Abstract
Background
Airway management remains a fundamental component of optimal care of the severely injured patient, with endotracheal intubation representing the definitive strategy for airway control. However, multiple studies document an association between early intubation and increased mortality.
Objectives
To explore the relationship between intubation attempts and outcome across sites participating in the Resuscitation Outcomes Consortium (ROC).
Methods
The ROC Epistry – Trauma, an epidemiologic database of prehospital encounters with critically injured trauma victims, was used to identify EMS-treated patients with Glasgow Coma Scale (GCS) score ≤ 8. Multiple logistic regression was used to explore the association between intubation attempts and vital status at discharge adjusting for the following covariates: age, gender, GCS score, hypotension, mechanism of injury, and ROC site. Sites were then stratified by frequency of intubation attempts and chi-square test for trend used to associate the frequency of intubation attempts with outcome.
Results
1,555 patients were included in this analysis; intubation was attempted in 758 (49%) of these. Patients in whom intubation was attempted had higher mortality (adjusted odds ratio 2.91, 95% CI 2.13–3.98, p<0.01). However, sites with higher rates of attempted intubation had lower mortality across all trauma victims with GCS ≤ 8 (OR 1.40, 95% CI 1.15–1.72, p<0.01).
Conclusions
Patients in whom intubation is attempted have higher adjusted mortality. However, sites with a higher rate of attempted intubation have lower adjusted mortality across the entire cohort of trauma patients with GCS ≤ 8.
Keywords: prehospital intubation, traumatic brain injury, airway management, paramedic, outcomes, major trauma victim, ventilation
Introduction
Airway management is considered to be fundamental in early trauma care, with endotracheal intubation (ETI) considered definitive treatment. Indeed, the axiom “GCS 8, intubate” reflects the widespread belief that patients suffering traumatic coma should undergo early ETI, with potential benefits including airway protection from aspiration, improved oxygenation, and control of ventilation (1). The perceived importance of this intervention has led to the performance of ETI by out-of-hospital providers for over three decades, and many emergency medical services (EMS) systems have extended the use of neuromuscular blocking agents to out-of-hospital providers through rapid sequence intubation (RSI) protocols in an effort to optimize the process and increase ETI success rates (2).
Despite the widespread use of intubation in the out-of-hospital resuscitation of trauma patients, clinical evidence to support this approach is limited. Multiple investigators have demonstrated an association between out-of-hospital ETI and increased mortality for patients with severe traumatic brain injury (TBI) (3–8). This may reflect some degree of selection bias, with patients who are able to be intubated without RSI likely suffering devastating neurologic injury that is not adequately quantified by covariates such as Glasgow Coma Scale (GCS) score or head abbreviated injury score (AIS). However, it is conceivable that intubation itself may lead to physiological insults, such as a rise in intracranial pressure (ICP) with laryngoscopy, oxygen desaturation during intubation attempts, or hypocapnia-induced cerebral ischemia from inadvertent post-intubation hyperventilation (9–11). Thus, the true therapeutic benefit – or harm – with out-of-hospital ETI remains uncertain.
The Resuscitation Outcomes Consortium (ROC) is a large out-of-hospital research network, with over 200 participating EMS agencies serving a total population of almost 25 million (12). In addition, ROC analyses utilize systematically collected out-of-hospital records rather than relying on trauma registries derived from inpatient data not available to out-of-hospital personnel (13). Finally, the multicenter nature of the network allows novel comparisons that capitalize on inherent differences in EMS configuration and approach to care. In this study, we explore the association between out-of-hospital intubation attempts and outcome among trauma patients with GCS ≤ 8 using the ROC Epistry database.
Methods
Study Design
These observational data were collected prospectively as part of the ROC trauma registry (ROC Epistry – Trauma). A detailed description of the registry methodology has been previously published (13). This analysis was performed using an out-of-hospital, consecutive-patient cohort registry of trauma victims 15 years and older for whom out-of-hospital treatment was initiated and physiologic abnormality was present in the field. One hundred fifty-three Institutional Review Boards/Research Ethics Boards (127 hospital-based and 26 EMS agency-based) in both the U.S. and Canada reviewed and approved the ROC Epistry-Trauma project and waived the requirement for informed consent under minimal-risk criteria.
Setting
The 10 participating major regional research centers included 7 U.S. locations (Birmingham, AL; Dallas, TX; Iowa; Milwaukee, WI; Pittsburgh, PA; Portland, OR; Seattle and King County, WA) and 3 Canadian locations (Ottawa, ON; Toronto, ON; and Vancouver, BC) serving a combined population of almost 25 million persons. One ROC site was not included in the analysis. The variability across the 10 sites with regard to size, location, geographic diversity, and EMS system structure has been described elsewhere (12). Episodes for this analysis occurred between December 1, 2005 and May 31, 2007 and were cared for by more than 36,000 EMS providers representing 237 EMS agencies and transporting to 189 designated trauma receiving hospitals and 98 non-trauma hospitals. The analysis used data available through September 2008.
