Abstract
Gestational testosterone (T) treatment causes maternal hyperinsulinemia, intra-uterine growth retardation (IUGR), low birth weight, and adult reproductive and metabolic dysfunctions. Sheep models of IUGR demonstrate placental insufficiency as an underlying cause of IUGR. Placental compromise is likely the cause of fetal growth retardation in gestational T-treated sheep. This study tested if T excess compromises placental differentiation by its androgenic action and/or via altered insulin sensitivity. A comparative approach of studying gestational T (aromatizable androgen) against dihydrotestosterone (DHT; non-aromatizable androgen) or T plus androgen antagonist, flutamide, was used to determine whether the effects of T in placental differentiation were programmed by its androgenic actions. Co-treatment of testosterone with the insulin sensitizer, rosiglitazone, was used to establish whether the effects of gestational T on placentome differentiation involved compromised insulin sensitivity. Parallel cohorts of pregnant females were maintained for lambing and the birth weight of their offspring was recorded. Placental studies were conducted on days 65, 90, or 140 of gestation. Results indicated that 1) gestational T treatment advances placental differentiation, evident as early as day 65 of gestation, and culminates in low birth weight, 2) placental advancement is facilitated at least in part by androgenic actions of T and is not a function of disrupted insulin homeostasis, and 3) placental advancement, while helping to increase placental efficiency, was insufficient to prevent IUGR and low birth weight female offspring. Findings from this study may be of relevance to women with PCOS, whose reproductive and metabolic phenotype is captured by the gestational T-treated offspring.
Keywords: IUGR, pregnancy, placenta, testosterone, sheep
INTRODUCTION
The endocrine, nutritional, and metabolic environment of the fetus programs its anatomy and physiology, and these changes likely persist into postnatal life leading to adult pathologies (Barker 2004, Gluckman et al. 2008, Nijland et al. 2008, Gabory et al. 2011, Padmanabhan & Veiga-Lopez 2011). Exposure of the fetus to excess steroids in utero has been found to alter fetal developmental trajectory and induce adult reproductive and metabolic pathologies (Abbott et al. 2006, Padmanabhan & Veiga-Lopez 2011). Specifically, gestational testosterone (T) treatment was found to induce intrauterine growth retardation (IUGR) and low birth weight female offspring (Manikkam et al. 2004, Steckler et al. 2005, Godfrey et al. 2011), culminating eventually in adult dysfunctions manifested at both reproductive and metabolic levels in the female (Abbott et al. 2006, Padmanabhan & Veiga-Lopez 2011). Of translational relevance, IUGR and low birth weight have been identified as risk factors for many adulthood reproductive, metabolic, and endocrine disorders (Barker 2006, Phillips et al. 2006, Simmons 2009). IUGR is also associated with a 6–10 times increase in the risk of perinatal mortality in the U.S. (Ananth & Wilcox 2001, Gould et al. 2003).
Several sheep models of IUGR demonstrate placental insufficiency as an underlying cause of fetal growth retardation (Regnault et al. 2002, Louey et al. 2003, Morrison 2008). For instance, IUGR induced by mid-gestation hypothermia in sheep is associated with reduced placental mass, uterine and umbilical blood flow, transplacental amino acid flux, glucose, and oxygen transport capacity (Rees et al. 1998). In many of these IUGR models, the placenta undergoes advanced differentiation to increase efficiency in an effort to overcome fetal growth retardation (Penninga & Longo 1998, Gardner et al. 2002, Vonnahme et al. 2006). Failure to adequately compensate appears to underlie IUGR and low birth weight outcomes. Conceivably, similar placental insufficiency underlies the IUGR evidenced in gestational T-treated females.
Because T can be aromatized to estrogen, any impaired placental function in the gestational T-treated model may be mediated via androgenic or estrogenic actions of T. Alternatively, because gestational T treatment appears to disrupt maternal insulin homeostasis (Abi Salloum et al. 2012), effects of T may also involve metabolic perturbations. In support of this, histological and/or morphological changes in human placenta are evident in women with type 1 and gestational diabetes (Higgins et al. 2011, Rossi et al. 2012). This study was undertaken to test the following hypotheses: 1) gestational T excess compromises placental differentiation, 2) placental compromise is facilitated by androgenic actions of T, 3) effects of T on placenta involve altered insulin sensitivity, and 4) placental compromise in gestational T-treated females involves both androgenic and metabolic pathways.
MATERIALS AND METHODS
Animals and gestational treatments
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with National Research Council’s Guide for the Care and Use of Laboratory Animals. The study was conducted at the University of Michigan Research Facility (Ann Arbor, MI; 42°18′N) using multiparous Suffolk breed of sheep. Beginning ~3 weeks before the time of breeding, ewes were group fed daily with 0.5 kg of shelled corn and 1.0–1.5 kg of alfalfa hay per ewe to increase energy balance. After breeding, all ewes were housed in the same pasture and group-fed daily with 1.25 kg of alfalfa/brome mix hay/ewe, ensuring they meet the nutrient requirements for sheep as defined by the Committee on the Nutrient Requirements of Small Ruminants (NRC 2007). Pure bred registered Suffolk sheep rams obtained from the provider were raddled on their chests and housed with the females. Mating was detected by the presence of paint on the backs of the females as a result of mounting. Once mated, the females were randomly assigned to the different treatment groups, blocking for body condition and weight.
The treatment groups used in this investigation include the following: a control (C), a gestational T-treated (T), a gestational DHT-treated (DHT), a gestational T plus androgen antagonist-treated (TF), a gestational T plus insulin sensitizer-treated (TR) and a gestational T plus androgen antagonist and insulin sensitizer (TFR) group. Details of gestational T, DHT, and androgen antagonist (flutamide) treatments, husbandry, and nutrition of maternal sheep and their impact on reproductive function have been previously published (Manikkam et al. 2004, Steckler et al. 2007, Jackson et al. 2008). In brief, for the generation of gestational T- and DHT-treated groups, pregnant sheep were administered intramuscularly twice a week from days 30–90 of gestation with either 100 mg of T propionate (~1.2 mg/kg; Sigma Chemical Co., St. Louis, MO) or 100 mg of DHT propionate (Steraloids, Inc., Newport, RI) suspended in 2 ml of cottonseed oil (Manikkam et al. 2004, Steckler et al. 2007). The generation of the TF group involved a co-treatment of T with an androgen antagonist, flutamide (Sigma-Aldrich., St. Louis, MO; 15 mg/kg/day, oral) (Jackson et al. 2008). The generation of the TR group involved a co-treatment of T with an insulin sensitizer, rosiglitazone (Avandia, GlaxoSmithKline, Durham, NC); 8mg/kg/day, oral). The generation of the TFR group involved a co-treatment of T with both androgen antagonist and insulin sensitizer.
The T and DHT doses were chosen to simulate the doses used in earlier neuroendocrine and ovarian investigations of offspring from treated mothers (Manikkam et al. 2004, Steckler et al. 2005, Steckler et al. 2007, Jackson et al. 2008, Padmanabhan & Veiga-Lopez 2011). The concentrations of T achieved in maternal circulation with this mode of T delivery are in the range of adult males (Veiga-Lopez et al. 2011). The concentrations of T achieved in female fetuses with this mode of T delivery are also within range seen in the control fetal males (Veiga-Lopez et al. 2011). The chosen flutamide dose has been found to effectively negate the masculinizing effects of both endogenously produced and exogenously administered T (Jackson et al. 2008). The rosiglitazone dose chosen has been shown to be effective in restoring insulin sensitivity in adult prenatal T-treated females (Veiga-Lopez et al. 2010) and normalize the increase in insulin/glucose ratio seen in gestational T-treated females (Abi Salloum et al. 2012).
