Abstract
Extracellular matrix remodeling and tissue rupture contribute to the progression of emphysema. Lung tissue elasticity is governed by the tensile stiffness of fibers and the compressive stiffness of proteoglycans. It is not known how proteoglycan remodeling affects tissue stability and destruction in emphysema. The objective of this study was to characterize the role of remodeled proteoglycans in alveolar stability and tissue destruction in emphysema. At 30 days after treatment with porcine pancreatic elastase, mouse lung tissue stiffness and alveolar deformation were evaluated under varying tonicity conditions that affect the stiffness of proteoglycans. Proteoglycans were stained and measured in the alveolar walls. Computational models of alveolar stability and rupture incorporating the mechanical properties of fibers and proteoglycans were developed. Although absolute tissue stiffness was only 24% of normal, changes in relative stiffness and alveolar shape distortion due to changes in tonicity were increased in emphysema (P < 0.01 and P < 0.001). Glycosaminoglycan amount per unit alveolar wall length, which is responsible for proteoglycan stiffness, was higher in emphysema (P < 0.001). Versican expression increased in the tissue, but decorin decreased. Our network model predicted that the rate of tissue deterioration locally governed by mechanical forces was reduced when proteoglycan stiffness was increased. Consequently, this general network model explains why increasing proteoglycan deposition protects the alveolar walls from rupture in emphysema. Our results suggest that the loss of proteoglycans observed in human emphysema contributes to disease progression, whereas treatments that promote proteoglycan deposition in the extracellular matrix should slow the progression of emphysema.
Keywords: tissue stiffness, alveolar stability, glycosaminoglycan, network model
Clinical Relevance
The destruction of alveolar structure in emphysema has been thought to be governed by enzymatic digestion of extracellular matrix proteins. It is not known how proteoglycans (PGs) contribute to alveolar stability and tissue degradation in emphysema. In this study, from the response of tissue stiffness and alveolar deformation to changes in tonicity, we infer that PGs significantly contribute to alveolar stability in mice with emphysema. Furthermore, network modeling suggests a protective role for PGs in the progression of tissue destruction.
Pulmonary emphysema results from the destruction of septal walls (1) and terminal bronchioles (2), which leads to permanent enlargement of airspaces and collapse of the neighboring alveoli (3), diminishing the gas exchanging efficiency of the lung (4). Although proteoglycans (PGs) are known to change in emphysema (5–7), their contribution to tissue stiffness, alveolar stability, and disease progression is not understood.
Tissue stiffness is mostly determined by collagen and elastin, which are major mechanical load–bearing components of the extracellular matrix (ECM) (8, 9). These fibers are embedded in a matrix of PGs, which are composed of glycosaminoglycan (GAG) chains covalently linked to a protein core. The negatively charged GAGs generate repulsive electrostatic forces, which contribute to the compressive and shear resistance of the ECM (10). During tissue deformation, collagen and elastin fibers unfold and reorient; this process is opposed by the PGs surrounding these fibers. Hence, PGs contribute to the stress–strain properties of the ECM, especially at lower lung volumes, by preventing the alveolar structure from collapse in the normal lung (11).
The contribution of PGs to lung mechanics and stability depends on the ratio of the stiffness of the PGs and the fibers (11). In emphysema, the elastin and collagen fibers undergo digestion and remodeling (12), which decrease lung tissue stiffness (13, 14). Based on a decrease in fiber stiffness alone, the contribution of PGs is expected to increase. However, alterations in PG structure and/or content also occur in patients with emphysema (5–7) and in animal models of emphysema (15–17). Nevertheless, it is not known how the biophysical properties of PGs affect tissue stiffness and alveolar stability in emphysema.
Because fiber stiffness is reduced in emphysema (13, 14), we hypothesized that PGs play a more important role in lung function and alveolar stability in the emphysematous lung than in the normal lung. To test this, we studied lung mechanics, tissue stiffness, and alveolar structure and deformability in normal and porcine pancreatic elastase (PPE)-treated mice. To assess the contribution of PGs without altering the ECM structure using PG digestion, we varied the tonicity of the measurement condition because tonicity is known to affect the bulk modulus of the PGs via alteration in charge distribution (10) but not the stiffness of fibers (18, 19). The results were interpreted using computational models of lung structure and elasticity.
Materials and Methods
Detailed methods are provided in the online supplement.
Animal Preparation
All procedures were approved by the Animal Care and Use Committee of Boston University. C57BL/6J mice were used. The first group was treated with PPE (n = 14), and the other was left untreated (normal group; n = 14). Experiments were performed 30 days after treatment.
Respiratory Mechanics
The animals were anesthetized, tracheostomized, and ventilated. Respiratory impedance was measured using forced oscillations, from which airway resistance (R) and respiratory compliance (C) were estimated.
Tissue Processing
Two lobes were cut into strips (5 × 2 × 1 mm). Some strips were imaged, some were digested with chondroitinase ABC or hyalurodinase before imaging, and some strips were used in mechanics measurements. The other lobes were used to determine the wet-to-dry weight ratio or for Western blots or were fixed in formalin.
Morphometry
Lung sections were stained with hematoxylin and eosin. Randomly selected regions were imaged and segmented, and the area of the airspaces and the equivalent diameter (D) of airspaces were measured. The mean equivalent diameter (Dmean), the area weighted mean equivalent diameter (D2) (20), and tissue fraction were calculated (21).
Tissue Strip Mechanics
Uniaxial quasi-static stress–strain curves were obtained as previously described (11). The stress–strain curves were obtained in hypotonic (0.05 M), isotonic (0.15 M), and hypertonic (0.45 M) saline.
Alveolar Structure as a Function of Tonicity
Strips were held at 40% uniaxial strain submerged in saline. Based on autofluorescence, the alveolar structures were imaged using a confocal microscope in hypotonic and hypertonic solutions in random order. The aspect ratio (r) of airspaces was determined.
