Abstract
Cigarette smoke (CS) is the most common cause of chronic obstructive pulmonary diseases (COPD), including emphysema. CS exposure impacts all cell types within the airways and lung parenchyma, causing alveolar tissue destruction through four mechanisms: (1) oxidative stress; (2) inflammation; (3) protease-induced degradation of the extracellular matrix; and (4) enhanced alveolar epithelial and endothelial cell (EC) apoptosis. Studies in human pulmonary ECs demonstrate that macrophage migration inhibitory factor (MIF) antagonizes CS-induced apoptosis. Here, we used human microvascular ECs, an animal model of emphysema (mice challenged with chronic CS), and patient serum samples to address both the capacity of CS to alter MIF expression and the effects of MIF on disease severity. We demonstrate significantly reduced serum MIF levels in patients with COPD. In the murine model, chronic CS exposure resulted in decreased MIF mRNA and protein expression in the intact lung. MIF deficiency (Mif−/−) potentiated the toxicity of CS exposure in vivo via increased apoptosis of ECs, resulting in enhanced CS-induced tissue remodeling. This was linked to MIF’s capacity to protect against double-stranded DNA damage and suppress p53 expression. Taken together, MIF appears to antagonize CS-induced toxicity in the lung and resultant emphysematous tissue remodeling by suppressing EC DNA damage and controlling p53-mediated apoptosis, highlighting a critical role of MIF in EC homeostasis within the lung.
Keywords: macrophage migration inhibitory factor, emphysema, cigarette, endothelial, apoptosis
Clinical Relevance
Our results strongly support a novel role for macrophage migration inhibitory factor (MIF) as a determinant of disease severity in mice and humans by impacting pulmonary endothelial cell (EC) apoptosis and alveolar remodeling. This work establishes a role for MIF in controlling DNA damage and p53-derived apoptotic responses to cigarette smoke by regulating double-stranded DNA breaks and caspase-3 activation in microvascular ECs. The identification of this and other such factors may provide additional therapeutic targets directed at ameliorating tissue obliteration in chronic obstructive pulmonary diseases/emphysema.
In humans, pulmonary endothelial cell (EC) apoptosis represents an early event in cigarette smoke (CS)-induced lung pathology, preceding other physiologic manifestations of obstructive disease (1). Numerous studies have identified increased EC apoptosis in human chronic obstructive pulmonary diseases (COPD)/emphysema, and animal studies support a causal link between cell death and airspace remodeling (2–4). Importantly, the targeted induction of EC death is sufficient to promote apoptosis of both ECs and type II alveolar epithelial cells within the alveolar–capillary unit, with the net result being emphysematous tissue remodeling (5). Thus, there is a critical interdependence between alveolar epithelial and microvascular ECs in the maintenance of airspace structure, and loss of ECs within the lung directly contributes to emphysematous remodeling. Despite these clinical and preclinical observations, understanding of the molecular basis of CS-induced EC apoptosis remains incomplete. We sought to identify and characterize intrinsic molecular regulators of alveolar–capillary homeostasis and define their contribution to disease severity and CS-induced tissue destruction.
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine, hormone, and enzyme with tautomerase activity (6, 7). It is constitutively expressed by multiple cell types, including pulmonary microvascular ECs (8), vascular smooth muscle (9), fibroblasts (10), and bronchial epithelium (11) within the lung. Furthermore, it is produced by multiple leukocytes, including both B and T cells (12) and macrophages (13), and is secreted by both the anterior pituitary gland (14) and pancreas (15). MIF is found in the serum under basal conditions, and elevated circulating levels of MIF have been observed in humans in both acute and chronic inflammatory disease states (16). Extracellular MIF is capable of engaging multiple recognized cell surface receptors, functioning in an autocrine or paracrine manner to activate signaling kinases, such as extracellular signal–related kinase (ERK), AKT, and AMP-activated protein kinase. Each of these kinases has been linked to prosurvival pathways. In addition, intracellular pools of MIF modulate the stability of transcription factors, including hypoxia-inducible factor 1α (17), p53 (18), and, as recent research shows, possibly nuclear factor (erythroid-derived 2)–like 2 (19), leading to secondary effects on gene expression. These three transcription factors are critical regulators of cell fate in the context of environmental stimuli (i.e., hypoxia, DNA damage, and increased reactive oxygen species, respectively), and thus are critical determinants of homeostatic responses to intracellular or extracellular stressors. Animal models of ischemic cardiac injury, bronchopulmonary dysplasia, and radiation-induced lung injury (19–21) demonstrate that loss of MIF is associated with increased tissue damage. We have identified MIF as a modulator of the sensitivity of CS-induced human lung EC apoptosis in vitro (22) antagonizing p53-dependent activation of the mitochondrial apoptotic pathway. Thus, we hypothesize that MIF will impact on the severity of CS-induced EC injury in vivo and thus influence emphysematous tissue remodeling through its effects on alveolar–capillary homeostasis.
The present study identifies MIF as a novel modifier of disease severity in CS-induced injury and emphysematous remodeling, and provides evidence that MIF is a critical determinant of lung EC homeostasis in the face of oxidative injury. Furthermore, it shows that MIF acts predominantly in ECs within the alveolar–capillary unit to antagonize DNA damage and to suppress subsequent p53 induction, thus preventing EC cell death and apoptosis-mediated tissue remodeling. Here, we demonstrate, for the first time, that serum MIF is significantly decreased in patients with COPD, most markedly in patients with the most severe disease, implicating relative MIF deficiency (Mif−/−) in the severity and/or susceptibility of humans with CS-induced lung disease.
Materials and Methods
Reagents and Cells
Cell and molecular reagents are detailed in the Materials and Methods in the online supplement.
Human Subjects
All study protocols related to human studies were approved by the Institutional Review Board of the Johns Hopkins University. Written, informed consent was obtained from all participants at the time of sample collection. Patients with COPD were recruited from clinical populations at the Johns Hopkins Hospital, Johns Hopkins Bayview Medical Center, and from the National Heart, Lung, and Blood Institute Lung Tissue Research Consortium. Healthy, nonsmoking subjects supplied serum for control reference.
Animals and Treatments
All animal protocols were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. Mif−/− mice with a C57BL/6 background (generation N10) were generated as previously described (18, 23). At 8–10 weeks of age, mice were exposed to CS or filtered air for 3 days to 24 weeks. CS exposure was performed as previously described (24), with minimal adjustments (detailed in the online supplement).
Lung Morphometry
At 15 hours after the last CS exposure, mice were anesthetized with ketamine/xylazine (75 mg/kg, 15 mg/kg) and tracheostomized (detailed in the online supplement).
Bronchoalveolar lavage (BAL) was performed on the right lung, which was removed and flash frozen for RNA and protein analysis. The left lung was inflated with agarose under pressure (30 cm H2O) and fixed in buffered formalin (Z-fix; Anatech Ltd., Battle Creek, MI). Lung volumes were estimated by water displacement. The fixed left lungs were systematically divided into three sections as previously described (25), with minimal modifications (detailed in the online supplement). For morphometric analysis, lung slices were stained with hematoxylin and eosin, and systematic random sampling of each section was performed to obtain unbiased representative pictures of each lung. The indirect stereological method for quantifying mean chord length (Lm) and lung alveolar surface area (Sav) (26) was completed using freely available software (STEPanizer) (27) (detailed further in the online supplement).