Experimental Design
Study 1: Impact of excess T on placental differentiation
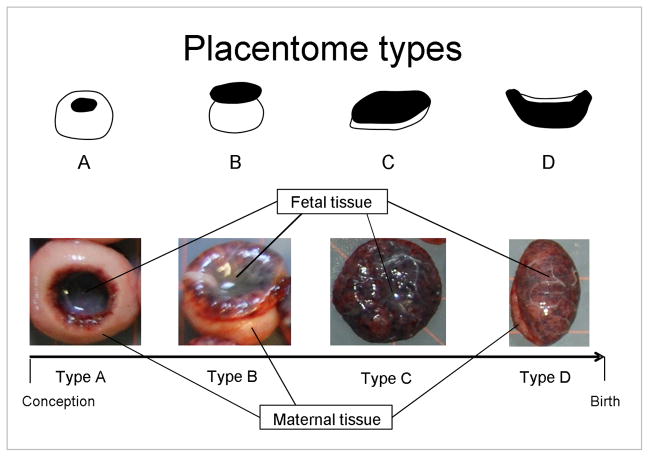
To determine the effects of gestational excess of T on placental differentiation, pregnant control (n = 20) and T-treated (n = 22) sheep were anesthetized as previously described (Veiga-Lopez et al. 2011) on day 65 (mean ± SEM: 64.9 ± 0.1) (C, n = 10; T, n = 10) of gestation (term: ~ 147 days) and uterus and fetuses removed. Fetal weight was recorded. After uterus removal, the cotyledonary-caruncular units (placentomes) of the placenta were dissected out from the uterine wall, and the placentome morphology was assessed as previously described (Vatnick et al. 1991). Placentomes were classified into four types (A, B, C, and D) based on their stage of development, which is determined by the relative predominance of the caruncular (maternal) versus the cotyledonary (fetal) compartment (see Figure 1 for details). During the first placentome stage (type A), the caruncular tissue is predominant and surrounds the cotyledonary tissue. As gestation progresses, the fetal tissue protrudes over the caruncular tissue (type B) until both compartments are flattened and occupy similar surface area (type C). In the final stage of differentiation (type D), the cotyledonary tissue occupies the majority of the surface area in view of an increased nutrient and oxygen demand at later gestational stages (Vatnick et al. 1991). For each uterus, placentome count and weight were recorded, and placental efficiency was calculated as the ratio between total fetal weight and the total placental weight.
Figure 1.
Schematics (top) and representative images (bottom) of the four placentome types. See text in Material and Methods for a more detailed description.
Study 2: Androgenic programming of placentome differentiation
A comparative approach of studying gestational T (aromatizable androgen) and DHT (non-aromatizable androgen) treatments was used to determine whether the effects of T on placentome differentiation were programmed by its androgenic actions. Pregnant control, T-treated, and DHT-treated females were studied on either day 90 (mean ± SEM: 89.9 ± 0.1; n = 7, 8, and 15, respectively) or day 140 (mean ± SEM: 139.9 ± 0.1; n = 6, 13, and 15, respectively) of gestation, at which point fetuses and uteri were harvested. Assessment of placental gross morphology was undertaken as described above.
Study 3: Androgenic vs. metabolic programming of placental differentiation
Since DHT (the androgen used in study 2) can be converted to 5α-androstane, 3β, 17β-diol (3β-diol) and therefore bind to estrogen receptor 2 (ESR2) (Handa et al. 2008), a co-treatment of T with the androgen antagonist was used to confirm androgenic mediation. To establish whether the effects of gestational T treatment on placentome differentiation may involve metabolic compromise, the insulin sensitizer, rosiglitazone, was used as a co-treatment with T. Two studies were performed. The first included control (n = 8), T (n = 11) and TF (T + androgen antagonist; n = 10) pregnant sheep. The second study included control (n = 12), T-treated (n = 12), TF (T + androgen antagonist; n = 10) and TR (T + insulin sensitizer; n = 10) pregnant sheep. The first study was undertaken on days 91.5 ± 0.1 (mean ± SEM) of gestation and the second 90.5 ± 0.1 of gestation. Assessment of placental changes was undertaken as described above.
To test whether both androgenic and metabolic pathways are involved in placental compromise of gestational T-treated sheep, control, T-treated, and TFR (T + androgen antagonist + insulin sensitizer)-treated pregnant females were studied on day 60 (mean ± SEM: 59.5 ± 0.1) (n = 9, 10, and 9, respectively) of gestation. Removal of fetuses and uteri and assessment of placental gross morphology were carried out as described above.
Weights of breeder animals, maternal weight at time of placentome collection, and birth weight of offspring from parallel cohorts
Weights of breeder animals at the time of mating (except study 1) and maternal weight at the time of placental collection (except study 2, day 90) were recorded. For the study of androgenic programming of placentome differentiation at day 90 and day 140 of gestation and combined androgenic and metabolic programming at day 60, when more animals were available, a parallel set of pregnant females was maintained for lambing. The birth weight of their offspring was recorded. Number of offspring born, treatment groups, and offspring gender distribution for each of these studies are as follows: androgenic programming at day 90 (females: [C: 3; T: 10; DHT: 6], males: [C: 3; T: 7; DHT: 8]), day 140 (females: [C: 18; T: 23; DHT: 6], males: [C: 26; T: 20; DHT: 9]), and combined androgenic and metabolic programming at day 60 (females: [C: 7; T: 3; TFR: 11], males: [C: 8; T: 5; TFR: 6]).
Statistical Analysis
Placental efficiency was calculated as a ratio of total fetal to placentome weight and excluded weights of both amniotic and chorioallantoic sacs. The differences in weight and number of each type of placentome, placental efficiency, and fetal weight between treatment groups were compared after appropriate transformations to account for heterogeneity of variances. To study the effect of excess T on placentome differentiation, an independent-samples T test was used to evaluate differences in number and weight of placentomes, placental efficiency, and fetal weight at gestational day 65.
To test the effects of androgens on placentome differentiation, the following analyses were carried out. ANOVA followed by Dunnett posthoc test was used to compare differences in number and weight of placentomes, placental efficiency, and fetal weight between control and treatment groups at day 90 and day 140. For gestational day 140, the same analysis was also performed separating females and male fetuses. The number of fetuses per dam was used as a covariate in the statistical model.
To test androgenic vs. metabolic programming of placental differentiation, the following analyses were carried out. Since two cohorts were used (see methods section for details), and no statistical differences within treatment group were found when comparing control, T, and TF groups between the two studies, both cohorts were merged for final analyses. A general linear model followed by Dunnett posthoc test was used to compare differences in weight of placentomes, placental efficiency, and fetal weight. For the placentome count, a general linear model using negative binomial distribution was used. The number of fetuses per dam and the cohort of origin were used as a covariate in the statistical model. A similar analysis was also conducted with the study testing the combined effects of androgen antagonist and insulin sensitizer.
Differences in birth weight by gender among treatment groups were analyzed using a generalized linear model with corrections for number of offspring, males, and females within the same mother. The same statistical model was used to test differences in fetal weight by gender for the study of androgenic vs. metabolic programming of placental differentiation at day 90. Weights of breeder animals at time of mating and maternal weight at the time of placental collection were analyzed using ANOVA followed by Dunnett posthoc test. Significance was defined as P < 0.05. Results are expressed as a mean ± SEM. All analyses were carried out using SPSS for Windows release 19.0.0.
RESULTS
Effects of excess gestational T on placental differentiation
On day 65 of gestation (midway of the gestational treatment regimen), total placentome number was similar between control and T-treated dams (Figure 2). Majority of placentomes were of type A with very few type B, C, and D placentomes. The number (P < 0.05) of type A placentomes was significantly lower in T treated dams compared to controls. The total weight of all placentomes was significantly lower in T-treated dams compared to the controls (P < 0.05). Total weight of type A placentomes was also lower in T-treated dams (P < 0.01). There were no differences in the number of fetuses per dam or fetal weight per dam. Placental efficiency was, however, significantly higher in dams treated with T (P < 0.01) compared to controls.