Alcian Blue Staining and Image Processing
Alcian Blue staining followed the standard protocol. The images were split into blue (representing acidic sulfate substances and hyaluronic acid) and pink (nuclei). The number of blue pixels and their intensity were determined. The mass per unit wall length, defined as the number of blue pixels weighed by their intensity, was also determined.
Western Blots
The samples were separated by PAGE and analyzed by Western blotting. Rabbit polyclonal anti-versican, rabbit or sheep polyclonal antidecorin, and mouse monoclonal anti–β-actin antibodies were used. The immune complexes were detected by chemiluminescence and quantified by computerized densitometry.
Spring Network Model
We extended a previously developed two-dimensional prestressed hexagonal network of linear elastic springs (11). Each spring represented the mechanical behavior of an alveolar wall, and the internal nodes were free to move. The network was stabilized by including a term in the total energy (Ea) that was a function of the change in the angle between two neighboring springs (θ):
| (1) |
where b is called the bond-bending constant (22), which is related to the compressive resistance of the PGs (11). The compliance of the network was calculated as the inverse of derivative of the total energy with respect to a small biaxial strain.
Statistical Analysis
All data are presented as mean ± SD. Different groups were tested with one-way or two-way ANOVA, unpaired t test, and Kolmogrov-Smirnoff test. Statistical significance was accepted at P < 0.05.
Results
Treatment with PPE resulted in a lower respiratory resistance (R) and a higher compliance (C) (Figures 1A and 1B, respectively) at positive end-expiratory pressures of 3 and 6 cm H2O. These functional changes were a result of airspace enlargement and its heterogeneity. The Dmean of airspaces was larger (42.1 ± 36.4 μm versus 32.3 ± 9.7 μm; P < 0.001), whereas tissue fraction, the ratio of the alveolar wall areas in a region to the total area of the region, was smaller (16.8 ± 3.2% versus 20.3 ± 3.7%; P < 0.01) in the treated mice. The D2, an index sensitive to alveolar heterogeneity (20), correlates with organ level function such as C (23). The D2 was substantially higher in PPE-treated mice (145.2 ± 48.5 μm) than in normal mice (37.5 ± 2.3 μm; P < 0.0001).
Figure 1.
Mean ± SD of respiratory system resistance (A) and compliance (B) as a function of positive end-expiratory pressure (PEEP) in normal mice (n = 7) and mice treated with porcine pancreatic elastase (PPE) (n = 7) 30 days before the experiments. Stars denote significant difference between groups at the same PEEP. *Significant interaction between PEEP and treatment at P < 0.05.
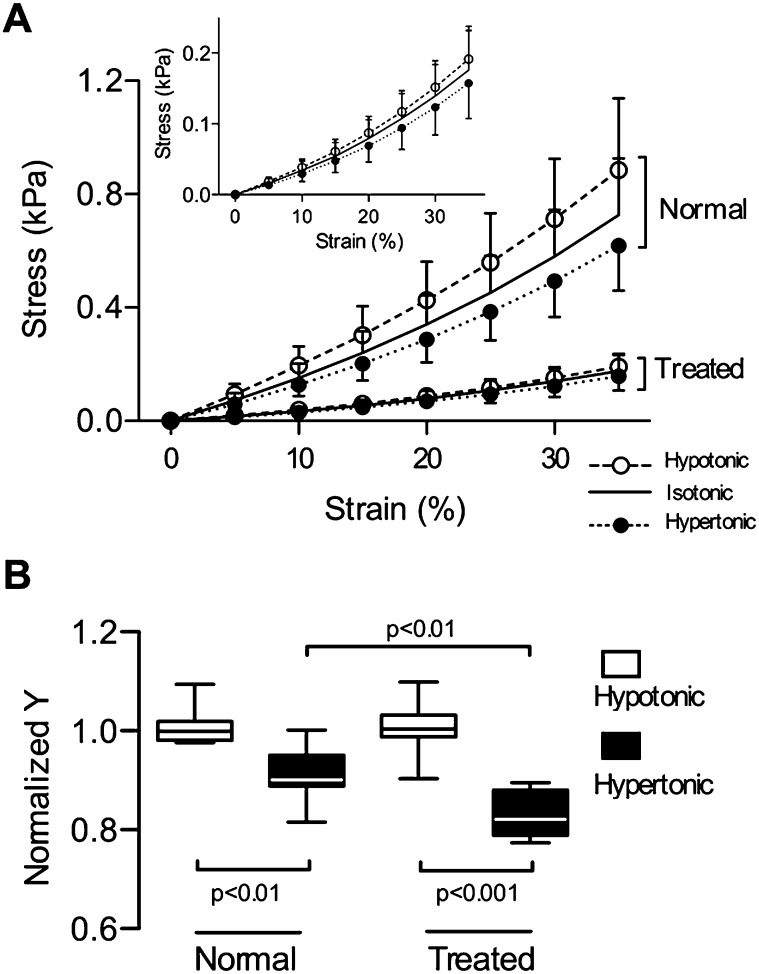
The functional changes at the tissue strip level were evaluated using quasistatic uniaxial stress–strain curves obtained under three tonicity conditions (Figure 2A). In isotonic saline (0.15 M), the stress at 25% strain in PPE-treated strips decreased to 24.4% of the stress in control tissues (0.45 ± 0.13 kPa versus 0.11 ± 0.04 kPa; P < 0.001). In hypotonic solution (0.05 M), the stress–strain curves shifted to the left with increased stiffness (Y), whereas in hypertonic solution (0.45 M) they shifted to the right with decreased Y. The stress significantly depended on the solution (P < 0.01) and the strain (P < 0.001), with an interaction (P < 0.001) in both groups. In hypertonic solution, Y, normalized to the mean Y in hypotonic solution, was lower in normal and in PPE-treated strips (P < 0.01 and P < 0.001, respectively) (Figure 2B). The drop in Y from hypotonic to hypertonic solution was larger in strips from treated mice (0.83 ± 0.05 versus 0.90 ± 0.06; P < 0.01). The dependence of Y on tonicity and treatment was similar at 35% strain (see Figure E1 in the online supplement). Thus, stiffness was more sensitive to changes in tonicity in the emphysematous tissue.
