Abstract
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract illnesses in infants worldwide. Both RSV-G and RSV-F glycoproteins play pathogenic roles during infection with RSV. The objective of this study was to compare the effects of anti–RSV-G and anti–RSV-F monoclonal antibodies (mAbs) on airway hyperresponsiveness (AHR) and inflammation after primary or secondary RSV infection in mice. In the primary infection model, mice were infected with RSV at 6 weeks of age. Anti–RSV-G or anti–RSV-F mAbs were administered 24 hours before infection or Day +2 postinfection. In a secondary infection model, mice were infected (primary) with RSV at 1 week (neonate) and reinfected (secondary) 5 weeks later. Anti–RSV-G and anti–RSV-F mAbs were administered 24 hours before the primary infection. Both mAbs had comparable effects in preventing airway responses after primary RSV infection. When given 2 days after infection, anti–RSV-G–treated mice showed significantly decreased AHR and airway inflammation, which persisted in anti–RSV-F–treated mice. In the reinfection model, anti–RSV-G but not anti–RSV-F administered during primary RSV infection in neonates resulted in decreased AHR, eosinophilia, and IL-13 but increased levels of IFN-γ in bronchoalveolar lavage on reinfection. These results support the use of anti–RSV-G in the prevention and treatment of RSV-induced disease.
Keywords: airway, inflammation, respiratory syncytial virus, anti–respiratory syncytial virus–G, anti–respiratory syncytial virus–F
Clinical Relevance
In comparison to targeting respiratory syncytial virus (RSV)-F glycoprotein, anti–RSV-G glycoprotein showed both preventative and therapeutic effects after primary or secondary reinfection. Targeting RSV-G glycoprotein can be an effective therapeutic strategy not only to prevent primary infection but also to benefit the consequences of reinfection.
Respiratory syncytial virus (RSV) is the leading cause of pneumonia and bronchiolitis in infants and young children worldwide. Almost all children are infected at least once by 2 years of age, and 5 to 10% of these young children experience severe lower respiratory tract disease (1). Repeated efforts to develop a vaccine against RSV have failed, and therapeutic options are limited (2). The development of new therapeutic or prophylactic agents remains a major challenge and clinical priority (3).
The protective immune response to RSV infection is primarily directed against two viral surface glycoproteins: the F (fusion) glycoprotein and G (attachment) glycoprotein (4). The F glycoprotein is highly conserved and is required to infect cells. In contrast, the G glycoprotein is highly variable and is not required to infect cells. Currently, the only licensed prophylactic agent for RSV infection in high-risk infants is palivizumab (Synagis), a neutralizing humanized monoclonal antibody (mAb) directed against RSV-F glycoprotein. However, this mAb has not proven to have significant efficacy when given postinfection and may even be deleterious when administered late (5).
Over the past decade, with recognition of its enhancing role in the pathogenesis of RSV infection, targeting G glycoprotein has gained attention (3). In mice, G glycoprotein has been linked to the induction of Th2 cytokines (6, 7) and to causing RSV-induced eosinophilia in a vaccine-enhanced disease model (8). G glycoprotein can modulate innate immunity by suppressing Toll-like receptor (TLR) 4 signaling triggered by F glycoprotein (9) and plays a critical role in cytotoxic T lymphocyte responses (10). In addition, G glycoprotein has been associated with increased levels of substance P and decreased respiratory rates in RSV-infected mice (11). Most of these activities were mapped to a conserved region in the G glycoprotein: a central cysteine-noose region, the CX3C chemokine motif at amino acid positions 182 to 186 (12). Fractalkine (CX3CL1) is a chemokine implicated in extravasation of antigen-specific killer T cells and natural killer (NK) cells (13). G glycoprotein competes with fractalkine for binding to the receptor CX3CR1 (14). Recent studies showed that anti–RSV-G mAbs had therapeutic effects in a postinfection treatment model, and they appeared to be superior to anti–RSV-F mAbs at reducing virus load and airway inflammation (15).
Given potential differences in the activities of these two surface glycoproteins, we compared the effects of anti–RSV-G and anti–RSV-F mAbs on airway hyperresponsiveness (AHR) and airway inflammation in primary and secondary RSV infection models in mice. The results demonstrated that anti–RSV-G mAbs exhibited both prophylactic and therapeutic effects on AHR and airway inflammation after primary RSV infection as well as protective effects on responses to secondary infection when administered during primary neonatal RSV infection. These results support the usefulness and potential advantages for anti–RSV-G mAbs in the prevention and treatment of RSV-induced disease. Some of the results of these studies have been previously reported in abstract form (16).
Materials and Methods
Antibodies
Anti–RSV-G mAbs (3G12 and 3D3) targeting the central conserved motif of RSV-G glycoprotein (15) and control human IgG were obtained from Trellis Bioscience (South San Francisco, CA). The 3D3 binding site was mapped to residues 164 to 172, and the 3G12 binding site was mapped to residues 167 to 176. Anti–RSV-F mAb (palivizumab) was purchased from MedImmune (Gaithersburg, MD).
Animals
BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under pathogen-free conditions at the Biological Resource Center, National Jewish Health. Mice were used under an experimental protocol approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Virus preparation
Stocks of purified human RSV (strain A2) were produced as previously described (17).
RSV Infection and Treatments
Mice were lightly anesthetized with inhaled isoflurane before intranasal inoculation with 106 PFU of purified RSV (in 25 μl of endotoxin-free phosphated buffered saline [PBS]/mouse) on Day 0 at the indicated age. Anti–RSV-G, anti–RSV-F, or control Abs were administered intraperitoneally one time at 15 mg/kg, on Days −1 or +2 in the adult primary infection model or 1 day before neonatal infection in the secondary reinfection model. Secondary RSV infection was performed 5 weeks after neonatal primary infection. Airway function and inflammation were assessed on Day 7 after either primary or secondary RSV infection.
Assessment of Airway Function
Airway function was assessed in anesthetized, mechanically ventilated animals by measuring changes in lung resistance in response to increasing doses of inhaled methacholine as described (18). Data are expressed as the percent change from baseline lung resistance obtained after inhalation of saline.
Airway Inflammation
Immediately after measurement of AHR, lungs were lavaged with 1 ml of HBSS through the trachea. Airway inflammation was assessed by total and differential counting of cells recovered in bronchoalveolar lavage (BAL) fluid.