Figure 2.
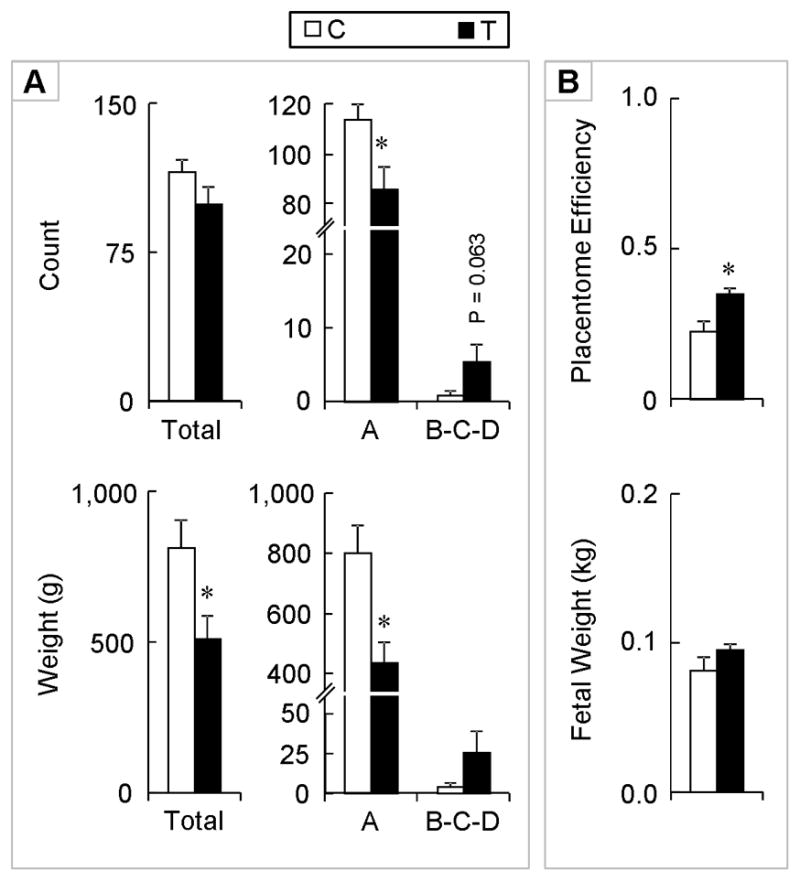
Panel A: Total number of placentomes, total number of type A, and types B + C + D placentomes on day 65 of gestation from control (open bars) and gestational T-treated dams (closed bars) are shown on the top. Total weight (g) and weights (g) of type A and types B+C+ D (bottom right) for the same animals are shown in the bottom. Panel B: Placental efficiency (top) and total fetal weight (bottom). Data presented are in mean ± SEM. Asterisks represent statistical differences between treatment groups (P < 0.05).
Androgenic programming of placentome differentiation
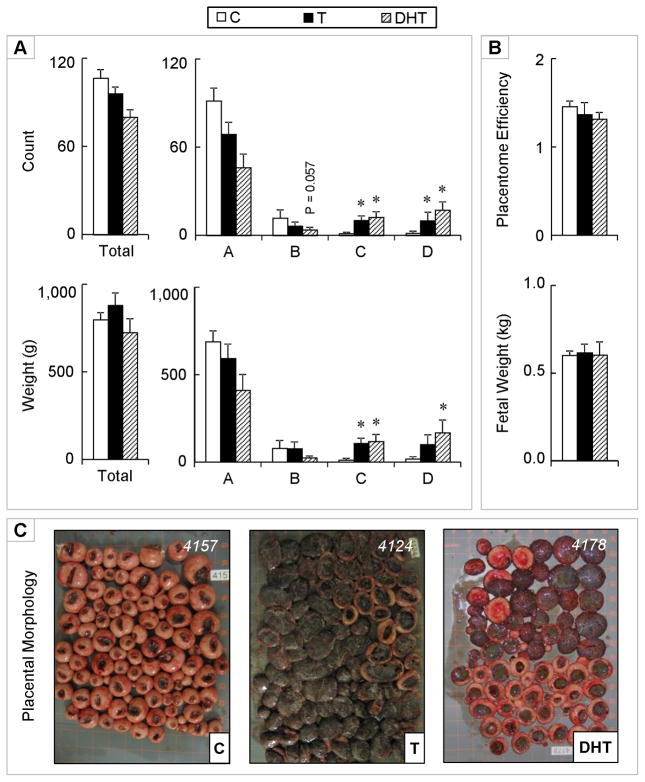
There were no differences in the total number or weight of placentomes between control, T, and DHT-treated dams (Figure 3). Similar to day 65 of gestation, type A placentomes were still predominant at day 90 of gestation (the end of the T treatment period), but with an increased proportion of types B, C, and D placentomes. No significant differences in the number and weight of type A placentomes were evident between T-treated and control dams at this time point. Conversely, the number (P < 0.01) and weight (P < 0.01) of type C placentomes were significantly higher in the dams that were treated with T relative to controls. A similar increase in number (P < 0.01), but not weight of type D placentomes, was also evident in T-treated dams. Similar effects, namely an increase in number and weight of type C and D placentomes were evident in DHT-treated dams. In addition, a tendency for a decrease (P = 0.057) in number of type B placentomes was evident in DHT-treated, but not T-treated dams. There were no differences in the number of fetuses born, total fetal weight, or placental efficiency at this time point.
Figure 3.
Panel A: Total (top left) and number of types A, B, C, and D (top right) placentomes on gestational day 90 from control (open bars), gestational T-treated (closed bars), and gestational DHT-treated dams (dashed bars). Total weight (g) (left) and weights of types A, B, C, and D (right) placentomes for the same set of animals are shown in the bottom. Panel B: Placental efficiency (top) and fetal weight (bottom). Panel C: Representative images of placentomes from each treatment. Data presented are in mean ± SEM. Asterisks represent statistical differences between treatment groups (P < 0.05). Body weights of breeder animals (mean ± SEM; kg) at the time of mating (C: 83.4 ± 2.9, T: 80.0 ± 4.2, and DHT: 80.3 ± 3.1) did not differ significantly amongst groups.