Figure 2.
(A) Mean ± SD of stress–strain curves in hypotonic (0.05 M), isotonic (0.15 M), and hypertonic (0.45 M) saline measured in lung tissue strips obtained from normal (n = 7) and PPE-treated (n = 7) mice. Inset shows data from the treated group. The stress significantly depended on strain (P < 0.001) and solution (P < 0.01), and there was an interaction between them (P < 0.01) in both groups. (B) Normalized Young’s modulus (Y) at 25% strain in hypotonic and hypertonic saline. The value of Y was set to 1 in hypotonic solution in both groups. Y in hypertonic saline was significantly lower in strips from treated mice.
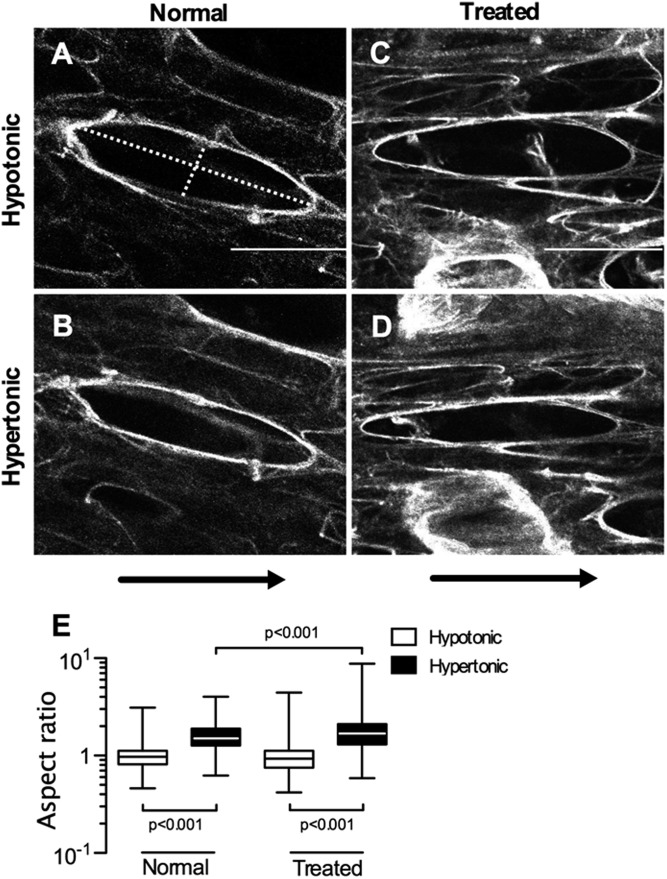
To shed light on the microscopic origin of the increased sensitivity in Y, we imaged the deformation of individual airspaces before and after changing tonicity. Representative images from normal and PPE-treated tissues at 40% strain are shown in Figure 3. The aspect ratio (r, major axis/minor axis of a fitting ellipse) in a normal sample (Figure 3A) in hypotonic solution was 4.25. After changing the solution to hypertonic, PGs collapsed, and their stiffness decreased, which resulted in a slightly collapsed airspace with r = 4.75 (Figure 3B). However, a similar size airspace in the emphysematous tissue was significantly more sensitive to changes in tonicity, with r increasing from 4.51 in hypotonic solution (Figure 3C) to 6.58 in hypertonic solution (Figure 3D). Overall, the mean increase in r with change in tonicity from hypotonic to hypertonic solution was significant in both groups (P < 0.001) (Figure 3E). However, the increase in r was significantly higher in samples from the PPE-treated mice (1.81 ± 0.69 versus 1.58 ± 0.54; P < 0.001). Thus, similar to Y, airspace stability was more sensitive to tonicity in the emphysematous tissue.
Figure 3.
Examples of autofluorescent confocal images of the alveolar walls at 40% uniaxial strain in the horizontal direction. The aspect ratio (r) is calculated as the ratio of the major axis and minor axis. (A) Normal lung tissue in hypotonic solution. The major and minor axes shown by dotted lines in the selected airspace with r = 4.25. (B) The same airspace in hypertonic solution with r increased to 4.75. (C) PPE-treated lung tissue in hypotonic solution with r = 4.51 (major and minor axis shown by dotted line). (D) The same airspace as in C in hypertonic solution (r = 6.58). Bar represents 50 μm. (E) Normalized r at 40% static strain in hypotonic and hypertonic saline. The mean value of r in hypotonic was set to 1 in both groups. The normalized r in hypertonic saline was significantly lower in the treated mice. Arrows show the direction of strain.
To verify that the sensitivity of tissue stiffness and alveolar deformation to changes in tonicity is related to the charge density of PGs in the septal walls, we quantified alveolar deformation after digestion of GAGs with chondroitinase ABC or hyaluronidase. Table 1 compares the relative change in r due to a change in tonicity. The data in the first row in Table 1 were obtained from reanalyzing the data in Figure 3E. After digestion with either enzyme, r showed a markedly decreased sensitivity to tonicity (i.e., a drop to one third of the predigestion value) in normal and PPE-treated tissues (Figure E2), providing evidence that the changes in stiffness and alveolar shape with tonicity are related to the charge density of PGs in the tissue.
Table 1.