Subjects
The study cohort consisted of consecutive injured adults (age 15 years and older) requiring activation of the emergency 9-1-1 system within predefined geographic regions at each ROC site. Patients included in the ROC Epistry – Trauma must have undergone evaluation and treatment by EMS personnel and met one or more of the following physiologic inclusion criteria at some time during their prehospital course: SBP ≤ 90 mmHg, respiratory rate <10 or >29 breaths/min, GCS ≤ 12, or attempts at invasive airway management (ETI, cricothyrotomy, supraglottic airway insertion). These were derived from physiological criteria contained in the Revised Trauma Score (14). “Injury” is broadly defined as any blunt, penetrating, or burn mechanism believed by EMS provider(s) to represent the primary clinical insult. For this analysis, only patients with GCS ≤ 8 were included. Patients without vital signs on EMS arrival, with unknown vital status, or for whom no resuscitative attempt was made were excluded from this analysis.
Methods of Measurement
This analysis included the following prehospital variables: age (years), gender, mechanism of injury (blunt versus penetrating), initial SBP (mmHg), initial respiratory rate (breaths/min), initial GCS prior to intubation, initial heart rate (beats/minute), intubation attempts (ETI or cricothyrotomy), the use of neuromuscular blocking agents as part of a rapid sequence intubation (RSI) protocol, and transport status (transported to ED, nontransported). The primary outcome of survival to hospital discharge was determined from ED and inpatient records for transported patients.
Data Collection and Processing
Research personnel at each ROC site screened EMS patient care records at regular intervals to identify eligible patients. Abstracted data were entered into standardized web-based data forms, matched to hospital outcomes, de-identified, and submitted to a central data coordinating center (DCC). Quality assurance processes at each ROC site included: training of ROC data abstractors in data collection and variable definition, routine data element range and consistency checks by the DCC, and annual site visits by DCC personnel to review original records for input accuracy. In addition, monthly ROC Epistry – Trauma case enrollment for each site and for individual EMS agencies was continually evaluated during data collection. Sites or agencies with substantially higher or lower case capture for a particular month relative to their average, as determined using a Poisson distribution with a 5% cutoff, were sent inquiries to determine whether such fluctuations were secondary to natural variation or some other identifiable trend or represented incomplete episode identification.
Outcome Measures
Survival to hospital discharge was the primary outcome measure for this analysis. While this does not allow evaluation of functional status at discharge, which is clearly important for outcomes following traumatic brain injury, more detailed hospital-based outcomes were not feasible due to limited resources available for hospital chart abstraction as well as approval of the study by IRBs under exception from documented written consent under minimal risk criteria limiting access to patient data.
Primary Data Analysis
The primary objective of this analysis was to explore the relationship between prehospital airway management practice and outcome for trauma patients with GCS ≤ 8. Logistic regression was to quantify the association between attempts at invasive airway management (ETI or cricothyrotomy) and vital status at discharge, adjusting for multiple known confounders of outcome from severe traumatic injury. Patients were stratified into “intubation attempt” and “no intubation attempt” cohorts. ROC did not collect the “success” of the intubation nor the number of attempts tried per patients but only that at least one attempt was made and the type of advanced airway attempted. The intubation cohort was defined by attempts at ETI, with or without use of RSI medications, or cricothyrotomy. Univariate analysis (two-sample t-test allowing for unequal variances, Mann Whitney U, likelihood-ratio chi-square test) was used to compare baseline characteristics between the patients with and without intubation attempts. Multivariate logistic regression was then performed using the following covariates: age (continuous in years), gender, initial pre-intubation GCS score, initial SBP <90 mmHg, mechanism of injury (blunt vs. penetrating), and ROC site. Odds ratios (95% confidence intervals) were used to quantify all associations.
Individual study sites were then stratified by compared with regard to the adjusted mortality for all trauma patients with GCS ≤ 8. The proportion of patients with GCS ≤ 8 undergoing intubation (ETI or cricothyrotomy) and overall mortality was then determined for each ROC site. The lowest GCS value recorded prior to advanced airway management was used. Sites were then stratified by the frequency of intubation among patients with GCS ≤ 8, with graphical analysis performed using the odds ratios calculated as part of the logistic regression analysis using the same covariates described above. In addition, chi-square test for linear trend used to assess the relationship between site-level intubation rate and mortality for the entire cohort of patients with GCS ≤ 8 and for intubated patients with GCS ≤ 8. This approach capitalizes on the natural inter-site variability with regard to airway management practices to determine whether an increased frequency of intubation attempts was associated with higher or lower mortality rates. During the time period for this analysis, EMS protocols at all ROC sites recommended consideration of advanced airway management strategies including intubation for patients with GCS ≤ 8. Selected sites also allowed the use of RSI by ground units to facilitate intubation, while all sites allowed use of RSI by air medical crews.
A p-value <0.05 was used to define statistical significance for all comparisons. All analyses performed for this manuscript used SAS v9.1 (The SAS Institute, Inc. Cary, NC) and R v2.4.0 and the rpart library v3.1–32 (Free Software Foundation Inc., Boston MA).
