Abstract
Plasma membrane Ca2+ influx, especially store-operated Ca2+ entry triggered by sarcoplasmic reticulum (SR) Ca2+ release, is a key component of intracellular calcium concentration ([Ca2+]i) regulation in airway smooth muscle (ASM). Agonist-induced Ca2+ oscillations in ASM that involve both influx and SR mechanisms have been previously demonstrated. In nonexcitable cells, [Ca2+]i oscillations involve Ca2+ influx via arachidonic acid (AA) –stimulated channels, which show similarities to store-operated Ca2+ entry, although their molecular identity remains undetermined. Little is known about AA-regulated Ca2+ channels or their regulation in ASM. In enzymatically dissociated human ASM cells loaded with the Ca2+ indicator, fura-2, AA (1–10 μM) triggered [Ca2+]i oscillations that were inhibited by removal of extracellular Ca2+. Other fatty acids, such as the diacylglycerol analog, 1-oleoyl-2-acetyl-SN-glycerol, oleic acid, and palmitic acid (10 μM each), failed to elicit similar [Ca2+]i responses. Preincubation with LaCl3 (1 μM or 1 mM) inhibited AA-induced oscillations. Inhibition of receptor-operated channels (SKF96,365 [10 μM]), lipoxygenase (zileuton [10 μM]), or cyclooxygenase (indomethacin [10 μM]) did not affect oscillation parameters. Inhibition of SR Ca2+ release (ryanodine [10 μM] or inositol 1,4,5-trisphosphate receptor inhibitor, xestospongin C [1 μM]) decreased [Ca2+]i oscillation frequency and amplitude. Small interfering RNA against caveolin-1, stromal interaction molecule 1, or Orai3 (20 nM each) reduced the frequency and amplitude of AA-induced [Ca2+]i oscillations. In ASM cells derived from individuals with asthma, AA increased oscillation amplitude, but not frequency. These results are highly suggestive of a novel AA-mediated Ca2+–regulatory mechanism in human ASM, reminiscent of agonist-induced oscillations. Given the role of AA in ASM intracellular signaling, especially with inflammation, AA-regulated Ca2+ channels could potentially contribute to increased [Ca2+]i in diseases such asthma.
Keywords: bronchial smooth muscle, calcium, influx, arachidonic acid, sarcoplasmic reticulum
Clinical Relevance
Arachidonic acid (AA) and its downstream metabolites are important in inflammatory conditions, such as asthma. Here, we present novel data on the expression and properties of a Ca2+ influx pathway regulated by AA in human airway smooth muscle. We believe that the basic science described here is highly relevant to understanding the mechanisms by which agonists and inflammation can enhance Ca2+ in airways and contribute to increased airway contractility.
In human airway smooth muscle (ASM), changes in intracellular calcium concentration ([Ca2+]i) are mediated by both Ca2+ influx/efflux and Ca2+ release/reuptake from the sarcoplasmic reticulum (SR) (1–4). We and others have previously shown a variety of Ca2+ influx mechanisms in ASM, including voltage-gated and receptor-operated channels (5–8). In addition, an important influx mechanism that occurs upon SR depletion (e.g., after agonist stimulation) is store-operated Ca2+ entry (SOCE), which aids SR Ca2+ refilling. We and others have demonstrated the presence of SOCE in ASM (9–11). However, one caveat in regard to SOCE is that physiologically relevant agonist concentrations do not necessarily deplete SR Ca2+ stores, raising the question of whether SOCE normally contributes to Ca2+ influx during agonist-induced airway contractions. We previously demonstrated in porcine ASM that physiologic concentrations of agonists result in the generation of repetitive and stable Ca2+ oscillations characterized by a rapid and transient release of submaximal Ca2+ from the SR, with concomitant rapid refilling of such intracellular Ca2+ stores via influx (5, 12). Such oscillations would prevent full depletion of SR Ca2+ stores, and thus mechanisms that mediate SOCE per se may not be as important in agonist-induced [Ca2+]i oscillations. Accordingly, the question is whether a noncapacitative influx pathway exists that may regulate Ca2+ oscillatory signaling in ASM.
Data in nonexcitable cells show the presence of a novel noncapacitative Ca2+ influx mechanism evoked by arachidonic acid (AA) stimulation (arachidonate-regulated Ca2+ [ARC] channels), that is independent of SR store depletion (13–16). Although initially thought to reflect AA effects on a noncapacitative influx pathway, the role of ARC channels is now considered much more diverse, with evidence that these channels are also involved in capacitive Ca2+ entry (17, 18). Evidence in nonexcitable cells for this connection comes from data that stromal interaction molecule (STIM) and Orai proteins, which are key regulators of SOCE, can also regulate ARC channels (18). However, what appears to distinguish SOCE from ARC channels is the role of a plasma membrane STIM1 in the regulation of ARC channels (18, 19) in contrast to endoplasmic reticulum STIM1 in SOCE. Furthermore, the ARC channel pore is thought to be formed by assembly of three plasma membrane proteins, Orai1 and two Orai3 subunits (18). There is now substantial evidence that plasma membrane Orai1, and STIM1 at the level of the SR, are mediators of SOCE in ASM (10, 11, 20–25). However, there is currently no information on the role of ARC channels in ASM, their molecular identity, or the mechanisms that may regulate such channels. Accordingly, we hypothesized that ARC channels exist in ASM, and the goal of the present study was to examine their regulation in human ASM cells.
Materials and Methods
Isolation of Human ASM Cells
In lung resections of patients undergoing surgery for focal tumors (excluding infectious pathologies, bullae, or widely disseminated cancers), normal third- to sixth-generation human bronchi distant from the tumor were identified and dissected. Patient histories were used to identify subjects with asthma versus those without asthma. Procedures were approved by the Mayo Clinic Institutional Review Board (minimal risk). ASM cells were enzymatically dissociated using papain/collagenase, and maintained in culture at 37°C (5% CO2, 95% air; less than fifth passage subculture; 4, 24). Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 10% FBS was used until approximately 75% confluence, with 48-hour serum deprivation before experimentation. Verification of ASM phenotype was performed as previously described (4), using Westerns or immunocytochemistry for presence of smooth muscle actin and myosin, but absence of fibroblast surface protein, and robust [Ca2+]i responses to acetylcholine (ACh).
Western Blot Analysis
Standard techniques involved SDS-PAGE, transfer to polyvinylidene fluoride membranes, blocking with 5% milk, overnight incubation at 4°C with 1 μg/ml primary antibody, exposure to horseradish peroxidase–conjugated secondary antibodies, and signal detection by enhanced chemiluminescence.
