Abstract
The mosquito Aedes aegypti is the main vector of Dengue and Yellow Fever flaviviruses. The organophosphate insecticide temephos is a larvicide that is used globally to control Ae. aegypti populations; many of which have in turn evolved resistance. Target site alteration in the acetylcholine esterase of this species has not being identified. Instead, we tracked changes in transcription of metabolic detoxification genes using the Ae. aegypti ‘Detox Chip’ microarray during five generations of temephos selection. We selected for temephos resistance in three replicates in each of six collections, five from México, and one from Perú. The response to selection was tracked in terms of lethal concentrations (LC50). Uniform upregulation was seen in the epsilon class glutathione-S-transferase genes (eGSTs) in strains from México prior to laboratory selection, while eGSTs in the Iquitos Perú strain became upregulated following five generations of temephos selection. While expression of many esterase genes (CCE) increased with selection, no single esterase was consistently upregulated and this same pattern was noted in the cytochrome P450 genes (CYP) and in other genes involved in reduction or oxidation of xenobiotics. Bioassays using GST, CCE and CYP inhibitors suggest that various CCE instead of GSTs are the main metabolic mechanism conferring resistance to temephos. We show that temephos selected strains show no cross resistance to permethrin and that genes associated with temephos selection are largely independent of those selected with permethrin in a previous study.
Keywords: Temephos resistance selection, Aedes aegypti, transcriptional expression, detoxification genes
Introduction
Dengue is the most prevalent mosquito-borne viral disease affecting humans globally, with the mosquito vector Aedes aegypti (L) found in nearly 100 tropical countries. Due to lack of vaccines or effective pharmaceutical treatments, dengue prevention is currently predicated on reduction of both larval and adult Ae. aegypti populations (Gubler, 2004). Adult control relies largely on formulations of pyrethroid insecticides. For larval control, the three most widely used compounds are Bacillus thuringiensis israelensis (Bti), methoprene, and temephos. Globally, temephos is the most used of these three due to its very low vertebrate toxicity and relatively low cost (WHO, 2009). Temephos is one of a few organophosphates (OP) registered to control Ae. aegypti larvae and it is an important management tool for mosquito abatement programs (EPA, US 2001).
Temephos was used for 30 years before initial reports of resistance appeared in 1995. Resistance ratios (RR) of two to ten were found in collections of Ae. aegypti from Venezuela (Mazzarri and Georghiou, 1995) and 17 Caribean countries (Rawlins and Wan, 1995). Since 2000, temephos resistance has been reported from Cuba and Venezuela (Rodriguez et al. 2001; Rodriguez et al. 2002), Thailand (Jirakanjanakit et al. 2007) and Brazil (Macoris et al. 2003; Braga et al. 2004; Lima et al. 2003, 2006, 2011; Beserra et al. 2007). Most recently reports have appeared from El Salvador (Lazcano et al. 2009), Martinique Island in the French West Indies (Marcombe et al. 2009), Argentina (Llinas et al. 2010; Seccacini et al. 2008), India (Tikar et al. 2009), Colombia (Ocampo et al. 2011), and Trinidad (Polson et al. 2010; 2011). Although resistance to temephos has been demonstrated in many areas of the world, it is the only remaining organophosphate larvicide with any appreciable use. As such, it is an important tool in managing resistance to the few alternative available larvicides.
Mechanisms of temephos resistance have been identified using chiefly bioassays with synergists, biochemical assays and most recently with microarrays. Two major mechanisms of OP resistance reported in mosquitoes involve target site mutations at the acetyl cholinesterase (AChE) and increased detoxification performed by three enzymatic systems: cytochrome P450 monooxygenases (CYP), glutathione-S-transferases (GST) and carboxyl/cholinesterase esterases (CCE). A common pattern of increased esterase activity, increased mortality with the esterase inhibitor DEF (S.S.S-tributlyphosphorotrithioate) and no evidence of insensitive AChE have by now been reported in multiple studies involving Ae. aegypti (Wirth and Georghiou 1999; Macoris et al. 2003; Lazcano et al. 2009; Montella et al. 2007; Rodriguez et al. 2007; Sousa-Polezzi and Bicudo 2004; Bisset et al. 2011; Melo-Santos, et al. 2010). However, some other studies also found differences in CYP and GST systems among resistant and susceptible populations (Braga et al. 2005; Melo-Santos et al. 2010; Ocampo et al. 2011; Polson et al. 2011; Rodriguez et al. 2001).
In the present study, we analyzed the response to laboratory temephos selection in five mosquito strains from México and in a strain from Iquitos, Perú. We monitored for changes in the lethal concentrations (LC50) and in the transcription profiles of the putative detoxification genes on the ‘Aedes Detox Chip’ DNA microarray v.2 (Strode et al. 2008). This microarray contains 318 70-mer probes representing 290 detoxification genes including 183 CYPs, 28 GSTs, 44 CCEs including nonspecific esterases, carboxyl/cholinesterase esterases, p- nitrophenyl acetate esterases, and acetylcholinesterases (AChE) and 35 additional enzymes potentially involved in response to oxidative stress in Ae. aegypti (RedOxs). This microarray has been widely used to follow changes in the expression of detoxification genes. Boyer et al. (2006) used the detox microarray to analyze the ability of Ae. aegypti larvae to tolerate temephos, Bti and toxic vegetable leaf litter. Both induction and selection were correlated with levels of larval detoxifying enzyme activities. Poupardin et al. (2008) tested the effect of exposure of Ae. aegypti larvae to sub-lethal doses of temephos on their subsequent tolerance to insecticides, detoxification enzyme activities and expression of detoxification genes. Overall, this study revealed the potential of xenobiotics found in polluted breeding sites to affect their tolerance to insecticides, possibly through the cross-induction of particular detoxification genes. Marcombe et al. (2009) investigated the molecular basis of insecticide resistance in Ae. aegypti collected in Martinique and found significantly elevated transcription of CYP, GST and CCE at both larval and adult stages. More recently, it was used to compare gene transcription in a temephos selected strain from Brazil and a decrease in expression of some of these genes following removal of temephos selection (Strode et al. 2012).
Recently we compared the gene expression profiles in six strains of Ae. aegypti during and after permethrin selection in adults (Saavedra-Rodriguez et al. 2012). Results indicated that many different genes respond to selection but consistency among strains was uncommon, even from geographically proximate strains. In the present study we report the response of this same collections following temephos selection. We compare gene expression at four levels with the ‘Aedes Detox Chip’ microarray. First, transcription patterns in the unselected FS0 strains were measured relative to New Orleans to identify patterns of differential expression already present in the field. Second, transcription patterns were compared between strains following one generation of selection (FS1) and FS0 strains to identify genes that respond rapidly to selection. Third, transcription was compared between FS1 and FS5 strains to identify genes that respond to five generations of selection. Fourth, transcription following five generations of selection was compared among all strains relative to New Orleans (FS5 versus NO) to identify genes commonly upregulated in all strains. Finally we performed cross resistance bioassays and compared temephos and permethrin expression profiles. Lack of cross resistance was evidenced by bioassays and different expression profiles for each insecticide selection experiment.
Results
Bioassays
Six Ae. aegypti lines collected from southern México and one collected from Iquitos, Perú (Table 1) were used in all experiments. Further each of these was divided into three replicate cages prior to selection and this replication was used to test for significance using the Limma analysis package in www.bioconductor.org. The New Orleans (NO) strain was used as a susceptible control.
Table 1.
Collection sites and temephos resistance before selection. LC50 in μg temephos/mL water. The LC50 resistance ratio was calculated relative the susceptible New Orleans (NO) strain.
| Country State | City Site | Coordinates (DDD.dddd) | Generation in which P0 was started | LC50 (FS0) ug ai/mL | 95% confidence intervals | RR (FS0) P1/NO | RR (FS5) FS5/NO |
|---|---|---|---|---|---|---|---|
| Perú | |||||||
| Iquitos | F2 | 0.029 | (0.0239–0.0355) | 5.8 | 129.0 | ||
| México | |||||||
| Quintana Roo | Chetumal | ||||||
| Calderitas | 18.5563°–88.2562° | F3 | 0.077 | (0.0583–0.1007) | 15.3 | 310.9 | |
| Lagunitas | 18.5199°–88.3339° | F3 | 0.237 | (0.1996–0.2974) | 47.5 | 251.4 | |
| Lázaro-Cárdenas | 18.5359°–88.3016° | F3 | 0.161 | (0.1147–0.2253) | 32.2 | 389.9 | |
| Solidaridad | 18.5284°–88.3027° | F3 | 0.027 | (0.0207–0.0363) | 5.5 | 205.0 | |
| Yucatán | Mérida | 20.9489°–89.6405° | F2 | 0.026 | (0.0215–0.0302) | 5.1 | 42.2 |
| United States | New Orleans | 0.005 | (0.0033–0.0074) | ||||
Offspring from field collections that had been in the laboratory for no more than three generations were designated FS0 (no prior selection). LC50 values were measured in each of the unselected FS0 lines (Table 1) using the CDC beaker bioassay http://www.cdc.gov/ncidod/wbt/resistance/assay/larval/step_2.htm. Moderate levels of resistance (RR50 > 5 –10) were detected in Iquitos, Solidaridad, and Mérida FS0 lines. High (RR50 >10) levels of resistance were detected in the FS0 of Calderitas, Lagunitas, and Lázaro-Cárdenas.
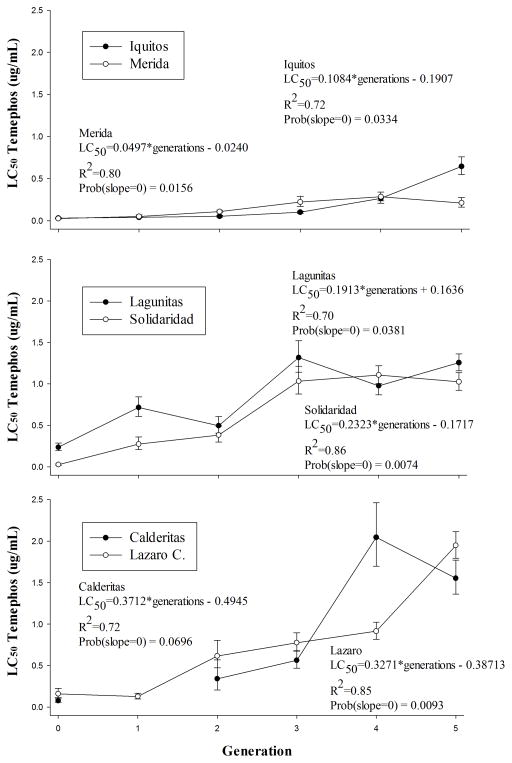
The general response to selection was an increase in LC50 in all mosquito lines (Fig. 1). Table 2 lists the realized heritability (h2) coefficients for LC50 during the selection process and the RR LC50 after five generations of selection. Supplement 1 shows the numbers of selected larvae in each biological replicate at each generation of selection. Out of 34 experiments, six showed significant difference among biological replicates, including Iquitos FS0 and FS3, Lagunitas FS4 and FS5, Mérida FS4 and Solidaridad FS4. Each strain exhibited a distinct response pattern. Iquitos and Mérida responded very gradually to selection and had the smallest h2 for LC50 suggesting that they had the least additive genetic variance in alleles conditioning resistance. The greatest response to selection was seen in Lázaro-Cárdenas and Calderitas and these had the largest h2 for LC50 suggesting that they had the largest amount of additive genetic variance. However, the increase was non-linear in both strains. Calderitas exhibited a large response to selection between generations 3 and 4 as did Lázaro-Cárdenas between generations 4 and 5. This may reflect the presence of recessive alleles that condition resistance to temephos but which were initially too low in frequency to breed recessive homozygotes. Solidaridad and Lagunitas evolved resistance at an intermediate rate and had intermediate h2 for LC50. The LC50 did not constantly increase in all strains. There were marked declines in LC50 in Calderitas and Lagunitas. These may have been attributable to lethal or deleterious recessive alleles that eventually increased sufficiently in frequency during the selection process to breed homozygotes. Using the experiment-wise error rate of α= 0.05, the h2 for LC50 was only significant in the Solidaridad and Mérida collections. Lack of significance in the other strains was largely due to the non-linear effects and a large variance among the three replicates within each generation.