Sensitivity of Alveolar Aspect Ratio in Tissue Strips to Changes in Tonicity in Normal and Porcine Pancreatic Elastase–Treated Mice*
| Normal | PPE Treated | |
|---|---|---|
| Undigested | 62.0 ± 15.3 | 94.1 ± 16.9† |
| Chondroitinase ABC | 19.9 ± 11.6‡ | 32.0 ± 15.3§ |
| Hyaluronidase | 29.7 ± 13.0§ | 28.4 ± 21.2‡ |
Definition of abbreviation: PPE, porcine pancreatic elastase.
The percent increase of the alveolar aspect ratio (r) is obtained by normalizing the difference between hypertonic r and hypotonic r with the hypotonic r. Data are presented as mean ± SD averaged over seven tissue strips in each group. There is a significant effect of PPE treatment groups (P < 0.01), enzyme treatment (P < 0.0001), and interaction between groups and enzymes (P < 0.05).
Significant difference between the normal and PPE-treated groups at P < 0.01.
Significant differences between digested and undigested groups at P < 0.001.
Significant differences between digested and undigested groups at P < 0.01.
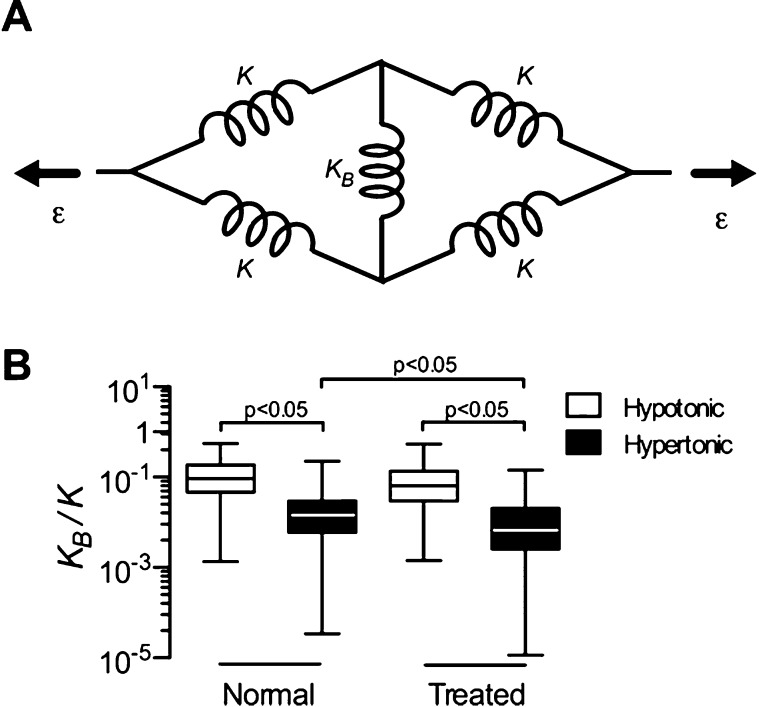
To better understand the role of PGs in alveolar shape changes, we developed a simple model of the deformation of a single isolated airspace. The model consists of five linear springs (Figure 4A). The four identical springs with constant K represent the fiber stiffness in the septal walls, whereas the middle spring with constant KB represents the compressibility of the PGs. When strain, ε, is applied at the two ends, the compressed spring, KB, stabilizes the shape, but when the solution is changed from hypotonic to hypertonic, KB decreases. Using geometric considerations and the force–length relation of springs, the stiffness ratio KB/K can be calculated for ε > 0 as:
| (2) |
where λ is the stretch ratio (λ = 1 + ε). Eliminating outliers (see additional information in the online supplement) and substituting r without normalization from Figure 3 with λ = 1.4 into Eq. 2, KB/K was computed (Figure 4B). In treated and normal strips, KB/K in hypotonic solution is around 0.1, suggesting that fibers are approximately 10 times stiffer than the effective compressive spring constant of the PGs. As expected, KB/K was smaller in hypertonic solution in both groups (P < 0.05). However, KB/K in hypertonic solution was significantly smaller in the samples from emphysematous tissue (P < 0.05). Furthermore, KB/K decreased with chondroitinase ABC and hyaluronidase digestion compared with undigested control (Figure E3).
Figure 4.
(A) A two-dimensional network model of a single airspace. The linear springs (K) account for the stiffness of the elastin and collagen fibers in the septal walls, whereas the linear spring in the middle (KB) represents the compressibility of the PGs. Changing osmolarity only affects KB but not K. ε indicates the local strain. (B) Box plot of the stiffness of PGs relative to that of the fibers, KB/K, obtained from the model in hypotonic and hypertonic solution in tissue strips from normal and treated mice.
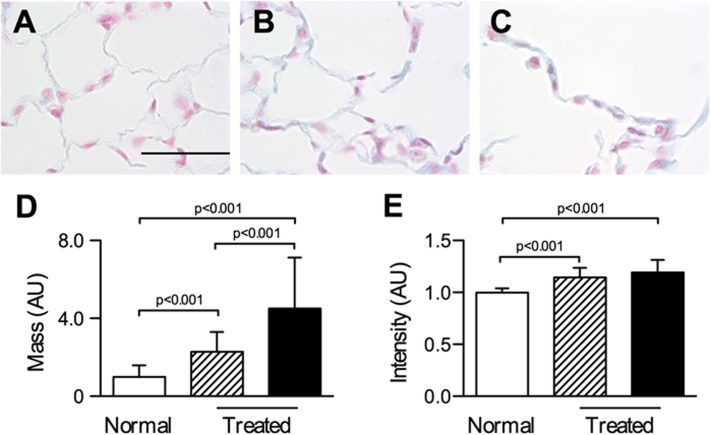
The origin of the increased sensitivity of PG stiffness to tonicity in emphysema was further studied by quantifying the GAG and core protein contents of the PGs. In comparison with normal tissue (Figure 5A), GAGs in PPE-treated lungs (Figures 5B and 5C) appeared denser. Quantification of GAG staining showed that the median intensity of blue pixels was higher in treated lungs than in normal lungs (P < 0.001) (Figure 5E). Also, the mass of blue pixels per unit length of wall (Figure 5D) was higher in treated mice, and this was higher in walls around large holes than in walls surrounding normal-sized airspaces (P < 0.001). Western blot analyses for versican and decorin, which are chondroitin/dermatan sulfate PGs, are shown in Figure 6. Versican expression increased (P < 0.001), whereas decorin expression decreased (P < 0.001), in the PPE-treated group, suggesting significant remodeling of the ECM.
