by EGF (P = 0.038 for ERK1 and P = 0.093 for ERK2; n = 4) (Figures 1D and E1D). Conversely, TGF-α significantly induced ERK2 but not ERK1 (P = 0.15 for ERK and P = 0.017 for ERK2; n = 4) (Figures 1E and E1E). Nrg had insignificant effects on ERK1 and ERK2 phosphorylation (n = 4) (Figures 1F and E1F). These results indicate that, in the absence of DHT, ErbB ligands inconsistently activated ERK1/2 in primary Type II cells.
As in fibroblasts, DHT treatment significantly increased responsiveness to all three ligands. DHT increased peak EGF-induced ERK1 phosphorylation by a factor of 2.2 (P = 0.035; n = 3) and ERK2 phosphorylation by a factor of 2.4 (P = 0.00028) (Figures 1D and E1D). Likewise, DHT significantly increased peak TGF-α–induced ERK1 phosphorylation by 4.2-fold (P = 0.018; n = 4) and ERK2 by 1.8-fold (P = 0.020) (Figures 1E and E1E). The Nrg-induced ERK1 and ERK2 phosphorylation was significantly higher in the presence of DHT (5.8-fold higher for ERK1 with P = 0.00054 [n = 3] and 3.4-fold higher for ERK2; P = 0.00034), and only in its presence did Nrg have a significant effect on ERK activation (P = 0.0023 for ERK1 and P = 0.0034 for ERK2) (Figures 1F and E1F). These results suggest that DHT potentiates ERK activation by EGF, TGF-α, and Nrg in fibroblasts and Type II epithelial cells.
DHT Regulation of ERK Activation Is Independent of Protein Translation
Sexual dimorphism occurs in the context of differential sex hormone exposure over the entire gestational period. However, androgens also have immediate effects on cell signaling. We therefore sought to determine if the observed potentiation of ERK1/2 activation requires prolonged DHT exposure.
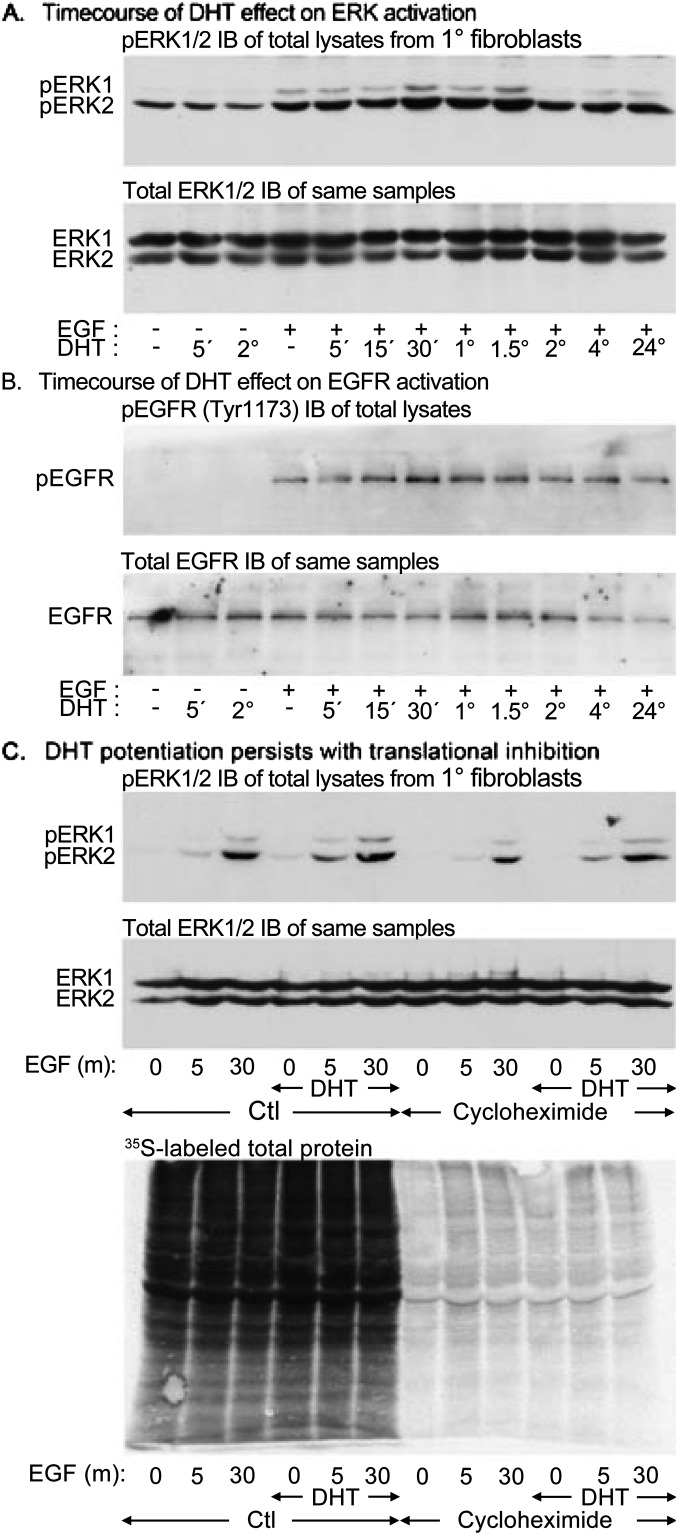
Primary fetal lung fibroblasts were isolated from untreated mice at E17 and exposed to DHT for periods of up to 24 hours. The cells were then uniformly stimulated with EGF 100 ng/ml for 5 minutes, and the subsequent ERK1/2 phosphorylation was assessed and normalized against the total ERK1/2 within each sample. DHT in the absence of EGF did not induce ERK activation (Figures 2A), but increasing DHT exposure did progressively potentiate EGF-induced ERK1 and ERK2 phosphorylation. Densitometric quantitation (Figure E2A) indicates that this potentiation became statistically significant at 1 hour of DHT exposure (P = 0.043 for ERK1 and P = 0.0071 for ERK2 by paired t test).