Figure 1.
LC50 response of mosquito lines to temephos selection over five generations. Regression analysis and significance is shown for each mosquito line.
Table 2.
Realized heritability for temephos LC50 in six field selected strains of Aedes aegypti.
| Strain | Realized h2 | p |
|---|---|---|
| Iquitos | 0.24 | 0.073 |
| Calderitas | 2.11 | 0.059 |
| Lagunitas | 0.55 | 0.225 |
| Lázaro-Cárdenas | 1.02 | 0.064 |
| Solidaridad | 0.82 | 0.018 |
| Mérida | 0.76 | 0.031 |
Microarray validation
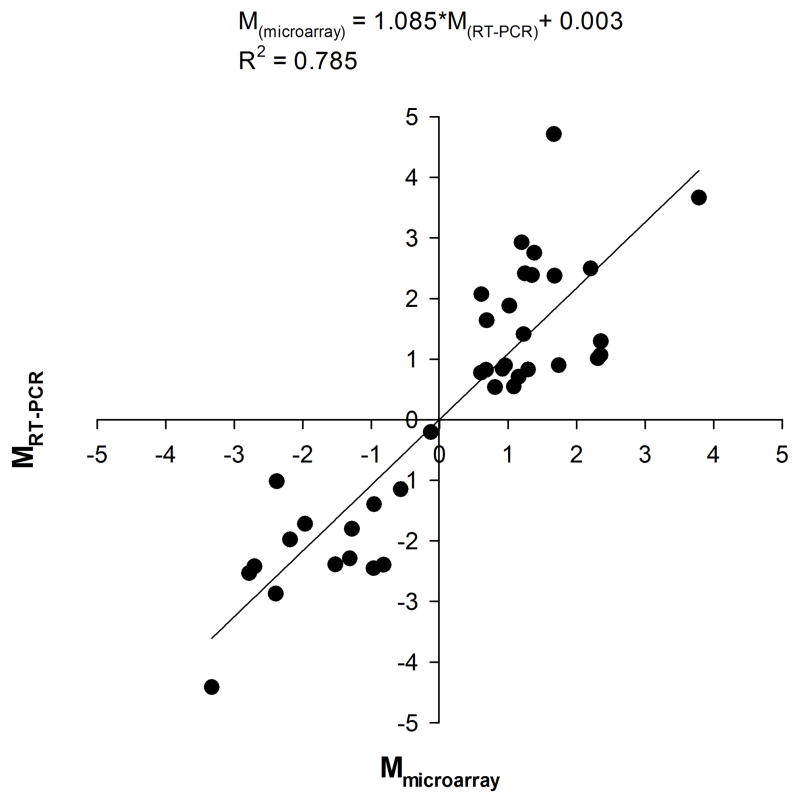
Expression ratios were calculated as M, the log2 of mean transcription ratios, where M = log2 (Cy5/Cy3), Cy5 is the adsorption at 649 nm and Cy3 is the adsorption at 532 nm. The expression ratios as measured by both microarray and quantitative-PCR were compared in twelve genes (GSTs1, CCae4C, COE-8, CYP325G3, CYP4H28, CYP4J13, CYP6Nae1, CYP9J22, CYP9J32, AAEL004388, AAEL004390 and AAEL010382). The same amplified RNA samples from 39 RNA collections were compared and a linear regression model explained 78.5% of the variance, had a slope significantly greater than zero (P<0.0001) that approximated one and an intercept not significantly less than zero (P=0.9851) (Fig. 2).
Figure 2.
Correlation between microarray and real time expression ratios. Ratios are display in a log2 scale.
Gene expression in unselected lines relative to New Orleans
Transcription patterns in five FS0 strains (RNA preparation failed in Solidaridad FS0) were measured relative to NO to identify any patterns of differential expression already present when they were collected in the field. A total of 39 genes were differentially expressed in two or more strains (Table 3). This included eight GSTs, fourteen CYPs, ten CCEs, and seven RedOx genes. Most notably, GSTe3 was 2–9 fold upregulated in all five field collections and GSTe7 was 2–4 fold upregulated in four collections. Among the CYPs, CYP9J26 and CYP9J32 were at least two fold upregulated in four strains. Many CCEs were differentially expressed but none had a consistent pattern of upregulation. Instead CCEs were significantly down regulated in the Iquitos strain and CCae4C was significantly down regulated in four strains. No trends were noted among RedOx genes. The numbers of genes differentially expressed varied among strains. From 15–21 genes were differentially expressed in Calderitas and Iquitos as compared with 46 in Mérida. From 30–40% of those differentially expressed genes were down regulated relative to NO in the Mexican collections as compared to 71% that were down-regulated in Iquitos.
Table 3.
| Table 3a. Genes differentially expressed in two or more unselected FS0 strains relative to the New Orleans strain. Ratio of the average expression of each comparison is displayed on a linear scale. Probability value is shown as a negative log10 scale. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Activity Gene | Vector Base ID | Iquitos | Calderitas | Lázaro-Cárdenas | Lagunitas | Mérida | |||||
|
| |||||||||||
| Ratio | p | Ratio | p | Ratio | p | Ratio | p | Ratio | p | ||
| GSTs | |||||||||||
| GSTd1-1 | AAEL001061 | 2.78 | 20.84 | 0.44 | 12.38 | ||||||
| GSTd1-2 | AAEL001061 | 2.70 | 5.43 | 4.17 | 5.50 | 2.26 | 7.71 | ||||
| GSTe2 | AAEL007951 | 5.59 | 16.69 | 2.70 | 5.21 | 4.87 | 23.92 | ||||
| GSTe3 | AAEL007947 | 2.68 | 7.41 | 7.07 | 14.86 | 7.18 | 16.83 | 8.77 | 9.94 | 5.87 | 20.36 |
| GSTe4 | AAEL007962 | 4.58 | 15.85 | 3.09 | 9.63 | 3.29 | 23.43 | ||||
| GSTe6 | AAEL007946 | 4.24 | 15.69 | 4.90 | 12.78 | 4.48 | 19.22 | ||||
| GSTe7 | AAEL007948 | 3.10 | 15.66 | 4.09 | 20.06 | 4.13 | 7.57 | 2.21 | 18.68 | ||
| GSTs1-2 | AAEL011741 | 0.48 | 4.57 | 0.34 | 8.52 | 0.48 | 12.20 | ||||
| CYPs | |||||||||||
| CYP6F3 | AAEL014684 | 2.76 | 16.69 | 3.36 | 13.40 | ||||||
| CYP4H28 | AAEL003380 | 0.27 | 14.55 | 0.18 | 18.48 | 0.19 | 21.21 | ||||
| CYP4J15 | AAEL013556 | 2.10 | 11.22 | 2.63 | 9.29 | 2.44 | 16.91 | ||||
| CYP6N11 | AAEL009138 | 0.34 | 10.29 | 0.38 | 14.14 | ||||||
| CYP6P12 | AAEL014891 | 0.43 | 5.90 | 0.49 | 11.17 | ||||||
| CYP6Z8 | AAEL009131 | 2.20 | 16.89 | 2.33 | 12.16 | 2.75 | 24.41 | ||||
| CYP9AE1 | AAEL003748 | 0.44 | 7.96 | 0.39 | 5.16 | ||||||
| CYP9M9 | AAEL001807 | 5.49 | 17.45 | 3.53 | 9.12 | 9.25 | 22.49 | ||||
| CYP9J15 | AAEL006795 | 0.48 | 16.71 | 0.35 | 14.14 | ||||||
| CYP9J19 | AAEL006810 | 2.23 | 10.20 | 2.19 | 7.92 | 3.77 | 16.84 | ||||
| CYP9J24 | AAEL014613 | 2.02 | 11.01 | 2.20 | 4.95 | ||||||
| CYP9J26 | AAEL014609 | 2.23 | 19.84 | 2.33 | 9.23 | 2.43 | 6.97 | 2.34 | 4.42 | ||
| CYP9J32 | AAEL008638 | 6.86 | 18.56 | 2.15 | 14.08 | 2.79 | 7.19 | 2.50 | 22.52 | ||
| CCEs | |||||||||||
| COE-1 | AAEL003201 | 0.25 | 5.83 | 4.26 | 8.00 | 2.32 | 19.83 | ||||
| COE-2 | AAEL002376 | 2.70 | 16.62 | 2.41 | 16.79 | ||||||
| COE-19 | AAEL005112 | 2.87 | 18.63 | 2.30 | 13.54 | ||||||
| CCae1C | AAEL003195 | 0.22 | 8.52 | 2.08 | 13.37 | 3.74 | 10.99 | 2.95 | 18.09 | ||
| CCae2C | AAEL003196 | 0.33 | 7.84 | 2.87 | 15.50 | 3.88 | 11.86 | 2.97 | 20.21 | ||
| CCae3o | AAEL011944 | 0.30 | 13.81 | 3.23 | 11.17 | ||||||
| CCae4C | AAEL003187 | 0.25 | 13.80 | 0.17 | 14.87 | 0.22 | 18.30 | 0.21 | 16.86 | ||
| CCae5C | AAEL003201 | 0.13 | 13.84 | 4.00 | 11.74 | ||||||
| CCae6C | AAEL003198 | 3.30 | 10.38 | 2.30 | 19.72 | ||||||
| CCEjhe2o | AAEL004323 | 0.42 | 11.86 | 0.34 | 12.00 | ||||||
| RedOx | |||||||||||
| Glutathione peroxidase 495 | AAEL000495 | 2.23 | 13.28 | 2.57 | 19.20 | ||||||
| Superoxide Dismutase (Cu-Zn) | AAEL006271 | 0.43 | 13.07 | 0.18 | 10.93 | 0.14 | 13.99 | ||||
| AAGE1005255 | AAGE1005255 | 0.44 | 17.09 | 0.44 | 16.31 | ||||||
| Heme peroxidase 6014 | AAEL006014 | 9.03 | 15.40 | 5.24 | 11.31 | 0.49 | 14.18 | ||||
| Thioredoxin peroxidase 4112 | AAEL004112 | 0.44 | 5.47 | 0.38 | 12.71 | ||||||
| Thioredoxin peroxidase 2309 | AAEL002309 | 0.39 | 9.50 | 0.41 | 8.01 | ||||||
| Catalase | AAEL013407 | 0.38 | 12.69 | 2.10 | 13.79 | ||||||
| Table 3b. Genes differentially expressed in one of the unselected FS0 strains relative to the susceptible New Orleans strain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GSTs | |||||||||||
| AaGSTd1-3 | AAEL001061 | 2.07 | 15.87 | ||||||||
| AaGSTu2 | AAEL000092 | 0.36 | 13.98 | ||||||||
| CYPs | |||||||||||
| CYP304C1 | AAEL014413 | 0.44 | 7.54 | ||||||||
| CYP325AA1 | AAEL004012 | 2.07 | 8.33 | ||||||||
| CYP325E3 | AAEL000338 | 0.43 | 3.70 | ||||||||
| CYP325X2 | AAEL005696 | 3.23 | 16.33 | ||||||||
| CYP4D23 | AAEL007816 | 2.5 | 18.47 | ||||||||
| CYP4G36 | AAEL004054 | 0.43 | 10.41 | ||||||||
| CYP4H31 | AAEL002085 | 0.41 | 4.36 | ||||||||
| CYP4J14 | AAEL013554 | 2.72 | 19.68 | ||||||||
| CYP6AG7 | AAEL006989 | 2.68 | 20.85 | ||||||||
| CYP6BB | AAEL014893 | 3.04 | 9.64 | ||||||||
| CYP6F2 | AAEL014678 | 0.48 | 16.28 | ||||||||
| CYP6N16 | AAEL010151 | 0.41 | 17.09 | ||||||||
| CYP6N6 | AAEL009126 | 2.09 | 3.44 | ||||||||
| CYP6Y3 | AAEL009132 | 2.31 | 17.92 | ||||||||
| CYP6Z9 | AAEL009129 | 0.40 | 8.71 | ||||||||
| CYP9J10 | AAEL014614 | 2.00 | 16.21 | ||||||||
| CYP9J20 | AAEL006814 | 2.60 | 8.68 | ||||||||
| CYP9J22 | AAEL006802 | 2.71 | 16.15 | ||||||||
| CYP9J8 | AAEL014608 | 0.46 | 7.83 | ||||||||
| AAGE01273771 | AAEL009124 | 2.20 | 12.25 | ||||||||
| CCEs | |||||||||||
| COE-17 | AAEL004341 | 0.33 | 9.15 | ||||||||
| COE-6 | AAEL005200 | 2.54 | 8.91 | ||||||||
| RedOx | |||||||||||
| Peroxidasin 376 | AAEL000376 | 2.12 | 14.19 | ||||||||
| Peroxinectin 3612 | AAEL003612 | 0.39 | 5.61 | ||||||||
| Peroxinectin 4386 | AAEL004386 | 0.49 | 4.46 | ||||||||
| Peroxinectin 4388 | AAEL004388 | 0.33 | 7.52 | ||||||||
| Peroxinectin 4390 | AAEL004390 | 0.29 | 5.51 | ||||||||
| Peroxinectin 4400 | AAEL004400 | 0.37 | 5.53 | ||||||||
| Ubiquinol-cytochrome c reductase | AAEL007868 | 0.48 | 17.80 | ||||||||
| Oxidase/peroxidase 13171 | AAEL013171 | 0.47 | 7.83 | ||||||||
| Thioredoxin peroxidase 13528 | AAEL013528 | 0.43 | 19.04 | ||||||||
| Thioredoxin 9351 | AAEL009351 | 0.46 | 13.99 | ||||||||
| Misc. | |||||||||||
| 60S ribosomal protein L44 | AAEL003942 | 0.48 | 11.65 | ||||||||
| Ribosomal S17 | AAEL004175 | 0.45 | 16.69 | ||||||||
Genes that respond to a single generation of selection
Transcription patterns were next compared between FS1 and FS0 strains to identify genes that responded to one generation of selection (Table 4). Fifteen genes responded in two or more strains. These included one sigma class GST, three CYPs, one each in the CYP4, CYP6, CYP9 families, three carboxyl-, four choline- and one juvenile hormone esterase. A heme- and a glutathione peroxidase and a superoxide dismutase were among the RedOx genes. From 27–29 genes were differentially expressed in Calderitas and Iquitos as compared with only two in Lagunitas and Mérida. From 56–62% of these genes in Calderitas and Iquitos were up-regulated as compared to 40% that were up-regulated in the other strains.
