Antiretroviral therapy (ART) has significantly reduced morbidity and increased life expectancy of individuals with HIV. Consequently, non-AIDS-defining malignancies are increasing in frequency, which necessitates concurrent use of antineoplastics and ART. While drug interactions are a major concern when combining these agents, there is currently limited guidance on dose adjustments required to maintain safe and efficacious drug exposure.
A CHANGING EPIDEMIOLOGIC LANDSCAPE
ART restores immune function, reduces opportunistic infection, lowers viral load, reduces the morbidity and mortality associated with AIDS-related complications, and increases life expectancy. As a result, the incidence of non-AIDS-defining malignancies (e.g., breast, head and neck, and lung cancer) is increasing whereas the incidence of AIDS-defining malignancies (e.g., Kaposi sarcoma, non-Hodgkin’s lymphoma, and cervical cancer) is decreased (Fig. 1). This shift in cancers in the AIDS population has been noted in resource-rich countries while AIDS-defining malignancies still predominate in resource-limited countries.1 Non-AIDS-defining malignancies in HIV patients are a significant comorbidity necessitating concurrent treatment with antineoplastic agents and ART.2
Figure 1.
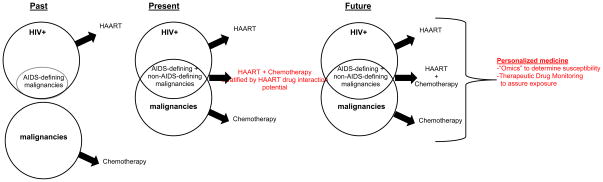
In the past, patients with HIV were unlikely to develop non-AIDS-defining malignancies. Presently, the efficacy of ART has increased the life expectancy of patients with HIV, resulting in an increase in the incidence of malignancies in this population. Concomitant therapy for HIV and cancer often results in drug-drug interactions (DDIs), prompting the Aids Malignancy Consortium to develop recommendations for dose-adjustments based on enzyme-induction strata. In the future, we envisage truly personalized medicine through characterization of the patient and the target with a variety of omics-based techniques, followed by dose-adjustments based on therapeutic drug monitoring, which will correct for the effects of comedication and all other covariates on exposure.
ANTIRETROVIRAL THERAPY
Currently recommended ART regimens typically consist of a combination of three active drugs to prevent resistance. Initial regimens in resource-rich countries include combinations of two nucleoside reverse transcriptase inhibitors (NRTIs) with a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with ritonavir, or an integrase strand transfer inhibitor (INSTI).3 A major concern with the use of many antiretrovirals is the potential for interactions mediated by drug metabolizing enzymes or transporters leading to altered drug exposure. NRTIs may be victims of transporter-mediated interactions as renal clearance is their major route of elimination. PIs and NNRTIs may be victims or perpetrators of enzyme-mediated interactions as they are extensively metabolized by, and may induce or inhibit the CYP450 system. The INSTI class cannot be generalized. Raltegravir is only metabolized by the phase II enzyme UDP glucuronosyltransferase (UGT) 1A1 and is unlikely to have major interactions. Elvitegravir and dolutegravir are metabolized by CYP3A and UGTs and consequently may interact with other agents. Elvitegravir is only available in a boosted combination pill with the potent CYP3A4 inhibitor cobicistat. The CCR5 antagonist maraviroc is a potential victim of drug interactions as it is a substrate of CYP3A and ABCB1, but does not alter metabolism or transport, and is unlikely to be a perpetrator. The fusion inhibitor enfuvirtide undergoes hydrolysis and to date, no drug interactions have been noted with this agent.