Figure 2.
DHT potentiation of EGF-induced ERK induction in fibroblasts requires relatively short DHT exposure and is independent of protein translation. DHT exposure potentiates ErbB responsiveness, but the mechanism is unclear. To begin characterization, the minimum duration of DHT exposure necessary to potentiate ERK activation was determined. Pulmonary fibroblasts were isolated from untreated E17 mice and exposed to 30 ng/ml DHT for up to 24 hours before uniform stimulation with 100 ng/ml EGF for 5 minutes. Phosphorylated ERK1/2 (pERK1 and pERK2) was detected by immunoblotting and normalized to total ERK1 and ERK2 content. (A) DHT potentiated EGF-induced ERK phosphorylation within 1 hour. Densitometric and statistical analyses are provided in Figure E2. (B) phosphorylated and total EGF receptor (pEGFR and EGFR) immunoblots of the same experiment. Normalized EGF-induced EGFR phosphorylation was also increased by 1 hour of DHT, consistent with receptor-level potentiation of EGF signaling. (C) The requirement for de novo protein synthesis in ERK potentiation by DHT was evaluated. Previously untreated primary pulmonary fibroblasts were exposed to the translational inhibitor cycloheximide (1 μg/ml) for 1 hour before the addition of 30 ng/ml DHT. After 1 hour, cells were stimulated with EGF, 100 ng/ml for 5 or 30 minutes. Top: pERK1/2 immunoblot of lysates showing that DHT potentiation of EGF-induced ERK phosphorylation persists in the presence of cycloheximide. The total ERK1/2 reprobe of the same blot is underneath. Bottom: Autoradiograph of resolved E17 fetal lung mesenchymal cell lysates treated identically with cycloheximide and DHT in the presence of 35S-methionine.
Potentiation of EGF signaling within 1 hour suggests that DHT acts via a transcriptionally independent signaling mechanism. One such mechanism is mediated by SRC, which is directly activated by DHT (32). Activated SRC phosphorylates the EGFR and potentiates its activation by EGF ligand. To determine if EGFR activation is potentiated by DHT in fetal lung cells, the samples described above were immunoblotted with an antibody against EGFR phosphorylated at Tyr1173 (Figure 2B). This site is autophosphorylated upon ligand binding to become a docking site for SHC1 (33), which mediates the activation of the ERK pathway downstream of EGFR. Ligand-induced EGFR phosphorylation was significantly increased by 60 minutes of DHT exposure (P = 0.0060 by paired t test) (Figure E2B). This finding is consistent with the hypothesis that DHT potentiation of ERK activation is mediated by the EGFR.
To confirm that the potentiation of ERK activation by DHT does not require de novo protein synthesis, E17 fetal mouse lung fibroblasts not previously exposed to DHT were treated with 1 μg/ml cycloheximide, an inhibitor of protein elongation (34), beginning 1 hour before DHT exposure and continuing through 1 hour of DHT and up to 30 minutes of EGF stimulation (Figure 2C, upper pair). In the absence of cycloheximide, DHT again potentiated EGF activation of ERK1 and ERK2 (P = 0.00045 and P = 0.00062 by ANOVA; n = 5) (Figure E2C), consistent with previous data. In the presence of cycloheximide, DHT continued to potentiate EGF-induced ERK1 and ERK2 phosphorylation (P = 0.00062 and P = 0.0022, respectively). The efficiency of translational inhibition by cycloheximide was confirmed by identically treating cells in the presence of 35S-methionine. Autoradiography confirmed that protein synthesis was abrogated by cycloheximide (Figure 2C, lower panel). These results confirm that DHT potentiation of EGF-induced ERK1/2 activation does not require protein synthesis.
DHT Potentiates SRC Activation to Increase ErbB1 Signaling
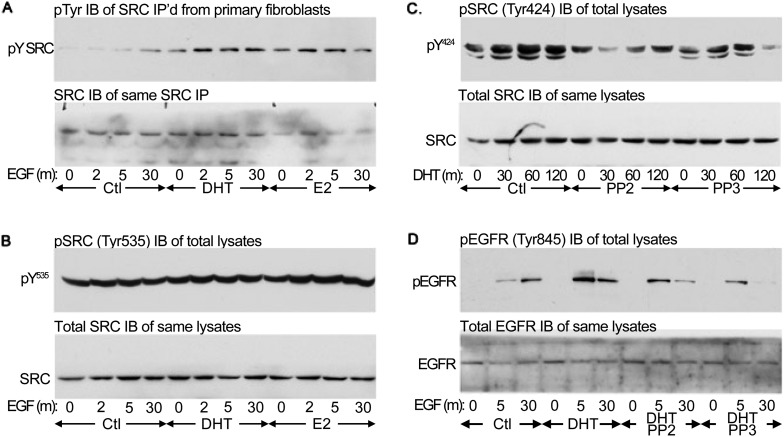
The foregoing data strongly suggest that ligand activation of the EGF signaling pathway is enhanced by DHT. In other systems, DHT has been shown to activate SRC, which phosphorylates EGFRs (32). To determine if SRC is activated by DHT, we examined SRC phosphorylation at Tyr424 (of murine SRC), which correlates with catalytic activity (35). Because SRC is also activated by estrogen receptors (36), we assessed EGF signaling in the presence of equimolar concentrations of estradiol (E2) to assess its potency relative to DHT. The relative potency of DHT and E2 on SRC activation should provide additional insight into the contribution of this pathway to the relative immaturity of the male fetal lung. Fibroblasts were isolated from E17 mice that were not previously treated with exogenous DHT or E2 and exposed to DHT 30 ng/ml, E2 30 ng/ml, or neither for 1 hour. The cells were then stimulated with 100 ng/ml EGF for up to 30 minutes. SRC Tyr424 phosphorylation was visualized by immunoblotting and normalized against total SRC within the same samples (Figure 3A). Constitutive SRC Tyr424 phosphorylation was not increased by DHT or E2. EGF-induced SRC phosphorylation was increased by DHT relative to controls (P = 0.0065) and to estradiol-treated cells (P = 0.031; n = 4) (Figure E3A).