Table 4.
| Table 4a. Genes differentially expressed in two or more FS1 strains relative to the unselected FS0. Ratio of the average expression of each comparison is displayed on a linear scale. Probability value is shown as a negative log10 scale.
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity Genes | Vector base ID | Iquitos | Calderitas | Lázaro-Cárdenas | Lagunitas | Solidaridad | Mérida | ||||||
|
| |||||||||||||
| Ratio | p | Ratio | p | Ratio | p | Ratio | p | Ratio | p | Ratio | p | ||
| GSTs | |||||||||||||
| GSTs1-2 | AAEL011741 | 0.34 | 13.72 | 0.47 | 6.33 | ||||||||
| CYPs | |||||||||||||
| CYP4H28 | AAEL003380 | 3.18 | 11.83 | 0.43 | 10.27 | 0.34 | 4.79 | ||||||
| CYP6F3 | AAEL014684 | 2.40 | 6.35 | 2.12 | 9.89 | ||||||||
| CYP9M9 | AAEL001807 | 1.99 | 6.16 | 2.32 | 8.93 | 2.03 | 6.90 | ||||||
| CCEs | |||||||||||||
| COE-1 | 7.89 | 9.98 | 2.92 | 11.09 | |||||||||
| COE-17 | AAEL004341 | 2.36 | 10.64 | 0.43 | 8.23 | ||||||||
| COE-20 | AAEL013543 | 2.86 | 9.31 | 2.25 | 5.14 | ||||||||
| CCae1C | AAEL003195 | 7.86 | 13.85 | 3.80 | 13.65 | 0.39 | 9.39 | ||||||
| CCae2C | AAEL003196 | 6.54 | 13.49 | 3.65 | 12.42 | 0.34 | 8.67 | ||||||
| CCae3o | AAEL011944 | 6.50 | 14.02 | 0.42 | 13.63 | ||||||||
| CCae6C | AAEL003198 | 4.18 | 13.15 | 2.02 | 6.65 | ||||||||
| CCEjhe2o | AAEL004323 | 2.38 | 9.75 | 2.15 | 6.95 | 0.28 | 7.51 | 5.36 | 6.12 | ||||
| RedOx | |||||||||||||
| Heme peroxidase 6014 | AAEL006014 | 2.53 | 10.08 | 0.20 | 15.09 | 3.68 | 13.56 | ||||||
| Glutathione peroxidase 495 | AAEL000495 | 2.77 | 11.40 | 0.41 | 13.63 | 0.47 | 8.57 | ||||||
| Superoxide Dismutase (Cu-Zn) | AAEL006271 | 4.16 | 12.29 | 0.45 | 11.91 | 0.27 | 8.14 | ||||||
| Misc. | |||||||||||||
| 60S ribosomal protein L44 | AAEL003942 | 0.39 | 10.97 | 0.49 | 4.91 | ||||||||
| Table 4b. Genes differentially expressed in one of the FS1 strains relative to the unselected FS0 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSTs | |||||||||||||
| GSTd1-1 | AAEL001061 | 0.33 | 15.82 | ||||||||||
| GSTd1-3 | AAEL001061 | 0.40 | 13.79 | ||||||||||
| GSTt3 | AAEL009020 | 0.34 | 8.46 | ||||||||||
| CYPs | |||||||||||||
| CYP18A1 | AAEL004870 | 0.41 | 5.68 | ||||||||||
| CYP306A1 | AAEL004888 | 0.47 | 9.81 | ||||||||||
| CYP4H31 | AAEL002085 | 6.54 | 11.00 | ||||||||||
| CYP4J15 | AAEL013556 | 0.42 | 1.43 | ||||||||||
| CYP6AG3 | AAEL007024 | 0.42 | 7.34 | ||||||||||
| CYP6AG4 | AAEL007010 | 0.42 | 9.28 | ||||||||||
| CYP6AG7 | AAEL006989 | 2.80 | 11.01 | ||||||||||
| CYP6Z9 | AAEL009129 | 0.47 | 7.86 | ||||||||||
| CYP9J22 | AAEL014619 | 2.14 | 8.40 | ||||||||||
| CYP9J24 | AAEL014613 | 2.09 | 7.46 | ||||||||||
| CYP9J28 | AAEL014617 | 0.35 | 14.58 | ||||||||||
| CYP9J8 | AAEL014608 | 2.04 | 10.32 | ||||||||||
| CYP9J9 | AAEL014605 | 2.06 | 7.24 | ||||||||||
| AAGE01273771 | AAEL009124 | 4.18 | 7.52 | ||||||||||
| CCEs | |||||||||||||
| COE-18 | AAEL005113 | 2.35 | 10.32 | ||||||||||
| COE-19 | AAEL005112 | 3.08 | 10.69 | ||||||||||
| COE-2 | AAEL002376 | 2.92 | 8.08 | ||||||||||
| COE-9 | AAEL012509 | 2.10 | 8.10 | ||||||||||
| CCae4C | AAEL003187 | 3.29 | 10.96 | ||||||||||
| CCae5C | AAEL003201 | 32.20 | 18.84 | ||||||||||
| RedOx | |||||||||||||
| Aldehyde oxidase 10384 | AAEL010384 | 0.48 | 7.44 | ||||||||||
| Aldehyde oxidase 14493 | AAEL014493 | 0.49 | 8.58 | ||||||||||
| Aldo-keto reductase 3154 | AAEL003154 | 2.01 | 8.29 | ||||||||||
| Aldo-keto reductase 7275 | AAEL007275 | 2.12 | 8.83 | ||||||||||
| Catalase | AAEL013407 | 3.02 | 11.16 | ||||||||||
| Dihydrolipoamide dehydrogenase | AAEL006928 | 0.49 | 5.76 | ||||||||||
| Heme peroxidase 4401 | AAEL004401 | 0.30 | 15.85 | ||||||||||
| Superoxide Dismutase (Mn-Fe) | AAEL004823 | 0.43 | 9.45 | ||||||||||
| Oxidase/peroxidase 5416 | AAEL005416 | 2.01 | 3.90 | ||||||||||
| Oxidase/peroxidase 11941 | AAEL011941 | 0.44 | 11.57 | ||||||||||
| Oxidase/peroxidase 12481 | AAEL012481 | 0.49 | 11.79 | ||||||||||
| Peroxinectin 3612 | AAEL003612 | 0.41 | 10.94 | ||||||||||
| Peroxiredoxin 37252 | AAEL037254 | 0.46 | 7.61 | ||||||||||
| Peroxiredoxin 7135 | AAEL007135 | 0.36 | 12.24 | ||||||||||
| Thioredoxin peroxidase 2309 | AAEL002309 | 4.35 | 7.03 | ||||||||||
| Thioredoxin peroxidase 8291 | AAEL008291 | 0.45 | 11.27 | ||||||||||
| Ubiquinol-cytochrome c reductase | AAEL007868 | 0.37 | 11.62 | ||||||||||
| Misc. | |||||||||||||
| 60S ribosomal protein P1 | AAEL005027 | 0.49 | 12.78 | ||||||||||
| Ribosomal S17 | AAEL004175 | 0.43 | 10.79 | ||||||||||
Genes that respond to four additional generations of selection
Transcription patterns were next compared between FS1 and FS5 generations to identify genes that respond to long term selection (Table 5). Sixteen genes responded in two or more strains. This included two GSTs, eight CYPs, one carboxyl-, one choline- and one juvenile hormone esterase. A heme- and a thioredoxin peroxidase and an aldehyde oxidase were among the RedOx genes. Most notably CYP4H28 was differentially expressed in all six strains but was down regulated in Iquitos, Lázaro-Cárdenas and Lagunitas and upregulated in Calderitas, Solidaridad and Mérida. Aldehyde oxidase 10382 was down regulated in four strains. The numbers of genes that responded to long term selection varied among strains. From 8–11 genes were differentially expressed in Iquitos, Calderitas, Lagunitas and Mérida. But 19 were differentially expressed in in Solidaridad and 23 in Lázaro-Cárdenas. In Iquitos, only four of the eight genes were upregulated and these were all epsilon GSTs.
Table 5.
| Table 5a. Genes differentially expressed in two or more FS5 strains relative to FS1. Ratio of the average expression of each comparison is displayed in a linear scale. Probability value is shown as a negative log10 scale.