Measurement of Cytokine Levels
Levels of IFN-γ and IL-4 were measured in BAL fluid using commercial ELISA kits according to the manufacturer’s instructions (eBioscience, San Diego, CA), as was IL-13 (R&D Systems, Minneapolis, MN).
Lung Viral Titers
Amounts of replicating virus in the lungs were quantitated (titers) by culture plaque assay combined with confirmatory immunostaining of syncytia for RSV as described previously (17).
Lung Cell Analysis
Mice were perfused with 10 ml of PBS before lung extraction. The lungs were minced and then digested with 2.5 mg/ml collagenase D (Roche, Carlsbad, CA) for 30 minutes at 37°C. EDTA was added to stop the digestion. The cells were collected with a glass Pasteur pipette and pressed through a 100-μm nylon strainer to provide a single-cell suspension. After digestion, erythrocytes were removed by means of hypotonic lysis with ACK lysis buffer. Single-cell suspensions from the lung were resuspended in 35% Percoll and centrifuged at 2,000 rpm for 20 minutes. Lung mononuclear cells were collected and washed with PBS. Cells were treated with Fc block (CD16/CD32; BD Biosciences, San Jose, CA) before labeling with fluorochrome-conjugated antibodies. Antibodies against the surface markers CD3, CD4, and CD8 were purchased from BD Biosciences. Anti-mouse CX3CR1 was obtained from R&D Systems. To assess IFN-γ production, cells from the lung and PBLN were incubated with a cell stimulation cocktail (plus protein transport inhibitors; eBioscience) for 6 hours. Cells were then stained with antibodies to CD3, CD4, CD8, and CX3CR1; fixed; permeabilized; stained with IFN-γ antibody (BD Biosciences); and analyzed by FACS. Appropriate isotype-matched control antibodies were obtained from BD Biosciences and R&D Systems. Flow cytometry was performed with the LSR-II (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical Analysis
All results were expressed as mean ± SD. Data were analyzed by ANOVA using the StatView 4.5 statistical analysis software package (Abacus Concepts, Piscataway, NJ). Differences between the groups were determined by multiple comparisons using Fisher protected least significant difference test. The P values for significance were set to 0.05 for all tests.
Results
Prophylactic Effects of Anti–RSV-G and Anti–RSV-F mAbs in the Response to Primary RSV Infection
Previous studies in a mouse model showed a prophylactic effect of palivizumab on AHR and airway inflammation (19). Mice were infected on Day 0 at 6 weeks of age; anti–RSV-G (3G12) or anti–RSV-F was administered intraperitoneally at 15 mg/kg on Day −1. Airway function and inflammation were assessed on Day +7 after infection. As shown in previous studies in this model, significant AHR and airway inflammation developed on RSV infection compared with sham control mice (17). Administration of either anti–RSV-G or anti–RSV-F significantly reduced AHR when compared with RSV-infected, control antibody-treated mice (Figure 1A). Similar results were seen when the antibodies were administered on Day 0, just before infection (data not shown).
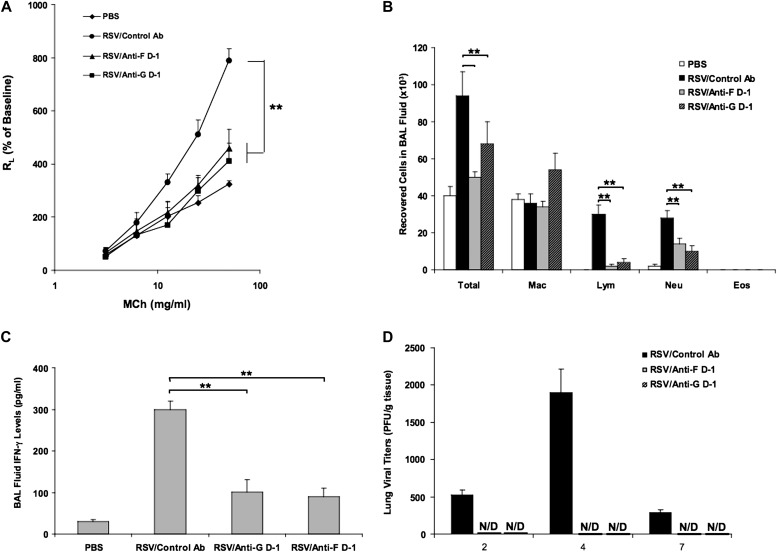
Figure 1.
Anti–respiratory syncytial virus (RSV)-G and anti–RSV-F monoclonal antibodies (mAbs) had comparable effects in preventing airway responses after primary RSV infection when administered on Day −1. (A) Airway responsiveness to inhaled MCh. (B) Bronchoalveolar lavage (BAL) cellularity. (C) BAL fluid IFN-γ levels. (D) Virus titers in the lung. Results from three independent experiments with 12 mice per group are expressed as mean ± SD. *P < 0.05, **P < 0.01. Eos, eosinophil; Lym, lymphocyte; Mac, macrophage; MCh, methacholine; Neu, neutrophil; PBS, phosphate buffered saline.
After primary RSV infection, the number of total cells, lymphocytes, and neutrophils recovered in BAL fluid were significantly increased compared with noninfected control mice. Treatment with anti–RSV-G or anti–RSV-F on Day −1 significantly reduced the number of total cells, lymphocytes, and neutrophils in BAL fluid (Figure 1B). Few eosinophils were detected in primary RSV-infected adult mice in any of the groups. As illustrated in Figure 1C, treatment with anti–RSV-G or anti–RSV-F significantly reduced the levels of IFN-γ in BAL fluid but had no effect on the low levels of IL-4 and IL-13 (data not shown).
Virus replication and clearance were examined by measuring the amounts of replicating virus recovered from lung tissue at different time points after inoculation. RSV titers peaked at Day +4 postinfection. Prophylactic administration of anti–RSV-F and anti–RSV-G equally inhibited RSV replication (Figure 1D).
Therapeutic Effects of Anti–RSV-G and Anti–RSV-F in the Response to Primary RSV Infection
Here, BALB/c mice were infected at 6 weeks of age and anti–RSV-G (3G12) or anti–RSV-F was administered intraperitoneally at 15 mg/kg on Day +2 after RSV infection, before the peak of viral replication and inflammatory response (17). Airway function and inflammation were assessed on Day +7 after infection. As shown in Figure 2A, when administered on Day +2 after RSV infection, anti–RSV-G–treated mice developed significantly decreased AHR, whereas anti–RSV-F–treated mice developed similar levels of AHR as control antibody-treated RSV-infected mice.