Results
A total of 1,575 trauma patients with GCS ≤ 8 were identified from the ROC Epistry over the 16-month study period. Intubation status and survival were documented in all patients, and first GCS score was missing in only 1.3% of patients, resulting in a study cohort for this analysis of 1,555 patients. Characteristics of patients with (n=758) and without (n=797) attempts at intubation are displayed in Table 1. Patients in the intubation cohort appeared to be more critically injured as evidenced by lower GCS scores, a higher incidence of hypotension (SBP<90 mmHg), and higher mortality. Penetrating trauma also tended to be more common in the intubation group. In addition, scene times were almost 6 minutes longer in the intubation cohort. As anticipated, intubation was associated with increased mortality when adjusted for increasing age, gender, lowest GCS score, hypotension, and site (OR 2.91, 95% CI 2.13–3.98, p<0.01) (Figure 1). With the use of neuromuscular blocking agents incorporated into the model, intubation without RSI was associated with increased mortality (OR 2.78, 95% CI 2.03–3.80, p<0.01). The association between intubation with RSI and mortality did not reach statistical significance (OR 1.33, 95% CI 0.78–2.26, p=0.30). Clinical characteristics of survivors (n=324) and non-survivors (n=434) among patients with intubation attempts are displayed in Table 2.
Table 1.
Demographic and clinical variables for intubated and non-intubated patients included in this analysis.
| Parameter | Intubation cohort | Non-intubation cohort | p-value |
|---|---|---|---|
| Number of subjects [#] | 758 | 797 | |
| Age in years [mean(sd)] | 42.1 (19.1) | 43.5 (19.3) | 0.16 |
| missing [#] | 0 | 0 | |
| Male [%] | 75.1 | 76.5 | 0.56 |
| missing [#] | 2 | 0 | |
| Mechanism | |||
| Blunt [%] | 77.3 | 82.7 | 0.008 |
| Penetrating [%] | 18.7 | 12.7 | 0.001 |
| Other [%] | 4.0 | 4.6 | 0.51 |
| Missing [#] | 0 | 0 | |
| Prehospital airway | |||
| ET [%] | 99.6 | 0.0 | <0.0001 |
| RSI [%] | 23.9 | ||
| Cricothyrotomy [%] | 0.7 | 0.0 | 0.007 |
| Supraglottic [%] | 4.0 | 3.8 | 0.90 |
| Missing [#] | 0 | 0 | |
| Prehospital times | |||
| Response [mean(sd)] | 6.4 (4.3) | 6.6 (5.3) | 0.52 |
| missing [#] | 36 | 21 | |
| Scene [mean(sd)] | 25.2 (13.8) | 19.5 (10.9) | <0.0001 |
| missing [#] | 53 | 52 | |
| Transport [mean(sd)] | 13.5 (11.9) | 10.7 (8.5) | <0.0001 |
| missing [#] | 72 | 73 | |
| Initial GCS [mean(sd)] | 4.3 (2.2) | 5.4 (2.9) | <0.0001 |
| missing [#] | 7 | 13 | |
| Initial SBP<90 [%] | 28.9 | 17.4 | <0.0001 |
| missing [#] | 183 | 124 | |
| Mortality [%] | 57.3 | 33.6 | <0.0001 |
| missing [#] | 0 | 0 | |
Figure 1.
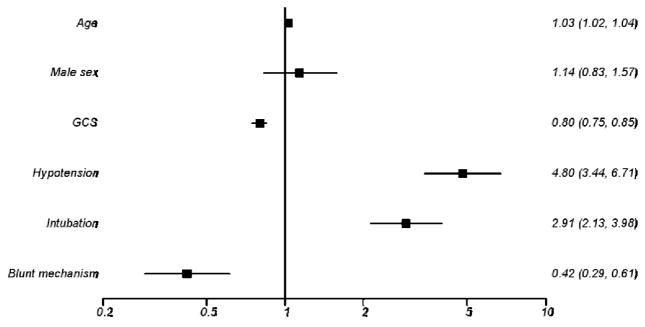
Logistic regression analysis with adjusted odds of mortality for various parameters.
Table 2.
Clinical variables for intubated survivors and intubated nonsurvivors.