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity Genes | Vector base ID | Iquitos | Calderitas | Lázaro-Cárdenas | Lagunitas | Solidaridad | Mérida | ||||||
|
| |||||||||||||
| Ratio | p | Ratio | p | Ratio | p | Ratio | p | Ratio | p | Ratio | p | ||
| GSTs | |||||||||||||
| GSTt3 | AAEL009020 | 0.48 | 18.30 | 0.39 | 14.71 | 3.01 | 10.32 | ||||||
| GSTu2 | AAEL000092 | 2.21 | 17.10 | 0.36 | 13.14 | ||||||||
| CYPs | |||||||||||||
| CYP4H28 | AAEL003380 | 0.10 | 15.75 | 2.13 | 10.83 | 0.47 | 9.11 | 0.44 | 8.64 | 2.42 | 11.39 | 13.80 | 15.12 |
| CYP9J10 | AAEL006798 | 0.38 | 14.96 | 2.46 | 11.31 | ||||||||
| CYP9J20 | AAEL006814 | 0.37 | 14.39 | 0.44 | 16.05 | ||||||||
| CYP9J22 | AAEL006802 | 0.41 | 13.52 | 2.48 | 10.31 | ||||||||
| CYP9J24 | AAEL014613 | 2.15 | 13.14 | 2.45 | 10.49 | 0.35 | 15.70 | ||||||
| CYP9J32 | AAEL008846 | 2.12 | 14.53 | 2.12 | 13.08 | ||||||||
| AAGE01273771 | AAEL009124 | 0.40 | 7.35 | 0.35 | 4.39 | ||||||||
| CCEs | |||||||||||||
| COE-8 | AAEL008757 | 2.34 | 11.75 | 0.35 | 12.80 | ||||||||
| CCae4C | AAEL003187 | 2.23 | 10.47 | 2.05 | 10.31 | ||||||||
| CCEjhe2o | AAEL004323 | 0.36 | 3.45 | 0.50 | 6.20 | ||||||||
| RedOx | |||||||||||||
| Heme peroxidase 6014 | AAEL006014 | 0.49 | 10.62 | 0.48 | 4.14 | 0.25 | 6.22 | ||||||
| Aldehyde oxidase 10382 | AAEL010382 | 0.47 | 5.77 | 0.35 | 11.16 | 0.37 | 6.07 | 0.40 | 12.22 | ||||
| Thioredoxin peroxidase | AAEL004112 | 2.68 | 13.54 | 0.50 | 5.07 | ||||||||
| Table 5b. Genes differentially expressed in one of the unselected FS5 lines relative to FS1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSTs | |||||||||||||
| GSTe2 | AAEL007951 | 2.45 | 18.27 | ||||||||||
| GSTe3 | AAEL007947 | 2.85 | 11.23 | ||||||||||
| GSTe4 | AAEL007962 | 3.07 | 22.70 | ||||||||||
| GSTe7 | AAEL007948 | 4.12 | 12.28 | ||||||||||
| GSTs1-1 | AAEL011741 | 0.44 | 14.49 | ||||||||||
| CYPs | |||||||||||||
| CYP304B3 | AAEL014411 | 0.42 | 6.04 | ||||||||||
| CYP304C1 | AAEL014413 | 0.44 | 10.40 | ||||||||||
| CYP325X2 | AAEL005696 | 2.29 | 12.71 | ||||||||||
| CYP329B1 | AAEL003763 | 2.65 | 13.60 | ||||||||||
| CYP4D23 | AAEL007816 | 0.46 | 13.09 | ||||||||||
| CYP4D24 | AAEL007815 | 0.48 | 13.23 | ||||||||||
| CYP6AG7 | AAEL006989 | 2.13 | 10.80 | ||||||||||
| CYP6Nae1 | AAEL009126 | 0.47 | 9.90 | ||||||||||
| CYP6Z7 | AAEL009130 | 2.21 | 8.52 | ||||||||||
| CYP9J8 | AAEL014608 | 0.34 | 15.45 | ||||||||||
| CYP9J9 | AAEL014605 | 0.36 | 15.39 | ||||||||||
| CYP9M9 | AAEL001807 | 2.31 | 12.23 | ||||||||||
| CCEs | |||||||||||||
| COE-2 | AAEL002376 | 2.51 | 12.88 | ||||||||||
| COE-6 | AAEL005200 | 0.44 | 6.42 | ||||||||||
| COE-19 | AAEL005112 | 2.43 | 11.84 | ||||||||||
| COE-20 | AAEL013543 | 2.35 | 15.16 | ||||||||||
| COE-22 | AAEL005101 | 2.29 | 4.93 | ||||||||||
| CCae3o | AAEL011944 | 0.36 | 11.91 | ||||||||||
| CCae5C | AAEL003201 | 2.60 | 12.74 | ||||||||||
| RedOx | |||||||||||||
| Glutathione peroxidase 495 | AAEL000495 | 2.90 | 15.67 | ||||||||||
| Aldo-keto reductase 4102 | AAEL004102 | 0.49 | 7.41 | ||||||||||
| Oxidase/peroxidase 5416 | AAEL005416 | 0.46 | 9.50 | ||||||||||
| Superoxide Dismutase(Cu-Zn) | AAEL006271 | 3.13 | 13.35 | ||||||||||
| Glutathione peroxidase 8397 | AAEL008397 | 0.50 | 11.08 | ||||||||||
| Heme peroxidase 13171 | AAEL013171 | 0.50 | 13.20 | ||||||||||
| Catalase | AAEL013407 | 0.47 | 13.03 | ||||||||||
| Thioredoxin peroxidase 2309 | AAEL002309 | 0.30 | 6.28 | ||||||||||
| Misc. | |||||||||||||
| Ribosomal_S17 | AAEL004175 | 2.54 | 10.27 | ||||||||||
Gene expression following five generations of selection relative to New Orleans
Transcription following five generations of selection was compared among all strains relative to NO to identify genes commonly upregulated in all strains (Table 6). The most evident trend in this analysis was the uniform upregulation of five of the epsilon class GSTs: GSTe2, -e3, -e4, -e6, and -e7. GSTe2 was upregulated from 2.2–3.2 fold in four strains, GSTe3 from 2.6–8.8 fold in five strains, and GSTe4 from 3.6–5.7 among three strains. GSTe6 and GSTe7 were upregulated in four strains from 2.2–3.8 and 2.7–7.0 respectively. All five were upregulated in Iquitos, Lázaro-Cárdenas and Lagunitas, three in Mérida and two in Calderitas.
Table 6.
| Table 6a. Genes differentially expressed in two or more FS5 strains relative to New Orleans. Ratio of the average expression of each comparison is displayed in a linear scale. Probability value is shown as a negative log10 scale.
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity Genes | Vector base ID | Iquitos | Calderitas | Lázaro-Cárdenas | Lagunitas | Mérida | |||||
|
| |||||||||||
| Ratio | p | Ratio | p | Ratio | p | Ratio | p | Ratio | p | ||
| GSTs | |||||||||||
| GSTd1-2 | AAEL001061 | 2.20 | 4.12 | 3.73 | 5.14 | ||||||
| GSTe2 | AAEL007951 | 3.12 | 22.49 | 3.12 | 25.13 | 3.18 | 15.23 | 2.16 | 14.82 | ||
| GSTe3 | AAEL007947 | 8.82 | 19.77 | 7.36 | 21.05 | 2.55 | 15.65 | 5.78 | 13.09 | 4.38 | 24.69 |
| GSTe4 | AAEL007962 | 5.74 | 19.56 | 4.11 | 27.92 | 3.63 | 20.23 | ||||
| GSTe6 | AAEL007946 | 2.23 | 11.48 | 2.87 | 25.27 | 3.81 | 19.57 | 2.99 | 20.58 | ||
| GSTe7 | AAEL007948 | 7.01 | 25.73 | 2.69 | 19.16 | 3.78 | 31.04 | 6.11 | 14.89 | ||
| GSTs1-1 | AAEL011741 | 2.58 | 21.88 | 0.23 | 24.17 | ||||||
| GSTs1-2 | AAEL011741 | 0.44 | 6.12 | 2.14 | 2.58 | 0.46 | 8.31 | ||||
| GSTt3 | AAEL009020 | 0.48 | 12.36 | 0.23 | 23.23 | 3.71 | 11.28 | ||||
| CYPs | |||||||||||
| CYP325X2 | AAEL005696 | 3.39 | 19.51 | 3.94 | 19.57 | ||||||
| CYP4H28 | AAEL003380 | 0.04 | 23.97 | 0.10 | 25.06 | 0.32 | 10.11 | 12.30 | 15.47 | ||
| CYP4J14 | AAEL013554 | 2.35 | 11.84 | 2.14 | 10.75 | ||||||
| CYP4J15 | AAEL013556 | 2.71 | 13.82 | 2.57 | 11.96 | ||||||
| CYP6AG7 | AAEL006989 | 0.50 | 15.17 | 2.87 | 16.58 | ||||||
| CYP6F3 | AAEL014684 | 2.64 | 21.23 | 2.53 | 21.67 | ||||||
| CYP6N11 | AAEL009138 | 0.46 | 6.72 | 0.49 | 6.70 | ||||||
| CYP6Z8 | AAEL009131 | 2.41 | 23.66 | 2.23 | 20.24 | ||||||
| CYP9J10 | AAEL014614 | 0.31 | 21.02 | 2.53 | 12.73 | ||||||
| CYP9J22 | AAEL006802 | 0.46 | 11.58 | 4.33 | 14.70 | ||||||
| CYP9J24 | AAEL014613 | 2.99 | 18.94 | 0.30 | 19.10 | ||||||
| CYP9J26 | AAEL014609 | 2.17 | 24.12 | 2.03 | 7.56 | ||||||
| CYP9J32 | AAEL008638 | 2.48 | 15.73 | 3.73 | 28.75 | ||||||
| CYP9J8 | AAEL014608 | 0.33 | 16.10 | 0.36 | 17.93 | ||||||
| CYP9J9 | AAEL014605 | 0.46 | 18.95 | 0.37 | 15.81 | ||||||
| CYP9M9 | AAEL001807 | 2.36 | 12.55 | 11.24 | 29.73 | 3.10 | 19.24 | ||||
| AAGE01273771 | AAEL009124 | 0.31 | 15.68 | 0.33 | 7.09 | ||||||
| CCEs | |||||||||||
| COE-1 | AAEL003201 | 0.28 | 6.14 | 3.29 | 8.41 | ||||||
| COE-2 | AAEL002376 | 2.25 | 17.65 | 3.41 | 18.88 | ||||||
| COE-8 | AAEL008757 | 2.11 | 15.79 | 0.35 | 12.79 | ||||||
| COE-19 | AAEL005112 | 2.08 | 8.97 | 3.20 | 18.79 | ||||||
| CCae1C | AAEL003195 | 0.43 | 4.58 | 4.79 | 15.17 | ||||||
| CCae2C | AAEL003196 | 6.06 | 17.68 | 2.58 | 19.82 | ||||||
| CCae3o | AAEL011944 | 0.49 | 8.76 | 0.45 | 8.83 | 2.06 | 6.46 | ||||
| CCae4C | AAEL003187 | 0.17 | 19.49 | 0.28 | 15.19 | ||||||
| CCae5C | AAEL003201 | 0.19 | 16.79 | 4.11 | 12.32 | 2.35 | 10.12 | ||||
| CCEjhe2o | AAEL004323 | 0.30 | 11.00 | 0.16 | 15.17 | 0.30 | 10.12 | ||||
| RedOx | |||||||||||
| Superoxide Dismutase (Cu-Zn) | AAEL006271 | 0.46 | 13.47 | 0.30 | 19.08 | 0.11 | 19.60 | ||||