Figure 3.
DHT increases SRC phosphorylation and potentiates the ligand-dependent activation of the EGFR. EGF-induced SRC and EGFR pathway activation is potentiated by DHT. Previously untreated primary fetal lung fibroblasts were stimulated with 100 mg/ml EGF for 0, 2, 5, or 30 minutes with or without a prior 1-hour exposure to 30 ng/ml DHT or 30 ng/ml estradiol (E2). Phosphoprotein blots are paired with total protein immunoblots of the same samples but not necessarily of the same membrane. (A, top) Immunoblot of SRC phosphorylated at Tyr424 in the kinase domain (Y424). Phosphorylation at this site correlates with SRC activation. (A, bottom) Total SRC immunoblot of the same lysates. Densitometric analyses are provided in Figure E3. EGF-induced SRC Tyr424 phosphorylation is significantly higher in DHT-treated cells than in controls (Ctl) or E2-treated cells. (B) EGF-induced EGFR phosphorylation is potentiated by DHT. Primary fibroblasts were similarly stimulated with EGF in the presence or absence of DHT or E2. Top: Immunoblot of EGFR phosphorylated at the Tyr1173 SHC1 binding site. Phosphorylation of SHC1 by EGFR mediates ERK1/2 activation. Bottom: Total EGFR immunoblot of the same lysates. EGF-induced EGFR phosphorylation is significantly higher in DHT-treated cells than in controls or E2-treated cells. (C) EGF-induced SHC1 phosphorylation and GRB2 association are increased by DHT. Fetal lung fibroblasts were treated with DHT and stimulated with EGF as previously described. The lysates were then equalized by protein content before SHC1 immunoprecipitation. The activation of SHC1, a substrate of EGFR and initiator of the ERK1/2 pathway, was then assessed by probing the precipitates for GRB2 and for phosphotyrosine. Top: Phosphotyrosine immunoblot showing phosphorylation of the p66, p52, and p46 SHC1 isoforms. Middle: GRB2 immunoblot of SHC1 immunoprecipitated from the same experiment. Bottom: SHC1 immunoblot of the same precipitates. EGF-induced p52SHC1 phosphorylation was significantly higher in DHT-treated cells relative to untreated or E2-treated cells. EGF-induced GRB2 coimmunoprecipitation, normalized to immunoprecipitated SHC1, was also increased by DHT relative to controls. (D) EGF-induced ERK1/2 phosphorylation is more potently potentiated by DHT than by E2. Fibroblasts were again stimulated with EGF with or without a prior 1-hour exposure to 30 ng/ml DHT or E2. ERK1 and ERK2 phosphorylation were assessed as previously described. Top: pERK1/2 immunoblot. Bottom: Total ERK1/2 immunoblot of the same samples. EGF-induced ERK1 phosphorylation was significantly higher in DHT-treated cells than in control or E2-treated cells. EGF-induced ERK2 phosphorylation was significantly higher in DHT-treated cells than in controls and approached significance compared with E2-treated cells (P = 0.055).
Subsequent EGF-induced receptor phosphorylation was assessed as previously described. Normalized EGF-induced Tyr1173 phosphorylation was increased by DHT relative to E2-treated fibroblasts (P = 0.015) and untreated controls (P = 0.010; n = 5) (Figure 3B).
To confirm that the increased ligand-induced EGFR phosphorylation noted in Figure 3A results in similarly accelerated substrate activation, the activation of SHC1 adapter proteins was assessed. SHC1 proteins are substrates of SRC and EGFRs and are expressed as alternatively spliced isoforms of 66, 52, and 46 kD. On phosphorylation, Tyr239/240 and Tyr317 of the p52SHC1 isoform become docking sites for GRB2 adapter proteins (37), enabling p52SHC1 to form a heterotrimer with GRB2 and the Sos guanine nucleotide exchange protein. The complex then translocates to the plasma membrane where it activates the Ras GTPase oncoprotein, which leads to the sequential phosphorylation of Raf, MEK, and ERK1/2 (38). To determine if this pathway is potentiated by DHT exposure, previously untreated primary fetal mouse lung fibroblasts were stimulated with 100 ng/ml EGF for 0, 1, 5, or 30 minutes with or without prior 1-hour exposure to DHT or estradiol. SHC1 function was then assessed by immunoprecipitating SHC1 from the lysates and subjecting the isolates to phosphotyrosine (Figure 3C, top) and GRB2 immunoblotting (Figure 3C, middle). Band densities were normalized against total SHC1 immunoprecipitates (Figure 3C, bottom). EGF-induced p52SHC1 phosphorylation was greater in DHT-treated cells relative to identical untreated control cells (P = 0.00025) or estradiol-treated cells (P = 0.037; n = 4) (Figure E3C, left). Similarly, GRB2 association with SHC1 is also increased by DHT exposure relative to controls (P = 0.0022; n = 4) but not by estradiol (P = 0.21) (Figure E3C, right). These data confirm that SHC1 activation is potentiated by DHT.
ERK1/2 phosphorylation was assessed in the same samples (Figure 3D). Again, DHT-treated cells significantly potentiated EGF-induced ERK1/2 phosphorylation (P = 0.0065 for ERK1 and P = 0.0068 for ERK2; n = 4) relative to control cells. Comparison with estradiol-treated cells showed significantly greater potentiation of ERK1 activation (P = 0.031) and a trend toward significance for ERK2 (P = 0.055) (Figure E3D). Taken together, these findings suggest that DHT activates SRC to quantitatively potentiate the ligand activation of the EGR receptor pathway leading to ERK1/2 and that this potentiation is more robust than that of estradiol.