Figure 5.
Examples of alveolar structure stained using Alcian blue and quantitative analysis of glycosaminoglycan (GAG) content. GAGs are shown in blue and nuclei are pink. Representative sections show airspace regions from a normal mouse (A) and from PPE treated mouse with normal airspace sizes (B) and enlarged airspace sizes (C). Bar represents 50 μm. (D) Total mass of GAGs computed as the intensity weighted sum of all blue pixels per unit septal wall length from normal mice and treated mice from regions of normal (shaded) and enlarged (black) airspace sizes. (E) Median blue pixel intensity per image from normal and PPE-treated mice from regions of normal (shaded) and enlarged (black) airspace sizes. The intensity and the total mass were higher in lungs from treated mice. Additionally, the enlarged airspace areas in treated lungs had more GAG mass per unit length than the normal airspace size areas.
Figure 6.
Versican and decorin each normalized by their loading control in normal (n = 5) and treated (n = 5) lung homogenates. Right upper images are example Western blots for versican and decorin together with their loading control.
Next, we assessed the dry weight and wet weight of the tissues. Although dry weight in normal and treated lungs was not different (5.4 ± 0.5 mg versus 5.6 ± 0.7 mg), there was a significant difference in wet weight (31.8 ± 3.3 mg versus 36.9 ± 4.5 mg; P < 0.05), suggesting that the emphysematous tissue held more water as a consequence of increased GAG content.
To integrate these physiological and molecular findings, network simulations were used to evaluate the effects of PGs on structure and function. In the network model, we assumed a higher probability of rupture for walls under a larger stretch. As springs mimicking septal walls were gradually eliminated (Figure 7A), C and D2 of the networks increased as a function of the bond-bending parameter (b), representing the compressive stiffness of PGs. The rate of increase of C and D2 diminished for higher values of b (Figure 7B) despite the fact that the number of eliminated springs was held constant. The network configurations at the eighth time step (Figure 7A) demonstrate that for b = 0.1 the network is highly heterogeneous, whereas for b = 1 the destruction pattern is nearly homogeneous. We also plotted how C changes with time (Figure 7C), from which we calculated the time required to double C (td). For larger values of b, td increased significantly (Figure 7C, inset), implying that strong bond bending protects the network by slowing the rate of degradation.
Figure 7.
Network model simulations showing the effects of the bond-bending parameter (b) on compliance (C) and the area weighted mean diameter (D2) during mechanical force–induced breakdown of the network. (A) Representative images of the network configurations are shown at the end of eight iterations for different values of b. The color of a spring is proportional to the tensile force it carries (blue: low force; red: high force). (B) Sensitivity plot showing the % increase in compliance (∆Compliance, filled symbols) and D2 (∆D2, open symbols) between first and eighth iteration as a function of b. The solid lines and dotted lines are curve fits. (C) Network compliance as a function time (iteration) for several values of b. Simulations were performed until the compliance of the network doubled. The inset shows the doubling time (td) as a function of b.
Discussion
The main findings of this study are that, compared with the normal lung, (1) the stability of the alveolar network and the stiffness of lung tissue was more sensitive to charge density on the PGs in PPE-treated lungs; (2) the GAG amount and versican expression increased, whereas decorin expression decreased, in alveolar walls in emphysema; (3) a microstructural model showed that the stiffness of PGs relative to fibers was more sensitive to tonicity in emphysema; and (4) a network model predicted that increasing PG density slows the progression of emphysema.
The organ level functional changes in R and C due to PPE treatment were similar to those in tight-skin (24), SP-D–deficient (25), TNF-α–overexpressing (26), and elastase-treated (21, 23) mice. These functional changes have structural origins (21, 23, 27, 28). Tissue destruction in emphysema leads to a spatially heterogeneous structure (29) with variability in airspace sizes (20, 21, 30, 31).
The stress in the lung tissue at a given strain decreased after PPE treatment (Figure 2A), which is in agreement with previous studies (13, 14, 21, 32). The increase in C results from several factors, including the decrease in alveolar wall stiffness, tissue loss, and airspace heterogeneity. Indeed, tissue fraction decreased by 17.3%, and airspace size and heterogeneity characterized by the index D2 increased by nearly 4-fold. Although there are limitations of the tissue strip preparation (e.g., the lack of air–liquid interface, uniaxial deformation, and the lack of circulation and innervation), most changes reported in the emphysema are related to remodeling of the ECM (2, 12, 23, 32, 33). Animal models also suggest that surface tension is perhaps not very important in emphysema (23, 34). Indeed, PPE-induced changes in the pressure–volume curve are similar to the change in the stress–strain curve in emphysema (14).
PGs are known to contribute to the viscoelastic and failure properties of various tissues (11, 35–38). The negatively charged GAGs on the PGs influence the compressibility of the ground substance (10), tissue osmotic pressure (39, 40), and tissue swelling (40, 41). We found increased swelling by more water retention in the emphysematous lung. Furthermore, the sensitivity of the normalized stiffness to tonicity increased by 9% (Figure 2B). To understand the origin of this increased sensitivity, we determined the stability of the alveolar structure. The r value, which reflects the shape distortion of an alveolus, changed with tonicity (Figure 3). The compressive stiffness of PGs impedes the alignment of fibers relative to each, and hence r at a given strain is determined by the relative magnitudes of the fiber stiffness in stretch and the matrix stiffness in compression (11). Because surface charges on PGs are high, they are much more sensitive to changes in tonicity than collagen (19) and elastin (18); this finding is also supported by our GAG digestion studies (Table 1) and by recent data in cartilage mechanics (42). Thus, the increased sensitivity of the r to tonicity in emphysema indicates an increased role of PG stiffness in the stability of airspaces, which was further supported by the computational results (Figure 4B) obtained with the microstructural model of the shape change of a single alveolus (Figure 4A).