Western Blotting and Immunohistochemistry
Western blotting and immunohistochemical protocols and reagents are detailed in the Materials and Methods in the online supplement.
Statistical Analysis
For comparisons among groups of normally distributed data sets, Student’s t test or ANOVA with post hoc Bonferroni correction was used. For nonnormally distributed data, the Mann Whitney or Kruskal-Wallis test with post hoc Bonferonni correction was used. Values are presented as means (± SE).
Results
MIF Levels Are Significantly Reduced in Humans with COPD
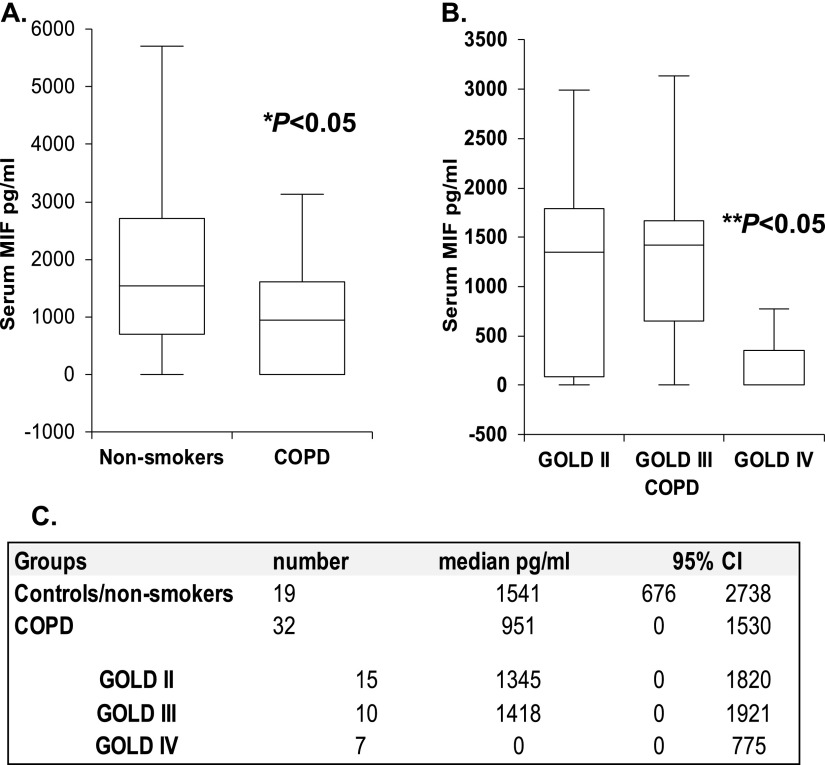
We have previously demonstrated that MIF modifies EC injury in vitro in response to acute CS exposure by antagonizing p53-mediated mitochondrial apoptosis (22). To explore if alterations in MIF occur in the context of human COPD, we quantified circulating MIF levels in the serum of normal control subjects (n = 18) and in subjects with COPD. This was defined by irreversible airflow obstruction (i.e., forced expiratory volume in 1 s/forced vital capacity ratio of < 0.7 and a forced expiratory volume in 1 s <90% predicted; n = 32). Patient demographics are in Table E1 in the online supplement. Serum MIF levels were significantly lower in patients with COPD compared with healthy, nonsmoking control subjects (950 pg/ml versus 1,541 pg/ml; P = 0.03; Figure 1A).
Figure 1.
Serum macrophage migration inhibitory factor (MIF) concentrations are reduced in patients with severe chronic obstructive pulmonary diseases (COPD). Serum MIF levels were significantly reduced in subjects with COPD when compared with nonsmokers (A). In patients with COPD, serum MIF was significantly reduced in severe, GOLD (Global Initiative for Chronic Obstructive Lung Disease) IV disease (B). Values are expressed as median and 95% confidence interval (CI) (C). *P < 0.05, nonsmokers versus COPD; **P < 0.05, GOLD IV versus GOLD II and III.
To begin to address the relationship between MIF and disease severity, the GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria was used to further classify patients in a COPD cohort, as previously described (28). In this cross-sectional analysis, patients with severe COPD (GOLD stage IV) had significantly lower circulating MIF than subjects with less severe COPD (stages II and III; P = 0.03; Figure 1B). Thus, we observed significantly lower circulating MIF in patients with COPD, and this was most pronounced in those with severe disease, implicating MIF in human disease severity.
Chronic Smoke Alters MIF Expression in the Intact Lung
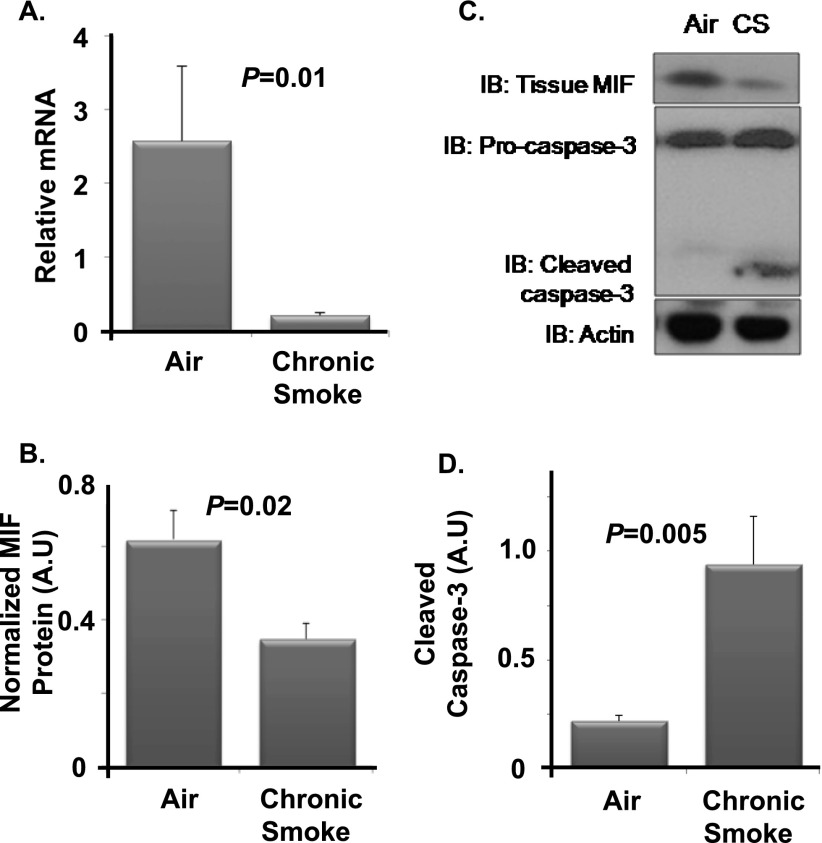
To address the effects of chronic CS exposure on MIF expression in vivo, wild-type C57BL/6 mice were randomized to exposure with filtered air or CS for 6 months. Whole-lung homogenates were analyzed for MIF mRNA and protein. Chronic CS exposure resulted in a significant decrease in total lung MIF mRNA (Figure 2A). Similarly, MIF protein expression was significantly decreased with chronic CS relative to air controls (Figures 2B and 2C). These changes in MIF expression were associated with enhanced activation of caspase-3 (Figure 2D), a marker of CS-induced apoptosis in the lung. Thus, MIF mRNA and protein expression were significantly decreased in the setting of chronic CS exposure in the intact murine lung coincident with activation of the apoptotic pathway.