Knockdown by Small Interfering RNA
Cells were transfected using 20 nM STIM1 small interfering RNA (siRNA; GCCUAUAUCCAGAACCGUtt) (Ambion, Inc., Lafayette, CO), or caveolin-1 siRNA (CUAAACACCUCAACGAUGAUU) or Orai3 siRNA (GCACCUCUUUGCACUCAUG) (Thermo Fisher Scientific, Pittsburgh, PA) using 1 μl/ml lipofectamine in DMEM F/12 lacking FBS. After 6 hours, fresh growth medium was added and cells maintained overnight. Transfection complexes were then aspirated and fresh DMEM/F12 containing 10% FBS added for an additional 24 hours. Serum deprivation was then maintained for 48 hours, followed by experimentation. Western blots verified specific protein knockdown.
[Ca2+]i Imaging
Techniques for real-time [Ca2+]i fluorescence imaging of human ASM cells have been previously described (4, 24). Fura-2/AM (Invitrogen, Carlsbad, CA) –loaded cells were visualized using a Metafluor-based imaging system on a Nikon microscope (Nikon, Melville, NY). Software regions of interest encompassing substantial intracellular areas were delineated for individual cells (multiple cells per visual field), and fluorescence changes calibrated to nM Ca2+ using established techniques. Estimated “noise” levels were roughly 10 nM, and a valid [Ca2+]i oscillation was considered at greater than 40-nM increase above baseline (measured immediately before initiation of an oscillation). Initial observations suggested temporal summation of oscillations immediately upon AA exposure. For consistency, oscillations were analyzed after 2 minutes of AA exposure, and all valid oscillations considered as individual events. Oscillation frequency was calculated as number of valid events over a defined time period (typically 20 min).
Materials
Chemicals were from Sigma-Aldrich (St. Louis, MO) unless mentioned otherwise. Tissue culture reagents (DMEM F/12, FBS) were from Invitrogen, zileuton from R&D Systems Inc. (Minneapolis, MN), xestospongin C (XeC) and ryanodine from Millipore Inc. (Billerica, MA), caveolin-1 and secondary antibodies from Santa Cruz Biotechnology, Inc. (Dallas, TX), STIM1 antibody from Novus (Littleton, CO), and Orai3 antibody from Abcam (Cambridge, MA).
Statistical Analysis
From ASM cells of five different patients (those with asthma or those without), [Ca2+]i experiments were performed using at least 20 cells per patient with three repeats per protocol. Statistically significant (P < 0.05) effects of drugs and/or siRNA were evaluated using Student’s t test or two-way ANOVA, as appropriate, with Bonferroni’s corrections for repeated measures, and Tukey’s test for post hoc analyses. Values are expressed as means (±SE).
Results
[Ca2+]i Responses of Human ASM Cells to AA
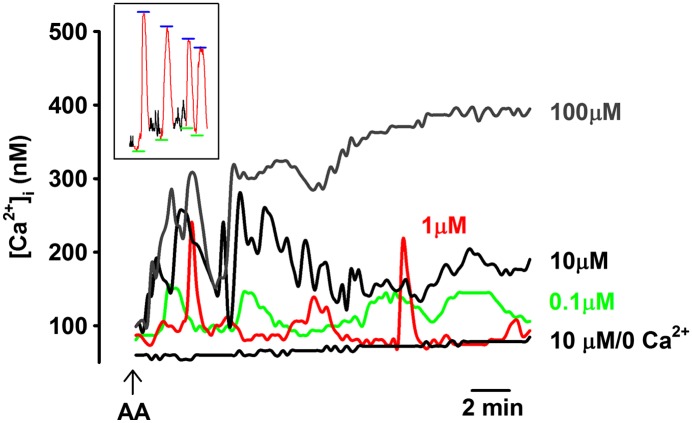
In untreated cells (from subjects without asthma), baseline [Ca2+]i levels before any AA exposure ranged between 80 and 120 nM, comparable to our previous reports (5). No spontaneous [Ca2+]i oscillations were observed at baseline. Exposure to AA (0.1–10 μM) in the presence of 2 mM extracellular Ca2+ resulted in [Ca2+]i oscillations (Figure 1). Interestingly, there was no systematic relationship between AA concentration and oscillation features (Table 1; see inset of Figure 1 for evaluation of baseline and peak of valid oscillations). For example, 0.1 and 1 μM AA induced slow [Ca2+]i oscillations of comparable amplitude and frequency (Figure 1), whereas 10 μM AA showed oscillations at a significantly higher frequency of 0.015 (± 0.005) Hz (which subsided as [Ca2+]i concentrations rose to a higher level; Figure 1; P < 0.05 compared with lower AA concentrations). Exposure to 10 nM AA did not result in [Ca2+]i oscillations, and inconsistently elevated the baseline [Ca2+]i levels in roughly 10% of the cells (data not shown; in these cells, average baseline at 20 min after 10 nM AA was 175 ± 24 nM). On the other hand, exposure to 100 μM AA resulted in an initial [Ca2+]i response similar to that produced by 10 μM AA, but rapidly progressed to a sustained plateau absent of any oscillations.
Figure 1.
Arachidonic acid (AA) induces intracellular calcium concentration ([Ca2+]i) oscillations in human airway smooth muscle (ASM). In the presence of 2 mM extracellular Ca2+, exposure of human ASM cells to different concentrations of AA (0.1–100 μM) rapidly resulted in [Ca2+]i oscillations. Removal of extracellular Ca2+ eliminated these AA-induced [Ca2+]i responses. These data suggest the presence of arachidonate-regulated Ca2+ (ARC) channels in human ASM cells, which modulate Ca2+ influx. Inset shows approach to evaluation of baseline Ca2+ (green) and peak Ca2+ (blue) for individual oscillations. See Table 1 for summary and statistics.
Table 1.
Summary of Intracellular Calcium Concentration Responses of Human Airway Smooth Muscle Cells to Arachidonic Acid
| |
Oscillation Amplitude |
Oscillation Frequency |
|---|---|---|
| (nM Ca2+) | (Hz) | |
| Baseline | No oscillations | — |
| 0.1 μM AA | 101+14 | 0.003 ± 0.0004 |
| 1 μM AA | 118 ± 20 | 0.002 ± 0.0004 |
| 10 μM AA | 150 ± 20* | 0.027 ± 0.007* |
| 100 μM AA | No oscillation, 350 ± 48 peak* | — |
Definition of abbreviation: AA, arachidonic acid
Values are means ± SE.
Significant difference from lower AA concentration (P < 0.05).