Various pathological ECM turnover processes remodel the alveolar wall tissue with excessive deposition of ECM molecules (33, 43). The hyaluronan content in the alveolar walls of mice exposed to cigarette smoke was reported to increase (15). In humans, versican in the alveolar walls increased progressively with the severity of emphysema (6), whereas there was a decrease in decorin and biglycan staining in the peribronchial area (7); this finding is similar to our data (Figure 6). Furthermore, these results are consistent with the possibility that PG remodeling was driven by pulmonary fibroblasts because versican and decorin levels in cultured fibroblasts isolated from normal and PPE-treated mice (Figure E4) were similar to those in Figure 6. Additionally, heparan sulfate in the alveolar basement membrane decreased in severe emphysema (7), and decreased hyaluronic acid with a reduced chain length was also reported in human emphysema (5, 44). Although the structural role of these core proteins in tissue stability is not understood, they might contribute indirectly to disease progression because loss of decorin, for example, results in abnormal collagen fibril formation (45).
In our study, we focused on the structural role of PGs based on their elastic resistance related to charge density on the GAGs. Through the repulsive forces from the negative surface charge, the increased GAGs in the treated mice contribute to alveolar stabilization. Because the overinflated airspace areas compress neighboring small alveoli (3), the increased GAG content resists this deformation, and this mechanism serves to stabilize alveoli and improve function. The network modeling in Figure 7 was performed to evaluate whether this mechanism can also influence the rate of tissue degradation.
Although computer models have been used to study structure–function relations in emphysema (23, 46), the role of PGs has not been evaluated. Our network simulations (Figure 7) aimed to clarify how alterations in PGs affect structure and function characterized by D2 and C, respectively, under mechanical force–based destruction. The GAG content was incorporated into the network model using bond bending (b) that resists angular changes of neighboring springs. The model included two major assumptions (23): (1) structural destruction occurred via rupturing springs representing alveolar walls, and (2) the probability of a wall to rupture increased with the strain on it. Using the current model, we first confirmed that if mechanical forces are neglected, b does not have a significant effect on the rate of network breakdown (Figure E1). Mechanical forces were then included in modeling tissue destruction by eliminating springs carrying high force, which resulted in a decrease in C and D2. Additionally, although adding nonlinearity affects C for small b, it does not alter the results qualitatively (Figure E2) compared with the current linear spring model. The structure–function predictions in Figure 7 should thus be generally valid for any treatment or species.
The rate of change in D2 and C was the largest when b was between 0.1 and 1 (Figure 7B). A similar increase in the doubling time of C occurred in this range of b. When b decreased from 1 (Figure 7A, right panel) to 0.1 (Figure 7A, left panel), C and D2 significantly increased by 22.3 and 12.5%, respectively, but the number of springs eliminated from the network did not change. A higher b stores part of the elastic energy in the bond-bending springs, representing PG compression. Consequently, after rupture, springs fold less, and the distribution of energy remains more homogeneous. Alternatively, a network with a lower b easily breaks down because springs carry a larger part of the elastic energy. In this case, after rupture, the energy is distributed among the neighboring elements. Because springs easily fold, the redistribution of energy after rupture unloads certain elements while adding extra energy by stretching others (29). Those elements that receive extra energy have an increased probability to rupture, and hence the process amplifies itself around a “seed” (i.e., the first rupture), where cellular remodeling sufficiently weakened the ECM fibers. As a result, breakdown in the presence of a lower b significantly increases heterogeneity, and hence D2, as well as C.
How do these simulation results compare with experimental data? In rats treated with PPE, it was found that the alveoli showed a decrease in heparan sulfate and GAG content of the lung, whereas the GAG concentration in urine was increased during the first 4 days (47). It was also reported that the increase in urinary GAG content positively correlated with the extent of emphysema at 40 days. In another study, rats were treated with PG synthesis inhibitor, and 40 days later considerable parenchymal destruction occurred, which correlated well with the 15-fold increase in urinary GAG at Day 1 (48). In a human study, there was a progressive loss of PGs in the peribronchial area with increasing severity of emphysema and a general loss of heparan sulfate in the alveolar septal walls (7). These results support our simulations showing that a loss of PGs generally leads to a more progressive destruction of the parenchyma.
Could these results be used to alter the progression of emphysema? Recently, a protective role of aerosolized hyaluronan against elastase- or smoke-induced emphysematous lung damage including airspace enlargement was reported (49, 50). The mechanism behind this improvement has been attributed to the protective shield of hyaluronan against degradation of elastic fibers. Our results offer a potential novel network level mechanism via the protective role of increased PG stiffness that stabilizes the alveoli and reduces the heterogeneity of mechanical forces within the network of septal walls, which in turn slows the overall destruction of lung structure.
In conclusion, we investigated how PGs contribute to the mechanical properties of the whole lung, the tissue strip, and the ECM of the parenchyma in normal and PPE-treated mice. We also developed a general network model that offers an explanation of why increasing deposition of PGs can protect the lung from destruction. Hence, a loss of PGs in human emphysema contributes to disease progression, whereas treatments that promote PG deposition in the ECM should slow the progression of emphysema.
Footnotes
This work was supported by National Institutes of Health grant HL-098976.