DHT Induces SRC Activation
The foregoing data indicate that DHT exposure rapidly potentiates EGFR-induced ERK activation in a translation-independent manner. Sex hormones can modulate the EGF pathway by activating SRC (39), which then interacts synergistically with EGFRs (40). In its inactive state, SRC’s constitutive kinase activity is sterically inhibited by intramolecular interactions (41). The SRC SH1 kinase domain is located behind the phosphotyrosine-binding SH3 and SH2 domains and ahead of the C-terminal tail, which contains an inhibitory phosphorylation site at murine Tyr535 (human Tyr 530). When latent, the SRC SH2 domain folds over the kinase domain to bind Tyr535, whereas the SH3 domain binds to the SH1 domain. Both interactions obstruct the kinase domain and prevent SRC from interacting with its substrates. SRC may be activated by dephosphorylating Tyr535 or by disrupting its intramolecular bonding, which is the putative mechanism by which androgen and estrogen receptors activate SRC (39). In the presence of ligand, estrogen receptors bind the SH2 domain to prevent its association with Tyr535, and androgen receptors bind the SH3 domain to unmask the kinase domain.
Releasing this steric inhibition enables SRC autophosphorylation, which leads to further activation. Specifically, autophosphorylation of Tyr424 in the SH1 domain (analogous to chicken Tyr416 and human Tyr419) is necessary for full activation (41). Activated SRC then associates with the EGFR (42) and, in the presence of EGF ligand, induces its phosphorylation at Tyr845, a critical regulatory residue in the EGFR catalytic domain (40). Phosphorylation of this site potentiates EGFR activity and thereby represents a mechanism by which androgens can regulate the amplitude of EGF signaling.
To determine if DHT potentiates SRC activation in primary fetal lung fibroblasts, we first assessed the EGF-induced tyrosine phosphorylation of SRC immunoprecipitated from primary cells exposed to DHT only for 1 hour before EGF stimulation (Figure 4A). In the presence of DHT, SRC was tyrosine phosphorylated within 2 minutes of EGF stimulation, and peak phosphorylation was increased 2.4-fold (P = 0.000014; n = 4) (Figure E4A). EGF-induced SRC phosphorylation was also assessed in cells treated with equimolar concentrations of E2. In these cells, EGF-induced SRC tyrosine phosphorylation was 2.5-fold greater than in cells not exposed to sex hormones (P = 0.019; n = 4) but was only 75% of that of cells treated with DHT (P = 0.0021; n = 4).
Figure 4.
SRC activation by DHT is consistent with direct binding. SRC may be activated by dephosphorylating Tyr535 or by disrupting its intramolecular bonding. Androgen receptors putatively bind the SH3 domain to unmask the kinase domain. Releasing this inhibition enables SRC to autophosphorylate on multiple residues. Of these, Tyr424 is necessary for full activation. Activated SRC then associates with the EGFR and, in the presence of EGF ligand, induces its phosphorylation at Tyr845. To assess the mechanism of activation, specific and global tyrosine phosphorylation of SRC and its EGFR substrate were assessed. The corresponding densitometric analyses are presented in Figure E4. Primary fibroblasts were exposed to DHT or E2 for 1 hour before stimulation with up to 30 minutes EGF as previously described. The SRC was immunoprecipitated, and the isolates were immunoblotted for phosphotyrosine. (A) Phosphotyrosine blot of SRC immunoprecipitates (IP) (top) and SRC immunoblot (IB) of the same samples (bottom). EGF-induced SRC tyrosine phosphorylation, and, hence, activation is increased by DHT and, to a lesser extent, by E2. Global tyrosine phosphorylation parallels Tyr424 phosphorylation. (B) Immunodetection of SRC Tyr535 (top) and SRC immunoblot of the same samples (bottom). Phosphorylation of Tyr535 is not decreased by DHT or E2. (C) PhosphoTyr424 immunoblot (top) and SRC immunoblot (below) of cells exposed to DHT in the absence of EGF and in the presence of the SRC inhibitor PP2 or its control, PP3. DHT significantly increases Tyr424 phosphorylation independently of EGF in control cells and in cells exposed to PP3 but not in cells treated with PP2. This suggests that SRC is activated by DHT in a manner that requires SRC kinase activity. (D) EGFR Tyr845 immunoblot of cells exposed to DHT in the presence of PP2 or PP3 and then stimulated with EGF. EGF-induced EGFR Tyr845 phosphorylation is potentiated by DHT only in the absence of PP2 or PP3. Because PP3 attenuates EGFR activity, this result confirms that SRC and EGFR kinase activities are both necessary for DHT potentiation.
We next sought to determine if androgen or estrogen exposure was associated with dephosphorylation of SRC’s inhibitory C-terminal Tyr535 site (Figure 4B). Dephosphorylation of this site could indicate that sex hormones activate a protein phosphatase that de-represses SRC. Neither DHT nor E2 stimulation significantly reduced SRC Tyr535 phosphorylation (Figure 4B); rather, DHT insignificantly increased Tyr535 phosphorylation (P = 0.10; n = 4), whereas E2 increased Tyr535 phosphorylation by 2.4-fold (P = 0.000027; n = 4) (Figure E4B). The effects of sex hormones on Tyr535 appear, on balance, to be inhibitory rather than activating.