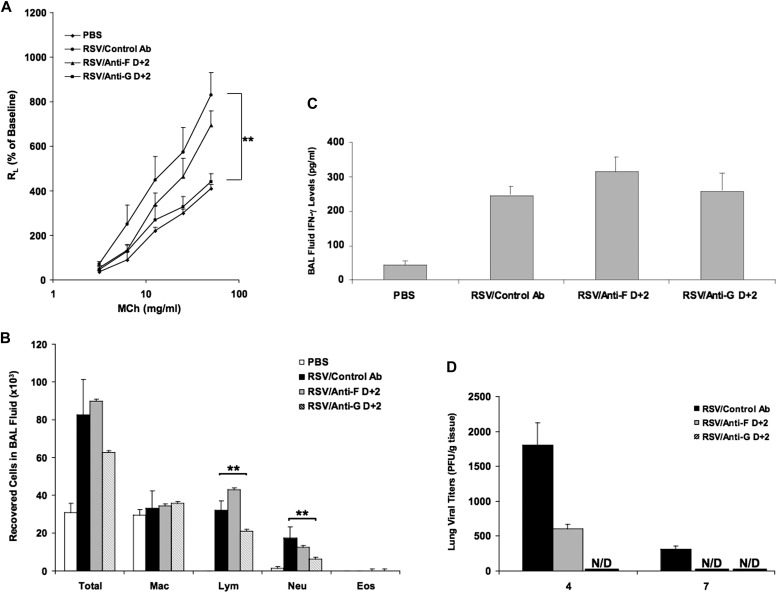
Figure 2.
Anti–RSV-G was effective in preventing AHR and airway inflammation after primary RSV infection when administered 2 days after infection. (A) Airway responsiveness to inhaled MCh. (B) BAL cellularity. (C) BAL fluid IFN-γ levels. (D) Virus titers in the lung. Results from three independent experiments with 12 mice per group are expressed as mean ± SD. *P < 0.05, **P < 0.01.
As shown in Figure 2B, treatment with anti–RSV-F on Day +2 had no effect on BAL cellularity, whereas treatment with anti–RSV-G on Day +2 significantly reduced numbers of lymphocytes and neutrophils. After RSV infection, the levels of IFN-γ in BAL fluid were significantly increased, but treatment with anti–RSV-G or anti–RSV-F did not alter the levels (Figure 2C).
At Day +4 postinfection, there was a marked decrease in viral titers in mice treated with anti–RSV-F, and the virus titer was below limits of detection in anti–RSV-G–treated mice. On Day +7 after infection, the levels of virus were below the limits of detection in both anti–RSV-F– and anti–RSV-G–treated mice (Figure 2D).
Impact of Anti–RSV-G and Anti–RSV-F Treatment on Day +2 on the Kinetics of the Responses after Primary RSV Infection
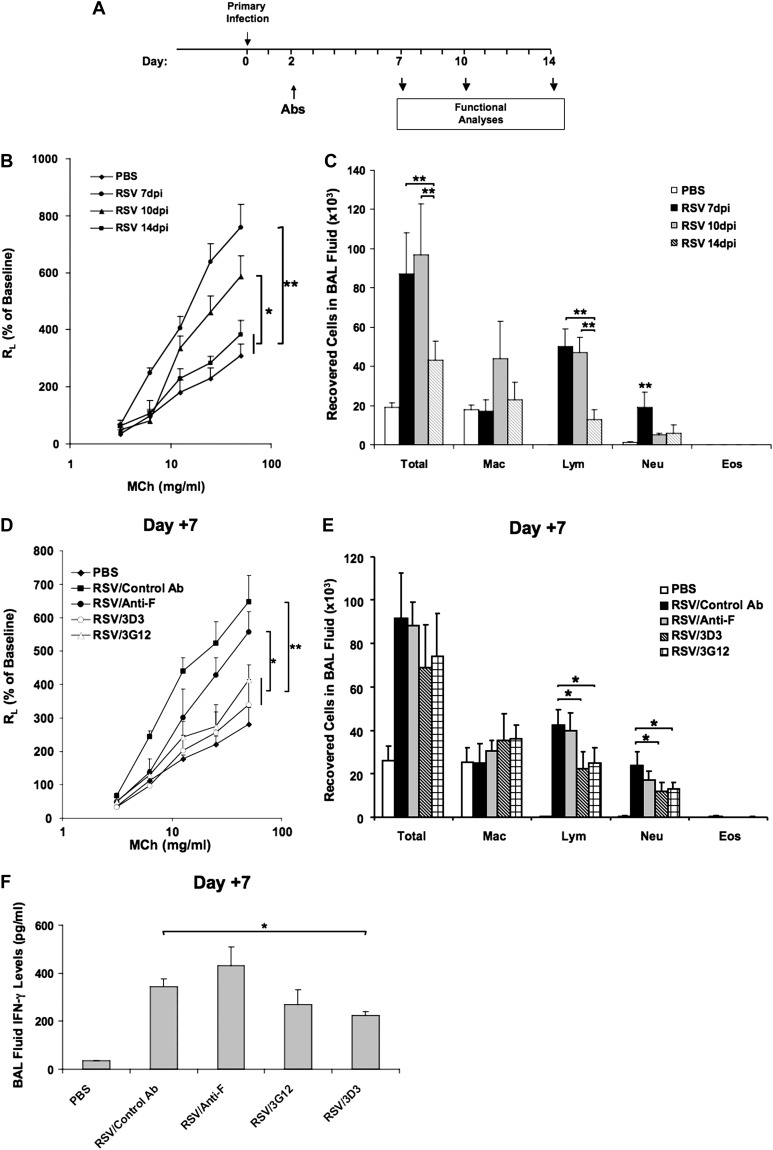
In light of the therapeutic benefit of anti–RSV-G on AHR and airway inflammation when administered on Day +2 after RSV infection, we determined the effects of anti–RSV-G and anti–RSV-F treatment on the natural history of the responses to primary RSV infection. BALB/c mice were infected at 6 weeks of age, and anti–RSV-G or anti–RSV-F was administered intraperitoneally at 15 mg/kg on Day +2 after RSV infection. Airway function and inflammation were assessed on Day +7, +10, and +14 after infection (Figure 3A).