| Aldehyde oxidase 10382 | AAEL010382 | 2.19 | 16.41 | 0.22 | 13.77 | 0.38 | 11.81 | ||||
| Aldo-keto reductase 4102 | AAEL004102 | 2.11 | 14.99 | 0.48 | 5.82 | 0.48 | 5.82 | ||||
| Glutathione peroxidase 495 | AAEL000495 | 2.14 | 15.02 | 8.17 | 29.64 | 2.16 | 12.56 | 2.01 | 15.00 | ||
| Heme peroxidase 6014 | AAEL006014 | 0.47 | 9.61 | 5.31 | 15.21 | 0.44 | 3.89 | ||||
| Heme peroxidase 13171 | AAEL013171 | 0.34 | 18.85 | 0.41 | 17.65 | ||||||
| Thioredoxin peroxidase 4112 | AAEL004112 | 2.46 | 12.67 | 0.48 | 8.51 | 0.40 | 8.12 | ||||
| Thioredoxin peroxidase 2309 | AAEL002309 | 0.50 | 8.82 | 0.30 | 11.47 | ||||||
| Table 6b. Genes differentially expressed in one of the FS5 strains relative to New Orleans. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GSTs | |||||||||||
| GSTd1-1 | AAEL001061 | 2.39 | 22.65 | ||||||||
| GSTu2 | AAEL000092 | 0.34 | 6.95 | ||||||||
| GSTu3 | AAEL010500 | 2.73 | 23.83 | ||||||||
| CYPs | |||||||||||
| CYP304C1 | AAEL014413 | 0.49 | 6.88 | ||||||||
| CYP325AA1 | AAEL004012 | 2.22 | 14.86 | ||||||||
| CYP329B1 | AAEL003763 | 3.78 | 19.18 | ||||||||
| CYP4C38 | AAEL012266 | 0.35 | 6.26 | ||||||||
| CYP4D23 | AAEL007816 | 2.85 | 9.61 | ||||||||
| CYP4H29 | AAEL007830 | 0.34 | 15.44 | ||||||||
| CYP4H31 | AAEL002085 | 0.43 | 4.46 | ||||||||
| CYP6BB2 | AAEL014893 | 2.43 | 20.82 | ||||||||
| CYP6Nae1 | AAEL009126 | 0.44 | 10.29 | ||||||||
| CYP6P12 | AAEL014891 | 0.45 | 6.81 | ||||||||
| CYP6Z6 | AAEL009123 | 0.36 | 22.65 | ||||||||
| CYP6Z7 | AAEL009130 | 2.53 | 9.47 | ||||||||
| CYP6Z9 | AAEL009129 | 0.39 | 9.54 | ||||||||
| CYP9J15 | AAEL006795 | 0.42 | 15.64 | ||||||||
| CYP9J20 | AAEL006814 | 0.29 | 18.87 | ||||||||
| CYP9J23 | AAEL014615 | 2.07 | 3.49 | ||||||||
| CCEs | |||||||||||
| COE-11 | AAEL010389 | 2.73 | 13.27 | ||||||||
| COE-17 | AAEL004341 | 0.36 | 10.83 | ||||||||
| COE-18 | AAEL005113 | 2.30 | 19.32 | ||||||||
| COE-20 | AAEL013543 | 3.84 | 23.30 | ||||||||
| RedOx | |||||||||||
| Superoxide Dismutase (Cu-Zn) | AAEL000274 | 0.40 | 19.71 | ||||||||
| Heme peroxidase 4388 | AAEL004388 | 0.44 | 5.83 | ||||||||
| Heme peroxidase 4390 | AAEL004390 | 0.40 | 5.36 | ||||||||
| Heme peroxidase 4400 | AAEL004400 | 0.43 | 7.07 | ||||||||
| Oxidase/peroxidase 5416 | AAEL005416 | 0.41 | 10.17 | ||||||||
| Dihydrolipoamide dehydro. | AAEL006928 | 0.40 | 18.61 | ||||||||
| Glutathione peroxidase 8397 | AAEL008397 | 0.40 | 15.65 | ||||||||
| Ubiquinol-cytochrome c reductase | AAEL010801 | 0.49 | 13.49 | ||||||||
| Oxidase/peroxidase 11941 | AAEL011941 | 2.13 | 17.25 | ||||||||
| Glutathione peroxidase 12069 | AAEL012069 | 2.77 | 17.28 | ||||||||
| Glutaredoxin 14064 | AAEL014064 | 0.45 | 13.73 | ||||||||
| Aldehyde oxidase 14493 | AAEL014493 | 0.45 | 17.91 | ||||||||
| Catalase | AAEL013407 | 0.41 | 15.00 | ||||||||
| Peroxiredoxin 7135 | AAEL007135 | 0.36 | 16.95 | ||||||||
| Thioredoxin 10777 | AAEL010777 | 2.51 | 19.77 | ||||||||
| Thioredoxin reductase 2886 | AAEL002886 | 0.47 | 12.99 | ||||||||
No gene or group of genes emerged from analysis of the 33 CYP genes found differentially expressed in one or more strains. CYP4H28 was differentially expressed in four strains but was down regulated in three and upregulated in one. CYP9M9 was upregulated in three strains. A similar trend was noted among the 13 esterase genes found differentially expressed in one or more strains. CCEjhe2o was down regulated in three strains. Similarly, among 24 RedOx genes that were differentially expressed in one or more strains, only glutathione peroxidase 495 was upregulated in four strains.
Cross resistance
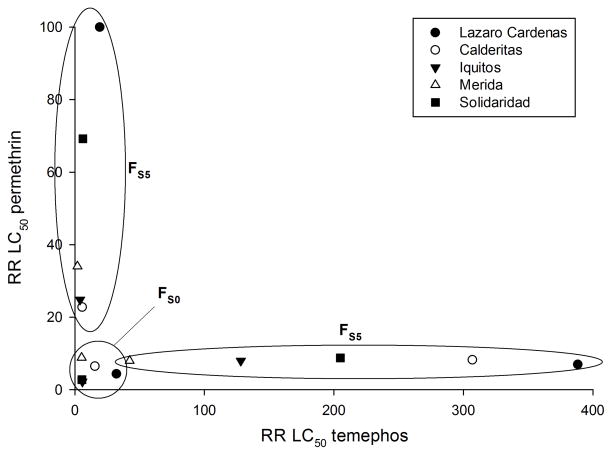
The LC50 and RR with respect to NO for temephos was calculated for all FS0 and FS5 mosquitoes using NO as a standard susceptible control (Table 1). Our previous study (Saavedra-Rodriguez et al. 2012) calculated the LC50 and RR for permethrin in the FS0 and permethrin selected FS5 mosquitoes. Figure 3 plots the RR of the LC50 with temephos along the abscissa and the RR of the LC50 with permethrin along the ordinate axis. Points in the circle close to the origin represent resistance in FS0 mosquitoes. Points within the oval oriented along the abscissa represent resistance in the temephos selected FS5 mosquitoes ranged over an order of magnitude from ~40 for the Mérida strain to ~400 for the Lázaro-Cárdenas strain. Points within the vertical oval oriented along the ordinate represent resistance in the permethrin selected FS5 mosquitoes. These varied five-fold from ~20 for the Calderitas strain up to ~100 for the Lázaro-Cárdenas strain. Cross resistance would be manifested as points located between the two ovals and would appear somewhere in the center of this graph but instead all points are near the abscissa or ordinate.
Figure 3.
Resistance ratio (RR) of the LC50 with temephos along the abscissa and the RR of the LC50 with permethrin along the ordinate axis. Points in the circle close to the origin represent resistance in FS0 mosquitoes. Points within the oval along the abscissa represent resistance in the temephos selected FS5 mosquitoes from (Saavedra-Rodriguez et al. 2012). Points within the vertical oval oriented along the ordinate all represent resistance in the permethrin selected FS5 mosquitoes.
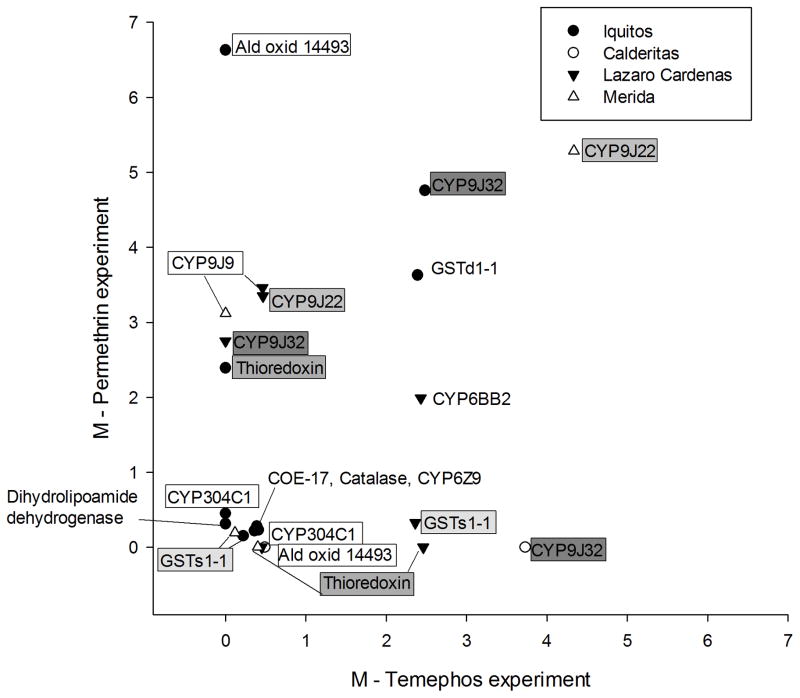
All of the genes in Table 6 were compared with all of the genes with significant differential expression between the FS5 vs. NO adults used in the permethrin experiment (Saavedra-Rodriguez et al. 2012) to identify genes with differential expression in both permethrin and temephos experiments. Of the 85 differentially transcribed genes in the present experiment (Table 6) and the 70 differentially transcribed genes in the permethrin experiment, there were 13 genes with differential expression in both experiments but only in four of the collections. M values for these genes are plotted in Figure 4. Eight of the 13 were regulated in the same direction in both experiments. These were: 1) GSTs1-1 which was downregulated in both Mérida and Iquitos, 2) GSTd1-1 and 3) CYP9J32 which were upregulated in Iquitos 4) CYP6Z9, 5) Catalase and 6) COE-17 which were downregulated in Iquitos, 7) CYP6BB2 which was upregulated in Lázaro-Cárdenas in both experiments and 8) CYP9J22 which was upregulated in Mérida. However, in each of these 8 cases the trend is inconsistent. For example Figure 4 shows that GSTs1-1 in Lázaro-Cárdenas was upregulated by temephos selection but was downregulated with permethrin. CYP9J32 was upregulated with permethrin selection but unchanged by temephos selection in Lázaro-Cárdenas. Conversely CYP9J32 was upregulated with temephos selection but unchanged by permethrin selection in Calderitas. CYP9J22 was upregulated with permethrin selection but downregulated by temephos selection in Lázaro-Cárdenas. In general, there was little or no consistent evidence of cross selection for the majority of the differentially transcribed genes in both experiments.
Figure 4.
The expression ratios (M) of genes with differential expression in both permethrin (Saavedra-Rodriguez et al. 2012) and the current temephos selection experiments. There are thirteen genes with differential expression in both experiments but only in four of the collections. Genes represent in more than one collection appear in boxes. Boxes were shaded in grade to assist in identification of the same gene.