These findings suggest that androgen receptors most likely activate SRC by disrupting its intramolecular bonding, consistent with previous reports (39). To confirm that DHT directly activates SRC, primary fibroblasts were exposed to 30 ng/ml DHT for increasing durations in the presence or absence of PP2, a pyrazolopyrimidine that potently inhibits SRC family kinases by competing with ATP (43–45) or its analog PP3, which lacks activity against SRC but inactivates EGFRs (46). Primary fetal lung fibroblasts were exposed to 10 μM PP2 or PP3 for 60 minutes before DHT exposure. In this experiment, cells were not stimulated with EGF before immunoblotting for SRC Tyr424 phosphorylation; in the absence of EGF, this phosphorylation was much lower than in EGF-stimulated cells and required more sensitive techniques than used in Figure 3. As shown in Figure 4C, SRC Tyr424 phosphorylation increased significantly over 120 minutes of DHT exposure in the absence of inhibitor (P = 0.031; n = 7) or in the presence of 10 μM PP3 (P = 0.035; n = 7) (Figure E4C). However, Tyr424 phosphorylation was abrogated by 10 μM PP2. These results suggest that DHT activates SRC in the absence of growth factors.
To ensure that this abrogation did not result from PP2 toxicity, the mortality associated with PP2 and PP3 exposure at the indicated times and concentrations was assessed. Primary fibroblasts were treated with no inhibitor, 10 μM PP2, or 10 μM PP3 for 90 minutes before staining with annexin V and propidium iodide. By fluorescent microscopy, neither annexin V nor propidium iodide staining differed overtly between control and PP2- or PP3-treated cells, and staining was substantially less than that of cells exposed to 10 μM H2O2 (Figures E6A–E6D). However, this method is not cumulative and does not reliably detect lysed cells. We therefore confirmed the lack of PP2 toxicity by performing MTT assays on similarly treated cells (47). Relative to untreated cells, cell mortality was decreased 5.3 ± 0.077% by PP2 and increased 0.4 ± 0.141% by PP3 (P = 0.94). Mortality from PP2 did not significantly differ from that of untreated cells (P = 0.17; n = 4) or cells treated with PP3 (P = 0.36). Taken together, these findings suggest that DHT directly activates SRC, as assessed by its autophosphorylation on Tyr424.
Finally, we evaluated EGFR phosphorylation at the regulatory Tyr845 site targeted by SRC. In the absence of EGF stimulation, Tyr845 was not phosphorylated (Figure 4D). When stimulated with EGF for up to 30 minutes, Tyr845 became rapidly phosphorylated. This phosphorylation was increased by pretreatment with 30 ng/ml DHT for 60 minutes, confirming that EGFR activation is potentiated by DHT (P = 0.09; n = 4) (Figure E4D). Exposure to the SRC inhibitor PP2 (10 μM) abrogated this induction (P = 0.01), as did treatment with the EGFR inhibitor PP3 (10 μM) (P = 0.047). The failure of DHT to potentiate EGF-induced Tyr845 phosphorylation in the presence of PP2 tends to rule out the direct association of androgen receptors with EGFRs (48). Rather, these results indicate that DHT induces the formation of a mutually phosphorylating SRC-EGFR complex that potentiates the activation of the EGFR by its ligand.
Src Inhibitors Prevent DHT from Increasing EGF Signaling Activity
To confirm that potentiation of ERK activation by DHT requires functional SRC, three independent methods were used to abrogate SRC activity in previously untreated primary lung fibroblasts isolated from E17 fetal mice. First, primary fetal lung fibroblasts were treated with PP2 beginning 1 hour before DHT treatment and continuing through EGF stimulation. ERK phosphorylation was assessed by immunoblotting and normalized against total ERK expression within the same samples (Figure 5A). In the absence of PP2, DHT potentiated EGF-induced ERK phosphorylation (P = 0.0075 for ERK1 and P = 0.00021 for ERK2; n = 5) (Figure E5A). In the presence of PP2, however, DHT did not increase EGF-induced ERK phosphorylation, and ERK activation associated with DHT was significantly lower in PP2-treated cells (P = 0.0074 for ERK1 and P = 0.0014 for ERK2). Similarly, normalized EGFR activation was potentiated by DHT only in the absence of PP2 (P = 0.018; n = 5) (Figure 5B), and DHT-associated EGFR phosphorylation was significantly lower in its presence (P = 0.045) (Figure E5B). Finally, the normalized phosphorylation of SRC Tyr424 was assessed (Figure 5C). DHT potentiated EGF-induced SRC Tyr424 phosphorylation (P = 0.028) only in the absence of PP2, and the SRC Tyr424 phosphorylation associated with DHT was significantly lowered by PP2 (P = 0.0010) (Figure E5C). These data suggest that SRC function is necessary for the potentiation of the ERK pathway by DHT.
Figure 5.
Src inhibition prevents DHT from enhancing the activity of the EGFR. (A–C) PP2 inhibition of SRC prevents DHT from potentiating EGF signaling. Previously untreated primary fetal lung fibroblasts were exposed to PP2 before DHT treatment and EGF stimulation as previously described. Lysates were immunoblotted for phosphorylated and total signaling intermediates; densitometric analyses are presented in Figure E5. (A) Phosphorylated ERK1/2 and total ERK1/2 immunoblots of the same samples. ERK activation is potentiated by DHT in the absence but not in the presence of PP2. (B) EGFR phosphoTyr1173 and total EGFR immunoblots of the same samples. EGFR activation is potentiated by DHT in the absence but not in the presence of PP2. (C) SRC phosphoTyr424 and total SRC immunoblots of the same samples. SRC activation is potentiated by DHT in the absence but not in the presence of PP2. (D) Src silencing prevents DHT from enhancing EGF signaling. Primary fetal lung fibroblasts were transfected with small interfering RNA (siRNA) against Src or Gapdh before DHT treatment and EGF stimulation. Lysates were immunoblotted for phosphorylated and total ERK1/2 (top pair of images) and for phosphorylated EGFR Tyr1173 and total EGFR (bottom pair of images). EGF-induced ERK phosphorylation was potentiated by DHT only in cells transfected with Gapdh siRNA, and DHT-associated ERK phosphorylation was significantly lower in cells transfected with Src siRNA. Similarly, EGF-induced receptor phosphorylation was potentiated by DHT only in the presence of Gapdh silencing and was significantly lower when Src was silenced. The efficiency of Src silencing was confirmed by immunoblotting the same samples for SRC (bottom). By densitometry, SRC content was reduced by 58% (SD = 9%). (E) overexpression of dominant–negative (DN) SRC prevents DHT from potentiating EGF signaling. Primary fibroblasts were infected with adenoviruses encoding green fluorescent protein (GFP) or a DN Src. Cells were inoculated 2 days before exposure to DHT and EGF as previously described. DN Src expression was confirmed by SRC immunoblotting (bottom). DHT potentiated EGF-induced ERK activation in cells expressing GFP but not in cells expressing DN Src. EGF-induced ERK2 phosphorylation in the presence of DHT was significantly lower in cells expressing DN Src than in GFP controls. ERK1 phosphorylation was also decreased by DN Src but did not achieve significance (P = 0.062).