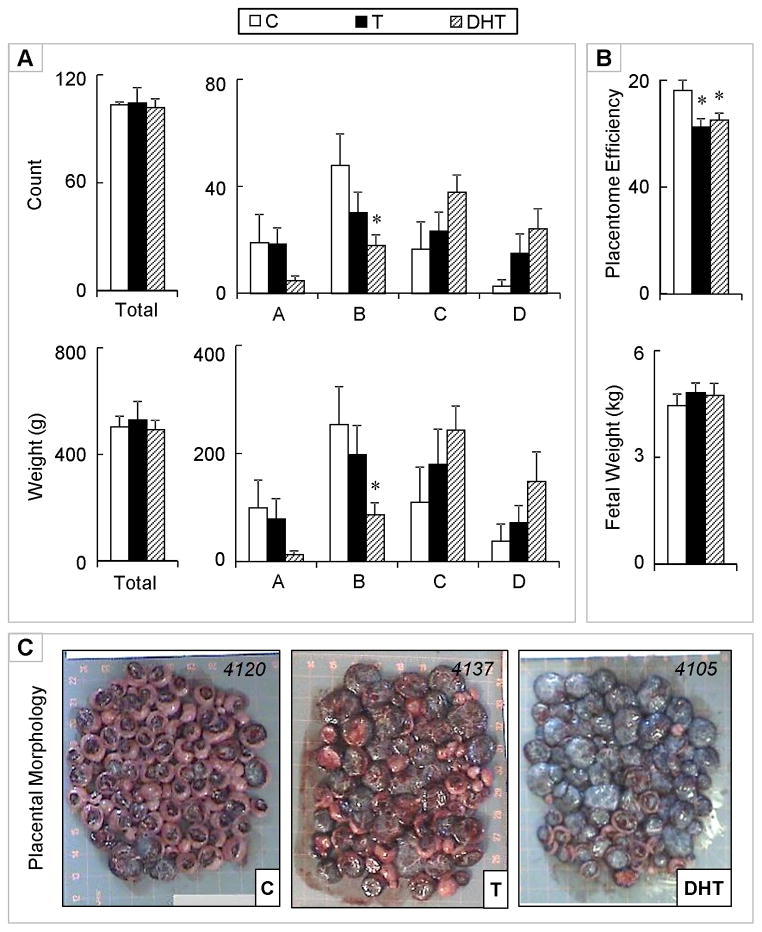
A week prior to lambing (gestational day 140) and approximately 50 days after the conclusion of gestational T treatment, a shift towards more advanced placentome types (B, C, and D) was evident in the control group compared to earlier gestational time points. There were no differences between control and T-treated females relative to total number and total weight, nor in the distribution of placental types (Figure 4). The only difference noted was in the DHT-treated group. This was reflected as a decrease in the number (P < 0.05) and weight (P < 0.05) of type B placentomes compared to the control group. There were no differences in total fetal number per dam, nor in the total fetal weight per dam. When female fetuses were analyzed separately, fetal weight was lower in T females compared to controls, but did not reach statistical significance with the small sample size. Placental efficiency, however, was significantly lower in T-treated (P < 0.05), as well as in DHT-treated (P < 0.05) dams, compared to controls.
Figure 4.
Panel A: Total (top left) and number of types A, B, C, and D (top right) placentomes on gestational day 140 from control (open bars), gestational T-treated (closed bars), and gestational DHT-treated dams (dashed bars). Total weight (g) (left) and weights of types A, B, C, and D (right) placentomes for the same set of animals are shown in the bottom. Panel B: Placental efficiency (top) and fetal weight (bottom). Panel C: Representative images of placentomes from each treatment. Data presented are in mean ± SEM. Asterisks represent statistical differences between treatment groups (P < 0.05). Body weights of breeder animals (mean ± SEM; kg) at the time of mating (C: 78.0 ± 4.3, T: 80.2 ± 2.9, and DHT: 81.9 ± 2.8; mean ± SEM) did not differ amongst groups. Maternal body weights (kg) at the time of placental collection (C: 82.1 ± 3.3, T: 83.1 ± 2.9, and DHT: 81.4 ± 2.2) also did not differ amongst treatment groups.
Androgenic and metabolic mediation of placentome differentiation
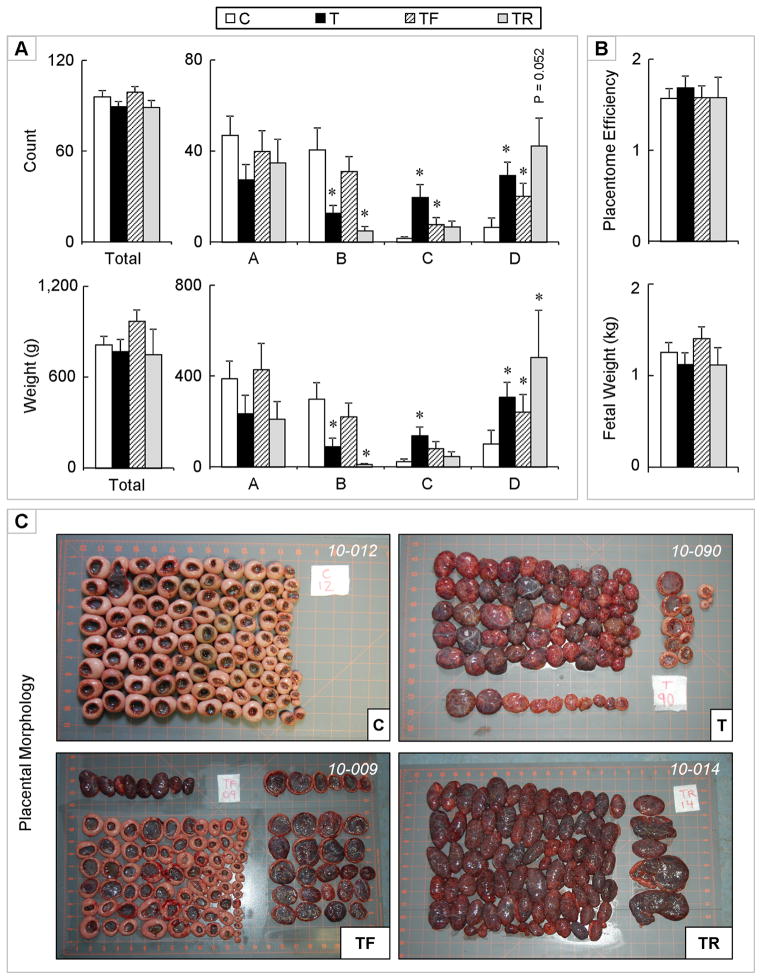
The impact of androgenic vs. metabolic mediation was studied at gestational day 90 (end of the T treatment). No differences were evident in total number and weight of placentomes across treatment groups (Figure 5). T treatment decreased the number (P < 0.05) and weight (P < 0.05) of type B placentomes and increased the number (P < 0.01) and weight (P < 0.01) of type C and D placentomes, relative to the control group.
Figure 5.
Panel A: Total (top left) and number of types A, B, C, and D (top right) placentomes on gestational day 90 from control (open bars), gestational T-treated (closed bars), gestational T + androgen antagonist treated (dashed bars), and gestational T + insulin sensitizer treated (grey bars) sheep. Total weight (g) (left) and weights of types A, B, C, and D (right) placentomes for the same set of animals are shown in the bottom. Panel B: Mean placental efficiency (top) and fetal weight (bottom). Panel C: Representative images of placentomes from each treatment. Data presented are in mean ± SEM. Asterisks represent statistical differences between treatment groups (P < 0.05). Body weights of breeder animals (mean ± SEM; kg) at the time of mating (C: 79.0 ± 2.8, T: 77.0±3.1, TF: 81.0 ± 3.1, and TR: 76.2 ± 5.0; mean ± SEM) did not differ amongst groups. Maternal body weights (kg) at the time of placental collection (C: 79.5 ± 2.5, T: 79.8 ± 3.0, TF: 83.9 ± 3.1, and TR: 79.9 ± 5.8) also did not differ amongst treatment groups.
Androgen antagonist treatment reversed the effects T on the number and weight of type B placentomes, while insulin sensitizer co-treatment had no effect. The increase in the number and weight seen in type C placentomes was partially reversed by both androgen antagonist and insulin sensitizer co-treatments. Number and weight of C placentomes were both similar to the control group in TR-treated females while the number (P < 0.05), but not weight of type C placentomes was significantly higher in TF-treated compared to control females.
In type D placentomes, the total number (P < 0.01) and weight (P < 0.01) was higher in T-treated animals compared to controls. Similarly, the count and weight of type D placentomes was also significantly higher in both TF-treated (P < 0.05 for both variables) and TR-treated (P = 0.052 and P < 0.01, respectively) dams. There were no differences in total number and total weight of fetuses or placental efficiency among the 4 treatment groups.