These interesting data raised the issue of what AA concentration to use in examining the potential role of ARC channels in ASM. Based on our observations, a lack of difference between 1 and 10 μM AA, previous studies in other cell types using greater than 1 μM AA (15, 16, 26), and indications that endogenous, active AA levels are in the tens of micromolar range (27), further studies were conducted using 10 μM AA.
Characterization of ARC Channels in Human ASM
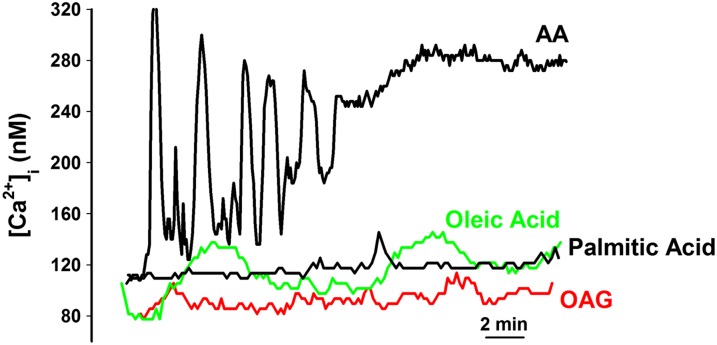
In contrast to the consistent effect of 10 μM AA on ASM cells reflected by [Ca2+]i oscillations, exposure to other fatty acids, such as the monounsaturated oleic acid, the saturated palmitic acid, and the diacylglycerol analog, 1-oleoyl-2-acetyl-SN-glycerol (OAG) (10 μM each), had minimal effects on [Ca2+]i levels with occasional, extremely slow oscillations induced by OAG and oleic acid, but no effects of palmitic acid (Figure 2, Table 2). Oscillations induced by OAG or oleic acid were significantly smaller and slower compared with those by 10 μM AA (Table 2; P < 0.05). With palmitic acid and OAG, baseline Ca2+ levels at 20 minutes were not significantly increased compared with that before exposure to these fatty acids (110 ± 19 nM versus 125 ± 25 nM, respectively). Exposure to oleic acid resulted in a slow increase in baseline to 180 (± 39) nM.
Figure 2.
Effect of other fatty acids on [Ca2+]i responses in ASM cells. In contrast to the consistent AA-induced [Ca2+]i oscillations, other fatty acids, such as the lipid diacylglycerol analog, 1-oleoyl-2-acetyl-SN-glycerol, the saturated fatty acid, palmitic acid, or the monounsaturated oleic acid did not elicit any [Ca2+]i oscillations. Indeed, only oleic acid induced any significant increase in [Ca2+]i in human ASM cells. These data suggest that the observed [Ca2+]i responses are specific to AA. See Table 2 for summary and statistics.
Table 2.
Characterization of Arachidonic Acid–Regulated Ca2+ Channels in Human Airway Smooth Muscle Cells
| |
Oscillation Amplitude |
Oscillation Frequency |
|---|---|---|
| (nM Ca2+) | (Hz) | |
| 2 mM Ca2+, AA | 178 ± 19 | 0.018 ± 0.007 |
| Zero Ca2+, AA | No oscillations | — |
| β-escin permeabilized, pCa 7.0 AA | No oscillations | — |
| 10 μM OAG | 33 ± 9* | 0.009 ± 0.003* |
| 10 μM Oleic acid | 42 ± 7* | 0.002 ± 0.007* |
| 10 μM Palmitic acid | No oscillations | — |
| 10 μM SKF96,365 + AA | 182 ± 12 | 0.021 ± 0.009 |
| 1 μM LaCl3 + AA | 110 ± 17* | 0.024 ± 0.003 |
| 1 mM LaCl3 + AA | 33 ± 9* | 0.002 ± 0.006* |
| 60mM KCl +AA | 132 ± 33 | 0.021+0.002 |
| Lipofectamine + AA | 155 ± 15* | 0.022 ± 0.007 |
| Nonsense siRNA + AA | 138+40 | 0.022+0.002 |
| STIM1 siRNA + AA | 91 ± 9† | 0.010 ± 0.001† |
| Caveolin-1 siRNA + AA | 101 ± 17† | 0.005 ± 0.005† |
| Orai3 siRNA + AA | 117 ± 22 | 0.002 ± 0.001† |
| Zileuton + AA | 130 ± 10 | 0.014 ± 0.001 |
| Indomethacin + AA | 122 ± 15 | 0.016 ± 0.001 |
| Asthmatic ASM, AA | 301 ± 90* | 0.013+0.001 |
Definition of abbreviations: AA, arachidonic acid; ASM, airway smooth muscle; OAG, 1-oleoyl-2-acetyl-SN-glycerol; pCa, calcium potential; siRNA, small interfering RNA; STIM, stromal interaction molecule
Values are means ± SE. All AA experiments were at 10-μM concentration.
Significant difference from 10 μM AA (P < 0.05).
Significant difference from 10 μM AA effects on lipofectamine-transfected cells (P < 0.05).
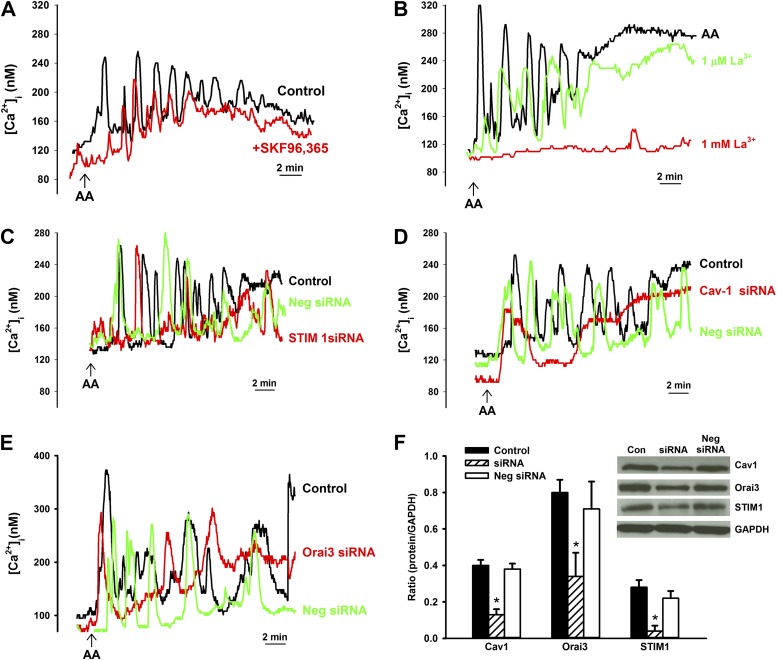
In ASM cells perfused with zero extracellular Ca2+, 10 μM AA–induced oscillations were completely abolished (Figure 1, Table 2), suggesting an influx-mediated mechanism. This was further confirmed in β-escin–permeabilized ASM cells (28) in calcium potential (pCa) 7.0, 6.3, or 6.0 solutions, where 10 μM AA did not induce any oscillations (also see below). To investigate whether the AA-induced increase in [Ca2+]i concentration is due to receptor-operated Ca2+ influx, [Ca2+]i responses to AA were repeated in the presence of 10 μM SKF96365 (Figure 3A). However, 10 μM AA–induced increases in baseline, average oscillation amplitude, or frequency were unaffected by inhibition of receptor-operated influx (Figure 3, Table 2).