| Parameter | Survivors | Non-survivors | Difference (p-value) | ||
|---|---|---|---|---|---|
| N | Mean (sd) or N (%) | N | Mean (sd) or N (%) | ||
| Demographics | |||||
| Age | 324 | 38.6 (16.7) | 434 | 44.7 (20.3) | 6.1 (<0.0001) |
| Male n(%) | 322 | 242 (75.2) | 434 | 327 (75.4) | 0.2 (0.95) |
| Mechanism n (%) | |||||
| Blunt | 324 | 278 (85.8) | 434 | 308 (71.0) | −14.8 (<0.0001) |
| Penetrating | 324 | 32 (9.9) | 434 | 110 (25.4) | 15.5 (<0.0001) |
| Other | 324 | 14 (4.3) | 434 | 16 (3.7) | −0.6 (0.66) |
| GCS | |||||
| Initial | 322 | 5.1 (2.4) | 429 | 3.6 (1.6) | −1.5 (<0.0001) |
| Worst | 324 | 4.1 (1.7) | 434 | 3.3 (1.0) | −0.8 (<0.0001) |
| Systolic BP (mmHg) | |||||
| Initial | 288 | 127.4 (34.2) | 287 | 82.2 (62.5) | −45.2 (<0.0001) |
| Lowest | 299 | 117.9 (31.7) | 289 | 75.7 (59.6) | −42.2 (<0.0001) |
| Respiratory Rate (breaths/min) | |||||
| Initial | 298 | 17.1 (9.1) | 345 | 9.6 (9.8) | −7.5 (<0.0001) |
| Lowest | 305 | 14.2 (9.0) | 351 | 7.1 (8.3) | −7.1 (<0.0001) |
| Highest | 305 | 18.9 (9.0) | 351 | 11.5 (10.6) | −7.4 (<0.0001) |
| Pulse (beats/min) | |||||
| Initial | 308 | 96.7 (26.7) | 367 | 68.8 (46.6) | −27.9 (<0.0001) |
| Lowest | 316 | 88.8 (27.2) | 376 | 57.5 (46.2) | −31.3 (<0.0001) |
| Highest | 316 | 104.6 (26.3) | 375 | 83.2 (49.2) | −21.3 (<0.0001) |
| RTS | |||||
| Initial | 270 | 4.9 (1.2) | 261 | 2.8 (2.1) | −2.1 (<0.0001) |
| Prehospital times (min) | |||||
| Response | 304 | 6.5 (4.5) | 418 | 6.4 (4.2) | −0.1 (0.76) |
| Scene | 298 | 27.7 (14.4) | 407 | 23.4 (13.1) | −4.2 (<0.0001) |
| Transport | 308 | 15.4 (14.0) | 378 | 11.9 (9.4) | −3.5 (0.0002) |
| Neuromuscular blocking agents used (%) | |||||
| Yes | 324 | 131 (40.4) | 434 | 50 (11.5) | −28.9 (<0.0001) |
Substantial variability was observed between study sites with regard to the proportion of trauma patients with GCS ≤ 8 undergoing intubation attempts as well as overall survival (Table 3). Sites with higher intubation attempt rates had lower mortality for all trauma patients with GCS ≤ 8 (p=0.018) and for those undergoing intubation attempts (p<0.0001). These results were unchanged with exclusion of patients with absent vital signs on initial EMS evaluation. The five sites with the lowest intubation rate among patients with GCS ≤ 8 had higher mortality than the five sites with the highest frequency of intubation (50% vs. 41%, OR 1.40, 95% CI 1.15–1.72, p<0.01). Figure 2 presents the adjusted odds ratio of mortality among all patients with GCS ≤ 8, with sites ordered from highest to lowest frequency of intubation.
Table 3.
Site-level analysis exploring the relationship between intubation rate and outcome for all patients with GCS ≤ 8 (left) and for all intubated patients with GCS ≤ 8 (right).
| Site | n | Intubation Attempt Rate | OverallMortality N=1555 | Chi- Square Trend p- value | n | Mortality in those Intubated N=758 | Chi- Square Trend p- value |
|---|---|---|---|---|---|---|---|
| TOR | 186 | 18.3 | 45.7 | 0.018 | 34 | 67.7 | <0.0001 |
| MLW | 105 | 29.5 | 50.5 | 31 | 80.7 | ||
| DAL | 150 | 37.3 | 62.0 | 56 | 78.6 | ||
| IWA | 84 | 41.7 | 39.3 | 35 | 57.1 | ||
| OTT | 137 | 44.5 | 49.6 | 61 | 73.8 | ||
| ARC | 199 | 45.7 | 38.2 | 91 | 61.5 | ||
| PTL | 160 | 50.6 | 31.3 | 81 | 48.2 | ||
| PGH | 78 | 53.9 | 47.4 | 42 | 50.0 | ||
| VAN | 202 | 67.8 | 58.9 | 137 | 63.5 | ||
| SKC | 254 | 74.8 | 34.7 | 190 | 39.0 |
Figure 2.
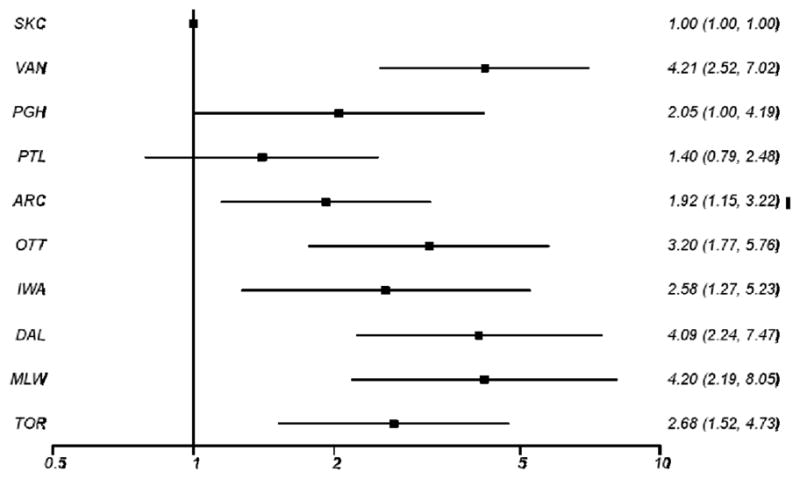
Site-level analysis exploring the relationship between intubation rate and outcome for all patients with GCS ≤ 8. Sites are arranged from highest attempted intubation rate on top to lowest attempted intubation rate on bottom. Adjusted odds of mortality are displayed.