Figure 3.
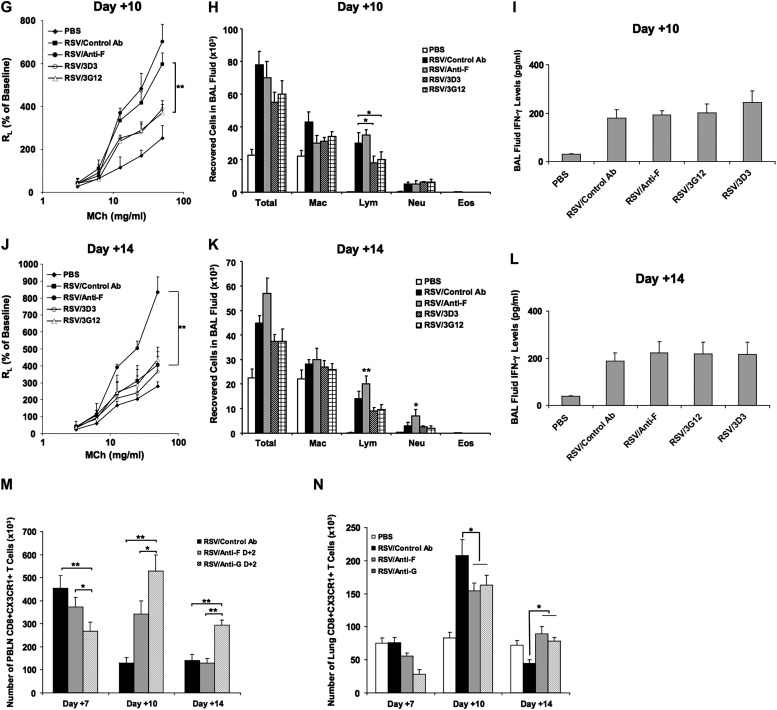
The effects of anti–RSV mAbs on the kinetics of airway responses. (A) Experimental protocol. (B) AHR kinetics after primary RSV infection. (C) BAL cellularity kinetics. (D–F) Airway responsiveness to inhaled MCh (D), BAL cellularity (E), and BAL fluid IFN-γ levels (F) at Day +7 postinfection. (G–I) Airway responsiveness to inhaled MCh (G), BAL cellularity (H), and BAL fluid IFN-γ levels (I) at Day +10 postinfection. (J–L) Airway responsiveness to inhaled MCh (J), BAL cellularity (K), and BAL fluid IFN-γ levels (L) at Day +14 postinfection. The number of CD8+CX3CR1+ T cells in the PBLN (M) and in the lung (N) on Days +7, +10, and +14 were determined by flow cytometry. Results from three independent experiments with 12 mice each group per time point are expressed as mean ± SD. *P < 0.05, **P < 0.01.
As shown in Figure 3B, AHR peaked on Day +7 postinfection and declined by Day +10 but remained significantly higher than uninfected control mice. At Day +14, AHR returned to baseline levels, comparable to uninfected control mice. Total cell numbers and lymphocyte numbers recovered in BAL fluid were significantly increased after RSV infection and were highest at Days +7 and +10 postinfection. Peak numbers of neutrophils were detected at Day +7 postinfection. The number of total cells, lymphocytes, and neutrophils all decreased by Day +14 postinfection (Figure 3C).
At Day +7 postinfection, administration of anti–RSV-G (3G12) or a second anti–RSV-G mAb (3D3) resulted in significantly reduced AHR when compared with either anti–RSV-F–treated or control antibody-treated RSV infected mice (Figure 3D). The number of lymphocytes and neutrophils recovered in BAL fluid were lower in anti–RSV-G (3G12 and 3D3)-treated mice, whereas anti–RSV-F treatment showed no effect on BAL cellularity (Figure 3E). A decrease in BAL fluid IFN-γ levels was observed in the 3D3-treated group (Figure 3F). At Day +10 postinfection, even though AHR in the control antibody-treated group was lower than at Day +7 postinfection, it was higher than in the anti–RSV-G–treated group. Strikingly, the anti–RSV-F–treated group showed increased AHR, even higher than control mice (Figure 3G), and this trend became more obvious at Day +14, when AHR in all other groups returned to baseline values (Figure 3J). Anti–RSV-G treatment reduced the number of lymphocytes at Day +10 postinfection (Figure 3H), whereas the anti–RSV-F–treated group showed higher numbers of lymphocytes and neutrophils at Day +14 (Figure 3K). The levels of IFN-γ in BAL fluid were highest at Day +7 postinfection and decreased at Days +10 and +14 postinfection. Anti–RSV-G– or anti–RSV-F–treated groups had similar levels of IFN-γ in BAL fluid as the control antibody-treated group at Days +10 (Figure 3I) and +14 postinfection (Figure 3L).
To determine whether particular cell types were targeted by anti–RSV-G treatment, cells from the lung and PBLN were stained and analyzed for expression of CD3, CD4, CD8, and CX3CR1. We determined that 30 to 40% of CD8+ T cells expressed CX3CR1, whereas only 1 to 3% of CD4+ T cells expressed CX3CR1. In PBLN, the number of CD8+CX3CR1+ T cells in anti–RSV-G–treated group was significantly higher than in control antibody or anti–RSV-F–treated groups at Days +10 and +14; on Day +7, the numbers were lower in the anti–RSV-G–treated group than the other groups (Figure 3M). In the lung, the number of CD8+CX3CR1+ T cells peaked on Day +10 in all RSV-infected groups, and numbers were higher than in noninfected mice (Figure 3N). Similar numbers of CD8+CX3CR1+ T cells were detected in both the anti–RSV-G– and anti–RSV-F–treated groups and were lower than in the control antibody-treated group. By Day +14, the number of CD8+CX3CR1+ T cells in the anti–RSV-G– or anti–RSV-F–treated groups decreased to levels comparable to noninfected mice. In control antibody-treated mice, this number was significantly lower than in the other groups. When IFN-γ production in CD8+CX3CR1+ T cells was examined by intracellular staining, we found the number of CD8+CX3CR1+ IFN-γ+ T cells in all RSV-infected groups increased on Days +7 and +10 and were significantly higher than in naive mice. However, no differences in the numbers of IFN-γ–producing CD8+CX3CR1+ T cells between antibody-treated groups were detected in the PBLN or lung (data not shown).