Figure 3.
Mechanisms of AA-induced Ca2+ influx. (A) Role of receptor-operated Ca2+ entry in AA-induced Ca2+ influx. In the presence of the receptor-operated channel inhibitor, SKF96365 (10 μM), AA-induced [Ca2+]i responses were not significantly altered, suggesting that the observed Ca2+ influx was not via this mechanism. (B) Role of store-operated Ca2+ entry (SOCE) in AA-induced Ca2+ influx. La3+ (1 μM; which inhibits SOCE) slightly decreased the amplitude of AA-induced [Ca2+]i oscillations, but did not affect oscillation frequency. In contrast, 1 mM La3+ completely eliminated [Ca2+]i responses, suggesting that the observed influx is unlikely to be SOCE, especially because sarcoplasmic reticulum (SR) stores were not depleted during these experiments. (C) Role of stromal interaction molecule (STIM) 1 in AA-induced [Ca2+]i responses. In the presence of STIM1 small interfering RNA (siRNA), AA-induced elevations in [Ca2+]i levels were reduced and [Ca2+]i oscillations decreased compared with lipofectamine (vehicle) control, whereas negative siRNA was without effect. These data suggest that STIM1 modulates AA-induced [Ca2+]i responses. (D) Role of caveolae/caveolin-1 in AA-induced [Ca2+]i responses. Suppression of caveolin-1 using siRNA eliminated AA-induced [Ca2+]i oscillations, whereas lipofectamine or negative siRNA were without effect. These data suggest that caveolin-1 modulates AA-induced Ca2+ influx in human ASM. (E) Role of Orai3 on AA-induced [Ca2+]i responses. Orai3 siRNA reduced the frequency of AA-induced [Ca2+]i oscillations and slightly altered the amplitudes of [Ca2+]I compared with lipofectamine or negative siRNA. These data suggest a role for Orai 3 in modulating AA-induced [Ca2+]i responses. (F) Western analysis confirmed siRNA knockdown of STIM1, caveolin-1, and Orai3, and lack of effect of negative siRNA. See Table 2 for summary and statistics of Ca2+. *Significant siRNA effect (P < 0.05). Cav1, caveolin-1; Con, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Neg, negative.
Although the above protocols did not specifically involve depletion of intracellular Ca2+ stores, it was important to determine whether La3+-sensitive influx pathways (reflecting SOCE at lower concentrations [9]) were influenced by AA. Experiments on AA exposure were performed in the presence of 1 μM or 1 mM La3+. Oscillation frequency was maintained in the presence of 1 μM La3+, but amplitude is decreased, whereas 1 mM La3+ completely abolished oscillations as well as any change in baseline with AA (Figure 3B, Table 2; P < 0.05). Conversely, clamping membrane potential using 60 mM KCl increased baseline Ca2+ to 230 (± 49) nM, but did not induce any oscillations or substantially influence AA-induced [Ca2+]i oscillations (Table 2), suggesting that voltage-gated influx channels were not contributory in this phenomenon.
STIM1 is an important regulatory mechanism in SOCE (22, 23, 29). We previously demonstrated that STIM1 in human ASM is mostly intracellular (e.g., at SR) and, to a lesser extent, within the plasma membrane (23). However, other investigators (17, 18) demonstrated that, in nonexcitable cells, STIM1 is located at the plasma membrane. To further investigate whether STIM1 plays a role in AA effects, AA-induced [Ca2+]i responses were evaluated in ASM cells transfected with STIM1 siRNA (Figure 3C). STIM1 siRNA significantly decreased the frequency and amplitude AA-induced [Ca2+]i oscillations, but did not eliminate them. Baseline [Ca2+]i levels were not significantly influenced by STIM1 siRNA (102 ± 31 nM versus 121 ± 20 nM in lipofectamine controls). In this regard, we previously demonstrated the importance of caveolae and its constituent protein, caveolin-1, in [Ca2+]i regulation in human ASM (4), further showing that plasma membrane STIM1 is within caveolae (23). Based on the above data showing an effect of STIM1 on AA-induced oscillations, to investigate the role of caveolae, we performed experiments in caveolin-1 siRNA–transfected ASM cells. Compared with lipofectamine control, caveolin-1 siRNA significantly blunted both amplitude and frequency of AA-induced [Ca2+]i oscillations (Figure 3D, Table 2; P < 0.05). Baseline [Ca2+]i levels were decreased by caveolin-1 siRNA, but not significantly (76 ± 21 nM versus 111 ± 22 nM in lipofectamine controls).
Although the identity of ARC channels is still under investigation, previous work in nonexcitable cells (30, 31) suggests plasma membrane Orai3. In human ASM cells transfected with Orai3 siRNA, AA-induced [Ca2+]i oscillation frequency was significantly reduced (Figure 3E, Table 2; P < 0.05). However, there was no significant effect on oscillation amplitude, or baseline Ca2+ levels.
For STIM1, caveolin-1, and Orai3 siRNA experiments, nonsense siRNAs did not substantially influence AA-induced [Ca2+]i oscillations, nor did lipofectamine alone (vehicle control). Western analysis confirmed substantial knockdown of these three proteins by the specific siRNAs, with lack of effect of the nonsense siRNAs (Figure 3F).