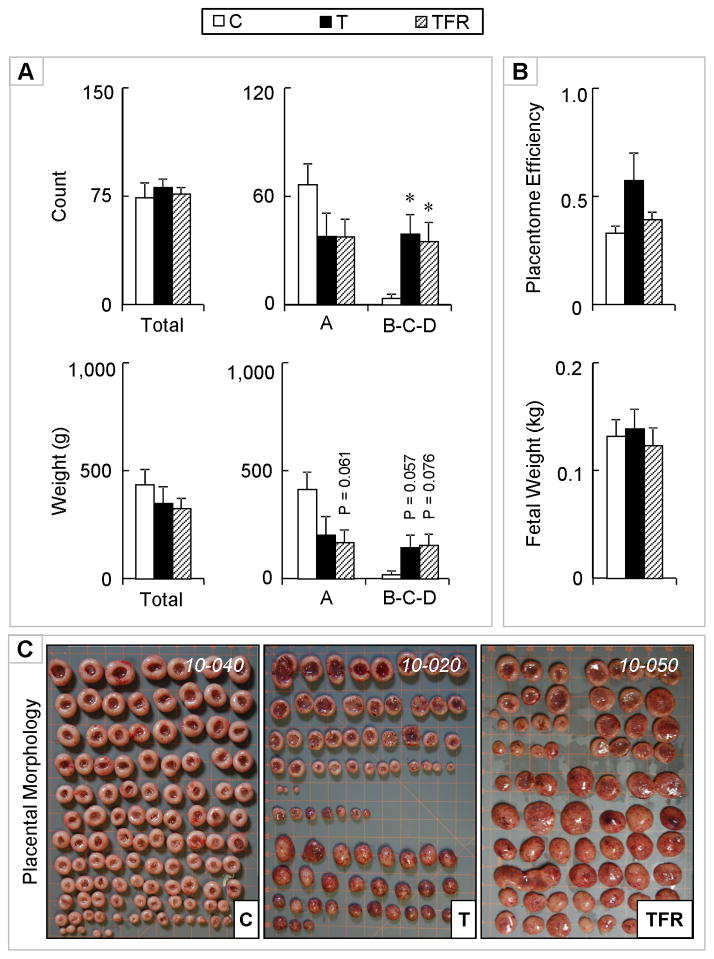
Combined treatment with androgen antagonist and insulin sensitizer treatment could not rescue the control phenotype in terms of advanced placental differentiation in T-treated females (Figure 6) on day 65 of gestation. T, as well as TFR-treated females showed similar advancement in placental number and weight (tendency) with higher number of B + C + D placentomes present in both groups relative to controls.
Figure 6.
Panel A: Total number of placentomes, total number of type A, and types B + C + D placentomes on day 65 of gestation from control (open bars), gestational T-treated (closed bars) and gestational T + androgen antagonist + insulin sensitizer treated dams (dashed bars) are shown on the top. Total weight (g) and weights (g) of type A and types B + C + D (bottom right) for the same animals are shown in the bottom. Panel B: Placental efficiency (top) and total fetal weight (bottom). Panel C: Representative images of placentomes from each treatment. Data presented are in mean ± SEM. Asterisks represent statistical differences between treatment groups (P < 0.05). Body weights of breeder animals (mean ± SEM; kg) at the time of mating were (C: 81.7 ± 3.9, T: 81.4 ± 2.7, and TFR: 82.2 ± 2.2; mean ± SEM) did not differ amongst groups. Maternal body weights (kg) at the time of placental collection (C: 79.9 ± 3.4, T: 79.1 ± 2.5, and TFR: 80.7 ± 2.5) also did not differ amongst treatment groups.
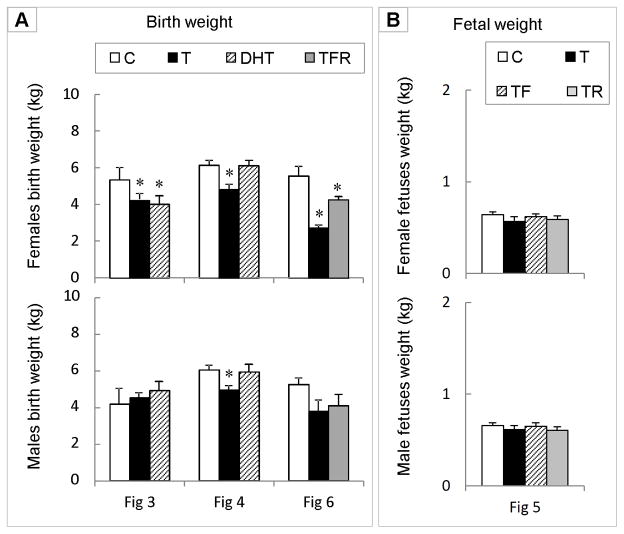
Impact of T/DHT treatment and intervention on birth weight
Gestational T treatment produced low birth weight female offspring in 3 of the cohorts where subsets of animals were maintained in parallel until delivery (corresponding data from group of animals assigned for placental and fetal measures are shown in Figures 3, 4, and 6). DHT treatment reduced birth weight in one set (Panel A, top left), but not in the other (Panel A, top middle) (Figure 7). Co-treatment with androgen antagonist plus insulin sensitizer did not completely normalize birth weight (Panel A, top right panel, grey bar). T treatment reduced birth weight of male offspring in one cohort (Panel A, lower middle panel), but not the other two (Panel A, lower left and right panels). Fetal weight of T-, TF-, and TR-treated female and male fetuses was similar to controls at day 90 of gestation (Panel B).
Figure 7.
Panel A: Birth weight of female and male offspring from 3 of the cohorts used in the placental studies. Placental and fetal data from the subset of animals from these same breeding cohorts raised in parallel are shown in Figures 3, 4, and 6. Panel B: Fetal weights of day 60 fetuses segregated by sex (corresponding Figure 5).
DISCUSSION
Findings from this study provide evidence that gestational T treatment advances placental differentiation and that this advancement is facilitated at least in part by androgenic actions of T and is not a function of disrupted insulin homeostasis. Advanced placental differentiation was sufficient to maintain placental efficiency during early stages of gestation, but not at later stages, culminating in IUGR and low birth weight mainly in the female offspring. The evidence in support of these conclusions and the implication of these findings are discussed below.
Testosterone, the gestational treatment used in this study, can induce a multitude of effects by directly signaling through the androgen receptor or via conversion to estrogen in tissues with aromatase activity, such as the placenta (France et al. 1987), thereby inducing estrogen-mediated actions. The conclusion that advanced placental differentiation is mediated in part by androgenic and not estrogenic action is supported by the finding that gestational treatment with the non-aromatizable androgen dihydrotestosterone, DHT, also advanced placental differentiation. That the effects of DHT are mediated by androgenic action and not by conversion of DHT to 3-beta-diol, which is capable of binding and signaling through estrogen beta receptor (Handa et al. 2008), is corroborated by the fact that co-treatment with flutamide, an androgen antagonist, partially prevented the advancement in placental differentiation. Incomplete reversal may be a function of inadequate blockade with androgen antagonist or suggestive of multiple mechanisms working in concert to advance placental differentiation. Our earlier finding that the dose of androgen antagonist used was sufficient to prevent phenotypic masculinization in male offspring of gestational T-treated animals (Jackson et al. 2008) suggests that dosage of flutamide used is appropriate. Androgen receptors have been shown to be present in the human and bovine placenta (Horie et al. 1992, Uzelac et al. 2010, Khatri et al. 2013). Furthermore, it is known that androgen receptor regulates gene networks involved in cell growth and differentiation (Martyniuk & Denslow 2012), including bone and muscle growth (Otto-Duessel et al. 2012). We have previously shown that gestational T excess reprograms the developmental trajectory of the IGF/IGFBP system in female fetuses to reduce IGF bioavailability during IUGR (Crespi et al. 2006). Considering the IGF system also plays a critical role in placental development (Forbes & Westwood 2008, Roberts et al. 2008, Bowman et al. 2010), accelerated placental differentiation may be function of altered IGF system, as appears to be the case in the fetus.