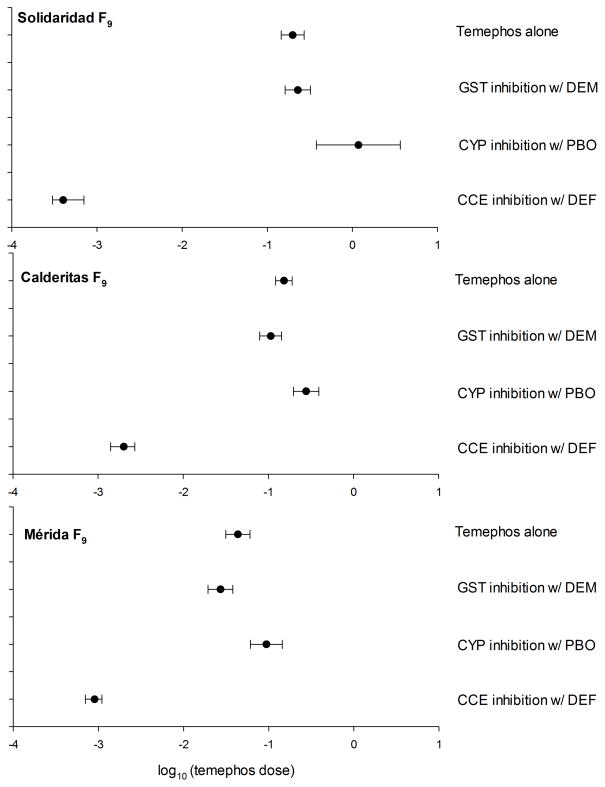
Bioassays using inhibitors
The F9 generation from three of the temephos selected strains (Solidaridad F9, Calderitas F9 and Mérida F9) that were released from selection after FS5 were bioassayed using inhibitors for CCE, CYP and GST systems. Releasing strains from temephos pressure resulted in an immediate reduction in temephos resistance for all tested strains. Mérida FS5 was 45 fold more resistant than NO while Mérida F9 was only 13 times more resistant than NO, resulting in a 32 fold resistance reduction. Calderitas FS5 was 105 fold more resistant than NO while Calderitas F9 was only 40 fold more resistant. Finally, Solidaridad FS5 to F9 resulted in a resistance reduction from 78 to 60 fold relative to NO.
We compared the LC50 values for temephos alone and for temephos + inhibitor in each of the resistant strains (Table 7). Figure 5 shows the LC50 obtained from Solidaridad F9, Calderitas F9 and Mérida F9 strains using different inhibitor treatments. LC50 decreased dramatically when using the temephos + DEF treatment in Solidaridad F9, Calderitas F9 and Mérida F9 (497, 76 and 48 fold respectively, relative to NO). CCE inhibition resulted in mortality within 45 minutes of exposure in Solidaridad F9 and Calderitas F9. In comparison, temephos alone caused mortality only after 2 hours of exposure. With the GST inhibitor (DEM), LC50 for Calderitas F9 and Mérida F9 decreased slightly (0.7 and 0.6 fold) and for Solidaridad F9 the LC50 was 1.14 fold higher. Applying the CYP inhibitor (PBO) LC50 values increased 5.9, 1.8, and 2.1 fold in Solidaridad F9, Calderitas F9 and Mérida F9, respectively.
Table 7.
Temephos LC50 (μg/ml) among larvae treated with a dose of temephos as compared with larvae pre-treated with one of three inhibitors followed by a dose of temephos. Synergism ratio (SRLC50) corresponds to temephos LC50 divided by the inhibitor-treatment LC50.
| Treatment | Inhibitor | Target Enzymes | Solidaridad F9 | Calderitas F9 | Mérida F9 | New Orleans | |
|---|---|---|---|---|---|---|---|
| Temephos | None | 0.1989 (0.1457–0.2715) | 0.1520 (0.1214–0.1901) | 0.0434 (0.0313–0.0602) | 0.0033 (0.0026–0.0041) | ||
| Temephos | DEM 150 μg/ml | GST | 0.2276 (0.1619–0.3199) | 0.1065 (0.0791–0.1432) | 0.0271 (0.0194–0.0378) | ||
| SRLC50 | 0.8739 | 1.4272 | 1.601 | ||||
| Temephos | PBO 5 μg/ml | CYP | 1.1754 (0.3769–3.664) | 0.2773 (0.1965–0.3913) | 0.0936 (0.0608–0.1440) | ||
| SRLC50 | 0.1692 | 0.5481 | 0.4636 | ||||
| Temephos | DEF 1.5 μg/ml | CCE | 0.0004 (0.0003–0.0007) | 0.0020 (0.0014–0.0027) | 0.0009 (0.0007–0.0011) | ||
| SRLC50 | 497.25 | 76.0 | 48.22 |
Figure 5.
Effect of inhibitors of esterases (DEF), cytochrome P450 oxidases (PBO) and glutathione transferases (DEM) on temephos LC50.
Discussion
In general, transcription of ‘Detox’ genes changes during temephos selection under laboratory conditions. Initially, moderate to high temephos resistance profiles were identified among unselected Ae. aegypti collections. This variation may reflect insecticide pressure implemented by local vector control campaigns in these regions. Transcriptional differences were observed among several CYP9, epsilon GSTs, CCE-C and RedOx genes in these FS0 collections. We found no evidence suggesting that higher levels of temephos resistance in the FS0 strains were associated with a larger number of up regulated or down regulated genes. For example, Mérida with the lowest LC50 exhibited similar patterns as the most resistant FS0 strains. This may reflect the presence of detoxification genes not yet annotated or included in the ‘Detox Chip’. It might also reflect the presence of an altered AChE. However, Flores et al. (2006) found no evidence of an altered AChE activity in these strains, Furthermore, altered AChE is rarely found in field collected Ae. aegypti. We amplified exon regions of the ace1 gene and identified only two non-synonymous point mutations at exon 1 (Reyes-Solis unpublished); however, we could not associate the frequency of these mutations with temephos resistance in our strains. It is still unknown if mutations in the ace2 gene are present in Ae. aegypti.
The responses to one generation of temephos selection varied greatly among strains. No differential transcription was detected in Mérida FS1 even though their LC50 were ~10x fold greater than NO. In contrast, the Iquitos FS1 responded with similar resistance ratio and exhibited upregulation of 18 genes and downregulation of nine genes. Other strains, like Calderitas exhibited a ~25 fold increase in resistance relative to NO and exhibited upregulation of nine CCE and three CYP9 genes. Lagunitas with ~145 fold higher resistance than NO actually exhibited down regulation of nine RedOx genes. Five strains exhibited up regulation of at least nine CCE gene members, GSTe3, CYP9M9, CYP9J32, CYP6F3 and at least three members of the CYP9J family.
After four additional generations of temephos selection we obtained 1.7 to 15.5 fold (42 – 380 fold relative to NO) levels of resistance. Nonetheless, genes responding to selection continued to vary among strains. For example, Mérida FS5 was the least responsive strain, with only four additional genes upregulated and four downregulated. Four epsilon GSTs were upregulated in Iquitos FS5. COE, CCE-4C, CYP9M9, CYP9J32 and two RedOx genes were upregulated in Lázaro-Cárdenas FS5.
In general, there was no common pattern of gene transcription among resistant strains. In an attempt to compare the transcriptional patterns among strains we made an indirect comparison using NO as a baseline. This analysis supports the role of epsilon-GSTs, CYP9J32, CYP9M9, COE and glutathione peroxidase 495 during temephos selection. Otherwise there was no common pattern of gene transcription among resistant strains.
Complex transcriptional changes among members of the CYP, CCE and GST gene families may reflect the many metabolic pathways involved in temephos toxicology, including mechanisms of activation and detoxification (Wilkinson, 1971; Dauterman, 1971; Hemingway and Karunaratne 1998; Roberts and Hutson, 1999). Further, these results suggest that evolution of resistance may reflect interactions among all three metabolic groups.
The three gene families responding to temephos selection belong to the CYP9J, CCE-C and epsilon GST. Members of the CYP9J family were upregulated previous to selection in the Mexican strains. These genes belong to a genomic cluster at the q arm of chromosome III and some gene products have demonstrated pyrethroid metabolizing activity (e.g. CYP9-J24, -J26, -J28) (Stevenson et al. 2012). On the other hand CYP9J32 and CYP9M9 are located at chromosome II. CYP9J32 has been shown to play a role in pyrethroid metabolism (Stevenson et al. 2012) but this gene was also consistently upregulated during temephos selection. Its role in temephos detoxification requires further study.
A second gene family appearing at different times of selection was the alpha esterase CCE-C family. This group consists in 6 genes (−1C to −6C) organized sequentially in the same genomic region (supercontig 1.81). The role of these genes in insecticide resistance is still unknown. Two CCE genes previously associated with OP resistance in Culex mosquito’s worldwide (Mouches et al. 1990; Vaughan et al. 1997) have clear orthologous in Ae. aegypti (CCEae1D and 2D) (Strode et al. 2008), however, we did not found expression changes in any of these genes. Finally, a much more specific pattern was noted with the eGSTs. This family was upregulated in the Mexican strains before temephos selection, however, Iquitos responded to selection with consistent upregulation of GSTe2, -e3, -e4, -e6, and -e7. Epsilon GSTs are a large, insect-specific class that is known to play an important role in insecticide detoxification (Huang et al. 1998; Ortelli et al. 2003; Wei et al. 2001). Most recently, Lumjuan et al. 2007, characterized the eight epsilon GST genes in Ae. aegypti. These genes are sequentially arranged over ~54 kb on chromosome II and GSTe2 was particularly associated with resistance to DDT. Lumjuan et al. (2011) examined the activity of recombinant eGSTs and the inhibition activity against the model substrate 1-chloro-2,4-dinitrobenzene (CDNB) with eleven different insecticides including temephos. GSTE3, -E4, -E5, -E7 and -E8 were inhibited with temephos by 100, 35, 77, 26 and 17% respectively. These results suggest that temephos may bind at the CDNB active site and this binding may in turn lead to sequestration of temephos followed by export from cells or tissues as a mechanism of resistance (Kostaropoulos et al. 2001). On the other hand, o-dealkylation by glutathione s-alkyl transferase (GSAT) has been reported as a major metabolic pathway for organophosphate metabolism in mammals, insects and plants (Hollingworth, 1971; Dauterman, 1971) and GSTs with glutathione peroxidase activity can protect against insecticide induced oxidative stress. We found glutathione peroxidase 495 to be upregulated in four strains.
We performed inhibitor bioassays to identify the role of GST, CYP and CCE in three of the temephos selected strains. Contrary to our expectations, GST inhibition using diethyl maleate (DEM) did not significantly reduce the LC50. This result suggests eGSTs are not involved in primary detoxification but might instead be involved in conjugation of secondary metabolites. Inhibition of the CYP system using PBO resulted in a 2–6 fold increase in temephos LC50. This suggests the possibility that temephos is activated by the CYP system. For instance CYP inhibition would result in lesser amounts of toxic temephos (temephos sulfoxide) and lower AChE inhibition. Whether activation or detoxification is implemented by different members of CYP needs further research.
CCE inhibition by DEF resulted in a 48–470 fold LC50 decrease among resistant strains, strongly supporting CCE as the main protection mechanism. This CCE activity can be correlated with upregulation of the CCE-C and COE gene members at different points of selection, however, we did not detect a clear upregulation pattern on these genes in the FS5 comparison. Whether resistance results from coordinated overexpression of CCE-C genes that occur in gene clusters or from specific CCE/COE genes with high catalytic activity requires further attention.
We found no evidence for cross resistance between permethrin and temephos. Neither the patterns of resistance ratios of permethrin against temephos selected lines nor resistance of temephos against permethrin selected lines exhibited a cross resistance phenotype (Fig. 3). Furthermore, there was little or no consistent evidence of cross selection between permethrin and temephos for the majority of the genes differentially transcribed in both experiments (Fig. 4). This result might seem unremarkable because the two insecticide have different modes of action and most pyrethroid resistance appears to be associated with two amino acid substitutions (Ile1,016 and Cys1,534) in the voltage-gated sodium channel (Harris et al. 2010; Saavedra-Rodriguez et al. 2007, 2008). However, we are surprised that at least some of the many genes identified in the current study are not involved in pyrethroid sequestration or degradation following knockdown. For example, Lumjuan et al. (2011) using the same methodology described above showed that GSTE3, -E4, -E5 were inhibited 75, 27, and 54 respectively by 10 mM permethrin. On the other hand CYP9J32 which metabolizes permethrin was upregulated in Iquitos FS5 selected with both temephos and permethrin.