Figure 2.
Chronic smoke alters MIF expression in the intact lung. Wild-type C57BL/6 mice were exposed to cigarette smoke (CS) or filtered air. Gene expression and Western blotting were used to study alterations in MIF and cleaved caspase-3. Lung MIF mRNA was significantly reduced after exposure to CS (A). Tissue MIF was significantly decreased with chronic CS relative to air controls (B). The resulting protein intensities were normalized to β-actin (C). Chronic CS was associated with enhanced cleavage of caspase-3 relative to air controls (D). n = 5 per arm. Values are expressed as means ± SEM.
MIF Suppresses CS-Induced DNA Damage In Vivo
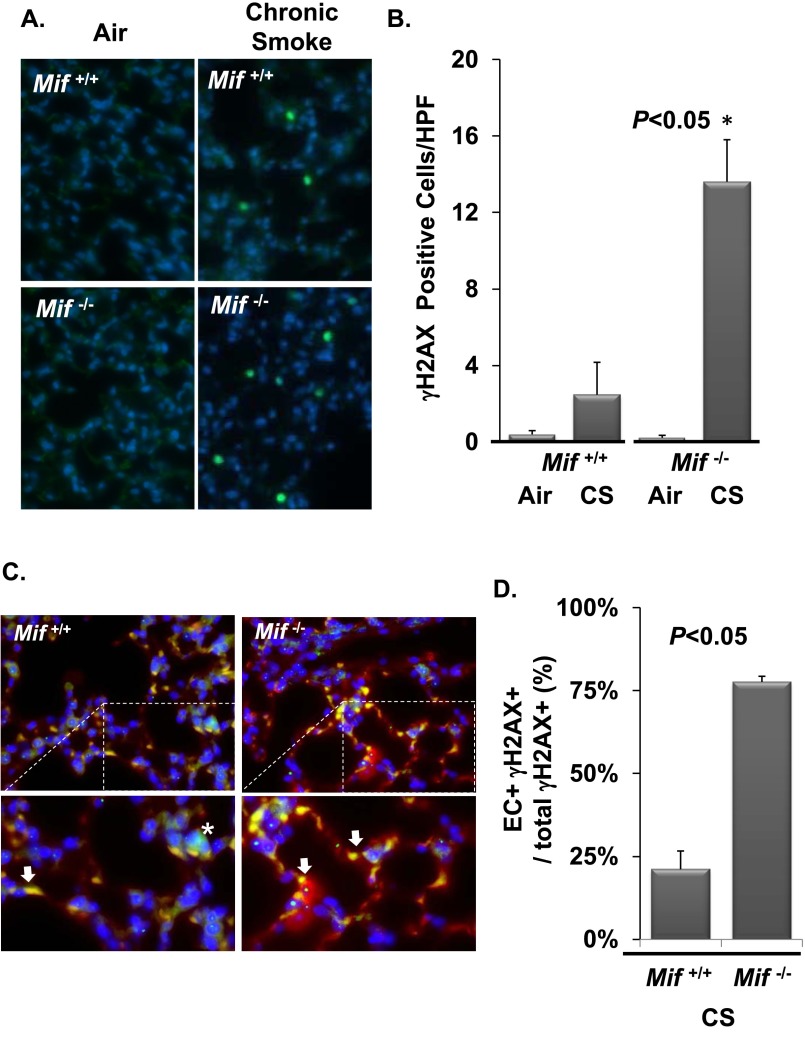
Cell culture, animal data (29), and human pathology (30) demonstrate double-stranded DNA breaks (DSBs) as one mechanism of CS-induced cellular injury in the lung. DNA damage results in cell cycle arrest, senescence, or cell death in the form of apoptosis, orchestrated by the transcription factor, p53, a major effector of the DNA damage response (31). In response to DSBs, histone H2AX is phosphorylated (termed γH2AX) by the ataxia telangiectasia mutated (ATM) kinase, making γH2AX a marker of both DSBs and ATM kinase activity. To test if MIF functions to suppress DNA damage in the face of CS, Mif+/+ and Mif−/− mice were randomized to air or CS for 0.5 months, and lung γH2AX levels were analyzed by immunohistochemistry and Western blot. In air-exposed mice, basal in situ staining for γH2AX was minimal and similar between genotypes. In contrast, Mif−/− animals demonstrated significantly increased numbers of γH2AX-positive cells in lung parenchyma compared with Mif+/+ mice after exposure to CS (13.6 ± 2.2 versus 2.5 ± 1.7 cells/high power field; P < 0.05; Figures 3A and 3B). This was confirmed by Western blot analysis (Figures E1A and E1B).
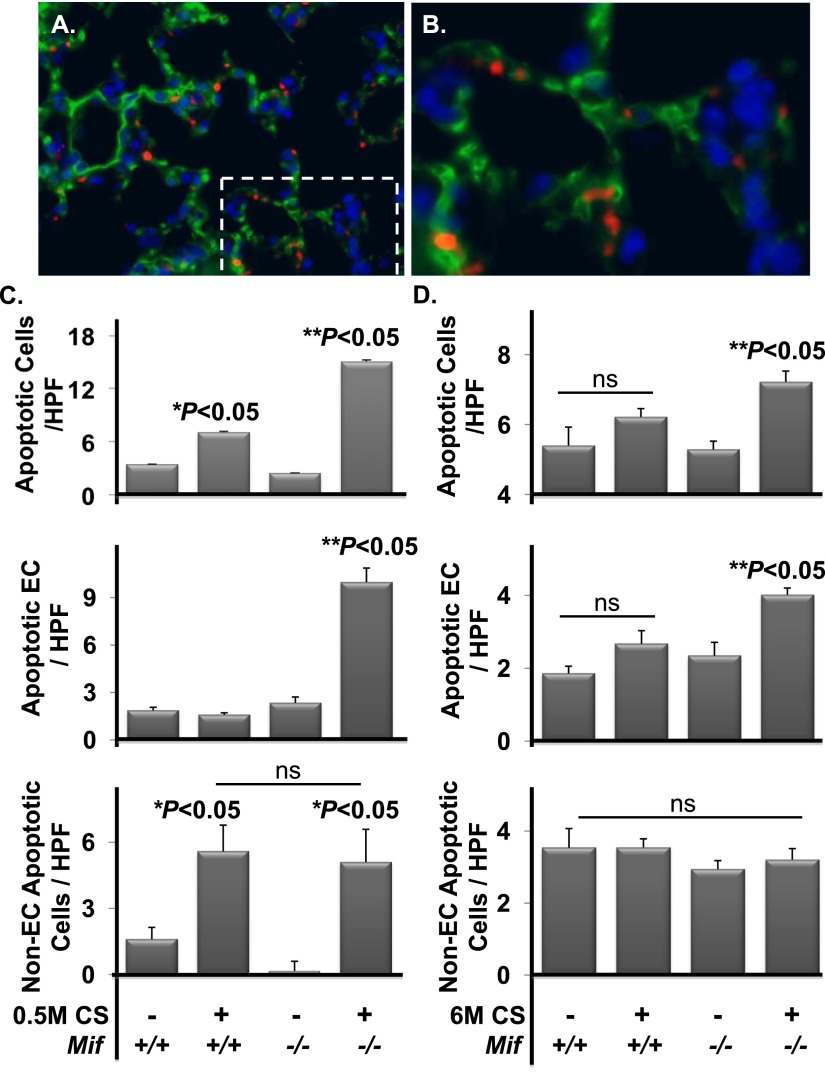
Figure 3.