Prophylactic Effects of Anti–RSV-G and Anti–RSV-F on the Responses to Secondary RSV Infection
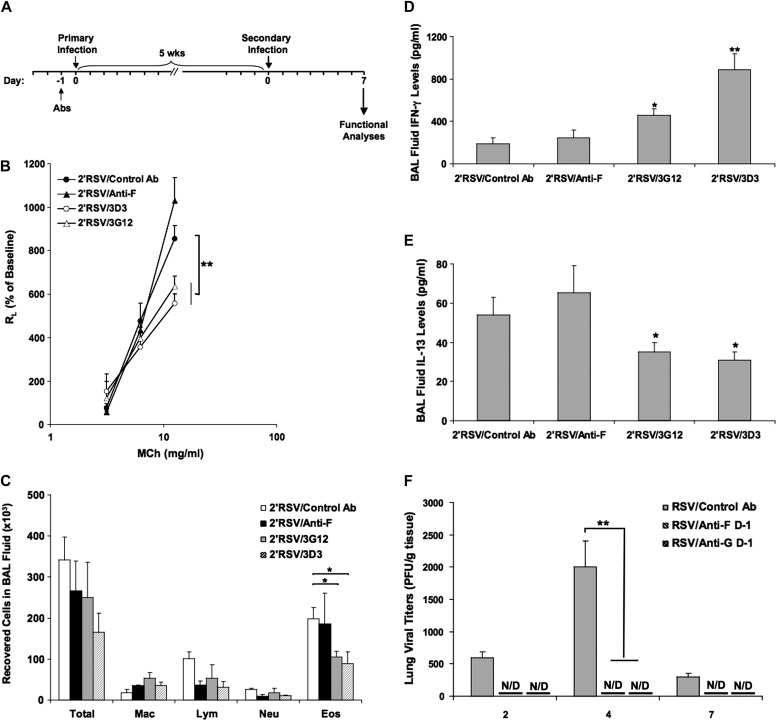
To determine whether the prophylactic administration of anti–RSV-G or anti–RSV-F during primary RSV infection reduced airway responses to RSV reinfection, mice were initially infected with RSV as neonates and reinfected 5 weeks later, a time when no residual AHR or airway inflammation was detected after recovery from primary infection (Figure 4A). Airway function and inflammation were assessed on Day +7 after secondary infection. Anti–RSV-G or anti–RSV-F mAbs were administered 1 day before neonatal primary infection. As illustrated in Figure 4B, mice initially infected as neonates developed enhanced AHR and airway eosinophilia on reinfection with RSV. Administration of anti–RSV-G but not anti–RSV-F during primary neonatal infection resulted in decreased AHR on reinfection to levels observed in mice receiving primary infection at this age. In parallel, anti–RSV-G but not anti–RSV-F significantly reduced the number of eosinophils in the BAL fluid; there were no differences in the numbers of macrophages, lymphocytes, or neutrophils in the treated and untreated groups (Figure 4C). In BAL fluid, anti–RSV-G but not anti–RSV-F during primary infection increased IFN-γ levels (Figure 4D) and decreased IL-13 levels (Figure 4E) in reinfected mice.
Figure 4.
Anti–RSV-G mAb reduces the response to secondary RSV infection when administered during neonatal primary infection. (A) Experimental protocol. (B) Airway responsiveness to inhaled MCh. (C) BAL cellularity. (D) BAL fluid IFN-γ levels. (E) BAL fluid IL-13 levels. (F) Virus titers in the lung after primary neonatal RSV infection. Results from three independent experiments with 12 mice per group are expressed as mean ± SD. *P < 0.05, **P < 0.01 indicate significant differences between anti–RSV-G antibody-treated mice and control antibody-treated mice. N/D, not detected.
Virus replication and clearance were examined by measuring the amounts of replicating virus recovered from lung tissue at different time points after primary neonatal RSV infection. Similar to infection of adult mice, RSV titers peaked at Day +4 postinfection; there were no significant differences detected between the two age groups, indicating similar rates of viral replication and clearance. Prophylactic administration of anti–RSV-F and anti–RSV-G equally inhibited RSV replication (Figure 4F).
Discussion
To date, no RSV subunit or live virus vaccine has proven effective in human clinical trials. The most widely used intervention today is palivizumab, a humanized murine mAb against the RSV-F glycoprotein. Although effective in reducing hospitalizations in susceptible infants and young children, there remains breakthrough disease when used prophylactically. Moreover, palivizumab has not been effective as postinfection therapy and may even be deleterious when administered postinfection (5). Emerging data support targeting of the G glycoprotein (3). In the present study, comparisons of anti–RSV-F (palivizumab) and anti–RSV-G were examined in both prophylactic and postinfection approaches as well as in a secondary, reinfection model. The anti–RSV-G mAbs were derived from B lymphocytes of recovering patients and targeted the conserved but poorly immunogenic central motif of the G glycoprotein (15). Relative to palivizumab, anti–RSV-G showed comparative efficacy when administered 1 day before infection, preventing airway inflammation and development of RSV-induced AHR. These data correspond to their viral neutralization potency (20). However, when administered 2 days postinfection, the anti–RSV-G showed superiority over anti–RSV-F in all measures of airway inflammation and AHR.
The surface glycoproteins G and F are the major glycoproteins involved in RSV infection. Two major antigenic subtypes, A and B, are defined primarily on the basis of the differences in the G glycoprotein. Deletion of G glycoprotein substantially reduces virus replication in vivo (21). Detailed study of G glycoprotein structure revealed a CX3C motif located in the cysteine noose central region of the RSV-G glycoprotein and flanking N- and C-terminal residues spanning residues 148 to 198 that are highly conserved across all strains (12). In the current study, two anti–RSV-G mAbs, screened from human B cells by a high-throughput single-cell phenotyping technology (CellSpot) were used (15). Both mAbs recognized tightly clustered epitopes limited to fewer than 20 residues in the conserved central cysteine-noose region and were shown to improve viral neutralization potency in vitro and enhance reduction of infectious virus in both prophylaxis and postinfection treatment models in mice (15).