Author Contributions: A.T., A.M., E.B.-S., and B.S. conceived and designed the study, and drafted the manuscript. A.T. performed the study and data analysis. E.B.-S. performed tissue staining and Western blot analysis. A.M. and B.S. developed network models. A.M. and H.P. helped with image analysis. All authors revised and approved the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0179OC on January 22, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Snider GL. Chronic obstructive pulmonary disease: a definition and implications of structural determinants of airflow obstruction for epidemiology. Am Rev Respir Dis. 1989;140:S3–S8. doi: 10.1164/ajrccm/140.3_Pt_2.S3. [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Senior RM. Chronic obstructive pulmonary disease - part 2: pathology and biochemistry of emphysema. Thorax. 2002;57:830–834. doi: 10.1136/thorax.57.9.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkler T, Suki B. Emergent structure-function relations in emphysema and asthma. Crit Rev Biomed Eng. 2011;39:263–280. doi: 10.1615/critrevbiomedeng.v39.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [accessed 2013 Feb]. Available from: http://www.goldcopd.org
- 5.Konno K, Arai H, Motomiya M, Nagai H, Ito M, Sato H, Satoh K. A biochemical study on glycosaminoglycans (mucopolysaccharides) in emphysematous and in aged lungs. Am Rev Respir Dis. 1982;126:797–801. doi: 10.1164/arrd.1982.126.5.797. [DOI] [PubMed] [Google Scholar]
- 6.Merrilees MJ, Ching PS, Beaumont B, Hinek A, Wight TN, Black PN. Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir Res. 2008;9:41. doi: 10.1186/1465-9921-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Straaten JF, Coers W, Noordhoek JA, Huitema S, Flipsen JT, Kauffman HF, Timens W, Postma DS. Proteoglycan changes in the extracellular matrix of lung tissue from patients with pulmonary emphysema. Mod Pathol. 1999;12:697–705. [PubMed] [Google Scholar]
- 8.Fung YC.Biomechanics: mechanical properties of living tissues. New York: Springer-Verlag; 1993 [Google Scholar]
- 9.Mecham RP.The extracellular matrix an overview: biology of extracellular matrix. Berlin; Heidelberg; New York: Springer; 2011. xiv [Google Scholar]
- 10.Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- 11.Cavalcante FS, Ito S, Brewer KK, Sakai H, Alencar AM, Almeida MP, Andrade JS, Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol (1985) 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 12.Finlay GA, O’Donnell MD, O’Connor CM, Hayes JP, FitzGerald MX. Elastin and collagen remodeling in emphysema: a scanning electron microscopy study. Am J Pathol. 1996;149:1405–1415. [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, Ingenito EP, Brewer KK, Black LD, Parameswaran H, Lutchen KR, Suki B. Mechanics, nonlinearity, and failure strength of lung tissue in a mouse model of emphysema: possible role of collagen remodeling. J Appl Physiol (1985) 2005;98:503–511. doi: 10.1152/japplphysiol.00590.2004. [DOI] [PubMed] [Google Scholar]
- 14.Kononov S, Brewer K, Sakai H, Cavalcante FS, Sabayanagam CR, Ingenito EP, Suki B. Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am J Respir Crit Care Med. 2001;164:1920–1926. doi: 10.1164/ajrccm.164.10.2101083. [DOI] [PubMed] [Google Scholar]
- 15.Bracke KR, Dentener MA, Papakonstantinou E, Vernooy JH, Demoor T, Pauwels NS, Cleutjens J, van Suylen RJ, Joos GF, Brusselle GG, et al. Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke-exposed mice. Am J Respir Cell Mol Biol. 2010;42:753–761. doi: 10.1165/rcmb.2008-0424OC. [DOI] [PubMed] [Google Scholar]
- 16.Karlinsky JB. Glycosaminoglycans in emphysematous and fibrotic hamster lungs. Am Rev Respir Dis. 1982;125:85–88. doi: 10.1164/arrd.1982.125.1.85. [DOI] [PubMed] [Google Scholar]
- 17.Lafuma C, Moczar M, Lange F, Robert L. Biosynthesis of hyaluronic acid, heparan sulfate and structural glycoproteins in hamster lung explants during elastase induced emphysema. Connect Tissue Res. 1985;13:169–179. doi: 10.3109/03008208509152395. [DOI] [PubMed] [Google Scholar]
- 18.Chalmers GW, Gosline JM, Lillie MA. The hydrophobicity of vertebrate elastins. J Exp Biol. 1999;202:301–314. doi: 10.1242/jeb.202.3.301. [DOI] [PubMed] [Google Scholar]
- 19.Fratzl P, Daxer A. Structural transformation of collagen fibrils in corneal stroma during drying: an x-ray scattering study. Biophys J. 1993;64:1210–1214. doi: 10.1016/S0006-3495(93)81487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of airspace enlargement in emphysema. J Appl Physiol (1985) 2006;100:186–193. doi: 10.1152/japplphysiol.00424.2005. [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol (1985) 2004;97:204–212. doi: 10.1152/japplphysiol.01246.2003. [DOI] [PubMed] [Google Scholar]
- 22.Arbabi S, Sahimi M. Elastic properties of three-dimensional percolation networks with stretching and bond-bending forces. Phys Rev B Condens Matter. 1988;38:7173–7176. doi: 10.1103/physrevb.38.7173. [DOI] [PubMed] [Google Scholar]
- 23.Hamakawa H, Bartolák-Suki E, Parameswaran H, Majumdar A, Lutchen KR, Suki B. Structure-function relations in an elastase-induced mouse model of emphysema. Am J Respir Cell Mol Biol. 2011;45:517–524. doi: 10.1165/rcmb.2010-0473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito S, Bartolák-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B. Early emphysema in the tight skin and pallid mice: roles of microfibril-associated glycoproteins, collagen, and mechanical forces. Am J Respir Cell Mol Biol. 2006;34:688–694. doi: 10.1165/rcmb.2006-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins RA, Ikegami M, Korfhagen TR, Whitsett JA, Sly PD. In vivo measurements of changes in respiratory mechanics with age in mice deficient in surfactant protein D. Pediatr Res. 2003;53:463–467. doi: 10.1203/01.PDR.0000049464.46191.BF. [DOI] [PubMed] [Google Scholar]