MIF antagonizes CS-induced endothelial cell (EC) double-stranded DNA breaks (DSBs) in vivo. Lungs from Mif+/+ and Mif−/− animals exposed to filtered air or CS for 0.5 months were harvested and sectioned for immunohistochemistry (A and C). MIF-deficient mice exposed to CS demonstrated a significant increase in γH2AX-positive parenchymal cells relative to air-exposed Mif−/− and CS-challenged Mif+/+ mice (A and B). Mif−/− mice had significantly higher γH2AX-positive (green) ECs as detected by colocalization of isolectin staining (red) (C and D). Area of magnification denoted with dashed lines (C). Arrow identifies double positive cells, * identifies H2AX positive/isolectin negative cells. n = 5 per arm. Values are expressed as means ± SEM. A.U., arbitrary unit; IB, immunoblot.
Microvascular ECs are susceptible to CS-induced DSBs in vitro (29); thus, we evaluated the extent of DNA damage in ECs in vivo. Using lectins, which bind alveolar (microvascular) and not extra-alveolar (macrovascular) ECs, we costained microvascular ECs and γH2AX by immunofluorescent microscopy (32). In vivo, the majority of cells with CS-induced DSBs in Mif−/− mice were microvascular ECs (Figures 3C and 3D). This differed significantly with Mif+/+ both in absolute numbers and as a proportion of damaged cells identified (P < 0.05). DSBs were not observed in large blood vessels (identified on phase contrast). Therefore, MIF deficiency potentiated CS-induced DSBs and ATM kinase activation within microvascular ECs in the intact lung.
MIF Suppresses CS-Mediated p53 Expression In Vivo
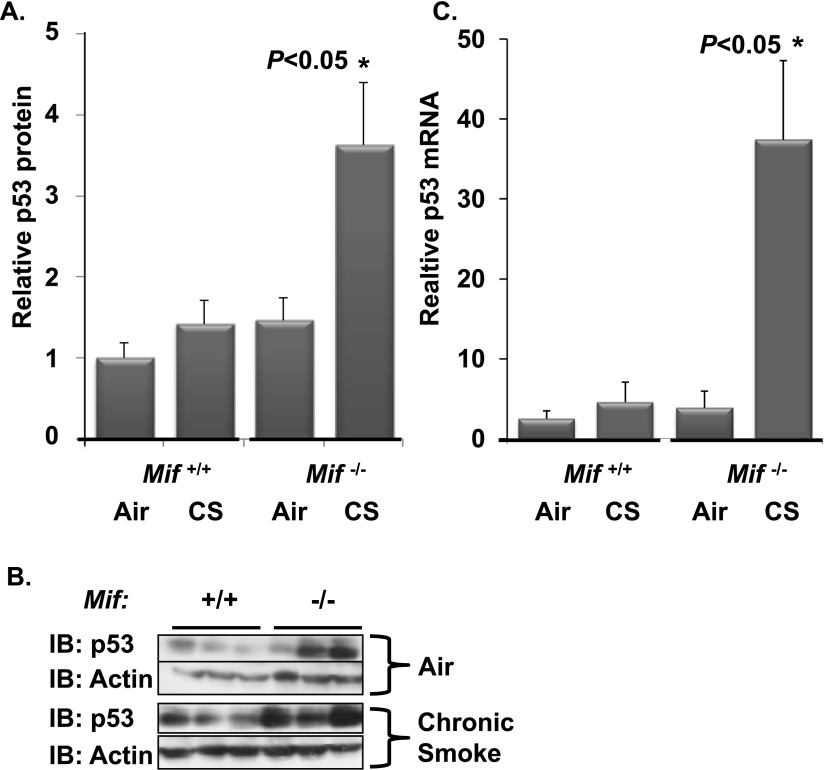
ATM kinase is a well established positive regulator of p53 expression. We next asked if the enhanced ATM kinase activity, as reflected by increased γH2AX (Figure 3), was associated with increased p53 expression in vivo. Protein and mRNA were extracted from the lung of CS-exposed Mif−/− and Mif+/+ mice, and p53 expression was quantified via Western blotting and quantitative PCR (Figure 4). Both were significantly enhanced in CS-exposed Mif−/− mice relative to CS-exposed Mif+/+ or air-exposed animals. CS-induced p53 expression is ATM kinase–dependent (29), and p53 triggers activation of proapoptotic bcl-2–like protein and EC death via the mitochondrial apoptotic pathway (22). MIF functions upstream of ATM kinase; thus, ATM kinase provides a molecular link between MIF effects in response to CS and p53 expression.
Figure 4.
CS-induced p53 expression is increased in the absence of MIF. Lungs from Mif+/+ and Mif−/− animals exposed to filtered air or CS for 0.5 months were harvested and homogenized for Western blotting (A and B) and gene expression (C). Relative p53 protein expression was increased in Mif−/− mice exposed to CS (A). Representative Western blot (B). n = 5–6 per arm. There was a significant increase in p53 mRNA in Mif−/− animals in response to CS as assessed by comparative quantitative PCR (C). *P < 0.05 Mif−/− CS versus air and Mif+/+ CS.
MIF Suppresses CS-Induced Apoptosis In Vivo
Based on the observed effects of MIF on p53-dependent human EC apoptosis in response to CS in vitro (22), we rationalized that increased p53 would be linked to increased cell apoptosis in vivo. To test this, lung sections from air- and CS-exposed (0.5 and 6 mo) mice were stained for cleaved caspase-3, the enzymatic effector of apoptosis (Figures 5A and 5B). In situ quantification demonstrated a significantly increased number of cleaved caspase-3–positive cells in the parenchyma of Mif−/− mice challenged with CS compared with Mif+/+ animals exposed in parallel (Figures 5C and 5D, upper panels). There was no difference in the frequency of cleaved caspase-3–positive cells between genotypes under basal conditions (air exposed) when comparing age-matched mice. In the presence of MIF, short-term exposure to CS was sufficient to increase apoptosis above air-exposed, age-matched mice (3.4/HPF versus 7.1/HPF; P = 0.02; Figure 5C, upper panel). This increase in cell death was lost by 6 months of CS exposure (5.4/HPF versus 6.2/HPF; P = 0.2; Figure 5D, upper panel), with basal frequencies of apoptosis being higher in the lungs of these older mice. In contrast, there was a significant increase in CS-induced apoptosis within the parenchyma of Mif−/− mice at 0.5 months (2.5/high-powered field [HPF] versus 15/HPF; P < 0.0001; Figure 5C, upper panel), which trended down by 6 months (Figure 5D, upper panel), but remained elevated above air-exposed littermates (5.2/HPF versus 7.3/HPF; P = 0.002). Thus, the absence of MIF was associated with increased and persistent cell death after CS exposure without demonstrable differences in air-exposed animals, further implicating MIF in cytoprotective responses in the lung after oxidative challenge.