High variability in ‘Detox’ gene expression profiles among temephos resistant strains prevent us from pinpointing a universal set of candidate genes to be used as operational markers for temephos resistance. This argues that general bioassays and biochemical assays may still serve as the best general predictors of temephos resistance. Possibly QTL mapping and deep sequencing will aid in the identification of CCE, GST and CYP genes involved in temephos resistance. Further research is needed to determine the extent to which the CCE contribute to temephos resistance relative to general CYP and GST activities. But the results of this study, and the many corroborating studies cited above, suggest that use of CCE inhibitors may effectively extend the use of temephos. It remains the only affordable OP larvicide with any appreciable use against Ae. aegypti and other container breeding vectors of the Dengue and Yellow Fever flaviviruses.
Methodology
Collection sites and colony rearing conditions
We used six Ae. aegypti lines collected from southern México and one collected from Iquitos, Perú (Table 1). Mexican collections were from two states: Quintana Roo and Yucatán. In Quintana Roo, four geographically proximate collections were obtained: Lázaro-Cárdenas, Solidaridad, Calderitas and Lagunitas. Mérida is located in the Yucatán Peninsula. Larvae were collected by the Universidad Autónoma de Nuevo León and Universidad Autónoma de Yucatán. The Iquitos, Perú collection was kindly provided by Amy Morrison (UC Davis). F1 or F2 eggs were obtained from field collected parents and mailed to CSU. We reared an additional F2 or F3 generation to provide enough larvae for bioassays, DNA isolation and selection experiments. Larvae were reared to adults using brewer’s yeast for larval food. Adults were provided 10%(w/v) sucrose solution and were bloodfed on citrated sheep blood in an artificial membrane feeder every three days. Incubators were set to a 14:10 photoperiod, 30°C water temperature for larvae and 28°C for adult with a relative humidity of 85%.
Bioassays and temephos selection
F2 or F3 offspring from the field constituted the FS0 generation in the selection experiments. FS0 larvae were bioassayed to estimate the temephos concentration at which 50% of larvae die (LC50) (Chem Service, West Chester, PA). Bioassays were performed in plastic cups containing 100 ml of water with five different concentrations of temephos, Ethanol (1 mL) was used as solvent. Approximately 25 3rd-instar were gently pipetted into each cup. Mortality was recorded every 15 minutes up to two hours. All larvae were then transferred into clean water and mortality was scored at 24 hours. Each bioassay was performed in triplicate to obtain ~75 larvae per concentration. LC50 and confidence limits were calculated using the IRMA quick calculator software (http://sourceforge.net/projects/irmaproj/files/) (Lozano-Fuentes et al. 2012) using logistic regression.
For some mosquito lines in certain generations, a lower temephos concentration was used for selection, depending on the damage inflicted by temephos exposure (i.e. low rate of mosquitoes emerging, continuous mortality). Terminology for the selected lines starts with the name of the line followed by FS1, FS2, FS3, FS4 and FS5 referring to the number of generations of temephos selection. Selection proceeded in three replicate lines for each of the six collections for five generations. In the first round of selection 250–700 3rd instar larvae from each of the 18 lines were exposed to their corresponding LC50. Temephos exposure time was two hours and ~50 larvae were placed in each temephos container. Larvae were then transferred to clean water and mortality was recorded at 24 hours. Surviving larvae were transferred to 1 cubic foot rearing cages (BugDorm-1, Mega View Science, Co.), raised to adults which were then bloodfed to obtain FS1 eggs. We performed an initial bioassay using a pool of larvae from the three biological replicates in each of the subsequent FS1 - FS5 generations to calculate the new LC50. From 300 – 700 larvae from each replicate were then exposed to the new LC50. Realized heritability coefficients (h2) to measure the ratio or response to selection relative to selection differential were calculated using the method of Tanaka and Noppun (1989) with software and modifications previously described by (Saavedra-Rodriguez et al. 2012).
Inhibitor bioassays
The toxicity of temephos using inhibitors for CCE, CYP and GST systems was assessed separately in three temephos selected strains: Solidaridad F9, Calderitas F9 and Mérida F9. Biological replicates from each strain were pooled and released from temephos selection following generation FS5. The level of resistance of these strains was re-assessed by bioassay using the New Orleans (NO) strain as a reference. Inhibition of CCE was performed using DEF (SSS-tributyl-phosphorotrithioate; Chem Service PS-562). CYPs were inhibited with PBO (piperonyl butoxide, Chem Service PS-100) and GSTs were inhibited using DEM (diethyl maleate; Chem Service O-482). Different concentrations of inhibitors were tested to obtain the following sublethal concentrations: 1.5 μg/ml for DEF, 5.0 μg/ml for PBO and 150 μg/ml for DEM. Larvae of third to early fourth instar were pre-treated with these concentrations during four hours. Following pre-treatment, different concentrations of temephos were applied and mortality was counted at 15 minute intervals during 2 hours and again at 24 hours of continuous exposure to both insecticide and inhibitor. Three controls were run simultaneously: water/acetone, temephos alone and inhibitor alone. Five to six temephos concentrations were used and the experiment was triplicated. LC50 was calculated as above.
Differential expression profiles
The ‘Aedes Detox Chip’ v.2 DNA microarray developed by Strode et al. (2008) was used to follow changes in the expression of detoxification genes. The three biological replicates from each mosquito line were processed separately. RNA isolation, cDNA synthesis and labeling reactions were performed independently for each biological replicate. Total RNA was extracted from batches of 30 3rd instar larvae using the RNeasy ®Midi Kit (Qiagen) according to manufacturer’s instructions. Total RNA quantity and quality was assessed on a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). cDNA synthesis, labeling reactions and procedures for array hybridization follow Strode et al. (2008). Spot finding, signal quantification and spot superimposition for both dye channels were performed using Genepix 5.1 software (Axon Instruments, Molecular Devices, Union City, CA, USA). Spots not satisfying conditions described by Strode et al. (2008) were excluded from analysis. Normalization and statistical analysis were performed using the Limma 1.9 software package for R 2.3.1, available from the CRAN repository (http://www.r-project.org) according to Muller et al. (2007). Expression ratios for biological replicates at each strain were normalized and averaged in the Limma program. Only those genes showing significantly similar coefficients among replicates in the associated t tests were included in the final list of candidate genes. Results are expressed as log2 of mean transcription ratios (M = log2(Cy5/Cy3)). An arbitrary two-fold threshold (log2 scale=1) was used to identify differentially expressed genes. The probability threshold was set at 0.001 (–log10 =3). Direct comparisons between three different points of selection were performed using Limma. These were 1) unselected-FS0 relative to the NO susceptible reference; 2) Temephos selected FS1 relative to unselected-FS0; and 3) temephos selected FS5 relative to temephos selected FS1. A fourth indirect comparison was performed with Limma between FS5 and NO to identify genes commonly upregulated in all strains relative to one standard strain.
Quantitative PCR for microarray validation
Transcription profiles of eight differentially expressed genes in the FS5 lines were validated by real-time quantitative PCR using the same RNA that was extracted for microarray experiments. Four micrograms of total amplified RNA (RiboAmpTM RNA amplification kit) were used for cDNA synthesis with Superscript Reverse Transcriptase III (Invitrogen) and oligo-(dT)15–18 primer (Invitrogen), according to manufacturer’s instructions. Resulting cDNAs were diluted 100 fold for real-time quantitative PCR. Primer pairs used for quantitative PCR were optimized and tested to determine if they provided unique amplification products as determined by agarose gel electrophoresis and a melting curve analysis at the end of the real-time quantitative PCR run. Reactions (20 μL) were performed in triplicate on a CFX-96 (BioRad-Hecules CA) system using iQ SYBR Green Supermix (BioRad, Hercules CA), 0.3μM of each primer and 5 μL of diluted cDNAs. For each gene analyzed, a cDNA dilution scale from 1 to 1,000,000 was performed to assess PCR efficiency. Data analysis was performed according to the ΔCT method and taking into account PCR efficiency (Pfaffl 2001; Pfaffl and Hageleit 2001). The gene encoding the ribosomal protein L8 (Vector Base ID AAEL000987) was used for normalization.
Supplementary Material
Acknowledgments
Larva was collected by collaborators at the Universidad Autónoma de Nuevo León and Universidad Autónoma de Yucatán. This work was funded in part by ICIDR: 5U01AI088647 and Fogarty Training Grant 5D43TW001130.