However, PP2 inhibits LCK and FYN as well as SRC and has significant activity against EGFRs (44). SRC function was therefore also abrogated by silencing Src mRNA. Previously untreated primary fetal lung fibroblasts were transfected with siRNA against Src or Gapdh 2 days before 1 hour of DHT pretreatment and 5 minutes of EGF stimulation as described previously. Silencing was confirmed by SRC Western blot (Figure 5D, bottom); densitometric quantitation showed that silencing reduced SRC content by 58% (SD = 9%). DHT potentiated EGF-induced ERK phosphorylation in the presence of Gapdh siRNA (P = 0.015 for ERK1 and P = 0.049 for ERK2; n = 4) but not Src siRNA (Figures 5D, top, and E5D, top), and DHT-associated ERK activation was significantly lower with Src silencing (P = 0.0052 for ERK1 and P = 0.028 for ERK2). Similarly, DHT potentiated EGF-induced phosphorylation of its receptor at Tyr1173 in cells transfected with siRNA against GAPDH (P = 0.0024; n = 3) but not against Src (Figure 5D, bottom). DHT-potentiated, EGF-induced EGFR Tyr1173 phosphorylation was significantly higher in cells transfected with Gapdh siRNA than in cells transfected with Src siRNA (P = 0.00043). These data again suggest that SRC directly potentiates EGFR signaling by DHT. Finally, SRC signaling was abrogated by infecting primary fetal fibroblasts with an adenovirus encoding a Src construct (DN Src) containing K296R and Y528F mutations that confer a dominant-negative effect (32). Cells were inoculated with 5 × 107 pfu per 2 cm2 well (multiplicity of infection = 20) 2 days before exposure to DHT for 1 hour and EGF stimulation for 5 or 30 minutes as previously described. DN Src overexpression was confirmed by SRC immunoblotting (Figure 5E, bottom). DHT potentiated EGF-induced ERK activation in cells infected with a control adenovirus expressing green fluorescent protein but not in cells infected with DN Src. EGF-induced ERK2 phosphorylation in the presence of DHT was significantly lower in cells expressing DN Src than in green fluorescent protein–expressing controls (P = 0.0094; n = 3). ERK1 phosphorylation was also decreased by DN Src but did not achieve significance (P = 0.062). Taken together, these results suggest that SRC function is necessary for the potentiation of ErbB signaling by DHT.
Discussion
These results suggest that DHT potentiates the EGF pathway at the level of the EGFR. EGF-induced receptor phosphorylation, SHC1 phosphorylation, SHC1–GRB2 association, and ERK activation are all increased by DHT. This potentiation, first identified in cells chronically exposed to DHT, is observed with as little as 30 minutes of DHT exposure and is independent of protein translation. The interaction between DHT and the EGF pathway is abrogated by three independent methods of SRC inactivation and is consistent with observations in Sertoli cells (32).
The EGF stimulation time necessary to achieve peak ERK activation varied within cells obtained from the same animal, necessitating multiple time points and multiple repetitions of each experiment. In particular, ERK phosphorylation associated with longer periods of EGF stimulation (e.g., 30 min) was quite variable. Given the multiplicity of functions ascribed to ERK, developmental heterogeneity within the lung, and differences in isolation efficiency, such variability is not unexpected. The ability to detect DHT potentiation against this background speaks to the magnitude of its effect on ERK activation. Conversely, we did not observe a consistent effect of DHT on the time course or variability of EGF-induced ERK activation. Similarly, the relative induction of ERK1 and ERK2 varied from experiment to experiment, often in ways that could not be predicted by the relative expression of each isoform. We do not believe that DHT disproportionately potentiated a specific ERK isoform.
Interpretation of this data is complicated by the fact that the Src and ErbB pathways share many components. For example, SRC directly phosphoryates SHC1 proteins to activate ERK1/2 (49). However, short-term DHT exposure did not activate ERK in the absence of EGF. Rather, DHT appears to induce low-level SRC activation that facilitates the formation of a mutually phosphorylating SRC–EGFR complex that amplifies the effect of EGF ligand.
Previous studies in the lung have correlated ERK activation with accelerated epithelial maturation and surfactant synthesis (50). Moreover, chronic DHT exposure inhibits late-gestation EGFR expression and activation (19, 20). In contrast, we demonstrate that DHT, an inhibitor of epithelial maturation, potentiates EGF-induced activation of the EGFR and downstream ERK activation in fibroblasts. DHT also increases ERK activation by Nrg in epithelial Type II cells, presumably by a similar mechanism. These results are opposite to those predicted by the existing literature. Interestingly, DHT has been shown to stimulate lung morphogenesis in ways that are consistent with these findings (51).
The source of this paradox may lie in our assessment of acute ERK pathway responsiveness as opposed to its constitutive activation. In this study, EGF-induced ERK phosphorylation was assessed in primary cells that were first subjected to serum starvation. This measures the magnitude of response to a single stimulus and minimizes counter-regulatory effects. EGFR responsiveness in situ is limited by feedback regulation that is dependent on the duration of ligand exposure. DHT-enhanced EGF pathway stimulation may up-regulate one or more negative feedback mechanisms that reduce the sensitivity of that pathway. Increased pathway responsiveness can thereby result in long-term pathway down-regulation.