Not all sites had complete data in the first several months and some data were incomplete in the final months of the study period. A sensitivity analysis was performed by restricting the episode dates to March 2008 through November 2008. This actually amplified the relationships reported here with stronger odds ratios and smaller p-values.
Discussion
While recommendations to intubate comatose trauma patients are ubiquitous, these are based primarily on the theoretical benefits of preventing or correcting hypoxemia and protecting from aspiration, with little empirical data to support this approach (1). We analyzed the ROC Epistry – Trauma database to explore the association between prehospital intubation and outcome. An expected increase in mortality was observed for patients undergoing intubation attempts, even after adjusting for known multiple prehospital confounders. However, study sites with higher rates of attempted intubation also had lower adjusted mortality for all trauma patients with GCS ≤ 8. These data, which capitalize upon the natural variability in practice across ROC sites, supporting a potential benefit to prehospital intubation.
Previous regression analyses have documented an association between prehospital intubation and increased mortality (3–7). Most prior investigations have utilized data from trauma registries with limited prehospital data, identifying eligible patients using a head AIS score of 3 or greater and adjusting for a combination of early clinical and injury severity data. Despite the inclusion of tens of thousands of patients and adjustment for dozens of variables affecting outcome from TBI, the association between prehospital intubation and mortality has persisted, raising questions about the benefit – or potential harm – with routine application of this procedure by EMS personnel (15). Our study results are unique in that our cohort of patients is defined by prehospital GCS score rather than data available only following transport to the ED. However, none of these analyses – including our own – avoid the possibility that a patient who is determined by prehospital personnel to require intubation is likely to have suffered a devastating neurologic injury that is not adequately quantified by measures such as GCS score or head AIS. Thus, selection bias may very well explain the finding that prehospital intubation is associated with increased mortality in trauma.
Two prospective studies using different methodological approaches both failed to demonstrate a benefit with prehospital intubation. Gauche et al performed a prospective, controlled trial in Los Angeles County to assess the impact of a ETI protocol vs. bag-valve-mask (BVM) ventilation alone on outcome in pediatric patients requiring ventilatory assistance for both traumatic and medical conditions (16). After a brief training session that included practice with both BVM ventilation and pediatric ETI, paramedics attempted intubation or performed BVM ventilation alone on alternating days. The authors observed no difference in overall survival between the two cohorts, with increased mortality among certain pre-specified subgroups exposed to the ETI protocol. However, it is difficult to determine the efficacy of prehospital ETI from their study, as the low intubation success rate (42%) in the ETI cohort and the heterogeneity of diseases included may have masked or diluted a true positive or negative effect. Furthermore, the subsequent ventilation patterns were not assessed.. Stiell et al conducted a study utilizing a before-and-after methodology (8). They explored the impact of introducing paramedics as part of an Advanced Life Support (ALS) protocol on outcomes from various patients in the multiphase before-after Ontario Prehospital Advanced Life Support (OPALS) study. With regard to traumatic injury, the authors observed no overall difference in mortality in the pre- versus post-ALS periods. However, mortality was increased during the ALS period in the subgroup of patients with GCS ≤ 8. The authors speculate that the introduction of ETI may have been responsible for the increase in mortality. Again, they were unable to quantify the appropriateness of ventilation.
The only evidence supporting prehospital non-RSI ETI in traumatic coma came from Winchell and Hoyt utilizing data from the San Diego County trauma registry. These patients were stratified by GCS (3 versus 4–8) and by isolated TBI vs. multi-system trauma using extracranial AIS (17). They observed an absolute survival benefit of over 20 percent with prehospital ETI in patients with isolated TBI. However, there was no adjustment for different covariates, and the rate of prehospital intubation was lower for patients with GCS 3 versus GCS 4–8, possibly reflecting some form of selection bias. Two other studies documented improved outcomes among patients with severe TBI following prehospital use of neuromuscular blocking agents to facilitate ETI (18, 19). However, in both studies the comparison groups consisted largely of patients intubated without RSI medications, making it difficult to determine the true impact of intubation on outcome.