Figure 5.
Caspase-3 expression is increased in the absence of MIF with subacute and chronic CS exposure. Lungs from Mif+/+ and Mif−/− animals exposed to filtered air or CS for 0.5 or 6 months were harvested and sectioned for immunohistochemistry. Representative fluorescent microscopy images are shown of cleaved caspase-3 (red) and thrombomodulin (green) in the lung (A and B). Area of magnification denoted with dashed lines (A). The frequency of cleaved caspase-3–positive parenchymal cells was significantly increased in Mif−/− versus Mif+/+ mice exposed to 0.5 months of CS (P < 0.05) ([C] upper panel), and with 6 months of CS (P < 0.05) ([D] upper panel). The majority of caspase-3–positive cells in Mif−/−exposed to 0.5 or 6 months of CS were ECs (both with **P < 0.05) (middle panels [C and D]). Non-ECs were enhanced with 0.5 months with exposure (*P < 0.05), and this did not differ with genoptype (lower panels [C and D]). No differences were observed under basal conditions independent of genotype (n = 5–8 per arm). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Values are expressed as means ± SEM. ns, not significant. *P < 0.05 air versus CS. **P < 0.05 Mif+/+ versus Mif−/−.
MIF Antagonizes CS-Induced Endothelial Apoptosis in the Intact Lung
To address the contribution of MIF to EC apoptosis, we performed coimmunohistochemistry for both cleaved caspase-3 and the EC marker, thrombomodulin (33) (Figures 5C and 5D, middle panels). In the presence of MIF (Mif+/+ animals), there was a relative resistance of microvascular ECs after subacute exposures to CS, with no significant difference in EC apoptosis observed relative to age-matched air-exposed animals. After prolonged exposure to CS (6 mo), there was a trend toward an increase in microvascular EC apoptosis, which did not meet statistical significance (1.8/HPF versus 2.7/HPF; P = 0.07; Figure 5D, middle panel). In the absence of MIF, there were no differences at baseline (air exposed) after 0.5- or 6-month exposure relative to Mif+/+ mice (Figure 5D, middle panel). In contrast, exposure to both short-term and prolonged CS resulted in significant increases in microvascular EC apoptosis in Mif−/− mice when compared with Mif+/+ mice (1.8/HPF versus 9.8/HPF at 0.5 mo [P = 0.001] and 2.6/HPF versus 4.1/HPF at 6 mo [P = 0.02]; Figures 5C and 5D, middle panels).
Non-EC apoptosis was significantly increased after 0.5 months of CS exposure (1.6/HPF versus 5.5/HPF; P = 0.01); however, there was no difference between genotypes (5.5/HPF versus 5.1/HPF; P = 0.8). Despite the apparent lower mean in the knockout mice, there was not a statistically significant difference between genotypes at baseline (1.6/HPF versus 0.2/HPF; P = 0.07; Figure 5C, lower panel). After 6 momths, the frequency of apoptosis in non-ECs did not differ between exposures or genotypes (Figure 5D, lower panel). Thus, MIF deficiency preferentially enhanced microvascular EC sensitivity to CS-induced apoptosis in the intact lung.
MIF Deficiency Enhances CS-Induced Tissue Remodeling
To test the hypothesis that MIF modifies the severity of CS-induced emphysematous tissue remodeling, Mif+/+ and Mif−/− mice were randomized to prolonged CS exposure (6 mo, 5 d/wk) or filtered air for the same duration of time. Lung tissue was subjected to morphometric analysis under basal conditions (air-exposed, age-matched animals) and in the setting of chronic CS. Using systematic random sampling, caudal, middle, and cranial left lung regions were analyzed for alterations in tissue architecture, with quantification of airspace enlargement defined by increased Lm.
Baseline analysis revealed a modest, but statistically significant, increase in the cranial and middle Lm in Mif−/− mice relative to age-matched Mif+/+ mice (29.7 versus 32.0 [P = 0.01] and 30.2 versus 32.4 [P = 0.02], respectively; Figure E2). Thus, in the absence of MIF, there were statistically significant differences in airspace morphology under basal conditions, which may reflect a contribution of MIF in normal lung development and/or in the maintenance of lung structure with aging.
In response to CS, airspace enlargement was visually detected in caudal regions regardless of genotype (Figures 6A–6D, Figure E3), and morphometric analysis confirmed that tissue remodeling in response to CS predominated in the caudal regions, highlighting a heterogeneous distribution of airspace enlargement and enhanced lower-lung damage due to CS in this model (Figures 6A–6D). In response to CS, airspace enlargement in Mif+/+ mice was significant in the cranial and middle lung regions (29.7 versus 31 [P = 0.03] and 30.3 versus 32.5 [P = 0.04], respectively; Figures E2 and E3), and tended to be enlarged in the caudal regions (31.0 versus 33.2; P = 0.07) relative to air controls.
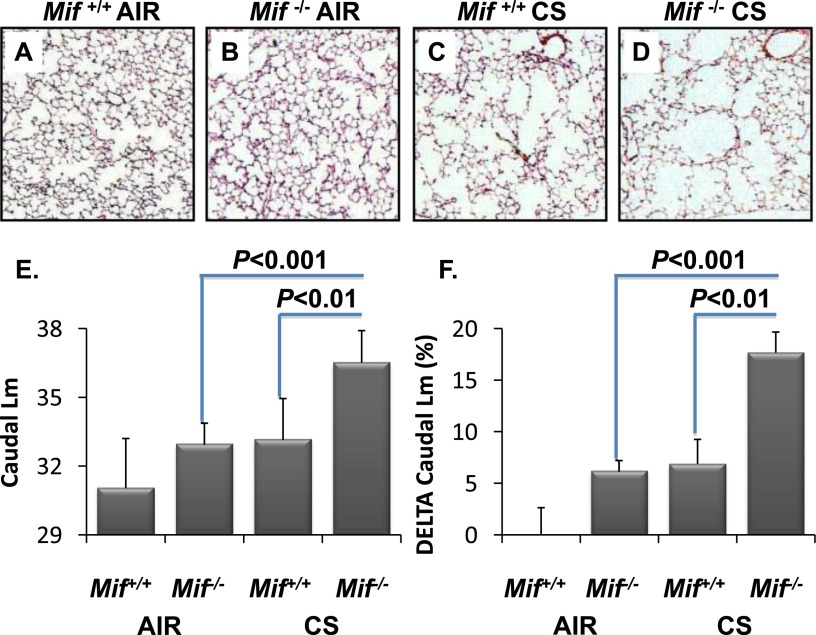
Figure 6.