The pathogenesis of RSV lower respiratory tract infection is not completely understood, but it is likely mediated both by the direct effects of the virus and by the host inflammatory response. Many features of the response to RSV are shared in humans and mice. In humans, neutrophils predominate in the BAL fluid and infiltrate the small airways in severe bronchiolitis (22). However, the lower airways have not been sampled in milder cases. The dynamics of innate neutrophil and adaptive T-cell responses in infants with severe RSV infection has been studied and showed a peak of blood neutrophilia between Days 7 and 9 after the onset of symptoms, whereas a robust CD8+ T-cell response peaked between 11 and 15 days after the onset of symptoms (23). In the mouse, RSV infection induces primarily a lymphocytic inflammatory response. However, higher doses of RSV induce airway neutrophilia in the early stages of infection (24). In the present model, RSV infection induced neutrophil recruitment in the lungs at Days 2 to 4 postinfection and the peak of CD8+ T cells appeared at Day +10.
There are few if any comparative data examining the response in infants after primary and secondary infection, which is feasible in mice, as reported here. Direct viral cytopathology played an initial role in the pathogenesis of RSV-induced disease in the model (25). By limiting RSV replication, direct cytopathology induced by virus was reduced. At the same time, by decreasing the viral antigen load, the magnitude of the inflammatory response, which contributes to the severity of the illness, was diminished. It is expected that anti–RSV-F and anti–RSV-G had prophylactic effects on airway inflammation by reducing viral load.
Both RSV-G and RSV-F glycoproteins can initiate host inflammation through direct interactions with host inflammatory cells, for example, F glycoprotein binding to TLR4 (26) and G glycoprotein binding to CX3CR1 (14). Neutralizing antibodies can directly block these interactions and lead to reduced numbers of airway infiltrating cells (15, 19, 27, 28). Airway inflammation, initiated either by innate immune responses or neurogenic responses, contribute to development of AHR (29). In both humans (30) and mice (31), inoculation of RSV induces an inflammatory response characterized by airway neutrophilia. Although not necessarily linked, anti–RSV-F or anti–RSV-G administered 1 day before infection resulted in reductions in AHR, which were associated with reduced airway inflammation. Thus, in a prophylactic mode, both targeted antibodies were effective.
Differences in efficacy were more obvious when treatment was initiated after infection. Anti–RSV-F failed to demonstrate therapeutic effects in RSV disease in animals (19) or humans (5), even though the viral titer was lower after treatment. These results suggested that once infection was established, host responses continued to contribute to disease pathogenesis. Host responses can be divided into those deemed protective or pathogenic. In general, the protective responses are cell-mediated immune responses characterized by Th1 cytokine production, whereas pathogenic responses involve Th2 responses associated with airway eosinophilia, goblet cell metaplasia, mucus production, and AHR. Consequently, effective therapy may require not only antiviral potency but also activity to enhance protective host response elements. A number of studies have implicated RSV-G glycoprotein in the pathogenesis of RSV disease through effects on the profile of lymphocyte cytokine and chemokine expression. BALB/c mice immunized with vaccinia virus expressing G glycoprotein or purified G glycoprotein produce an exaggerated CD4+ T-cell response with increased Th2-type cytokine production and airway eosinophilia when challenged with RSV (8). The underlying mechanisms are not fully elucidated but may be related to the CX3C chemokine motif. This motif is located in the central conserved cysteine-noose region and appears to mimic CX3C chemokine. CX3C–CX3CR1 interactions facilitate virus infection and are capable of modifying pulmonary leukocyte migration and activation (14). A nonneutralizing anti–RSV-G glycoprotein mAb (131-2G) has been shown to inhibit RSV-G glycoprotein binding to CX3CR1 and RSV-G glycoprotein-induced chemotaxis (14). This antibody has been shown to reduce virus replication through antibody-dependent cell cytotoxicity and decrease airway inflammation independent of Fc interactions or effects on virus replication (32).
Based on these observations, we hypothesized that anti–RSV-G might have better treatment outcomes than anti–RSV-F. When administered 2 days after RSV infection, anti–RSV-G–treated mice developed significantly lower levels of AHR and significantly reduced numbers of lymphocytes and neutrophils in BAL fluid; in contrast, anti–RSV-F treatment showed no alterations in these outcomes. These results are similar to previous studies demonstrating that when administered 3 days postinfection, anti–RSV-G was more potent in reducing lower respiratory tract infection, pulmonary inflammation, and proinflammatory cytokine production (15, 32).
The superiority of anti–RSV-G became more apparent when administered 2 days postinfection and followed for up to 14 days postinfection. In nontreated mice, AHR and airway inflammation peaked at 7 days and declined to baseline at 14 days postinfection. The efficacy of anti–RSV-G was shown at 7 days and persisted though 14 days. Strikingly, after anti–RSV-F treatment on Day +2, AHR and airway inflammation did not subside but persisted and were even enhanced with time. Given the natural decline in untreated but infected mice, it suggests that anti–RSV-F interfered with a protective pathway or resulted in the persistence of a pathogenic pathway. Because F glycoprotein can trigger release of “protective” cytokines such as IL-12, IL-6, and TNF-α through TLR4 activation (26), the immunomodulatory effects of the F glycoprotein may have been attenuated by anti–RSV-F treatment administered late. Of interest, mutations in TLR4 that reduce receptor activity were overrepresented in infants hospitalized with RSV (33). On the other hand, RSV-G glycoprotein modulates suppressor of cytokine signaling family of proteins (SOCS) expression that negatively regulates cytokine expression and results in inhibition of type 1 IFN and IFN-stimulated gene-15 expression (34). Targeting the G glycoprotein, as in this study, resulted in reduction of AHR and airway inflammation. A second potential benefit of anti–RSV-G is that RSV-G competes with fractalkine (CX3CL1) for binding to CX3CR1 (14). CD8 T cells expressing CX3CR1 play a major role in the cytotoxic response to RSV infection. CX3CR1 has been shown to be expressed preferentially on polarized Th1 compared with Th2 cells (35). The number of CD4+CX3CR1+ and CD8+CX3CR1+ T cells in the BAL was significantly higher at Day +12 postinfection in mice infected with RSV mutant viruses lacking the G or G protein CX3C motif compared with wild-type infected mice. However, this increase was not detected at Day +6 postinfection. These results indicate the potential for a long-term effect of RSV G protein on migration or survival of CD4+CX3CR1+ and CX3CR1+ CD8+ T cells in the lung (10). By analogy, blocking RSV-G glycoprotein may have enhanced the numbers of CX3CR1+ cytotoxic T cells accumulating in the lung, thereby limiting the consequences of infection even when administered after infection. In our study, we found that the number of PBLN CD8+CX3CR1+ T cells in the anti–RSV-G–treated group was significantly higher than in the control antibody- or anti–RSV-F–treated groups at Days +10 and +14 (Figure 3M). These results suggested that anti–RSV-G treatment enhanced the migration of CD8+CX3CR1+ T cells from the circulation to the PBLN. On Day +7, the results showed an opposite trend, which may have been related to the timing of soluble G protein release. In the lung, the situation was more complicated than in PBLN (Figure 3N). It seems that at later time points, CX3CR1 might serve as the major chemokine receptor responsible for CD8+ T cell migration to the lung after RSV infection as the number of CD8+CX3CR1+ T cells in all RSV-infected groups peaked on Day +10 and was significantly higher than in naive mice. However, anti–RSV-G treatment did not show any differences from anti–RSV-F treatment, and both treatments resulted in lower CD8+CX3CR1+ T cell accumulation than in the control antibody-treated group on Day +10. These results suggested the effects of anti–RSV-G might not be solely on the migration of T cells but might have other effects, for example, on G glycoprotein–mediated increases in levels of substance P and decreased respiratory rates in RSV-infected mice (11). As with TLR4 polymorphisms, human polymorphisms associated with reduced activity of CX3CR1 were overrepresented in infants with severe RSV disease (36). Because CX3CR1 is also expressed on dendritic cells (37) and NK cells (38) in addition to T cells, whether blocking RSV-G glycoprotein has other effects through these cell types is unknown at this point.