Stratifying the ROC study sites based on intubation attempt rates reveals decreased mortality among all trauma patients with GCS ≤ 8 in sites with more frequent intubation attempts. Although our data are not suitable for confirming a direct causal relationship, as intubation may be a surrogate marker for other variables affecting outcome, it does suggest a potential benefit to a more aggressive airway management approach. It is also possible that a higher frequency of intubation leads to greater familiarity with and better performance of the procedure and avoidance of “mistakes” that have been previously linked to survival, such as multiple attempts, oxygen desaturation, or unrecognized esophageal intubation (20–26). Indeed, the importance of skills maintenance and the potential impact on survival is becoming recognized as a critical issue in EMS (11, 20–22). Sites with higher intubation rates may also have a lower incidence of hyperventilation, which may occur to a lesser degree in EMS systems with greater experience with ETI and subsequent ventilation (10, 11, 23–26). The adverse hemodynamic effects of positive-pressure ventilation, hypocapnia-induced ischemia, and the rapid rise in pro-inflammatory cytokines as a result of injurious ventilation may overwhelm any potential benefits of ETI with regard to airway protection or improved oxygenation (27–40). Lastly, the potential for harm caused by hyperoxemia has only recently been recognized but may potentially be responsible for a portion of the adverse outcomes associated with intubation (41–43).
Despite the large sample size and relatively comprehensive dataset, we acknowledge multiple study limitations must be considered when interpreting these results. The ROC Epistry – Trauma does not include inpatient assessment data such as AIS that could allow better models to adjust for injury severity. The absence of SpO2, ventilation rate, or end-tidal CO2 data eliminates our ability to account for some of the most important covariates associated with prehospital airway management. We could not separate decreased level of consciousness due to TBI vs. hypotension due to traumatic shock, which may have very different responses to intubation and mechanical ventilation. In addition, we did not have morbidity information, with the main outcome limited to mortality.
We included both non-RSI and RSI patients in the site-level analysis. An EMS system using neuromuscular blocking agents would be expected to have a higher intubation rate and may include providers with greater experience or more intense training. In addition, implementing paramedic RSI may be part of what defines a more aggressive EMS system. The absence of paramedic RSI protocols in most sites may explain the relatively low overall intubation rate despite protocols suggesting that all trauma patients with GCS ≤ 8 should undergo attempts at intubation. We were unable to quantify the number of intubations for each provider and did not calculate the number of intubations per paramedic in each site, both of which may be important predictors of intubation performance and outcome. We also made the decision not to consider supraglottic airway insertion as an attempt at intubation. However, it is certainly possible that the benefits of endotracheal intubation can be achieved with fewer complications using these less invasive devices. Finally, the ROC Epistry – Trauma does not record the success of intubation but merely whether attempts were made. While this does not allow us to determine the impact of successful intubation on outcome from traumatic coma, it offers the more operational metric of an association between intubation attempts and outcome, which may help guide prehospital protocols. In addition, prior data document fairly high rates of intubation success once attempts are initiated (49).
Conclusions
Our results from a major trauma registry with protocol-driven data collection demonstrate a decrease in adjusted mortality for trauma patients with GCS ≤ 8. While causation cannot be inferred, these data support a more aggressive approach to prehospital airway management. Randomized trials are needed to better define the role of prehosptial intubation for patients with severe traumatic injuries.
Acknowledgments
FUNDING
The Resuscitation Outcome Consortium (ROC) was supported by a series of cooperative agreements to 10 regional clinical centers and one data Coordinating Center (5U01 HL077863, HL077881, HL077871 HL077872, HL077866, HL077908, HL077867, HL077885, HL077877, HL077873) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, and the American Heart Association.
Contributor Information
Daniel P. Davis, UCSD Center for Resuscitation Science, Department of Emergency Medicine, San Diego CA.
Kent M. Koprowicz, University of Washington, Seattle WA, Axio Research Corp, Seattle WA.
Craig D. Newgard, Center for Policy and Research in Emergency Medicine, Department of Emergency Medicine, Oregon Health & Science University, Portland, Oregon.
Mohamud Daya, Center for Policy & Research in Emergency Medicine, Department of Emergency Medicine, Oregon Health & Science University, Portland, Oregon.
Eileen M. Bulger, Associate Professor of Surgery, University of Washington, Seattle, WA.
Ian Stiell, Department of Emergency Medicine, University of Ottawa.
Graham Nichol, Clinical Trials Center, University of Washington.
Shannon Stephens, Department of Surgery, University of Alabama at Birmingham.
Jonathan Dreyer, Division of Emergency Medicine, University of Western Ontario.
Joseph Minei, UT Southwestern Medical Center, Parkland Memorial Hospital.
Jeffrey D. Kerby, Department of Surgery, University of Alabama at Birmingham.