MIF deficiency leads to increased sensitivity to emphysematous remodeling in vivo. Lungs from Mif+/+ and Mif−/− animals exposed to filtered air or CS for 6 months were sectioned for morphometry. Differences in alveolar remodeling in Mif+/+ versus Mif−/− mice are visually apparent in CS-exposed caudal lung regions (C and D, respectively) compared with air-exposed mice (A and B, respectively). Mif−/− mice had significantly higher mean chord length (Lm) in the caudal lung regions than Mif+/+ when exposed to CS (E), and the change in Lm from baseline was significant in Mif−/− mice (F). Values are expressed as means ± SEM.
Regional remodeling was significantly enhanced in the absence of MIF. In Mif−/− mice, the caudal lung demonstrated significant increases in Lm when compared with CS-exposed Mif+/+ mice (33.2 versus 36.5; P = 0.007) or air-exposed littermates (32.9 versus 36.5; P = 0.002; Figure 6, Figure E3). Even after adjusting for multiple comparisons, Mif−/− mice exposed to CS demonstrated a significant increase in the absolute Lm relative to smoke-challenged Mif+/+ mice (P < 0.008; Figure 6, Figure E3). In addition to the effects observed in absolute Lm in response to CS, quantification of the relative change in Lm highlighted the effects of both MIF deficiency and CS exposure on airspace enlargement (Figure 6F). The calculated mean whole-lung Sav (34) did not differ between genotypes at baseline, and was lower in Mif−/− mice after smoke. This did not reach statistical significance secondary to large variability in the replicates (P = 0.09; Table E2). Importantly, the validity of the calculated Sav requires homogeneity of remodeling throughout the lung, a condition present at baseline, but not observed after CS-induced remodeling.
Discussion
Emphysema, a common form of COPD, contributes to an estimated 2.5 million deaths and a health care cost approaching 38.8 billion dollars annually in the United States alone (35). It is a debilitating disease characterized by the irreversible destruction of the lung architecture, with enlargement of the airspaces driven by enhanced apoptosis (34). Targeted EC apoptosis is sufficient to induce emphysema (5), whereas pharmacologic blockade prevents disease, arguing that EC apoptosis is both sufficient and necessary for development of emphysema (33, 36). Furthermore, loss of EC viability is sufficient to induce apoptosis of type II alveolar epithelial cells (5), highlighting the contribution of EC homeostasis in the maintenance of normal alveolar structure. We set out to identify new molecular determinants of EC apoptosis and survival in response to CS (22, 29), predicting that such factors could represent novel determinants of disease severity and pathologic tissue remodeling. The capacity of MIF to regulate the sensitivity of human pulmonary ECs to CS in vitro made it an attractive candidate. Our work provides evidence of altered MIF expression in murine and human CS-induced lung disease, and links enhanced disease severity to the loss of MIF’s capacity to suppress DNA damage and apoptosis in microvascular ECs within the lung, potentiating CS-induced tissue remodeling.
Here, we provide evidence that patients with COPD have diminished serum MIF relative to normal control subjects (nonsmokers). Furthermore, we demonstrate that patients with severe disease (GOLD stage IV COPD) have markedly lower circulating MIF than those with milder disease. It remains to be determined if this is a cause or consequence in human disease, which cannot be specifically addressed in a cross-sectional study design such as this. However, in our animal and human EC studies, MIF levels inversely correlate with cellular injury and tissue remodeling, implicating a causal relationship in humans rather than an epiphenomenon. Our results suggest the possibility that individual variability in MIF expression, which is genetically determined (37), could account for the clinical heterogeneity in susceptibility to CS-induced emphysematous pathology in humans.
In our animal model, we have demonstrated CS-induced DNA damage in the lung parenchyma that is antagonized by MIF. Furthermore, our data indicate that MIF functions predominantly to maintain homeostasis of microvascular ECs. In the absence of MIF, there was an increase in DSBs, leading to increased ATM kinase–dependent p53 expression. As a consequence, MIF deficiency exacerbated CS-induced lung cell death. The cytoprotective effects of MIF in the context of CS impact on the microvascular ECs within the lung, as these are the primary targets of DSBs and apoptosis in the absence of MIF. CS-induced EC apoptosis was observed rapidly (0.5 mo), at a higher frequency (approaching fivefold greater), and persistently (6 mo) in Mif−/− mice compared with Mif+/+ animals. This occurred without evidence of basal differences in age-matched mice. Importantly, there was no difference between the frequencies of CS-induced non-EC death between genotypes, again pointing to the enhanced dependence of microvascular ECs on MIF in the setting of CS. The enhanced EC apoptosis was linked to increased emphysematous tissue destruction manifested by a significant increase in Lm in the lower lung zones (i.e., middle and caudal regions).
The alveolar–capillary structure is composed of multiple cell types. In addition to the contribution of microvascular ECs, the alveolar wall is composed predominantly of type I and II epithelial cells (pneumocytes). Homeostasis of both ECs and pneumocytes are necessary for maintenance of normal alveolar structure. Both cell types are also destroyed during human emphysematous remodeling. Our in vivo data indicate that microvascular ECs represent the major cellular target of DNA damage and apoptosis in the absence of MIF. Others have shown that targeted killing of ECs in the lung is sufficient to trigger apoptosis of type II pneumocytes (5). Thus, it is possible that loss of these cells in the alveolus may be a consequence of EC death in our model or a direct effect of CS exposure. Importantly, although we observed apoptosis in other cell types in the lung parenchyma, this did not differ as a function of MIF expression. MIF deficiency has also been linked to decreased macrophage viability (38), and macrophage numbers are significantly altered in both patients with COPD and in animal models of CS-induced lung disease (39). Thus, we specifically assessed macrophage numbers, activation status, and/or viability. Although we observed an increase in macrophage frequency in the BAL fluid after short-term CS exposure, this did not differ between genotypes (Figure E4). Viability of both naive (CD11b+) and activated (CD11c+) f4/80+ macrophages in the BAL fluid and lung tissue increased after CS (Figure E5), and, again, was not altered by MIF expression. Thus, the effects of MIF on macrophage number, viability, or activation status do not contribute to CS-induced tissue injury or remodeling in our model.
Under normal physiologic conditions, MIF is detectable in the circulation. It is produced and secreted by numerous cell types and tissues, and functions via autocrine and paracrine effects. Although we demonstrate that MIF expression decreases in the lung with chronic smoke, and is lower in the circulation of patients with COPD, at this point it remains to be determined if MIF expression is reduced systemically or in a subset of cells/tissues in the context of disease. Such information will be critical to define the mechanism(s) by which MIF is decreased in this disease state. Irrespective of the basal cellular and tissue-derived sources of MIF, low circulating levels predictably impact ECs that are in direct contact with the blood. Our murine data indicate that MIF mRNA is globally reduced in the lung after chronic smoke exposure. There are few defined molecular mechanisms to suppress MIF transcription. MIF is, however, sensitive to epigenetic silencing. MicroRNA-451 can suppress MIF expression (40) and, intriguingly, this microRNA is up-regulated in the circulation of human smokers (41), suggesting one potential molecular mechanism of MIF suppression in COPD. In addition, the MIF promoter is repressed by histone deacetylase inhibitors (42). Both histone deacetylase expression and activity are reduced in human COPD (43), suggesting a second potential molecular mechanism to account for the observed decline in MIF.