Because studies with primates have found that gestational T treatment impairs maternal glucose tolerance (Abbott et al. 2010) and our preliminary findings in sheep found that gestational T excess induces maternal hyperinsulinemia (Abi Salloum et al. 2012), the possibility exists that the effects of excess T on placental advancement may be mediated also in part via perturbation of insulin-glucose homeostasis. That a complex relationship exists between androgenic stimulation and insulin signaling has been widely recognized and is evidenced in the placental tissue in both observational and interventional studies. For example, the placentae of gestational diabetes pregnancies have increased androgenic activity with reduced aromatase protein levels and increased abundance of androgen receptors (Uzelac et al. 2010). A direct effect of insulin on suppressing aromatase activity in the human trophoblast cells has been demonstrated (Nestler 1993). In addition, insulin sensitization indirectly affects the placenta via circulating adipokines, such as adiponectin (McDonald & Wolfe 2009), which regulates extensive gene expression networks including steroidogenic enzymes in the human placenta. However, findings from this study that the insulin sensitizer, rosiglitazone, did not alter the effects of T on placental development, indicates that perturbations in the insulin signaling pathway are not a major mediator behind the actions of T described here.
The advanced differentiation of placentomes is likely a compensatory approach to provide adequate nutrition for the fetus. Visual observation indicated that the fetal compartment of the placentome appear more vascular in T-treated females than controls. The finding that placental efficiency was increased at day 65 of gestation (Figure 2), comparable to that of controls at day 90 of gestation (Figure 3), and reduced at day 140 of gestation (Figure 4) is consistent with placental advancement being able to compensate during early pregnancy, but unable to do so as pregnancy progressed. Absence of change in placental efficiency in T-treated sheep compared to controls at day 90 of gestation (Figure 3), in the face of advanced placental differentiation, suggests that this advancement is a means by which placental efficiency is maintained at this age. In contrast, later in pregnancy, when fetal growth is at its maximum, this compensatory mechanism appears to be insufficient to meet the high demands of the growing fetus. This is supported by findings of reduced placental efficiency at day 140 of gestation (Figure 4). While no differences were evident in total fetal weight of control and gestational T-treated fetuses at day 140 (Figure 4), female offspring of gestational T-treated animals from the same cohort of animals maintained in parallel until delivery were of low birth weight. This was also the case with the other 2 cohorts maintained until birth (Figure 7). In sheep, the last week of fetal growth can account for as much as 10% or 17% of the total birth weight in single and twin pregnancies, respectively (Koong et al. 1975). Therefore, it is likely that the additional week of maturation time, when fetal growth is at its highest, accounts for the difference between the weights recorded at day 140 (similar weights between controls and T-treated) vs. at birth (lower weights in T-treated females). In all these studies, consistent with our earlier findings (Manikkam et al. 2004), the birth weight of female offspring born to T treated mothers were lower compared to controls (Figure 7).
Birth weight of males was not affected by gestational testosterone excess from days 30–90 of gestation, but for one cohort (Figure 7). It is unclear why IUGR was only restricted to this cohort in males. Low birth weight of male offspring has been reported when testosterone treatment was extended through day 120 of gestation (Recabarren et al. 2008). One possibility for the differential effects on male birth weight among cohorts is that the impact of steroid excess at the placental level was stronger in this cohort. In support of this, among all cohorts studied, placental efficiency was most significantly compromised in the pregnancy cohort where male birth weight was also affected (day 140; Figure 4). It is possible that the stronger effect on this cohort may have also been influenced by maternal body condition, which can affect placental function and fetal growth in sheep (Osgerby et al. 2003). Conceivably, male fetuses are more resilient to the homeostatic imbalance and placental disruptions induced by T. Previous evidence supports gender-specific resilience to placental changes (Misra et al. 2009).
While the focus of this discussion is IUGR/birth weight as it relates to placental efficiency, it needs to be recognized that developmental disruptions in organ systems may persist and manifest as adult disorders, even when advanced placentome development may be able to overcome IUGR and low birth weight. We have previously shown that female lambs born after exposure to excess T at the doses used here during the critical gestational period for reproductive organ differentiation develop reproductive and metabolic dysfunctions in adulthood, exemplifying the developmental programming of adult diseases (Padmanabhan et al. 2010, Padmanabhan & Veiga-Lopez 2013). From the evolutionary perspective, it may be more crucial to maintain normal gestational weight to ensure perinatal survival, than to protect the endocrine organs during fetal development, so compensatory mechanisms may not have evolved for the latter.
Compromised placental differentiation, intra-uterine growth restriction, and low birth weight are likely a function of inadequate nutrient support. During normal pregnancy, D-type placentome increases during late gestation to support the increasing demand of the growing fetus (Alexander 1964b). In response to maternal undernutrition (Symonds et al. 1998, Heasman et al. 1999) or hypoxia (Penninga & Longo 1998), an increase in D-type placentomes occurs early during pregnancy to increase nutrient transfer in order to compensate for the reduced nutritional intake. On the other hand, overnutrition of adolescent sheep (Wallace et al. 2000) leads to a predominance of A-type placentomes in late gestation, which is accompanied by a reduction in uterine and umbilical blood flow (Wallace et al. 2000). The finding that no difference in placentome numbers was evident at any gestational age, combined with a decline in placental weight at fetal day 65 (Figure 2) of gestational T-treated sheep, is consistent with previous findings, in that reduced placental weight appears to be the result of differences in mean weight of placentomes rather than a reduction in placentome numbers (Alexander 1964a, Bell et al. 1987, Wallace et al. 2000).
The compromised placental differentiation (likely function) and low birth weight (Figure 7) of gestational T-treated sheep may be a function of reduced progestogenic support to the developing fetus. Our preliminary findings in sheep found that gestational T treatment compromises progesterone production at day 90 of gestation (Abi Salloum et al. 2012). Progesterone is essential to support immunological adaptation to pregnancy (Szekeres-Bartho et al. 2001, Di Renzo et al. 2005) and create a protective immune environment. Studies in sheep have found that low progesterone levels during pregnancy are associated with low birth weight (Wallace et al. 1997). The finding of reduced progestogenic support and low birth weight female, but not male, offspring in gestational T-treated pregnancies is similar to findings in a human cohort study (Hartwig et al. 2013). Another study in sheep found that maternal periconceptional undernutrition increased maternal progesterone with no impact on birth weight (Debus et al. 2012). Evidence points to paternal/maternal genes influencing placental imprinting and, thus, having an impact on placental transfer of nutrients (Reik et al. 2003). In considering reduced progestogenic support as causal or consequence of placental insufficiency, it is important to recognize that gestational T treatment could alter progesterone metabolism at the level of the feto-placental unit (Carrizo et al. 1994, Grzesiak et al. 2014). Previous studies point to influence of stress on reduced progesterone levels (Parker & Douglas 2010). Gestational T excess may be viewed as a stressor in this regard.
Although not closely related phylogenetically, the sheep and the human placenta are of a structurally similar type. This is particularly reflected by the architecture of the fetal villous tree, with stem, intermediate, and terminal vessels. The terminal vessels or capillary complex, which is the functional unit of exchange, differ little between the sheep and human. In both, chorioallantoic villous tree build up to fetal placental cotyledons. In the sheep, one cotyledon penetrates the maternal septal system of the endometrial caruncle, both together forming one of numerous placentomes. In the human, cotyledons protrude into the extended maternal intervillous blood space of the single polycotyledonary placental disc (Kaufmann et al. 1985, Mossman 1987, Leiser 1991, Leiser & Kaufmann 1994, Leiser et al. 1997, Parker & Douglas 2010). As such, findings from this study may have implications relative to placental development in pregnant women with elevated androgen levels, such as polycystic ovary syndrome (PCOS) (Sir-Petermann et al. 2002, Sir-Petermann et al. 2012) or congenital adrenal hyperplasia (CAH) (Lo & Grumbach 2001). It is of interest in this regard that female offspring of gestational T-treated sheep manifest reproductive and metabolic features of women with PCOS (Padmanabhan et al. 2010, Padmanabhan & Veiga-Lopez 2011, Padmanabhan & Veiga-Lopez 2013). Pregnancy complications are common with PCOS pregnancies (Qin et al. 2013). In general, pregnancy complications arise from defective placentation or trophoblast invasion (Jindal et al. 2007, Longtine & Nelson 2011). Recent studies have found placental alterations (Palomba et al. 2013) and altered steroid production (Maliqueo et al. 2013) in PCOS pregnancies. Interestingly, gestational diabetic mothers also have high levels of androgens (Sir-Petermann et al. 2002, Sir-Petermann et al. 2012). Placental morphology is also altered in diabetic pregnancies, with increased androgen levels (Higgins et al. 2011).