References
- Beserra EB, Fernandes CRM, De Queiroga MDC, De Castro FP. Resistance of Aedes aegypti (L.) (Diptera : Culicidae) populations to organophosphates ternephos in the Paraiba State, Brazil. Neotropical Entomology. 2007;36:303–307. doi: 10.1590/s1519-566x2007000200019. [DOI] [PubMed] [Google Scholar]
- Bisset JA, Rodriguez MM, Ricardo Y, Ranson H, Perez O, Moya M, Vazquez A. Temephos resistance and esterase activity in the mosquito Aedes aegypti in Havana, Cuba increased dramatically between 2006 and 2008. Medical and Veterinary Entomology. 2011;25:233–239. doi: 10.1111/j.1365-2915.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- Boyer S, David JP, Rey D, Lemperiere G, Ravanel P. Response of Aedes aegypti (Diptera : Culicidae) larvae to three xenobiotic exposures: Larval tolerance and detoxifying enzyme activities. Environmental Toxicology and Chemistry. 2006;25:470–476. doi: 10.1897/05-267r2.1. [DOI] [PubMed] [Google Scholar]
- Braga IA, Lima JBP, Soares SD, Valle D. Aedes aegypti resistance to Temephos during 2001 in several municipalities in the states of Rio de Janeiro, Sergipe, and Alagoas, Brazil. Memorias Do Instituto Oswaldo Cruz. 2004;99:199–203. doi: 10.1590/s0074-02762004000200015. [DOI] [PubMed] [Google Scholar]
- Braga IA, Mello CB, Montella IR, Lima JBP, Junior AJM, Medeiros PFV, Valle D. Effectiveness of methoprene, an insect growth regulator, against temephos-resistant Aedes aegypti populations from different Brazilian localities, under laboratory conditions. Journal of Medical Entomology. 2005;42:830–837. doi: 10.1093/jmedent/42.5.830. [DOI] [PubMed] [Google Scholar]
- Dauterman WC. Biological and nonbiological modifications of organophosphorus compounds. Bull World Health Organ. 1971;44(1–3):133–150. [PMC free article] [PubMed] [Google Scholar]
- Flores AE, Grajales JS, et al. Mechanisms of insecticide resistance in field populations of Aedes aegypti (L.) from Quintana Roo, Southern Mexico. Journal of the American Mosquito Control Association. 2006;22(4):672–677. doi: 10.2987/8756-971X(2006)22[672:MOIRIF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comparative Immunology, Microbiology and Infectious Diseases. 2004;27(5):319–330. doi: 10.1016/j.cimid.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Harris AF, Rajatileka S, Ranson H. Pyrethroid Resistance in Aedes aegypti from Grand Cayman. American Journal of Tropical Medicine and Hygiene. 2010;83:277–284. doi: 10.4269/ajtmh.2010.09-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Karunaratne SH. Mosquito carboxylesterases: a review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Medical and veterinary entomology. 1998;12(1):1–12. doi: 10.1046/j.1365-2915.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- Hollingworth RM. Comparative metabolism and selectivity of organophosphate and carbamate insecticides. Bull World Health Organ. 1971;44(1–3):155–170. [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Hu NT, Yao YE, Wu CY, Chiang SW, Sun CN. Molecular cloning and heterologous expression of a glutathione S-transferase involved in insecticide resistance from the diamondback moth, Plutella xylostella. Insect Biochemistry and Molecular Biology. 1998;28:651–658. doi: 10.1016/s0965-1748(98)00049-6. [DOI] [PubMed] [Google Scholar]
- Jirakanjanakit N, Saengtharatip S, Rongnoparut P, Duchon S, Bellec C, Yoksan S. Trend of Temephos resistance in Aedes (Stegomyia) mosquitoes in Thailand during 2003–2005. Environmental Entomology. 2007;36:506–511. doi: 10.1603/0046-225x(2007)36[506:totria]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E. Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochemistry and Molecular Biology. 2001;31:313–319. doi: 10.1016/s0965-1748(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Lazcano JAB, Rodriguez MM, Martin JLS, Romero JE, Montoya R. Assessing the insecticide resistance of an Aedes aegypti strain in El Salvador. Revista Panamericana De Salud Publica-Pan American Journal of Public Health. 2009;26:229–234. [PubMed] [Google Scholar]
- Lima EP, de Oliveira AM, Lima JWD, Junior ANR, Cavalcanti LPD, Pontes RJS. Aedes aegypti resistance to temefos in counties of Ceara State. Revista Da Sociedade Brasileira De Medicina Tropical. 2006;39:259–263. doi: 10.1590/s0037-86822006000300006. [DOI] [PubMed] [Google Scholar]
- Lima EP, Paiva MHS, de Araujo AP, da Silva EVG, da Silva UM, de Oliveira LN, Santana AEG, Barbosa CN, Neto CCD, Goulart MOF, Wilding CS, Ayres CFJ, Santos MAVD. Insecticide resistance in Aedes aegypti populations from Ceara, Brazil. Parasites & Vectors. 2011;4 doi: 10.1186/1756-3305-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima JBP, Da-Cunha MP, Da Silva RC, Galardo AKR, Soares SD, Braga IA, Ramos RP, Valle D. Resistance of Aedes aegypti to organophosphates in several municipalities in the state of Rio de Janeiro and Espirito Santo, Brazil. American Journal of Tropical Medicine and Hygiene. 2003;68:329–333. [PubMed] [Google Scholar]
- Llinas GA, Seccacini E, Gardenal CN, Licastro S. Current resistance status to temephos in Aedes aegypti from different regions of Argentina. Memorias Do Instituto Oswaldo Cruz. 2010;105:113–116. doi: 10.1590/s0074-02762010000100019. [DOI] [PubMed] [Google Scholar]
- Lozano-Fuentes S, Saavedra-Rodriguez K, Black WC, IV, Eisen L. QCal: a software application for the calculation of dose-response curves in insecticide resistance bioassays. Journal of the American Mosquito Control Association. 2012;28:59–61. doi: 10.2987/11-6192.1. [DOI] [PubMed] [Google Scholar]
- Lumjuan N, Rajatileka S, Changsom D, Wicheer J, Leelapat P, Prapanthadara LA, Somboon P, Lycett G, Ranson H. The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochemistry and Molecular Biology. 2011;41:203–209. doi: 10.1016/j.ibmb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Lumjuan N, Stevenson BJ, Prapanthadara LA, Somboon P, Brophy PM, Loftus BJ, Severson DW, Ranson H. The Aedes aegypti glutathione transferase family. Insect Biochemistry and Molecular Biology. 2007;37:1026–1035. doi: 10.1016/j.ibmb.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Macoris MD, Andrighetti MTM, Takaku L, Glasser CM, Garbeloto VC, Bracco JE. Resistance of Aedes aegypti from the State of Sao Paulo, Brazil, to organophosphates insecticides. Memorias Do Instituto Oswaldo Cruz. 2003;98:703–708. doi: 10.1590/s0074-02762003000500020. [DOI] [PubMed] [Google Scholar]
- Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, Strode C, Brengues C, Yebakima A, Ranson H, Corbel V, David JP. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies) Bmc Genomics. 2009:10. doi: 10.1186/1471-2164-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarri MB, Georghiou GP. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. Journal of the American Mosquito Control Association. 1995;11(3):315–322. [PubMed] [Google Scholar]
- Melo-Santos MAV, Varjal-Melo JJM, Araujo AP, Gomes TCS, Paiva MHS, Regis LN, Furtado AF, Magalhaes T, Macoris MLG, Andrighetti MTM, Ayres CFJ. Resistance to the organophosphate temephos: Mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Tropica. 2010;113:180–189. doi: 10.1016/j.actatropica.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Montella IR, Martins AJ, Viana-Medeiros PF, Lima JBP, Braga IA, Valle D. Insecticide resistance mechanisms of Brazilian Aedes aegypti Populations from 2001 to 2004. American Journal of Tropical Medicine and Hygiene. 2007;77:467–477. [PubMed] [Google Scholar]
- Mouches C, Pauplin Y, Agarwal M, Lemieux L, Herzog M, Abadon M, Beyssat-Arnaouty V, Hyrien O, de Saint Vincent BR, Georghiou GP, et al. Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex. Proc Natl Acad Sci U S A. 1990;87(7):2574–2578. doi: 10.1073/pnas.87.7.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Donnelly MJ, Ranson H. Transcription profiling of a recently colonised pyrethroid resistant Anopheles gambiae strain from Ghana. Bmc Genomics. 2007:8. doi: 10.1186/1471-2164-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo CB, Salazar-Terreros MJ, Mina NJ, McAllister J, Brogdon W. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Tropica. 2011;118:37–44. doi: 10.1016/j.actatropica.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Ortelli F, Rossiter LC, Vontas J, Ranson H, Hemingway J. Heterologous expression of four glutathione transferase genes genetically linked to a major insecticide-resistance locus from the malaria vector Anopheles gambiae. Biochemical Journal. 2003;373:957–963. doi: 10.1042/BJ20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. Reregistration Eligibility Decision (RED) for temephos. 2001 ( http://www.epa.gov/oppsrrd1/REDs/temephos_red.htm)
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001:29. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Hageleit M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnology Letters. 2001;23:275–282. [Google Scholar]
- Polson KA, Rawlins SC, et al. Organophosphate Resistance in Trinidad and Tobago Strains of Aedes Aegypti. Journal of the American Mosquito Control Association. 2010;26(4):403–410. doi: 10.2987/10-6019.1. [DOI] [PubMed] [Google Scholar]
- Polson KA, Brogdon WG, Rawlins SC, Chadee DD. Characterization of insecticide resistance in Trinidadian strains of Aedes aegypti mosquitoes. Acta Tropica. 2011;117:31–38. doi: 10.1016/j.actatropica.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Poupardin R, Reynaud S, Strode C, Ranson H, Vontas J, David JP. Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: Impact on larval tolerance to chemical insecticides. Insect Biochemistry and Molecular Biology. 2008;38:540–551. doi: 10.1016/j.ibmb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Rawlins SC, Wan JO. Resistance in some Caribbean populations of Aedes aegypti to several insecticides. Journal of the American Mosquito Control Association. 1995;11(1):59–65. [PubMed] [Google Scholar]
- Roberts TR, Hutson DH. The Royal Soc Chem. Cambridge, UK: Metabolic Pathway of Agrochemicals Part II; 1999. Insecticides and Fungicides; pp. 490–493. [Google Scholar]
- Rodriguez MM, Bisset J, De Fernandez DM, Lauzan L, Soca A. Detection of insecticide resistance in Aedes aegypti (Diptera : Culicidae) from Cuba and Venezuela. Journal of Medical Entomology. 2001;38:623–628. doi: 10.1603/0022-2585-38.5.623. [DOI] [PubMed] [Google Scholar]
- Rodriguez MM, Bisset J, Ruiz M, Soca A. Cross-resistance to pyrethroid and organophosphorus insecticides induced by selection with temephos in Aedes aegypti (Diptera : Culicidae) from Cuba. Journal of Medical Entomology. 2002;39:882–888. doi: 10.1603/0022-2585-39.6.882. [DOI] [PubMed] [Google Scholar]
- Rodriguez MM, Bisset JA, Fernandez D. Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. Journal of the American Mosquito Control Association. 2007;23:420–429. doi: 10.2987/5588.1. [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I, Bisset J, Rodriguez M, Mccall PJ, Donnelly MJ, Ranson H, Hemingway J, Black WC. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007;16(6):785–798. doi: 10.1111/j.1365-2583.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Strode C, Flores Suarez A, Fernandez Salas I, Ranson H, Hemingway J, Black WC. Quantitative trait loci mapping of genome regions controlling permethrin resistance in the mosquito Aedes aegypti. Genetics. 2008;180(2):1137–1152. doi: 10.1534/genetics.108.087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Suarez AF, Salas IF, Strode C, Ranson H, Hemingway J, Black WC. Transcription of detoxification genes after permethrin selection in the mosquito Aedes aegypti. Insect molecular biology. 2012;21(1):61–77. doi: 10.1111/j.1365-2583.2011.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seccacini E, Lucia A, Zerba E, Licastro S, Masuh H. Aedes Aegypti Resistance to Temephos in Argentina. Journal of the American Mosquito Control Association. 2008;24:608–609. doi: 10.2987/5738.1. [DOI] [PubMed] [Google Scholar]
- Sousa-Polezzi RD, Bicudo HEMD. Effect of phenobarbital on inducing insecticide tolerance and esterase changes in Aedes aegypti (Diptera : Culicidae) Genetics and Molecular Biology. 2004;27:275–283. [Google Scholar]
- Stevenson BJ, Pignatelli P, Nikou D, Paine MJ. Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: developing new tools to combat insecticide resistance. PLoS neglected tropical diseases. 2012;6(3):e1595. doi: 10.1371/journal.pntd.0001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strode C, Wondji CS, David JP, Hawkes NJ, Lumjuan N, Nelson DR, Drane DR, Karunaratne SHPP, Hemingway J, Black WC, Ranson H. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochemistry and Molecular Biology. 2008;38:113–123. doi: 10.1016/j.ibmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Strode C, de Melo-Santos M, Magalhaes T, Araujo A, Ayres C. Expression profile of genes during resistance reversal in a temephos selected strain of the dengue vector, Aedes aegypti. PLoS One. 2012;7(8):e39439. doi: 10.1371/journal.pone.0039439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Noppun V. Heritability Estimates of Phenthoate Resistance in the Diamond-Back Moth. Entomologia Experimentalis Et Applicata. 1989;52:39–47. [Google Scholar]
- Tikar SN, Kumar A, Prasad GBKS, Prakash S. Temephos-induced resistance in Aedes aegypti and its cross-resistance studies to certain insecticides from India. Parasitology Research. 2009;105:57–63. doi: 10.1007/s00436-009-1362-8. [DOI] [PubMed] [Google Scholar]
- Vaughan A, Hawkes N, Hemingway J. Co-amplification explains linkage disequilibrium of two mosquito esterase genes ininsecticide-resistant Culex quinquefasciatus. Biochem J. 1997;325:359–365. doi: 10.1042/bj3250359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SH, Clark AG, Syvanen M. Identification and cloning of a key insecticide-metabolizing glutathione S-transferase (MdGST-6A) from a hyper insecticide-resistant strain of the housefly Musca domestica. Insect Biochemistry and Molecular Biology. 2001;31:1145–1153. doi: 10.1016/s0965-1748(01)00059-5. [DOI] [PubMed] [Google Scholar]
- WHO. Global insecticide use for vector-borne disease control. 4. 2009. “WHO/HTM/NTD/WHOPES/GCDPP/2009.6”. [Google Scholar]
- Wilkinson CF. Effects of synergists on the metabolism and toxicity of anticholinesterases. Bull World Health Organ. 1971;44(1–3):171–190. [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Georghiou GP. Selection and characterization of temephos resistance in a population of Aedes aegypti from Tortola, British Virgin Islands. Journal of the American Mosquito Control Association. 1999;15:315–320. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.