- 26.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171:1363–1370. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardi C, Martorana PA, de Santi MM, van Even P, Lungarella G. A biochemical and morphological investigation of the early development of genetic emphysema in tight-skin mice. Exp Mol Pathol. 1989;50:398–410. doi: 10.1016/0014-4800(89)90048-8. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell MD, O’Connor CM, FitzGerald MX, Lungarella G, Cavarra E, Martorana PA. Ultrastructure of lung elastin and collagen in mouse models of spontaneous emphysema. Matrix Biol. 1999;18:357–360. doi: 10.1016/s0945-053x(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 29.Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168:516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- 30.Baldi S, Miniati M, Bellina CR, Battolla L, Catapano G, Begliomini E, Giustini D, Giuntini C. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:585–589. doi: 10.1164/ajrccm.164.4.2010066. [DOI] [PubMed] [Google Scholar]
- 31.Russi EW, Bloch KE, Weder W. Functional and morphological heterogeneity of emphysema and its implication for selection of patients for lung volume reduction surgery. Eur Respir J. 1999;14:230–236. doi: 10.1034/j.1399-3003.1999.14a39.x. [DOI] [PubMed] [Google Scholar]
- 32.Sata M, Takahashi K, Sato S, Tomoike H. Structural and functional characteristics of peripheral pulmonary parenchyma in golden hamsters. J Appl Physiol (1985) 1995;78:239–246. doi: 10.1152/jappl.1995.78.1.239. [DOI] [PubMed] [Google Scholar]
- 33.Vlahovic G, Russell ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal walls in emphysema. Am J Respir Crit Care Med. 1999;160:2086–2092. doi: 10.1164/ajrccm.160.6.9706031. [DOI] [PubMed] [Google Scholar]
- 34.Mouded M, Egea EE, Brown MJ, Hanlon SM, Houghton AM, Tsai LW, Ingenito EP, Shapiro SD. Epithelial cell apoptosis causes acute lung injury masquerading as emphysema. Am J Respir Cell Mol Biol. 2009;41:407–414. doi: 10.1165/rcmb.2008-0137OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Jamal R, Roughley PJ, Ludwig MS. Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am J Physiol Lung Cell Mol Physiol. 2001;280:L306–L315. doi: 10.1152/ajplung.2001.280.2.L306. [DOI] [PubMed] [Google Scholar]
- 36.Bader DL, Kempson GE, Egan J, Gilbey W, Barrett AJ. The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim Biophys Acta. 1992;1116:147–154. doi: 10.1016/0304-4165(92)90111-7. [DOI] [PubMed] [Google Scholar]
- 37.Ebihara T, Venkatesan N, Tanaka R, Ludwig MS. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis: temporal aspects. Am J Respir Crit Care Med. 2000;162:1569–1576. doi: 10.1164/ajrccm.162.4.9912011. [DOI] [PubMed] [Google Scholar]
- 38.Ritter MC, Jesudason R, Majumdar A, Stamenovic D, Buczek-Thomas JA, Stone PJ, Nugent MA, Suki B. A zipper network model of the failure mechanics of extracellular matrices. Proc Natl Acad Sci USA. 2009;106:1081–1086. doi: 10.1073/pnas.0808414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chahine NO, Chen FH, Hung CT, Ateshian GA. Direct measurement of osmotic pressure of glycosaminoglycan solutions by membrane osmometry at room temperature. Biophys J. 2005;89:1543–1550. doi: 10.1529/biophysj.104.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han EH, Chen SS, Klisch SM, Sah RL. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J. 2011;101:916–924. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanir Y. Osmotic swelling and residual stress in cardiovascular tissues. J Biomech. 2012;45:780–789. doi: 10.1016/j.jbiomech.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Canal Guterl C, Hung CT, Ateshian GA. Electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage. J Biomech. 2010;43:1343–1350. doi: 10.1016/j.jbiomech.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snider GL, Lucey EC, Stone PJ. Animal models of emphysema. Am Rev Respir Dis. 1986;133:149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- 44.McDevitt CA, Beck GJ, Ciunga MJ, O’Brien J. Cigarette smoke degrades hyaluronic acid. Lung. 1989;167:237–245. doi: 10.1007/BF02714952. [DOI] [PubMed] [Google Scholar]
- 45.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med. 2007;176:617–623. doi: 10.1164/rccm.200611-1739OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Lest CH, Versteeg EM, Veerkamp JH, van Kuppevelt TH. Digestion of proteoglycans in porcine pancreatic elastase-induced emphysema in rats. Eur Respir J. 1995;8:238–245. doi: 10.1183/09031936.95.08020238. [DOI] [PubMed] [Google Scholar]
- 48.van Kuppevelt TH, van de Lest CH, Versteeg EM, Dekhuijzen PN, Veerkamp JH. Induction of emphysematous lesions in rat lung by beta-D-xyloside, an inhibitor of proteoglycan synthesis. Am J Respir Cell Mol Biol. 1997;16:75–84. doi: 10.1165/ajrcmb.16.1.8998082. [DOI] [PubMed] [Google Scholar]
- 49.Cantor JO, Cerreta JM, Ochoa M, Ma S, Liu M, Turino GM. Therapeutic effects of hyaluronan on smoke-induced elastic fiber injury: does delayed treatment affect efficacy? Lung. 2011;189:51–56. doi: 10.1007/s00408-010-9271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantor JO, Turino GM. Can exogenously administered hyaluronan improve respiratory function in patients with pulmonary emphysema? Chest. 2004;125:288–292. doi: 10.1378/chest.125.1.288. [DOI] [PubMed] [Google Scholar]