References
- 1.Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury. Prehosp Emerg Care. 2008;12(Suppl 1):1–52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 2.McDonald CC, Bailey B. Prehospital use of neuromuscular-blocking agents in theUnited States. Prehosp Emerg Care. 1998;2(1):29–32. doi: 10.1080/10903129808958836. [DOI] [PubMed] [Google Scholar]
- 3.Bochicchio GV, Ilahi O, Joshi M, Bochicchio K, Scalea TM. Endotracheal intubation in the field does not improve outcome in trauma patients who present without lethal traumatic brain injury. J Trauma. 2003;54(2):307–11. doi: 10.1097/01.TA.0000046252.97590.BE. [DOI] [PubMed] [Google Scholar]
- 4.Davis DP, Peay J, Sise MJ, et al. The impact of prehospital endotracheal intubation on outcome in moderate-to-severe traumatic brain injury. J Trauma. 2005 doi: 10.1097/01.ta.0000162731.53812.58. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein M, Chan L, Schneir A, Palmer R. Effect of prehospital advanced life support on outcomes of major trauma patients. Journal of Trauma. 2000;48(4):64–8. doi: 10.1097/00005373-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Murray JA, Demetriades D, Berne TV, et al. Prehospital intubation in patients with severe head injury. Journal of Trauma. 2000;49(6):1065–70. doi: 10.1097/00005373-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Wang HE, Peitzman AD, Cassidy LD, Adelson PD, Yealy DM. Prehospital endotracheal intubation and outcome after traumatic brain injury. Ann Emerg Med. 2004;44(5):439–50. doi: 10.1016/j.annemergmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Stiell I, Nesbitt LP, Pickett W, et al. The OPALS major trauma study: impact of advanced life-support on survival and morbidity. Can Med Assoc J. 2008;178(9):1141–52. doi: 10.1503/cmaj.071154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozeman WP, Idris AH. Intracranial pressure changes during rapid sequence intubation: a swine model. J Trauma. 2005;58(2):278–83. doi: 10.1097/01.ta.0000152536.71932.85. [DOI] [PubMed] [Google Scholar]
- 10.Warner KJ, Cuschieri J, Copass MK, Jurkovich GJ, Bulger EM. The impact of prehospital ventilation on outcome after severe traumatic brain injury. J Trauma. 2007;62(6):1330–8. doi: 10.1097/TA.0b013e31804a8032. [DOI] [PubMed] [Google Scholar]
- 11.Davis DP, Dunford JV, Hoyt DB, Ochs M, Holbrook T, Fortlage D. The impact of hypoxia and hyperventilation on outcome following paramedic rapid sequence intubation of patients with severe traumatic brain injury. Journal of Trauma. 2004 doi: 10.1097/01.ta.0000135503.71684.c8. [submitted] [DOI] [PubMed] [Google Scholar]
- 12.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of emergency medical service systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11(4):369–82. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 13.Newgard CD, Sears GK, Rea TD, et al. The Resuscitation Outcomes Consortium Epistry -Trauma: design, development, and implementation of a North American epidemiologic prehospital trauma registry. Resuscitation. 2008;78(2):170–8. doi: 10.1016/j.resuscitation.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champion HR, Sacco WJ, Copes WS, et al. A revision of the trauma score. J Trauma. 1989;29:623–9. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Davis DP. Should invasive airway management be done in the field? Can Med Assoc J. 2008;178(9):1171–3. doi: 10.1503/cmaj.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gausche M, Lewis RJ, Stratton SJ, et al. Effect of prehospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. Jama. 2000;283(6):783–90. doi: 10.1001/jama.283.6.783. [see comments] [DOI] [PubMed] [Google Scholar]
- 17.Winchell RJ, Hoyt DB. Endotracheal intubation in the field improves survival in patients with severe head injury. Trauma Research and Education Foundation of San Diego. Archives of Surgery. 1997;132(6):592–7. doi: 10.1001/archsurg.1997.01430300034007. [DOI] [PubMed] [Google Scholar]
- 18.Bulger EM, Copass MK, Sabath DR, Maier RV, Jurkovich GJ. The use of neuromuscular blocking agents to facilitate prehospital intubation does not impair outcome after traumatic brain injury. J Trauma. 2005;58(4):718–23. doi: 10.1097/01.ta.0000159239.14181.bc. [DOI] [PubMed] [Google Scholar]
- 19.Cudnik MT, Newgard CD, Daya M, Jui J. The impact of rapid sequence intubation on trauma patient mortality in attempted prehospital intubation. J Emerg Med. 2008:36. doi: 10.1016/j.jemermed.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Dunford JV, Davis DP, Ochs M, Doney M, Hoyt DB. Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid sequence intubation. Annals of Emergency Medicine. 2003;42(6):721–8. doi: 10.1016/s0196-0644(03)00660-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang HE, O’Connor RE, Schnyder ME, Barnes TA, Megargel RE. Patient status and time to intubation in the assessment of prehospital intubation performance. Prehospitet almergency Care. 2001;5(1):10–8. doi: 10.1080/10903120190940254. [DOI] [PubMed] [Google Scholar]
- 22.Wang HE, Sweeney TA, O’Connor RE, Rubinstein H. Failed prehospital intubations: an analysis of emergency department courses and outcomes. Prehospitet almergency Care. 2001;5(2):134–41. doi: 10.1080/10903120190939995. [DOI] [PubMed] [Google Scholar]