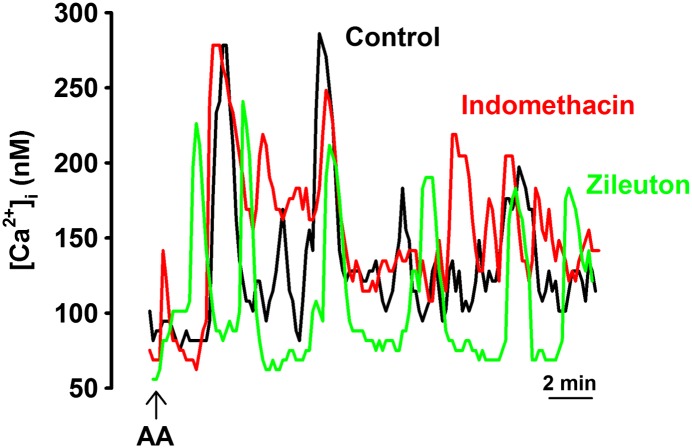
AA can serve as a substrate for the lipoxygenase (LOX), cyclo-oxygenase (COX), and CYP450 pathways, which produce metabolites, such as leukotrienes, prostaglandins, epoxyeicosatrienoic acid, and hydroxyeicosatetraenoic acid. These metabolites can induce inflammation and alter Ca2+ influx (32). Additional experiments were performed in the presence of the LOX inhibitor, zileuton (10 μM), and the COX inhibitor, indomethacin (10 μM; Figure 4). Cells were incubated with inhibitor 15 minutes before AA. Neither inhibitor showed a significant effect on AA-induced [Ca2+]i oscillations (Figure 4, Table 2; P < 0.05). Based on these data showing lack of effect of LOX or COX inhibition on AA-induced oscillations, we did not explore the potential role of AA metabolites further.
Figure 4.
Role of lipoxygenase (LOX) and cyclo-oxygenase (COX) pathways in AA-mediated [Ca2+]i responses. AA can serve as a substrate for the LOX and COX pathways that produce metabolites (leukotrienes, prostaglandins, and prostacyclins), which can mediate inflammation and alter Ca2+ influx. Inhibitors of LOX (zileuton) and COX (indomethacin) did not significantly influence 10 μM AA–induced [Ca2+]i oscillations, suggesting that ARC channels are specifically modulated by AA, and not by products downstream to AA. See Table 2 for summary and statistics.
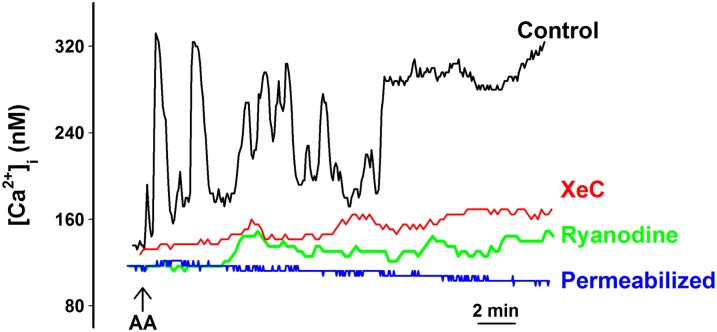
We previously demonstrated that agonist-induced [Ca2+]i oscillations represent repetitive SR Ca2+ release via ryanodine receptor (RyR) channels or inositol 1,4,5-trisphosphate (IP3) receptor channels (5, 12). To determine whether AA-induced [Ca2+]i oscillations also represented such repetitive Ca2+ release from intracellular stores, we repeated experiments in the presence of high, blocking concentrations of the RyR modulator, ryanodine (10 μM) or the IP3 receptor blocker, XeC (1 μM; Figure 5). In the presence of either ryanodine or XeC, AA-induced [Ca2+]i oscillations were abolished, and only minimally elevated [Ca2+]i levels were observed (Figure 5), demonstrating that AA effects do involve SR Ca2+ release, but likely due to indirect effects on the SR resulting from Ca2+ influx. This was supported by the lack of [Ca2+]i oscillations in the absence of extracellular Ca2+ (Figure 1) as well as in β-escin–permeabilized ASM cells in pCa 7.0 (corresponding to baseline Ca2+ of ∼ 100 nM; Figure 5). A similar lack of effect of AA was observed in permeabilized ASM cells in pCa 6.3 (∼ 500 nM; data not shown).
Figure 5.
Role of SR Ca2+ release in AA-induced Ca2+ oscillations. The presence of the cell-permeant inositol 1,4,5-trisphosphate (IP3) receptor blocker, xestospongin C (XeC; 1 μM) or blocking concentrations of ryanodine (10 μM) eliminated all [Ca2+]i oscillations, with only slight increases in [Ca2+]i levels. Separately, in β-escin–permeabilized cells in a pCa of 7.0 (corresponding to 100 nM Ca2+ as reflected by baseline Ca2+), AA was without effect on [Ca2+]i, indicating that AA-induced [Ca2+]i responses only indirectly modulate SR Ca2+ release. See Table 2 for summary and statistics.
AA Effects in ASM Cells from Subjects with Asthma
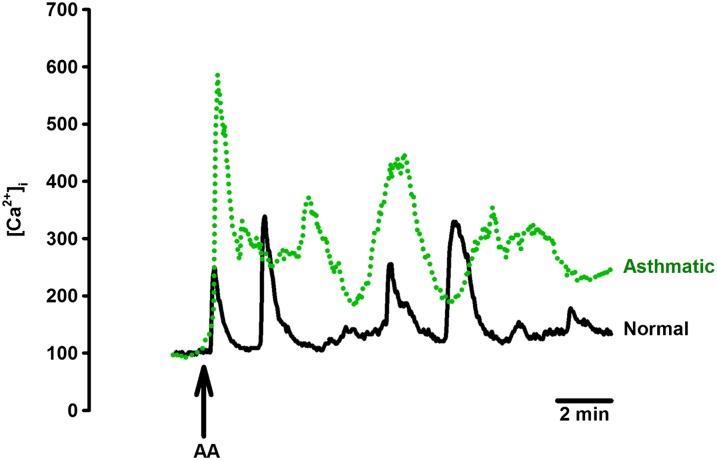
In general, baseline [Ca2+]i levels before AA exposure were not higher in ASM cells from subjects with moderate asthma (defined by forced expiratory volume in 1 second/forced vital capacity, daily use of short-acting bronchodilator). However, exposure of ASM cells from subjects with asthma to 10 μM AA resulted in higher oscillation amplitude compared with cells from subjects without asthma, but there was no difference in oscillation frequency (Figure 6, Table 2). Removal of extracellular Ca2+ prevented [Ca2+]i oscillations as with nonasthmatic cells.
Figure 6.
Effect of AA on [Ca2+]i in asthmatic ASM. In isolated ASM cells from patients with moderate asthma, 10 μM AA induced [Ca2+]i oscillations of higher amplitude compared with nonasthmatic ASM cells. However, oscillation frequency was not different. See Table 2 for summary and statistics.