MIF has well established proinflammatory functions, and has been implicated as a positive regulator of metalloproteases (MMPs), including collagenases (MMP13 [44] and MMP1 [45]) and gelatinases (MMP2 [46] and MMP9 [44]), but not elastase (MMP12). Of the known MMPs responsive to MIF, MMP2 and MMP9 are elevated in human COPD (47, 48) and animal models of emphysema (49). Despite these potential molecular targets of MIF in CS-induced disease, we were unable to identify significant differences of the proinflammatory cytokine, TNF-α, expression or MMP2/MMP9 in our model by quantitative PCR (data not shown) or zymography, respectively (Figure E6), based on genotype. Furthermore, we did not detect altered total BAL cell counts between genotypes, although lymphocyte numbers were differentially affected by CS (Figure E5). Thus, our loss-of-function and clinical data argue against a model in which the proinflammatory functions of MIF promote COPD pathology.
Airspace enlargement is a well documented age-related process (50, 51), and it is recognized that genetically engineered strains of mice, hypersensitive to CS, can also demonstrate evidence of accelerated age-dependent lung remodeling (52, 53). We observed differences in baseline Lm in Mif−/− compared with Mif+/+ mice housed in a filtered-air environment. This observation suggests that MIF may influence lung homeostasis and/or development. MIF protein decreases in the lungs of aging mice (19), indirectly implicating it in age-related changes. In neonatal mice exposed to hyperoxia, both excess and insufficient MIF alters neonatal alveolar development, suggesting that there is an ideal “dose” of MIF for optimal lung maturation (54). Despite the basal difference in airspace morphology, the effects of CS exposure on Mif−/− animals were more than an additive. Because all studies used age-matched controls, aging cannot account for the differences observed between treatment arms. Moreover, we did not observe differences in basal DSBs or apoptosis, indicating that the effects of MIF on DNA damage and EC apoptosis in adult life are unlikely to account for the basal differences in alveolar morphology. Further studies will be necessary to determine if the observed basal differences in the adult lung are a result of premature aging and/or abnormal lung development.
From a clinical and therapeutic perspective, our data suggest that relative MIF deficiency may predispose patients to COPD/emphysema, and that normalization of MIF could have a therapeutic advantage. Our preclinical and in vitro data (22) implicate the capacity of MIF to antagonize p53 expression in its cytoprotective effects against CS. Although p53-induced apoptosis predisposes to pathologic remodeling, p53 is also a tumor suppressor, and prolonged and/or global p53 suppression would predictably increase risk of malignancies, especially in the face of chronic exposure to carcinogens, such as CS. Importantly, the effects of MIF deficiency were observed in the microvascular ECs. This cell type rarely gives rise to tumors (i.e., angiosarcomas) in the lung, and these tumors are not epidemiologically linked to CS exposure. Moreover, our data indicate that MIF does not directly impact on p53, but functions upstream of CS-induced DSBs. Thus, we predict that restoring physiologic MIF would reduce CS-induced DNA damage, promoting homeostasis and eliminating the driving force for CS-induced p53 expression in ECs.
Limitations of our analysis are imparted by the animal model used, which must condense a prolonged exposure in humans into a restricted timeline. Consequently, airspace remodeling is relatively mild. Second, these studies were performed in a relatively resistant background strain, C57BL/6, to take advantage of a genetic model. Although MIF expression was suppressed by chronic CS in this strain, it remained detectable within the lung after 6 months. Thus, the reduced DSBs, delayed EC apoptosis (observed at 6 mo, not 0.5 mo), and a diminished airspace enlargement observed in Mif+/+ mice, relative to Mif−/−, demonstrate the persistent cytoprotective role of MIF in response to CS exposure.
From a methodologic standpoint, the most reliable and accurate stereologic methods to quantify emphysematous remodeling in small animal models are highly debated. We used an unbiased approach, including systematic random sampling and the indirect/point-counting method of unadulterated lung images to estimate Lm. This is in contrast to the mean linear intercept or direct method, which can substantially bias the Lm estimations, favoring larger values. Thus, in the face of heterogeneous tissue destruction, Lm derived from the point-counting method represents the more accurate assessment of regional alveolar remodeling.
Finally, our serum analysis has the following limitations: (1) it is a cross-sectional analysis; (2) we have insufficient clinical data to address the relationship between MIF and other clinically relevant variables, such as emphysematous remodeling quantified by high-resolution chest tomography; and (3) it does not define MIF levels directly in the lungs of patients. Prior clinical studies suggest that the lung is a major source of circulating MIF (55), making it a reasonable clinical surrogate. Despite these limitations, this represents the first clinical link between MIF and COPD, and provides the framework for understanding the role of this potential modifier in disease severity.
Despite acknowledged environmental risks, it is well recognized that individual susceptibility and severity of COPD/emphysema is heterogeneous. Although rare heritable risk factors have been identified as typified by α1-antitrypsin deficiency, the contribution of other molecular antagonists of tissue destruction in emphysema is nascent. Our results provide strong support for a novel role for MIF as a determinant of disease severity in mouse and man, impacting on pulmonary EC apoptosis and alveolar remodeling. It establishes a role for MIF in controlling DNA damage and p53-derived apoptotic responses to smoke by regulating DSBs and caspase-3 activation in microvascular ECs. The identification of this and other such factors may provide additional therapeutic targets directed at ameliorating tissue obliteration in COPD/emphysema.
Acknowledgments
Acknowledgments
The authors thank Dr. Robert Wise for his support and generosity.
