Abstract
Background
Blood transfusions represent a major therapeutic option in acute management of sickle cell disease (SCD). Few data exist documenting trends in transfusion among children with SCD, particularly during hospitalization.
Procedure
This was an analysis of cross-sectional data of hospital discharges within the Kid’s Inpatient Database (years 1997, 2000, 2003, 2006, 2009). Hospitalizations for children (0–18 years) with a primary or secondary SCD-related diagnosis were examined. The primary outcome was blood transfusion. Trends in transfusion were assessed using weighted multivariate logistic regression in a merged dataset with year as the primary independent variable. Co-variables consisted of child and hospital characteristics. Multivariate logistic regression was conducted for 2009 data to assess child and hospital-level factors associated with transfusion.
Results
From 1997 to 2009, the percentage of SCD-related hospitalizations with transfusion increased from 14.2% to 28.8% (P <0.0001). Among all SCD-related hospitalizations, the odds of transfusion increased over 20% for each successive study interval. Hospitalizations with vaso-occlusive pain crisis (OR 1.35, 95% CI 1.27–1.43) or acute chest syndrome/pneumonia (OR 1.24, 95% CI 1.13–1.35) as the primary diagnoses had the highest odds of transfusion for each consecutive study interval. Older age and male gender were associated with higher odds of transfusion.
Conclusions
Blood transfusion is increasing over time among hospitalized children with SCD. Further study is warranted to identify indications contributing to the rise in transfusions and if transfusions in the inpatient setting have been used appropriately. Future studies should also assess the impact of rising trends on morbidity, mortality, and other health-related outcomes.
Keywords: epidemiology, health care utilization, outcomes research, sickle cell disease
INTRODUCTION
Sickle cell disease (SCD) is an inherited hemoglobinopathy that occurs in 1 in every 375 African-American births [1]. Clinical manifestations of SCD include vaso-occlusive pain crises (VOC), anemic episodes, infections, and stroke [2,3]. While hydroxyurea has emerged as a major therapeutic option in the management of SCD [4], transfusion remains an essential and life-saving component of clinical care [5–7]. Transfusion of red blood cells lowers the percentage of HbS and reduces new sickle erythrocyte production secondary to suppressed erythropoietin release [8–10]. In acute care settings such as hospitalization, transfusions are used to treat life threatening anemia, ischemic stroke, acute chest syndrome (ACS), and other indications [5,7,11,12]. Transfusion may be additionally employed among children with SCD in the peri-operative period for surgery [13].
Historically, complications associated with transfusion have limited its use in SCD management [5,14–16]. Several laboratory and pharmacologic advances, including improved red cell phenotyping and matching, more sensitive microbiological testing of blood donors, and the advent of effective iron chelating agents, have mitigated concerns regarding transfusion [14]. These advances have reduced barriers to an extent that transfusion usage may be increasing. In the United States, approximately 50% of adults with SCD receive intermittent or chronic transfusion therapy [17]. In a single institution study of adults in the UK, the percentage of patients transfused each year increased from 15% in 2000 to 19% in 2009 [18]. Increased use of blood transfusions presupposes knowledge of growing needs and that an adequate blood supply exists to meet this growing demand. However, there is little available data to identify trends in transfusion therapy among children, particularly during hospitalization. Knowledge of such trends may be particularly important in the inpatient setting where transfusion may impact projections for needed blood supply, clinical outcomes, length of stay, and hospital and other transfusion related costs.
The primary aims of this study were to describe trends in transfusion among hospitalized children with SCD and determine factors associated with transfusion. Using data from the Kids’ Inpatient Database, we assessed trends between 1997 and 2009 among a nationally representative sample of hospitals. We hypothesized that transfusions would increase over time, even with controlling for socio-demographic variables and hospital characteristics.