- 23.Davis DP, Douglas DJ, Buono C, Dunford JV. The incidence of desaturations and hyperventilation associated with air medical rapid sequence intubation. Prehosp Emerg Care. 2005 [submitted] [Google Scholar]
- 24.Davis DP, Dunford JV, Ochs M, Heister R, Hoyt DB. Ventilation patterns following paramedic rapid sequence intubation of patients with severe traumatic brain injury. Neurocritical Care. 2005;2(2) doi: 10.1385/NCC:2:2:165. [DOI] [PubMed] [Google Scholar]
- 25.Davis DP, Dunford JV, Ochs M, Park K, Hoyt DB. The use of quantitative end-tidal capnometry to avoid inadvertent severe hyperventilation in head-injured patients following paramedic rapid sequence intubation. Journal of Trauma. 2003 doi: 10.1097/01.ta.0000100217.05066.87. in press. [DOI] [PubMed] [Google Scholar]
- 26.Davis DP, Idris AH, Sise MJ, et al. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med. 2006;34:1202–8. doi: 10.1097/01.CCM.0000208359.74623.1C. [DOI] [PubMed] [Google Scholar]
- 27.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 29.Pepe P, Raedler C, Lurie KG, Wigginton JG. Emergency ventilatory management in hemorrhagic states: elemental or detrimental? J Trauma. 2003;54(6):1048–55. doi: 10.1097/01.TA.0000064280.05372.7C. [DOI] [PubMed] [Google Scholar]
- 30.Slutsky AS, Ranieri VM. Mechanical ventilation: lessons from the ARDSNet trial. Respir Res. 2000;1:73–77. doi: 10.1186/rr15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremblay LN, Miatto D, Hamid Q, Govindarajan A, Slutsky AS. Injurious ventilation induces widespread pulmonary epitheliet alxpression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit Care Med. 2002;30:1693–1700. doi: 10.1097/00003246-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282:L892–L896. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 34.Wilson MR, Choudhury S, Goddard ME, O’Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol. 2003;95:1385–1393. doi: 10.1152/japplphysiol.00213.2003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Downey GP, Suter PM, Slutsky AS, Ranieri VM. Conventional mechanical ventilation is associated with bronchoalveolar lavage-induced activation of polymorphonuclear leukocytes. Anesthesiology. 2002;97:1426–1433. doi: 10.1097/00000542-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Bao Y, Jiang J, Zhu C, Lu Y, Cai R, Ma C. Effect of hyperventilation on brain tissue oxygen pressure, carbon dioxide pressure, pH value and intracranial pressure during intracranial hypertension in pigs. Chin J Traumatol. 2000;3(4):210–213. [PubMed] [Google Scholar]
- 37.Forbes ML, Clark RS, Dixon CE, et al. Augmented neuronal death in CA3 hippocampus following hyperventilation early after controlled cortical impact. J Neurosurg. 1998;88(3):549–56. doi: 10.3171/jns.1998.88.3.0549. [DOI] [PubMed] [Google Scholar]
- 38.Manley GT, Hemphill JC, Morabito D, et al. Cerebral oxygenation during hemorrhagic shock: perils of hyperventilation and the therapeutic potential of hypoventilation. Journal of Trauma. 2000;48(6):1025–33. doi: 10.1097/00005373-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Skippen P, Seear M, Poskitt K, et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25(8):1402–9. doi: 10.1097/00003246-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Stringer WA, Hasso AN, Thompson JR, Hinshaw DB, Jordan KG. Hyperventilation-induced cerebral ischemia in patients with acute brain lesions: demonstration by xenon-enhanced CT. AJNR Am J Neuroradiol. 1993;14(2):475–84. [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu EH, Liu CS, Tan TY, Chang KC. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Arch Neurol. 2006;63(5):741–4. doi: 10.1001/archneur.63.5.741. [DOI] [PubMed] [Google Scholar]
- 42.Klinger G, Beyene J, Perlman M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Arch Dis Child Fetal Neonatet ald. 2005;90:F49–52. doi: 10.1136/adc.2003.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis DP, Meade W, Jr, Sise MJ, et al. Both hypoxemia and hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009 doi: 10.1089/neu.2009.0940. [in progress] [DOI] [PubMed] [Google Scholar]
- 44.Davis DP, Hoyt DB, Ochs M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma. 2003;54(3):444–53. doi: 10.1097/01.TA.0000053396.02126.CD. [DOI] [PubMed] [Google Scholar]
- 45.Davis DP, Ochs M, Stern J, et al. Factors associated with head-injury mortality following paramedic rapid sequence intubation: a final analysis of the San Diego Paramedic RSI Trial. J Trauma. 2005 doi: 10.1097/00005373-200508000-00037. [submitted] [DOI] [PubMed] [Google Scholar]
- 46.Spaite DW, Tse DJ, Valenzuela TD, et al. The impact of injury severity and prehospital procedures on scene time in victims of major trauma. Annals of Emergency Medicine. 1991;20(12):1299–305. doi: 10.1016/s0196-0644(05)81070-4. [DOI] [PubMed] [Google Scholar]
- 47.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. Journal of Trauma. 1993;34(2):216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61(5):1134–41. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 49.Davis DP, Fisher R, Buono C, et al. Predictors of intubation success, unrecognized esophageal intubations, and reversal of hypoxemia associated with paramedic airway management in a large, urban EMS system. Prehosp Emerg Care. 2006 doi: 10.1080/10903120600725751. [in press] [DOI] [PubMed] [Google Scholar]