CANCER THERAPY
Classically, cancer therapy has consisted of cytotoxic agents (e.g., antimetabolites, anti-microtubule agents, alkylators, platinating agents, and topoisomerase inhibitors). Subsequently, these were combined in a manner as to avoid overlapping toxicity profiles. Generally, these agents are not selective in cytotoxicity and have a narrow therapeutic index. Although the trend in anticancer drug development has been to move towards molecularly targeted agents, which have a wider therapeutic index, cytotoxic regimens still play a key role in cancer therapies. Because most anticancer agents will be used in combination, drug interactions are a major concern both during drug development and in clinical practice. For both cytotoxics and molecularly targeted agents, exposure-response relationships have been described, which suggest that suboptimal exposure results in therapeutic failure and excessive exposure is associated with increased toxicity. Achieving the right exposure is an important predictor of treatment success, and this may be compromised by coadministration of drugs perpetrating drug-drug interactions.4
COMBINING ANTIRETROVIRAL AND CANCER TREATMENT
As HIV patients live longer and develop non-AIDS-defining malignancies, guidance for cancer treatment in patients taking ART is needed. The timing of diagnoses of HIV and a malignancy may guide therapy decisions. If a patient is taking ART and is diagnosed with a curable malignancy, all attempts should be made to achieve proper exposure of the anticancer drug in these patients in order to increase the chance of cure and at the same time minimize any toxicity. If a patient is co-diagnosed with HIV and a malignancy, anticancer therapy should be started first until tolerability is known at which point a ART regimen with low potential pharmacokinetic and pharmacodynamic interaction can be initiated. Finally, considerations beyond changes in drug exposure, such as extent of overlapping toxicity profiles may be considered.
Assessing hepatic function in patients on antiretrovirals
Cancer agents are commonly studied in patients with varying degrees of liver dysfunction, which results in recommended dose decreases based on liver function tests, such as bilirubinemia. PIs such as atazanavir and indinavir may cause unconjugated hyperbilirubinemia. If no other signs of liver dysfunction exist, suggested dose modifications of anticancer drugs based on liver function tests may be ignored. The rarely utilized NRTIs didanosine, stavudine, and zidovudine can lead to hepatotoxicity associated with lactic acidosis, which cannot be ignored. Therefore, the less hepatotoxic NRTIs such as abacavir, emtricitabine, lamivudine, and tenofovir may be warranted and are the preferred NRTIs.
Pharmacodynamic interactions
Depending on the exact composition, ART may be associated with a variety of side effects. Molecularly targeted agents tend to have a different toxicity profile than the classic diarrhea, myelosuppression or peripheral neuropathy associated with cytotoxics, and therefore overlapping toxicity is less of a concern. However, molecularly targeted anticancer agents are not without toxicities, which include QT prolongation, rash, hepatotoxicity or hypertension. In combining ART and anticancer drug therapy, overlapping toxicity profiles should be avoided.
Because zidovudine is associated with severe neutropenia, it should not be combined with cytotoxic regimens that contain neutropenic agents. If the ART regimen cannot be altered, less myelosuppressive chemotherapy is preferred, and the patient should be monitored closely for myelosuppression.
The NRTIs didanosine and stavudine are associated with irreversible peripheral neuropathy, which is also a common side effect of platinating agents, taxanes, vinca-alkaloids and the proteasome inhibitor bortezomib. For chemotherapy-induced neuropathy, management consists of cumulative dose-reduction or lower dose-intensity regimens. To avoid irreversible peripheral neuropathy, treatment options include: 1) substituting an alternate NRTI or other appropriate antiretroviral, 2) temporarily discontinuing ART, or 3) selecting an alternative chemotherapy regimen.
Lastly, cardiac toxicity from QT prolongation is a growing concern. QT prolongation has been associated with the following PIs: atazanavir, ritonavir boosted lopinavir, and saquinavir. QT prolongation is increasingly common with the newer molecularly targeted anticancer agents including the tyrosine kinase inhibitors (e.g. lapatinib and nilotinib). Due to the potential for arrhythmias and sudden death, combinations of agents that can prolong the QT interval should be avoided.
Pharmacokinetic interactions
The drug interaction potential of ART in general is well documented but not specifically with traditional cytotoxics or targeted cancer agents. If a practitioner consults the package insert of an anticancer agent, concrete dose adjustment guidance is rarely provided and often antiretrovirals are outright excluded due to the extensive CYP450 mediated interactions associated with their use. Indeed, efavirenz and ritonavir represent the perpetrator extremes of enzyme induction and inhibition, potentially leading to decreased efficacy or increased toxicity, respectively, of the co-administered anticancer agents.