In summary, findings from this study support placental compromise as the mechanism responsible for the IUGR and low birth weight offspring in gestational T-treated animals. More in-depth studies are needed to delineate the underlying molecular mechanisms.
Acknowledgments
This work was supported by United States Public Health Service Grant P01 HD44232 (VP).
We thank Douglas Doop for his help with breeding, lambing, excellent animal care and facility management. We are also grateful to Carol Herkimer, Jim Lee, Jacob Moeller, Sindhu Halubai, Maria Cernea, Drs. Bachir Abi-Salloum and Chrysanthi Fergani, and all the undergraduates that helped throughout the study via the Undergraduate Research Opportunities Program at the University of Michigan for the help provided during prenatal treatment, surgery and/or placentome harvest.
Footnotes
Disclosure statement: Authors have nothing to disclose
References
- Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. American Journal of Physiology – Endocrinology and Metabolism. 2010;299:E741–E751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. Totowa, NJ: Humana Press Inc; 2006. pp. 259–272. [Google Scholar]
- Abi Salloum B, Veiga-Lopez A, Abbott DH, Padmanabhan V. Developmental programming: gestational exposure to excess testosterone, by its androgenic action, disrupts maternal steroidal and metabolic environment in sheep. 94th Annual Meeting of the Endocrine Society; Houston, TX. MON-15; 2012. p. 145. [Google Scholar]
- Alexander G. Studies on the Placenta of the Sheep (Ovis Aries L.). Effect of Surgical Reduction in the Number of Caruncles. Journal of Reproduction and Fertility. 1964a;7:307–322. doi: 10.1530/jrf.0.0070307. [DOI] [PubMed] [Google Scholar]
- Alexander G. Studies on the Placenta of the Sheep (Ovis Aries L.). Placental Size. Journal of Reproduction and Fertility. 1964b;7:289–305. doi: 10.1530/jrf.0.0070289. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Wilcox AJ. Placental abruption and perinatal mortality in the United States. American Journal of Epidemiology. 2001;153:332–337. doi: 10.1093/aje/153.4.332. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Adult consequences of fetal growth restriction. Clinical Obstetrics and Gynecology. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. Journal of Developmental Physiology. 1987;9:17–29. [PubMed] [Google Scholar]
- Bowman CJ, Streck RD, Chapin RE. Maternal-placental insulin-like growth factor (IGF) signaling and its importance to normal embryo-fetal development. Birth Defects Research: Part B, Developmental and Reproductive Toxicology. 2010;89:339–349. doi: 10.1002/bdrb.20249. [DOI] [PubMed] [Google Scholar]
- Carrizo DG, Rastrilla AM, Telleria CM, Aguado LI. Androstenedione stimulates progesterone production in corpora lutea of pregnant rats: an effect not mediated by oestrogen. Journal of Steroid Biochemistry and Molecular Biology. 1994;51:191–197. doi: 10.1016/0960-0760(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Steckler TL, Mohankumar PS, Padmanabhan V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. Journal of Physiology. 2006;572:119–130. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus N, Chavatte-Palmer P, Viudes G, Camous S, Rosefort A, Hassoun P. Maternal periconceptional undernutrition in Merinos d’Arles sheep: 1. Effects on pregnancy and reproduction results of dams and offspring growth performances. Theriogenology. 2012;77:1453–1465. doi: 10.1016/j.theriogenology.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Di Renzo GC, Mattei A, Gojnic M, Gerli S. Progesterone and pregnancy. Current Opinion in Obstetrics and Gynecology. 2005;17:598–600. doi: 10.1097/01.gco.0000191899.84567.4d. [DOI] [PubMed] [Google Scholar]
- Forbes K, Westwood M. The IGF axis and placental function. a mini review. Hormone Research. 2008;69:129–137. doi: 10.1159/000112585. [DOI] [PubMed] [Google Scholar]
- France JT, Mason JI, Magness RR, Murry BA, Rosenfeld CR. Ovine placental aromatase: studies of activity levels, kinetic characteristics and effects of aromatase inhibitors. Journal of Steroid Biochemistry. 1987;28:155–160. doi: 10.1016/0022-4731(87)90371-2. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Developmental programming and epigenetics. American Journal of Clinical Nutrition. 2011;94:1943S–1952S. doi: 10.3945/ajcn.110.000927. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Ward JW, Giussani DA, Fowden AL. The effect of a reversible period of adverse intrauterine conditions during late gestation on fetal and placental weight and placentome distribution in sheep. Placenta. 2002;23:459–466. doi: 10.1053/plac.2002.0830. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Seminars in Reproductive Medicine. 2011;29:257–265. doi: 10.1055/s-0031-1275518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould JB, Madan A, Qin C, Chavez G. Perinatal outcomes in two dissimilar immigrant populations in the United States: a dual epidemiologic paradox. Pediatrics. 2003;111:e676–e682. doi: 10.1542/peds.111.6.e676. [DOI] [PubMed] [Google Scholar]
- Grzesiak M, Knapczyk-Stwora K, Ciereszko RE, Golas A, Wieciech I, Slomczynska M. Androgen Deficiency During Mid- and Late Pregnancy Alters Progesterone Production and Metabolism in the Porcine Corpus Luteum. Reproductive Sciences. 2014 doi: 10.1177/1933719113518991. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta, 17beta-diol. Hormones and Behavior. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig IR, Pincus MK, Diemert A, Hecher K, Arck PC. Sex-specific effect of first-trimester maternal progesterone on birthweight. Human Reproduction. 2013;28:77–86. doi: 10.1093/humrep/des367. [DOI] [PubMed] [Google Scholar]
- Heasman L, Clarke L, Stephenson TJ, Symonds ME. The influence of maternal nutrient restriction in early to mid-pregnancy on placental and fetal development in sheep. Proceedings of the Nutrition Society. 1999;58:283–288. doi: 10.1017/s0029665199000397. [DOI] [PubMed] [Google Scholar]
- Higgins M, Felle P, Mooney EE, Bannigan J, McAuliffe FM. Stereology of the placenta in type 1 and type 2 diabetes. Placenta. 2011;32:564–569. doi: 10.1016/j.placenta.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Human Reproduction. 1992;7:1461–1466. doi: 10.1093/oxfordjournals.humrep.a137595. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal P, Regan L, Fourkala EO, Rai R, Moore G, Goldin RD, Sebire NJ. Placental pathology of recurrent spontaneous abortion: the role of histopathological examination of products of conception in routine clinical practice: a mini review. Human Reproduction. 2007;22:313–316. doi: 10.1093/humrep/del128. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Bruns U, Leiser R, Luckhardt M, Winterhager E. The fetal vascularisation of term human placental villi. II. Intermediate and terminal villi. Anatomy and Embryology. 1985;173:203–214. doi: 10.1007/BF00316301. [DOI] [PubMed] [Google Scholar]
- Khatri P, Hoffmann B, Schuler G. Androgen receptor is widely expressed in bovine placentomes and up-regulated during differentiation of bovine trophoblast giant cells. Placenta. 2013;34:416–423. doi: 10.1016/j.placenta.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Koong LJ, Garrett WN, Rattray PV. A description of the dynamics of fetal growth in sheep. Journal of Animal Science. 1975;41:1065–1068. doi: 10.2527/jas1975.4141065x. [DOI] [PubMed] [Google Scholar]
- Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Experimental and Clinical Endocrinology. 1994;102:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- Leiser R, Kosanke G, Kaufmann P. Human placental vascularization. Structural and quantitative aspects. In: Soma H, editor. Placenta: Basic Research for Clinical Application. Basel: Karger; 1991. pp. 32–45. [Google Scholar]