Discussion
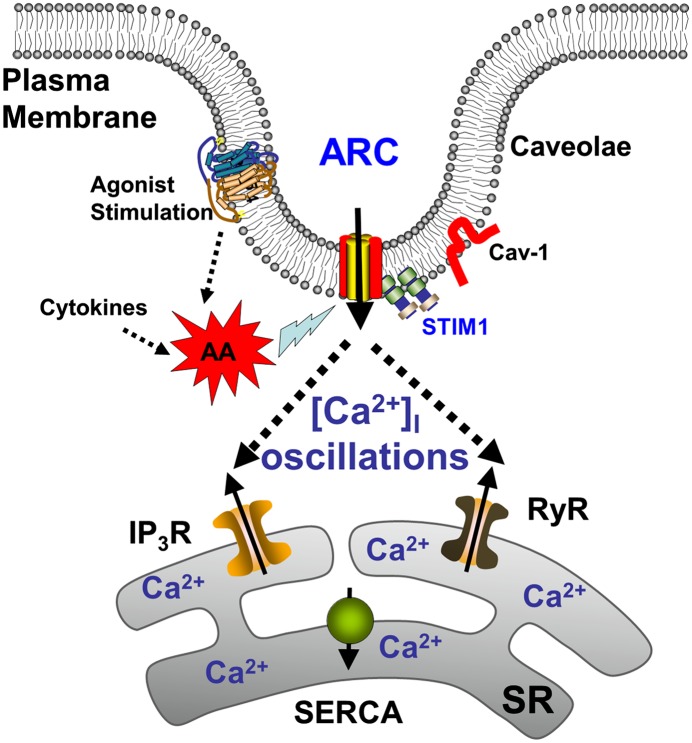
Ca2+ influx through a variety of mechanisms is a key aspect of [Ca2+]i regulation in ASM. In this regard, capacitative Ca2+ entry in response to intracellular store depletion (i.e., SOCE) is an important mechanism in ASM (9–11, 25). However, there is increasing evidence in nonexcitable cells for an additional noncapacitative Ca2+ influx mechanism regulated by AA (13, 33). What is less clear is the molecular identity of such ARC channels, with data in other cell types suggesting a role for Orai proteins that are also involved in SOCE. The data in the current study strongly suggest the existence of ARC channels in human ASM, and such channels involve Ca2+ influx and are mediated via Orai3 proteins. However, other modulatory mechanisms, such as STIM1 and caveolin-1, also appear to be important. Although AA generation is known to occur in human ASM in response to agonist stimulation and in inflammation, our novel results suggest that such AA, by itself, may act through a plasma membrane caveolar mechanism, perhaps Orai3 with a role for plasma membrane STIM1, to induce [Ca2+]i oscillations (Figure 7), akin to those induced by agonists, such as ACh (5). Such AA-induced oscillations appear to involve intracellular Ca2+ release as well, and may thus also partly result from store-operated mechanisms (but not receptor-operated influx). Furthermore, AA-induced [Ca2+]i responses appear to be enhanced in asthmatic ASM. Overall, these data indicate that ARC channels may be an important Ca2+-regulatory mechanism in human ASM, and may contribute to modulation of [Ca2+]i during agonist stimulation or with inflammation.
Figure 7.
Proposed model of ARC channels in human ASM. Regardless of the source (agonist- or cytokine-induced production), AA activates plasma membrane ARC channels that are within caveolae. Such activation is facilitated by plasma membrane STIM1, and involves repeated SR Ca2+ release. IP3R, IP3 receptor; RyR, ryanodine receptor; SERCA, sarco/endoplasmic reticulum calcium ATPase.
Although ARC channels have been demonstrated in a variety of nonexcitable cells (16, 34), their existence and role in ASM has not been studied. In terms of smooth muscle, a previous study reported AA-sensitive Ca2+ influx pathways in A7R5 vascular smooth muscle cells (35). In the current study, we demonstrate that concentrations of AA in the micromolar range induce [Ca2+]i responses, including oscillations. This effect is completely abolished upon removal of extracellular Ca2+, demonstrating the importance of Ca2+ influx in responses of ASM cells to AA. An interesting observation was that there was no clear relationship between AA concentration and the amplitude or frequency of [Ca2+]i oscillations. Furthermore, [Ca2+]i responsiveness to AA in terms of oscillations seems to occur over a narrow concentration range (> 0.1 to < 100 μM), and involves just frequency modulation (Table 1). In this regard, it may be important to consider the appropriate concentrations of AA to examine. There is currently no information on levels in ASM per se. However, published literature (e.g., Ref. 27) suggests that the concentration of free AA in resting cells is “low.” Esterified (presumably inactive) AA levels in platelets are in the 5-mM range, with plasma membrane concentrations being even higher. Thus, even small amounts of release of this AA would provide 50 μM. Free AA levels in tissues or cells has been reported to be anywhere from 0.5–100 μM, and cells are thought to respond in the 1–10 μM AA range. Therefore, our use of 10 μM AA is consistent with previous data.
Although the underlying reasons for the lack of a classical dose–response curve to AA are not entirely clear, oscillations only at higher AA concentrations may represent a threshold within ASM cells to prevent inappropriate [Ca2+]i responses to AA. Accordingly, the higher [Ca2+]i responses to AA in asthmatic ASM may reflect a change in this threshold, contributing to an overall increase in [Ca2+]i that does occur in asthmatic ASM cells. Alternatively, because ARC channels are regulated by AA, but do not necessarily involve receptor-coupled mechanisms, perhaps a classical dose–response cannot be expected. Furthermore, it may be necessary to examine the precise relationships between AA-induced regulatory mechanisms in ASM, and the sensitivity of putative ARC channel elements, such as Orai3 or STIM1, to these mechanisms: topics for future studies.
Mignen and colleagues (36) demonstrated in HEK293, COS, and HeLa cells the existence and high selectivity of ARC channels for Ca2+ as well as AA itself. In comparison to AA, other monounsaturated and saturated fatty acids had no effect. In the current study using human ASM, we demonstrated that, in comparison to AA, the diacylglycerol analog, OAG, oleic acid, and palmitic acid had minimal effects on [Ca2+]i, and only occasionally induced [Ca2+]i oscillations. Furthermore, inhibition of LOX and COX pathways downstream to AA were without effect. These novel data are highly suggestive of an AA-specific influx pathway in ASM similar to that in nonexcitable cells.