In the present study, a secondary or reinfection model was used to compare prophylactic administration of anti–RSV-G or anti–RSV-F during primary infection on reinfection. This experimental approach was used for reasons that differentiate it from other single-infection approaches. First, it has clinical relevance. Given the fact that RSV infection does not lead to enduring immunity, many infants experience RSV reinfection, often with serious consequences (39). Currently, the anti-F antibody is licensed for prophylactic use for high-risk infants. We used the reinfection model to compare outcomes after second virus exposure after prophylactic use of these antibodies before initial exposure. Second, in this model, neonatal RSV infection predisposes to the development of airway eosinophilia and enhanced AHR via an IL-13–dependent mechanism after reinfection, whereas initial infection at a later age protects against the development of these altered airway responses after reinfection (17). This Th2-biased response in neonates at initial infection appeared mediated, at least in part, by increases in TSLP release, up-regulation of OX40 ligand expression on dendritic cells (40), and lower IFN-γ responses (41). Given purported associations between RSV infection and asthma (42), it is important to determine whether blocking RSV-G or RSV-F during neonatal infection has effects on responses after reinfection that could be linked to asthma development such as the cytokine milieu and excessive mucus production. The third reason for use of this model follows on the implications from the vaccine-enhanced disease models. Sensitization to G glycoprotein led to Th2 responses associated with pulmonary eosinophilia after challenge with RSV; on the other hand, sensitization to F glycoprotein led to a Th1-dominant response (43). These results suggested that G and F glycoproteins had distinct immunomodulatory roles on the consequences of RSV infection. When administered to neonates before initial infection, both anti–RSV-F and anti–RSV-G mAbs reduced the viral load. Although passive administration of antibodies to RSV prevents productive infection, it may not prevent abortive replication of the virus as indicated by detection of viral RNA by real-time PCR (44). Anti–RSV-G treatment during neonatal primary infection resulted, on reinfection, in lower AHR, and reduced airway eosinophilia and IL-13 levels. In contrast, anti–RSV-F treatment did not show any effect. IL-13 is essential to the development of mucus production and AHR in models of allergic airway inflammation (45). In the present model, IL-13 was required for development of the asthma-like phenotype after reinfection of mice initially infected as neonates (17). The decreased levels of IL-13 in anti–RSV-G–treated mice likely contributed to the lower AHR and reduced airway eosinophilia. IL-13 is produced from both CD4+ (Th2) and CD8+ (Tc2) T cells. In neonatally infected mice, the increased levels of IL-13 on reinfection suggest a bias toward Th2-like responses. It is known that neonatal mice exhibit an impaired IFN-γ response after primary RSV infection compared with their older counterparts (17). Anti–RSV-F might even accentuate this lower Th1 response, given the findings that the interaction of RSV-F–TLR4 contributes to a Th1 response (43). On the other hand, anti–RSV-G might block Th2 responses triggered by CX3C–CX3CR1 interactions. A second possibility is that anti–RSV-G interferes with the known TSLP release after initial RSV infection (40). As with allergen (46), RSV-G glycoprotein interactions with lung epithelial cells might induce TSLP release, initiating the cascade leading to Th2-like responses on reinfection. By intercepting this interaction, anti–RSV-G administered to infected neonates could limit activation of the TSLP-initiated cascade, preventing skewing of the immune response when RSV is reencountered later in life. Because neonatal mice have been shown to exhibit Th2 skewing of their immune responses, encounter with RSV-G glycoprotein but not the F glycoprotein may have further enhanced the Th2 phenotype as seen in earlier vaccination trials. By blocking the RSV-G glycoprotein, this skewing, initiated by the RSV-mediated epithelial cell release of TSLP, may have been attenuated.
In summary, this study demonstrates that anti–RSV-G and anti–RSV-F mAbs have similar prophylactic activities on RSV-induced AHR and airway inflammation. However, anti–RSV-G showed benefits when administered in a therapeutic mode. In addition, anti–RSV-G, but not anti–RSV-F, given before neonatal infection, significantly reduced airway inflammation, eosinophilia, and AHR on reinfection. The results support the use of these novel human anti–RSV-G mAbs in both prophylactic and therapeutic applications.
Acknowledgments
Acknowledgments
The authors thank Diana Nabighian for her help in preparing the manuscript.