- Leiser R, Krebs C, Ebert B, Dantzer V. Placental vascular corrosion cast studies: a comparison between ruminants and humans. Microscopy Research and Technique. 1997;38:76–87. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<76::AID-JEMT9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lo JC, Grumbach MM. Pregnancy outcomes in women with congenital virilizing adrenal hyperplasia. Endocrinology and Metabolism Clinics of North America. 2001;30:207–229. doi: 10.1016/s0889-8529(08)70027-6. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Seminars in Reproductive Medicine. 2011;29:187–196. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louey S, Cock ML, Harding R. Postnatal development of arterial pressure: influence of the intrauterine environment. Archives of Physiology and Biochemistry. 2003;111:53–60. doi: 10.1076/apab.111.1.53.15137. [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Lara HE, Sanchez F, Echiburu B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2013;166:151–155. doi: 10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Denslow ND. Exploring androgen-regulated pathways in teleost fish using transcriptomics and proteomics. Integrative and Comparative Biology. 2012;52:695–704. doi: 10.1093/icb/ics072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald EA, Wolfe MW. Adiponectin attenuation of endocrine function within human term trophoblast cells. Endocrinology. 2009;150:4358–4365. doi: 10.1210/en.2009-0058. [DOI] [PubMed] [Google Scholar]
- Misra DP, Salafia CM, Miller RK, Charles AK. Non-linear and gender-specific relationships among placental growth measures and the fetoplacental weight ratio. Placenta. 2009;30:1052–1057. doi: 10.1016/j.placenta.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clinical and Experimental Pharmacology and Physiology. 2008;35:730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- Mossman HW. Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology; Evolution; Phylogenetic Significance; Basic Functions; Research Opportunities. London: Macmillan Press, Ltd; 1987. [Google Scholar]
- Nestler JE. Regulation of the aromatase activity of human placental cytotrophoblasts by insulin, insulin-like growth factor-I, and -II. Journal of Steroid Biochemistry and Molecular Biology. 1993;44:449–457. doi: 10.1016/0960-0760(93)90249-v. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Current Opinion in Obstetrics and Gynecology. 2008;20:132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- NRC Committee on the Nutrient Requirements of Small Ruminant. Nutrient Requirements of Small Ruminants. Sheep, Goats, Cervids, and New World Camelids. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- Osgerby JC, Gadd TS, Wathes DC. Effect of maternal body condition on placental and fetal growth and the insulin-like growth factor axis in Dorset ewes. Reproduction. 2003;125:717–731. [PubMed] [Google Scholar]
- Otto-Duessel M, He M, Jones JO. Tissue-selective regulation of androgen-responsive genes. Endocrine Research. 2012;37:203–215. doi: 10.3109/07435800.2012.668254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. International Journal of Andrology. 2010;33:394–404. doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic versus estrogenic reprogramming. Seminars in Reproductive Medicine. 2011;29:173–186. doi: 10.1055/s-0031-1275519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Molecular and Cellular Endocrinology. 2013;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S, Russo T, Falbo A, Di Cello A, Tolino A, Tucci L, La Sala GB, Zullo F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Human Reproduction. 2013;28:2838–2847. doi: 10.1093/humrep/det250. [DOI] [PubMed] [Google Scholar]
- Parker VJ, Douglas AJ. Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. Journal of Reproductive Immunology. 2010;85:86–92. doi: 10.1016/j.jri.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Penninga L, Longo LD. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 1998;19:187–193. doi: 10.1016/s0143-4004(98)90008-x. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Jones A, Goulden PA. Birth weight, stress, and the metabolic syndrome in adult life. Annals of the New York Academy of Sciences. 2006;1083:28–36. doi: 10.1196/annals.1367.027. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reproductive Biology and Endocrinology. 2013;11:56. doi: 10.1186/1477-7827-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Rojas-Garcia PP, Recabarren MP, Alfaro VH, Smith R, Padmanabhan V, Sir-Petermann T. Prenatal testosterone excess reduces sperm count and motility. Endocrinology. 2008;149:6444–6448. doi: 10.1210/en.2008-0785. [DOI] [PubMed] [Google Scholar]
- Rees S, Mallard C, Breen S, Stringer M, Cock M, Harding R. Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1998;119:653–660. doi: 10.1016/s1095-6433(98)01001-0. [DOI] [PubMed] [Google Scholar]
- Regnault TR, Galan HL, Parker TA, Anthony RV. Placenta. Placental development in normal and compromised pregnancies-- a review. 2002;23(Suppl A):S119–S129. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. Journal of Physiology. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CT, Owens JA, Sferruzzi-Perri AN. Placenta. Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs. 2008;29(Suppl A):S42–S47. doi: 10.1016/j.placenta.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Rossi R, Scillitani G, Vimercati A, Fiore MG, Mastrodonato M, Resta L. Diabetic placenta: ultrastructure and morphometry of the term villi. Analytical and Quantitative Cytology and Histology. 2012;34:239–247. [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of adult disease. Pediatric Clinics of North America. 2009;56:449–466. doi: 10.1016/j.pcl.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Ladron de Guevara A, Villarroel AC, Preisler J, Echiburu B, Recabarren S. Polycystic ovary syndrome and pregnancy. Revista Medica de Chile. 2012;140:919–925. doi: 10.4067/S0034-98872012000700015. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Heasman L, Clarke L, Firth K, Stephenson T. Maternal nutrition and disproportionate placental-to-fetal growth. Biochemical Society Transactions. 1998;26:91–96. doi: 10.1042/bst0260091. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. International Immunopharmacology. 2001;1:1037–1048. doi: 10.1016/s1567-5769(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Uzelac PS, Li X, Lin J, Neese LD, Lin L, Nakajima ST, Bohler H, Lei Z. Dysregulation of leptin and testosterone production and their receptor expression in the human placenta with gestational diabetes mellitus. Placenta. 2010;31:581–588. doi: 10.1016/j.placenta.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Vatnick I, Ignotz G, McBride BW, Bell AW. Effect of heat stress on ovine placental growth in early pregnancy. Journal of Developmental Physiology. 1991;16:163–166. [PubMed] [Google Scholar]
- Veiga-Lopez A, Lee JS, Padmanabhan V. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology. 2010;151:4007–4017. doi: 10.1210/en.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biology of Reproduction. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme KA, Hess BW, Nijland MJ, Nathanielsz PW, Ford SP. Placentomal differentiation may compensate for maternal nutrient restriction in ewes adapted to harsh range conditions. Journal of Animal Science. 2006;84:3451–3459. doi: 10.2527/jas.2006-132. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Aitken RP, Cheyne MA, Humblot P. Pregnancy-specific protein B and progesterone concentrations in relation to nutritional regimen, placental mass and pregnancy outcome in growing adolescent ewes carrying singleton fetuses. Journal of Reproduction and Fertility. 1997;109:53–58. doi: 10.1530/jrf.0.1090053. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Palmer RM, Da Silva P, Cruickshank MA. Relationship between nutritionally-mediated placental growth restriction and fetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta. 2000;21:100–108. doi: 10.1053/plac.1999.0440. [DOI] [PubMed] [Google Scholar]