In the absence of negative feedback regulation, prolonged stimulation tends to saturate most systems; additional stimulation yields no corresponding increases in output because system output is already maximal. Human-designed control systems typically use negative feedback to maintain proportional responsiveness to additional stimuli. In principle, however, feedback may be used to dampen system responsiveness to levels that are lower than baseline. In biological systems, such dampening can be conferred by decreasing the expression or the efficiency of critical pathway components. We speculate that DHT paradoxically decreases the activity of the EGF pathway by increasing its sensitivity to EGF, thereby inducing negative feedback mechanisms that lower its responsiveness.
Acknowledgments
Acknowledgments
The authors thank William H. Walker of the University of Pittsburgh for generously providing the Src K296R, Y528F construct and for technical assistance. We also acknowledge the generous support of Changgong Li of the University of Southern California in providing critical reagents. This work was funded by grants HL037930 and HL085648 from the NIH, and by the Hastings Foundation.
Footnotes
This work was supported by National Institutes of Health grants HL037930 and HL085648 and by the Hastings Foundation.
Author Contributions: Acquisition of data: S.M.S., S.M., L.D.P. Conception and design: M.K.L., S.M.S., S.M., L.D.P., P.M., H.C.N. Analysis and interpretation: M.K.L., H.C.N. Drafting the manuscript for important intellectual content: M.K.L., S.M.S., H.C.N. Final approval: M.K.L., S.M.S., S.M., L.D.P., P.M., H.C.N.
Originally Published in Press as DOI: 10.1165/rcmb.2012-0179OC on January 21, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Torday JS, Nielsen HC, Fencl MM, Avery ME. Sex differences in fetal lung maturation. Am Rev Respir Dis. 1981;123:205–208. doi: 10.1164/arrd.1981.123.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Miller HC, Futrakul P. Birth weight, gestational age, and sex as determining factors in the incidence of respiratory distress syndrome of prematurely born infants. J Pediatr. 1968;72:628–635. doi: 10.1016/s0022-3476(68)80005-8. [DOI] [PubMed] [Google Scholar]
- 3.Farrell PM, Avery ME. Hyaline membrane disease. Am Rev Respir Dis. 1975;111:657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen HC, Zinman HM, Torday JS. Dihydrotestosterone inhibits fetal rabbit pulmonary surfactant production. J Clin Invest. 1982;69:611–616. doi: 10.1172/JCI110488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross I, Wilson CM, Ingleson LD, Brehier A, Rooney SA. The influence of hormones on the biochemical development of fetal rat lung in organ culture: I. Estrogen. Biochim Biophys Acta. 1979;575:375–383. doi: 10.1016/0005-2760(79)90106-1. [DOI] [PubMed] [Google Scholar]
- 6.Catterton WZ, Escobedo MB, Sexson WR, Gray ME, Sundell HW, Stahlman MT. Effect of epidermal growth factor on lung maturation in fetal rabbits. Pediatr Res. 1979;13:104–108. doi: 10.1203/00006450-197902000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Sundell HW, Gray ME, Serenius FS, Escobedo MB, Stahlman MT. Effects of epidermal growth factor on lung maturation in fetal lambs. Am J Pathol. 1980;100:707–726. [PMC free article] [PubMed] [Google Scholar]
- 8.Whitsett JA, Weaver TE, Lieberman MA, Clark JC, Daugherty C. Differential effects of epidermal growth factor and transforming growth factor-beta on synthesis of Mr = 35,000 surfactant-associated protein in fetal lung. J Biol Chem. 1987;262:7908–7913. [PubMed] [Google Scholar]
- 9.Higuchi M, Hirano H, Maki M. Effect of human epidermal growth factor on lung surfactant production in fetal rabbit. Tohoku J Exp Med. 1989;159:15–22. doi: 10.1620/tjem.159.15. [DOI] [PubMed] [Google Scholar]
- 10.Gross I, Dynia DW, Rooney SA, Smart DA, Warshaw JB, Sissom JF, Hoath SB. Influence of epidermal growth factor on fetal rat lung development in vitro. Pediatr Res. 1986;20:473–477. doi: 10.1203/00006450-198605000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Sen N, Cake MH. Enhancement of disaturated phosphatidylcholine synthesis by epidermal growth factor in cultured fetal lung cells involves a fibroblast-epithelial cell interaction. Am J Respir Cell Mol Biol. 1991;5:337–343. doi: 10.1165/ajrcmb/5.4.337. [DOI] [PubMed] [Google Scholar]
- 12.Dammann CE, Nielsen HC, Carraway KL., III Role of neuregulin-1 beta in the developing lung. Am J Respir Crit Care Med. 2003;167:1711–1716. doi: 10.1164/rccm.200205-468OC. [DOI] [PubMed] [Google Scholar]
- 13.Ryan RM, Mineo Kuhn MM, Kramer CM, Finkelstein JN. Growth factors alter neonatal type II alveolar epithelial cell proliferation. Am J Physiol. 1994;266:L17–L22. doi: 10.1152/ajplung.1994.266.1.L17. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen HC. Epidermal growth factor influences the developmental clock regulating maturation of the fetal lung fibroblast. Biochim Biophys Acta. 1989;1012:201–206. doi: 10.1016/0167-4889(89)90097-9. [DOI] [PubMed] [Google Scholar]
- 15.Floros J, Nielsen HC, Torday JS. Dihydrotestosterone blocks fetal lung fibroblast-pneumonocyte factor at a pretranslational level. J Biol Chem. 1987;262:13592–13598. [PubMed] [Google Scholar]
- 16.Torday JS. The sex difference in type II cell surfactant synthesis originates in the fibroblast in vitro. Exp Lung Res. 1984;7:187–194. doi: 10.3109/01902148409087912. [DOI] [PubMed] [Google Scholar]