The following classes of anticancer drugs undergo non-CYP450 routes of elimination and their pharmacokinetics is unlikely to be altered by ART: anthracyclines, antimetabolite agents, antitumor antibiotics, and platinating agents. Camptothecins and proteasome inhibitors are substrates but not inhibitors or inducers of CYP450 and UGT isozymes and are likely to be victims of ART mediated interactions. Bidirectional drug interactions could be anticipated in case of other classes of anticancer agents that are substrates of, and inhibit or induce CYP450s, including alkylating agents, corticosteroids, epipodophyllotoxins, taxanes, tyrosine kinase inhibitors, and vinca alkaloids. Newer antiretrovirals such as raltegravir may become standard of care in patients with multiple comorbidities due to their reduced interaction potential compared to NNRTIs and PIs. If a patient is already on a ART regimen, the anticancer regimen could be switched from a first-line anticancer regiment to a regimen containing a drug with less drug interaction potential.
Future of Antiretroviral Therapy and Targeted Anticancer Treatment
The AIDS Malignancy Consortium is conducting prospective clinical trials in patients on ART with stratification by perpetrator category of the antiretroviral component when the antineoplastic drug is prone to drug interactions (i.e., for a CYP3A4 substrate, the stratum would be enzyme-inducing (efavirenz), versus enzyme-inhibiting (e.g. ritonavir or cobicistat), versus other ART agents). The goal is to identify tolerable dosing regimens that can be applied to complex patient populations. These clinical activities are supported by preclinical in vitro and in vivo screening of agents for drug-drug interaction potential to aid in prioritization of clinical trial concepts. In the future, we envisage truly personalized medicine through characterization of the patient with a variety of omics-based techniques, followed by dose-adjustments based on therapeutic drug monitoring, which will correct for the effects of co-medication on exposure.
Conclusion
Detailed guidelines for the combination of anticancer and antiretroviral drugs are not readily available. While truly personalized therapy through therapeutic drug monitoring is not always possible or practical, we look forward to more concrete data to guide clinical decision making based on in vitro and in silico data and well-designed phase I trials. A better understanding of cancer chemotherapy and antiretroviral drug interactions is needed now that ART has turned AIDS into a chronic medical condition. Beyond the drug-drug interactions discussed here, it will be important to evaluate the effect of ART therapy on the outcome metrics of anticancer therapy, such as quality of life, toxicity, and ultimately survival. To address the challenges of combined antiretroviral and anticancer therapy presently and in the future, communication between treating infectious disease physicians, oncologists, and pharmacologists will be crucial.
Acknowledgments
The work was supported in part by a contract from the National Center Institute (NCI) N02-CM-62212 and supported by the following grants from the NCI: P30-CA047904, P30-CA006973, and U01-CA121947 (AIDS Malignancy Consortium).
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
J.H.B. is a consultant for Saladax Biomedical and Infinity Pharmaceutical and has received research funding from Bristol–Myers Squibb, Spectrum Pharmaceuticals, Abbvie, and Novartis. J.H.B.’s spouse is employed by GlaxoSmithKline. R.V is supported by a grant from United Therapeutics. R.V. has received travel funds from Sandoz. M.A.R. was on an Advisory Board for Galen, LTD. and has received research funding from Celgene Corporation. M.A.R.’s spouse is employed by Amplimmune, a subsidiary of AstraZeneca.
References
- 1.Deeken JF, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55:1228–1235. doi: 10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011;12:905–912. doi: 10.1016/S1470-2045(11)70056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services: Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed November 15, 2013];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013 Feb 12; Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 4.Beumer JH. Without therapeutic drug monitoring, there is no personalized cancer care. Clin Pharmacol Ther. 2013;93:228–230. doi: 10.1038/clpt.2012.243. [DOI] [PubMed] [Google Scholar]