METHODS
Study Design and Data Source
This was a cross-sectional analysis of pediatric hospitalizations for children with SCD in the U.S., using the 1997–2009 Kid’s Inpatient Database (KID). The database is sponsored by the Agency for Healthcare Research and Quality, as part of the Healthcare Cost and Utilization Project (HCUP) [19]. The KID, available every 3 years since 1997, was designed to report hospital use and outcomes for children. This database is the only U.S. pediatric inpatient database including data from all payers and multiple hospital types, and contains information on patient demographics, hospital characteristics, diagnoses, procedures, and length of stay.
The KID sampling frame was constructed using all U.S. short-term, non-federal, general, and specialty hospitals that had pediatric discharges defined as less than age 20 years from states participating in HCUP. The database does not include all discharges from participating institutions but instead a 10% sample of uncomplicated in-hospital births and an 80% sample of other pediatric discharges. The database contains more than 100 clinical and non-clinical variables included in hospital abstracts of all hospitalizations. The KID database is designed to provide national estimates for pediatric hospitalizations for both common and rare conditions. The database contains weighted discharge data for use in generating national estimates of total U.S. discharges for specific diagnoses and procedures. These weights are configured to produce rates that are comparable across years despite variation in the number of participating states. In order to protect hospital and patient confidentiality, identifying information on hospitals or patients are omitted in the database. For this study, all currently released datasets (1997, 2000, 2003, 2006, and 2009) were analyzed. The project was reviewed and approved by the Baylor College of Medicine Institutional Review Board.
Study Subjects
Pediatric hospitalizations for patients ≤18 years of age with a primary or secondary discharge diagnosis related to SCD were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 282.6, 282.60, 282.61, 282.62, 282.63, 282.64, 282.68, 282.69, 282.41, 282.42, 517.3, or 289.52. These codes represent all different genotypes of SCD (with or without crisis) and SCD defining complications—ACS and splenic sequestration. Hospitalizations coded for sickle cell trait (282.5) were excluded.
Primary Outcome Variable
The principal outcome was the proportion of hospitalizations among children with SCD that required transfusion. Both packed red blood cell and exchange transfusions were assessed. Packed blood cell transfusions were identified by ICD-9 code 99.04 and exchange transfusions by ICD-9 99.01.
Independent Variables
Child characteristics consisted of age, gender, race/ethnicity, payer type, and household income. Age was divided into <1 year (infant), 1–4 years (preschool age), 5–12 years (school age), and 13–18 years (adolescence). Race/ethnicity as collected by hospitals was categorized as White, Black, Hispanic, or other. Children for which no race/ethnicity was collected were categorized as Unknown. Payer type was classified as private, public, or other (uninsured). Insurance type was grouped into the following payers: private, public, and other. Income was divided by the KID into four groups based on the median household income for the child’s zip code of residence: first quartile ($0–25,000), second quartile ($25,001–30,000), third quartile ($30,001–35,000), and fourth quartile (>$35,000). Hospital characteristics consisted of individual identification code, region, hospital size, teaching status, and hospital ownership. U.S. region was grouped into four categories (Northeast, Midwest, South, and West). Hospital size was defined as small, medium, or large. Hospital teaching status was categorized as teaching/urban and non-teaching/urban. Hospital ownership consisted of children’s general hospital, children’s unit in a general hospital, and hospitals not identified as children’s hospital (i.e., general hospital without a children’s unit) by the National Association of Children’s Hospitals and Related Institutions (NACHRI) hospital type.
In addition to these categorical variables, we also investigated specific surgical procedures and clinical diagnoses associated with SCD that may have directly influenced the receipt of transfusions. We specifically assessed principal diagnoses that were surgical procedures associated with SCD (e.g., cholecystectomy, splenectomy) and non-SCD-related procedures (e.g., appendectomy). SCD-related diagnoses examined consisted of VOC, ACS, and pneumonia.