- 17.Rosenblum DA, Volpe MV, Dammann CE, Lo YS, Thompson JF, Nielsen HC. Expression and activity of epidermal growth factor receptor in late fetal rat lung is cell- and sex-specific. Exp Cell Res. 1998;239:69–81. doi: 10.1006/excr.1997.3888. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen HC. Androgen receptors influence the production of pulmonary surfactant in the testicular feminization mouse fetus. J Clin Invest. 1985;76:177–181. doi: 10.1172/JCI111943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dammann CE, Nielsen HC. Regulation of the epidermal growth factor receptor in fetal rat lung fibroblasts during late gestation. Endocrinology. 1998;139:1671–1677. doi: 10.1210/endo.139.4.5934. [DOI] [PubMed] [Google Scholar]
- 20.Dammann CE, Ramadurai SM, McCants DD, Pham LD, Nielsen HC. Androgen regulation of signaling pathways in late fetal mouse lung development. Endocrinology. 2000;141:2923–2929. doi: 10.1210/endo.141.8.7615. [DOI] [PubMed] [Google Scholar]
- 21.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 22.Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Di Stasio R, Barbieri A, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26:6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio A, Castoria G, Auricchio F. Analysis of androgen receptor rapid actions in cellular signaling pathways: receptor/Src association. Methods Mol Biol. 2011;776:361–370. doi: 10.1007/978-1-61779-243-4_21. [DOI] [PubMed] [Google Scholar]
- 25.Migliaccio A, Castoria G, Giovannelli P, Auricchio F. Cross talk between epidermal growth factor (EGF) receptor and extra nuclear steroid receptors in cell lines. Mol Cell Endocrinol. 2010;327:19–24. doi: 10.1016/j.mce.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Fraslon C, Bourbon JR. Comparison of effects of epidermal and insulin-like growth factors, gastrin releasing peptide and retinoic acid on fetal lung cell growth and maturation in vitro. Biochim Biophys Acta. 1992;1123:65–75. doi: 10.1016/0005-2760(92)90172-r. [DOI] [PubMed] [Google Scholar]
- 27.Kim UH, Kim HS, Rhee SG. Epidermal growth factor and platelet-derived growth factor promote translocation of phospholipase C-gamma from cytosol to membrane. FEBS Lett. 1990;270:33–36. doi: 10.1016/0014-5793(90)81228-g. [DOI] [PubMed] [Google Scholar]
- 28.Fisher DA, Lakshmanan J. Metabolism and effects of epidermal growth factor and related growth factors in mammals. Endocr Rev. 1990;11:418–442. doi: 10.1210/edrv-11-3-418. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen HC, Kirk WO, Sweezey N, Torday JS. Coordination of growth and differentiation in the fetal lung. Exp Cell Res. 1990;188:89–96. doi: 10.1016/0014-4827(90)90281-e. [DOI] [PubMed] [Google Scholar]
- 30.Lee MK, Hwang C, Lee J, Slavkin HC, Warburton D. TGF-beta isoforms differentially attenuate EGF mitogenicity and receptor activity in fetal lung mesenchymal cells. Am J Respir Cell Mol Biol. 1997;273:L374–L381. doi: 10.1152/ajplung.1997.273.2.L374. [DOI] [PubMed] [Google Scholar]
- 31.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, Muller WJ, Moses HL. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 33.Okabayashi Y, Kido Y, Okutani T, Sugimoto Y, Sakaguchi K, Kasuga M. Tyrosines 1148 and 1173 of activated human epidermal growth factor receptors are binding sites of Shc in intact cells. J Biol Chem. 1994;269:18674–18678. [PubMed] [Google Scholar]
- 34.Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–217. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patschinsky T, Hunter T, Esch FS, Cooper JA, Sefton BM. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci USA. 1982;79:973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migliaccio A, Di Domenico M, Castoria G, De Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 37.Harmer SL, DeFranco AL. Shc contains two Grb2 binding sites needed for efficient formation of complexes with SOS in B lymphocytes. Mol Cell Biol. 1997;17:4087–4095. doi: 10.1128/mcb.17.7.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti Furga G, Pawson T, di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Migliaccio A, Castoria G, Di Domenico M, De Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeatman TJ, Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 42.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 43.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence DS, Niu J. Protein kinase inhibitors: the tyrosine-specific protein kinases. Pharmacol Ther. 1998;77:81–114. doi: 10.1016/s0163-7258(97)00052-1. [DOI] [PubMed] [Google Scholar]
- 45.Chong YP, Ia KK, Mulhern TD, Cheng HC. Endogenous and synthetic inhibitors of the Src-family protein tyrosine kinases. Biochim Biophys Acta. 2005;1754:210–220. doi: 10.1016/j.bbapap.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 46.Traxler P, Bold G, Frei J, Lang M, Lydon N, Mett H, Buchdunger E, Meyer T, Mueller M, Furet P. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J Med Chem. 1997;40:3601–3616. doi: 10.1021/jm970124v. [DOI] [PubMed] [Google Scholar]
- 47.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 48.Bonaccorsi L, Muratori M, Carloni V, Marchiani S, Formigli L, Forti G, Baldi E. The androgen receptor associates with the epidermal growth factor receptor in androgen-sensitive prostate cancer cells. Steroids. 2004;69:549–552. doi: 10.1016/j.steroids.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Esteban J, Wang Y, Gruppuso PA, Rubin LP. Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. Am J Respir Cell Mol Biol. 2004;30:76–83. doi: 10.1165/rcmb.2003-0121OC. [DOI] [PubMed] [Google Scholar]
- 51.Levesque BM, Vosatka RJ, Nielsen HC. Dihydrotestosterone stimulates branching morphogenesis, cell proliferation, and programmed cell death in mouse embryonic lung explants. Pediatr Res. 2000;47:481–491. doi: 10.1203/00006450-200004000-00012. [DOI] [PubMed] [Google Scholar]