Intracellular Ca2+ regulation in ASM involves interplay between plasma membrane Ca2+ influx/efflux and SR Ca2+ release/reuptake (1–4). Accordingly, overall [Ca2+]i regulation may be dependent on both physical proximity of regulatory elements as well as changes in Ca2+ gradients and fluxes induced by these regulatory elements. In this regard, our previous data, showing that agonists, such as ACh, induce repetitive [Ca2+]i oscillations representing cyclical SR Ca2+ release/reuptake with a supportive role for Ca2+ influx, are significant. Here, in addition to voltage-operated and receptor-operated Ca2+ influx, we and others have demonstrated the importance of SOCE in ASM (9–11). What is less clear is whether SOCE per se is important in agonist-induced [Ca2+]i oscillations, because store depletion may or may not occur, especially at lower agonist concentration. In this regard, data from nonexcitable cells show that, in contrast to SOCE, which relies on high agonist concentrations and almost complete SR Ca2+ depletion, ARC channel activity depends on low agonist concentrations (16, 34). Accordingly, the relevance of ARC channels in ASM cells may lie in providing a non-SOCE Ca2+ influx pathway after submaximal agonist stimulation typically associated with [Ca2+]i oscillations (37, 38). Here, the higher [Ca2+]i responses of asthmatic ASM cells to AA may contribute to the higher [Ca2+]i responses to bronchoconstrictor agonists in such cells.
Although it is clear that ARC channels involve Ca2+ influx (demonstrated by the abolition of responses to AA in zero extracellular Ca2+, by high levels of LaCl3, and the lack of responses to AA in β-escin–permeabilized cells), given the lack of specific pharmacological inhibitors, and only limited data suggesting actual molecular identities of these channels, it is difficult to state with complete confidence that the AA-sensitive [Ca2+]i responses observed in ASM cells in this study truly present ARC channels per se. However, data included in our study are generally consistent with previous work in nonexcitable cells, and indirectly suggest that ARC channels do exist in ASM. For example, because ARC channel activity can depend on agonist concentration, receptor-operated Ca2+ channels need to be eliminated as potential confounders. Our data showing lack of effect of the receptor-operated Ca2+ channel inhibitor, SKF96365 (consistent with data in nonexcitable cells [34]), would indeed eliminate this possibility. Another potential confounder is channels that mediate SOCE that occurs in response to SR Ca2+ depletion, thus serving as an important mechanism for SR refilling (9, 39–42). In nonexcitable cells, it has been shown that Ca2+ influx switches from ARC channels at low agonist concentrations to SOCE at higher agonist concentrations (16). Previous studies in ASM demonstrate SOCE is triggered by SR Ca2+ release via both IP3 receptor and RyR channels, and is modulated by agonists (9, 43). However, the sensing or activation mechanisms for SOCE, initially thought to involve transient receptor potential channels, including in ASM (4, 43), have been shown to involve STIM1 at the level of the SR and the plasma membrane Orai1 (44, 45). In this regard, our data (and those in nonexcitable cells) show that AA-mediated [Ca2+]i responses do not represent STIM1/Orai1–mediated SOCE. However, it is also important to note that both STIM and Orai are highly relevant to ARC channels. The Orai family of proteins (Orai1–3) is thought to be the “pore-forming” unit in the plasma membrane that interacts with the C terminus of STIM1 to initiate SOCE or to activate ARC channels (46). Shuttleworth (18) and Thompson and Shuttleworth (19) demonstrated in nonexcitable cells a role for plasma membrane STIM1 in ARC channels. This is in contrast to SOCE, where SR STIM1 appears to be important. We previously found that SR STIM1 is involved in ASM SOCE (23), but found only small amounts of plasma membrane STIM1. Our finding that AA-induced [Ca2+]i responses are substantially blunted in the presence of STIM1 siRNA now suggests a role for the latter. In addition, the units required for the assembly of SOCE channels in comparison to ARC channels are different (18). Whereas Orai1 is particularly important in SOCE (including in ASM), the ARC channel pore is thought to be formed by assembly of three Orai1 and two Orai3 subunits (18). Our observation that knockdown of Orai3 via siRNA results in a 50% decrease in AA-induced [Ca2+]i oscillations is entirely consistent with this scenario in human ASM. Thus, our data overall underline the existence of ARC channels in human ASM.
A novel aspect of our study is the finding that plasma membrane caveolae are important for ARC channels in human ASM. Caveolae are specialized forms of lipid rafts found in most cells types. Previous studies by our group and others (4, 47, 48) suggest that caveolae contain a variety of proteins that are important for [Ca2+]i signaling in ASM, including STIM1 and Orai proteins. Our data in this study, showing that caveolin-1 substantially blunts AA responses, suggests that the mechanisms that involve ARC channel activity, likely STIM1, Orai1, and Orai3, are located within caveolae. Thus, caveolae may facilitate interactions between plasma membrane STIM1 and Orai3, and thus be an important modulator of AA-induced Ca2+ influx.
An interesting aspect of AA-induced [Ca2+]i oscillations is their inhibition by blocking of SR Ca2+ release channels via ryanodine and XeC. Although ARC channels are clearly an influx mechanism, such influx appears to trigger SR Ca2+ release, perhaps by the well known Ca2+-induced Ca2+ release mechanism. What is less clear is whether any intermediate signaling pathways are involved. Furthermore, it is not clear whether AA or its metabolites separately affect IP3 channel or RyR to alter their opening probability or sensitivity to [Ca2+]i. Although AA effects on SR Ca2+ release channels are possible, the lack of AA-induced [Ca2+]i oscillations in β-escin–permeabilized cells would suggest otherwise.
In addition to effects on mechanisms, such as Orai3, which may be part of the ARC channel pore, AA itself is known to activate a number of channels, particularly a range of K+ channels (49), with both membrane depolarization and hyperpolarization being possible depending on whether AA or its metabolites are involved. Although we did not specifically examine the role of K+ channels in this study, the observation that clamping membrane potential with 60 mM KCl did not produce spontaneous [Ca2+]i oscillations or influence oscillations induced by AA would suggest that even if K+ channels were affected by AA, membrane potential itself may not influence ARC channel regulation. With regard to AA metabolites, the downstream CYP450, COX, and LOX pathways can produce a number of metabolites that may potentially mediate AA effects on [Ca2+]i. However, our observations that inhibition of COX or LOX did not substantially influence AA-induced oscillations would suggest that metabolites of at least these two enzymes are likely not involved. Lack of enhanced AA effects in the presence of inhibitors of COX or LOX would further suggest that diversion of AA to the CYP450 pathway and resultant metabolites also may not play a role.
The relevance of AA-induced [Ca2+]i responses and ARC channels in ASM lies in the potential role of this regulatory mechanism in inflammatory airway diseases, such as asthma, where AA-mediated pathways are thought to be important (50). Accordingly, the limited but interesting data that asthmatic ASM cells have a higher amplitude of [Ca2+]i responses to AA raise the question of whether ARC channels are potentially contributory to increased [Ca2+]i in asthmatic ASM. Although the mechanisms for such up-regulation were beyond the scope of this study, enhanced Orai3 expression (or activation) may be involved, and would be consistent with previous findings that related mechanisms, such as STIM1, Orai1, and even caveolin-1, are up-regulated in human ASM after inflammation (11, 24, 25). Furthermore, inflammation can result in enhanced expression of SR Ca2+ proteins and functionality, and AA-induced influx could produce greater SR Ca2+ responses, leading to greater contraction.