Footnotes
Supported by Trellis Bioscience and National Institutes of Health grants AI-77609 and HL-36577 (E.W.G.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Author Contributions: J.H. performed many of the experiments. K.T. helped design and analyze results. M.W., W.Z., and Y.J. provided advice and helped in cytokine assays. Y.S. and M.O. were involved in the initiation of these studies. A.D. helped in the propagation of RSV and experimental design. E.W.G. supervised the entire project, designed experiments, and analyzed data. All contributors participated in the drafting of the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0360OC on February 12, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco JC, Boukhvalova MS, Shirey KA, Prince GA, Vogel SN. New insights for development of a safe and protective RSV vaccine. Hum Vaccin. 2010;6:482–492. doi: 10.4161/hv.6.6.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauvar LM, Harcourt JL, Haynes LM, Tripp RA. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy. 2010;2:655–661. doi: 10.2217/imt.10.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshansky CM, Zhang W, Moore E, Tripp RA. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 2009;4:279–297. doi: 10.2217/fmb.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 6.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TR, Graham BS. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol. 1999;73:8485–8495. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 9.Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, Laham FR, Thumar B, Hendry RM, Melero JA, et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci USA. 2005;102:8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol. 2006;176:1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- 11.Tripp RA, Dakhama A, Jones LP, Barskey A, Gelfand EW, Anderson LJ. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J Virol. 2003;77:6580–6584. doi: 10.1128/JVI.77.11.6580-6584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA. 1987;84:5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umehara H, Bloom E, Okazaki T, Domae N, Imai T. Fractalkine and vascular injury. Trends Immunol. 2001;22:602–607. doi: 10.1016/s1471-4906(01)02051-8. [DOI] [PubMed] [Google Scholar]
- 14.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 15.Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, Harriman WD, Carroll SF, Ellsworth SL, Anderson LJ, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol. 2009;183:6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Takeda K, Okamoto M, Zeng W, Jia Y, Dakhama A, Gelfand EW. Comparison of the preventative, therapeutic, and prophylactic effects of anti-G and anti-F RSV glycoprotein antibodies in the response to primary and secondary RSV infection [abstract] Am J Respir Crit Care Med. 2011;183:A6206. [Google Scholar]
- 17.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, Takeda K, Gelfand EW. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol. 2005;175:1876–1883. doi: 10.4049/jimmunol.175.3.1876. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mejías A, Chávez-Bueno S, Ríos AM, Saavedra-Lozano J, Fonseca Aten M, Hatfield J, Kapur P, Gómez AM, Jafri HS, Ramilo O. Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob Agents Chemother. 2004;48:1811–1822. doi: 10.1128/AAC.48.5.1811-1822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connors M, Collins PL, Firestone CY, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teng MN, Whitehead SS, Collins PL. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology. 2001;289:283–296. doi: 10.1006/viro.2001.1138. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 23.Lukens MV, van de Pol AC, Coenjaerts FE, Jansen NJ, Kamp VM, Kimpen JL, Rossen JW, Ulfman LH, Tacke CE, Viveen MC, et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 27.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J Virol. 2010;84:9632–9636. doi: 10.1128/JVI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejías A, Chávez-Bueno S, Ríos AM, Aten MF, Raynor B, Peromingo E, Soni P, Olsen KD, Kiener PA, Gómez AM, et al. Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency. Antimicrob Agents Chemother. 2005;49:4700–4707. doi: 10.1128/AAC.49.11.4700-4707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dakhama A, Lee YM, Gelfand EW.Virus-induced airway dysfunction: pathogenesis and biomechanisms Pediatr Infect Dis J 200524S159–S169., discussion S166–S167 [DOI] [PubMed] [Google Scholar]
- 30.Smith PK, Wang SZ, Dowling KD, Forsyth KD. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health. 2001;37:146–151. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 31.van Schaik SM, Enhorning G, Vargas I, Welliver RC. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J Infect Dis. 1998;177:269–276. doi: 10.1086/514208. [DOI] [PubMed] [Google Scholar]
- 32.Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, Anderson LJ. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis. 2009;200:439–447. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- 33.Tal G, Mandelberg A, Dalal I, Cesar K, Somekh E, Tal A, Oron A, Itskovich S, Ballin A, Houri S, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 34.Oshansky CM, Krunkosky TM, Barber J, Jones LP, Tripp RA. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a toll-like receptor pathway. Viral Immunol. 2009;22:147–161. doi: 10.1089/vim.2008.0098. [DOI] [PubMed] [Google Scholar]
- 35.Fraticelli P, Sironi M, Bianchi G, D’Ambrosio D, Albanesi C, Stoppacciaro A, Chieppa M, Allavena P, Ruco L, Girolomoni G, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amanatidou V, Sourvinos G, Apostolakis S, Tsilimigaki A, Spandidos DA. T280M variation of the CX3C receptor gene is associated with increased risk for severe respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2006;25:410–414. doi: 10.1097/01.inf.0000214998.16248.b7. [DOI] [PubMed] [Google Scholar]
- 37.Bonduelle O, Duffy D, Verrier B, Combadière C, Combadière B. Cutting edge: protective effect of CX3CR1+ dendritic cells in a vaccinia virus pulmonary infection model. J Immunol. 2012;188:952–956. doi: 10.4049/jimmunol.1004164. [DOI] [PubMed] [Google Scholar]
- 38.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 39.Samson L. Prevention of respiratory syncytial virus infection. Paediatr Child Health (Oxford) 2009;14:521–532. doi: 10.1093/pch/14.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han J, Dakhama A, Jia Y, Wang M, Zeng W, Takeda K, Shiraishi Y, Okamoto M, Ziegler SF, Gelfand EW.Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand J Allergy Clin Immunol 20121301175–1186.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, Joetham A, Gelfand EW, Dakhama A. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2008;177:208–218. doi: 10.1164/rccm.200612-1890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everard ML. The relationship between respiratory syncytial virus infections and the development of wheezing and asthma in children. Curr Opin Allergy Clin Immunol. 2006;6:56–61. doi: 10.1097/01.all.0000200506.62048.06. [DOI] [PubMed] [Google Scholar]
- 43.Srikiatkhachorn A, Braciale TJ. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boukhvalova MS, Prince GA, Blanco JC. Respiratory syncytial virus infects and abortively replicates in the lungs in spite of preexisting immunity. J Virol. 2007;81:9443–9450. doi: 10.1128/JVI.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 46.Li DQ, Zhang L, Pflugfelder SC, De Paiva CS, Zhang X, Zhao G, Zheng X, Su Z, Qu Y.Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways J Allergy Clin Immunol 20111281318–1325.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]