Footnotes
This work was supported by Flight Attendants Medical Research Institute grants 072,129 and 110,627 (B.K. and R.D.) and National Institutes of Health grants K08HL088320 (R.D.) and R01HL049441 (P.M.H.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0371OC on February 3, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, Mezey JG, Crystal RG. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henson PM, Cosgrove GP, Vandivier RW. State of the art: apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:512–516. doi: 10.1513/pats.200603-072MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanazawa H, Yoshikawa J. Elevated oxidative stress and reciprocal reduction of vascular endothelial growth factor levels with severity of COPD. Chest. 2005;128:3191–3197. doi: 10.1378/chest.128.5.3191. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese F, Giacometti C, Beghe B, Rea F, Loy M, Zuin R, Marulli G, Baraldo S, Saetta M, Valente M. Marked alveolar apoptosis/proliferation imbalance in end-stage emphysema. Respir Res. 2005;6:14. doi: 10.1186/1465-9921-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem. 2008;283:29447–29460. doi: 10.1074/jbc.M804595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- 8.Damico RL, Chesley A, Johnston L, Bind EP, Amaro E, Nijmeh J, Karakas B, Welsh L, Pearse DB, Garcia JG, et al. Macrophage migration inhibitory factor governs endothelial cell sensitivity to LPS-induced apoptosis. Am J Respir Cell Mol Biol. 2008;39:77–85. doi: 10.1165/rcmb.2007-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Shen M, Xu M, Liu LL, Luo Y, Xu DQ, Wang YX, Liu ML, Liu Y, Dong HY, et al. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediators Inflamm. 2012;2012:840737. doi: 10.1155/2012/840737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Talwar A, Tsang D, Bruchfeld A, Sadoughi A, Hu M, Omonuwa K, Cheng KF, Al-Abed Y, Miller EJ. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol Med. 2012;18:215–223. doi: 10.2119/molmed.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, Lane SJ, Craft J, Nishihira J, Donnelly SC, et al. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci USA. 2005;40:14410–14415. doi: 10.1073/pnas.0507189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacher M, Metz CN, Calandra T, Mayer J, Chesney J, Lonoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 15.Waeber G, Calandra T, Roduit R, Haefliger JA, Bonny C, Thompson N, Thorens B, Temler E, Meinhardt A, Bacher M, et al. Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1997;94:4782–4787. doi: 10.1073/pnas.94.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morand EF. New therapeutic target in inflammatory disease: macrophage migration inhibitory factor. Intern Med J. 2005;35:419–426. doi: 10.1111/j.1445-5994.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 17.Oda S, Oda T, Nishi K, Takabuchi S, Wakamatsu T, Tanaka T, Adachi T, Fukuda K, Semenza GL, Hirota K. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS ONE. 2008;3:e2215. doi: 10.1371/journal.pone.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Muller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci USA. 2003;100:9354–9359. doi: 10.1073/pnas.1533295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew B, Jacobson JR, Siegler JH, Moitra J, Blasco M, Xie L, Unzueta C, Zhou T, Evenoski C, Al-Sakka M, et al. Role of migratory inhibition factor in age-related susceptibility to radiation lung injury via NF-E2–related factor-2 and antioxidant regulation. Am J Respir Cell Mol Biol. 2013;49:269–278. doi: 10.1165/rcmb.2012-0291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prencipe G, Auriti C, Inglese R, Devito R, Ronchetti MP, Seganti G, Rava L, Orzalesi M, De Benedetti F. A polymorphism in the macrophage migration inhibitory factor promoter is associated with bronchopulmonary dysplasia. Pediatr Res. 2011;69:142–147. doi: 10.1203/PDR.0b013e3182042496. [DOI] [PubMed] [Google Scholar]
- 21.Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, Young LH. Cardiac macrophage migration inhibitory factor inhibits jnk pathway activation and injury during ischemia/reperfusion. J Clin Invest. 2009;119:3807–3816. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Hassoun PM. p53 mediates cigarette smoke–induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol. 2011;44:323–332. doi: 10.1165/rcmb.2009-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, David JR. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke–induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitzner W, Fallica J, Bishai J. Anisotropic nature of mouse lung parenchyma. Ann Biomed Eng. 2008;36:2111–2120. doi: 10.1007/s10439-008-9538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol. 2010;108:412–421. doi: 10.1152/japplphysiol.01100.2009. [DOI] [PubMed] [Google Scholar]
- 27.Tschanz SA, Burri PH, Weibel ER. A simple tool for stereological assessment of digital images: the stepanizer. J Microsc. 2011;243:47–59. doi: 10.1111/j.1365-2818.2010.03481.x. [DOI] [PubMed] [Google Scholar]
- 28.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:502–506. doi: 10.1513/pats.200701-001FM. [DOI] [PubMed] [Google Scholar]
- 29.Kim BS, Serebreni L, Hamdan O, Wang L, Parniani A, Sussan T, Scott Stephens R, Boyer L, Damarla M, Hassoun PM, et al. Xanthine oxidoreductase is a critical mediator of cigarette smoke–induced endothelial cell DNA damage and apoptosis. Free Radic Biol Med. 2013;60:336–346. doi: 10.1016/j.freeradbiomed.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Pastukh VM, Zhang L, Ruchko MV, Gorodnya O, Bardwell GC, Tuder RM, Gillespie MN. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis. 2011;6:209–217. doi: 10.2147/COPD.S15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- 32.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7:53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the U.S.: a review and synthesis of the literature. COPD. 2006;3:211–218. doi: 10.1080/15412550601009396. [DOI] [PubMed] [Google Scholar]
- 36.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: The missing link. Am J Respir Cell Mol Biol. 2003;28:551–554. doi: 10.1165/rcmb.F269. [DOI] [PubMed] [Google Scholar]
- 37.Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, Wolfe F, Gregersen PK, Bucala R. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–176. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2013;19:95–102. doi: 10.1097/MCP.0b013e32835cfff5. [DOI] [PubMed] [Google Scholar]
- 40.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, et al. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol. 2013;272:154–160. doi: 10.1016/j.taap.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Lugrin J, Ding XC, Le Roy D, Chanson AL, Sweep FC, Calandra T, Roger T. Histone deacetylase inhibitors repress macrophage migration inhibitory factor (MIF) expression by targeting MIF gene transcription through a local chromatin deacetylation. Biochim Biophys Acta. 2009;1793:1749–1758. doi: 10.1016/j.bbamcr.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 44.Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, Minami A. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts: relevance to intracellular signaling pathways. J Biol Chem. 2002;277:7865–7874. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 45.Kong YZ, Huang XR, Ouyang X, Tan JJ, Fingerle-Rowson G, Bacher M, Mu W, Scher LA, Leng L, Bucala R, et al. Evidence for vascular macrophage migration inhibitory factor in destabilization of human atherosclerotic plaques. Cardiovasc Res. 2005;65:272–282. doi: 10.1016/j.cardiores.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Meyer-Siegler K. Macrophage migration inhibitory factor increases MMP-2 activity in DU-145 prostate cells. Cytokine. 2000;12:914–921. doi: 10.1006/cyto.2000.0682. [DOI] [PubMed] [Google Scholar]
- 47.Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61:1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999;159:1985–1991. doi: 10.1164/ajrccm.159.6.9809043. [DOI] [PubMed] [Google Scholar]
- 49.Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res. 2004;30:1–15. doi: 10.1080/01902140490252858. [DOI] [PubMed] [Google Scholar]
- 50.Papaioannou AI, Rossios C, Kostikas K, Ito K. Can we delay the accelerated lung aging in COPD? Anti-aging molecules and interventions. Curr Drug Targets. 2013;14:149–157. doi: 10.2174/1389450111314020003. [DOI] [PubMed] [Google Scholar]
- 51.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung: comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992;101:793–799. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]
- 52.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6–mediated TGFbeta activation causes MMP12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 53.Ruwanpura SM, McLeod L, Miller A, Jones J, Bozinovski S, Vlahos R, Ernst M, Armes J, Bardin PG, Anderson GP, et al. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am J Respir Cell Mol Biol. 2011;45:720–730. doi: 10.1165/rcmb.2010-0462OC. [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Choo-Wing R, Sureshbabu A, Fan J, Leng L, Yu S, Jiang D, Noble P, Homer RJ, Bucala R, et al. A critical regulatory role for macrophage migration inhibitory factor in hyperoxia-induced injury in the developing murine lung PLoS One 20138e60560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakuragi T, Lin X, Metz CN, Ojamaa K, Kohn N, Al-Abed Y, Miller EJ. Lung-derived macrophage migration inhibitory factor in sepsis induces cardio-circulatory depression. Surg Infect (Larchmt) 2007;8:29–40. doi: 10.1089/sur.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]