In conclusion, our study demonstrates the existence of ARC channels in human ASM similar to that in nonexcitable cells. The relevance of ARC channels in ASM lies in its potential contribution to Ca2+ influx during agonist stimulation, when SR store depletion may not occur, thus allowing a non-SOCE pathway for Ca2+ influx to replenish stores. Given the generation of AA after agonist stimulation and inflammation, ARC channels may be particularly important in contributing to the elevated [Ca2+]i levels observed in inflamed/asthmatic ASM, and thus to increased airway contractility.
Footnotes
This work was supported by National Institutes of Health R01 grants HL090595 (C.M.P.), and HL088029 and HL56470 (Y.S.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2013-0144OC on January 28, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007;30:114–133. doi: 10.1183/09031936.00147706. [DOI] [PubMed] [Google Scholar]
- 2.Pabelick CM, Sieck GC, Prakash YS. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol (1985) 2001;91:488–496. doi: 10.1152/jappl.2001.91.1.488. [DOI] [PubMed] [Google Scholar]
- 3.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L26–L34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1118–L1126. doi: 10.1152/ajplung.00136.2007. [DOI] [PubMed] [Google Scholar]
- 5.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol. 1997;272:C966–C975. doi: 10.1152/ajpcell.1997.272.3.C966. [DOI] [PubMed] [Google Scholar]
- 6.Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;298:L521–L530. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein–coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci USA. 2009;106:11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L909–L917. doi: 10.1152/ajplung.00317.2003. [DOI] [PubMed] [Google Scholar]
- 10.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash YS, Pabelick CM, Kannan MS, Sieck GC. Spatial and temporal aspects of ACh-induced [Ca2+]i oscillations in porcine tracheal smooth muscle. Cell Calcium. 2000;27:153–162. doi: 10.1054/ceca.1999.0106. [DOI] [PubMed] [Google Scholar]
- 13.Shuttleworth TJ. Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium. 2009;45:602–610. doi: 10.1016/j.ceca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- 15.Mignen O, Shuttleworth TJ. I(ARC), a novel arachidonate-regulated, noncapacitative Ca(2+) entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 16.Mignen O, Thompson JL, Shuttleworth TJ. Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein. J Physiol. 2005;567:787–798. doi: 10.1113/jphysiol.2005.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuttleworth TJ. STIM and Orai proteins and the non-capacitative ARC channels. Front Biosci (Landmark Ed) 2012;17:847–860. doi: 10.2741/3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson JL, Shuttleworth TJ. A plasma membrane-targeted cytosolic domain of STIM1 selectively activates ARC channels, an arachidonate-regulated store-independent Orai channel. Channels (Austin) 2012;6:370–378. doi: 10.4161/chan.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284:22501–22505. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1–mediated store-operated calcium entry in airway smooth muscle cell proliferation. J Appl Physiol. 2011;110:1256–1263. doi: 10.1152/japplphysiol.01124.2010. [DOI] [PubMed] [Google Scholar]
- 23.Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 regulation of store-operated Ca(2+) influx in human airway smooth muscle. Eur Respir J. 2012;40:470–478. doi: 10.1183/09031936.00090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathish V, Abcejo AJ, VanOosten SK, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 in cytokine-induced enhancement of intracellular Ca(2+) in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;301:L607–L614. doi: 10.1152/ajplung.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia L, Delmotte P, Aravamudan B, Pabelick CM, Prakash YS, Sieck GC. Effects of the inflammatory cytokines TNF-α and IL-13 on stromal interaction molecule-1 aggregation in human airway smooth muscle intracellular Ca2+ regulation. Am J Respir Cell Mol Biol. 2013;49:601–608. doi: 10.1165/rcmb.2013-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung-Yam-Wah V, Lee AK, Tse FW, Tse A. Arachidonic acid stimulates extracellular Ca(2+) entry in rat pancreatic beta cells via activation of the noncapacitative arachidonate-regulated Ca(2+) (ARC) channels. Cell Calcium. 2010;47:77–83. doi: 10.1016/j.ceca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol. 1997;272:L659–L664. doi: 10.1152/ajplung.1997.272.4.L659. [DOI] [PubMed] [Google Scholar]
- 29.Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuttleworth TJ. Orai3—the ‘exceptional’ Orai? J Physiol. 2012;590:241–257. doi: 10.1113/jphysiol.2011.220574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li WG, Yu Y, Zhang ZD, Cao H, Xu TL. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain. 2010;6:88. doi: 10.1186/1744-8069-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuttleworth TJ. Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J Biol Chem. 1996;271:21720–21725. doi: 10.1074/jbc.271.36.21720. [DOI] [PubMed] [Google Scholar]
- 34.Mignen O, Thompson JL, Yule DI, Shuttleworth TJ. Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells. J Physiol. 2005;564:791–801. doi: 10.1113/jphysiol.2005.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broad LM, Cannon TR, Taylor CW. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J Physiol. 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- 37.Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the non-capacitative arachidonate-regulated Ca2+ channels in HEK293 cells: comparison with endogenous store-operated channels. J Biol Chem. 2001;276:21365–21374. doi: 10.1074/jbc.M102311200. [DOI] [PubMed] [Google Scholar]
- 38.Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J Biol Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 39.Pabelick CM, Ay B, Prakash YS, Sieck GC. Effects of volatile anesthetics on store-operated Ca(2+) influx in airway smooth muscle. Anesthesiology. 2004;101:373–380. doi: 10.1097/00000542-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 41.Putney JW, Jr, McKay RR. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 42.Sieck GC, White TA, Thompson MA, Pabelick CM, Wylam ME, Prakash YS. Regulation of store-operated Ca2+ entry by CD38 in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L378–L385. doi: 10.1152/ajplung.00394.2007. [DOI] [PubMed] [Google Scholar]
- 43.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-α–enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 45.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shuttleworth TJ. Orai channels—new insights, new ideas. J Physiol. 2012;590:4155–4156. doi: 10.1113/jphysiol.2012.237552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med. 2008;8:741–753. doi: 10.2174/156652408786733720. [DOI] [PubMed] [Google Scholar]
- 49.Meves H. Arachidonic acid and ion channels: an update. Br J Pharmacol. 2008;155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnes PJ. Biochemical basis of asthma therapy. J Biol Chem. 2011;286:32899–32905. doi: 10.1074/jbc.R110.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]