Statistical Analysis
Data were weighted to generate national estimates using appropriately scaled weights provided by HCUP. Weighting within each study year accounted for hospital strata, clustering, and the volume of hospitals within each dataset. Summary statistics were performed to determine means, medians, and proportions. Frequencies were calculated for principal discharge diagnoses most often having transfusion as a co-diagnosis. Chi-square analysis was used to examine differences in the proportions of hospitalizations requiring transfusion between individual years. Trends in the proportion of hospitalizations with blood transfusions were assessed using weighted multivariate logistic regression in a merged dataset with survey year as the principal independent variable. Co-variables examined in multivariate logistic regression consisted of age, gender, race/ethnicity, payer type, household income, region, hospital size, teaching status, and hospital ownership. Discharges for which race/ethnicity was unknown were grouped into an “unknown” category for race/ethnicity and controlled for in analysis. Individual hospital identification codes were also controlled for in the analysis to account for clustering. Subgroup analyses were conducted for different principal diagnoses, age categories, and surgical procedures. For the purpose of analysis, we combined ACS and pneumonia into one principal diagnosis category—ACS/pneumonia—given that they collectively represent pulmonary complications of SCD. Logistic regression was also used to examine socio-demographic variables and hospital characteristics associated with transfusion (2009 only). Results are reported as odds ratios (OR) with 95% confidence intervals (CI). We performed analyses using SAS 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
SCD-Related Hospitalizations
The national weighted number of SCD-related hospitalizations per year ranged from 31,364 (2000) to 39,903 (2009). No trend was observed in the proportion of pediatric hospitalizations attributable to SCD. SCD-related hospitalizations accounted for less than 1% of all pediatric hospitalizations for all years, ranging from 0.43% to 0.55%. VOC as a principal diagnosis accounted for 56% of SCD-related hospitalizations where transfusions were administered. Other principal diagnoses accounting for more than 2% of hospitalizations with transfusions consisted of SCD unspecified (7.8%), Hb-SS without crisis (5.9%), and ACS/pneumonia (5.5%).
Trends in Transfusion
We assessed multiple trends in transfusion comparing the years 1997 and 2009. In 1997, transfusions were given in 14.2% of SCD-related hospitalizations. In 2009, the percentage of SCD-related hospitalizations with transfusion increased to 28.8% (P <0.0001). The increasing trend was notable for all years: 14.2% (1997), 16.8% (2000), 20.4% (2003), 24.7% (2006), and 28.8% (2009). According to transfusion type, the percentage of hospitalizations with packed red blood cell transfusions increased from 13.9% in 1997 to 27.8% in 2009 (P <0.0001). The percentage of hospitalizations with exchange transfusion decreased from 2.1% (n = 307) in 1997 to 1.7% (n = 451) in 2009 (P <0.0001). During this time period (1997–2009), the percentage of transfusions in all pediatric hospitalizations (minus SCD-related hospitalizations) increased from 0.8% in 1997 to 1.7% in 2009 (P <0.0001).
Trends in transfusion according to patient demographics and hospital characteristics are shown in Table I. For all years, children ages 4 and under accounted for approximately 20% of transfusions. For all years, males accounted for the majority of transfusions. Children in families where the household income was $30,000 or less accounted for over 50% of those transfused for all study years. According to insurance type, children insured by public insurance made up the majority of SCD-related hospitalizations where transfusion occurred. Most transfusions occurred in large hospitals, urban hospitals, and teaching hospitals. While the percentage of transfusions for ACS/pneumonia as a principal diagnosis was consistently below 10%, the percentage for VOC ranged from 45.7% to 65.6%.
TABLE I.
Characteristics of Individuals With SCD Who Received a Blood Transfusion, Stratified by Study Year
| 1997
|
2000
|
2003
|
2006
|
2009
|
|
|---|---|---|---|---|---|
| n (weighted %) | n (weighted %) | n (weighted %) | n (weighted %) | n (weighted %) | |
| Age | |||||
| <1 | 39 (1.5) | 67 (2.7) | 83 (2.0) | 161 (3.1) | 226 (2.9) |
| 1–4 | 434 (18.8) | 471 (16.6) | 705 (16.6) | 1,050 (19.8) | 1,570 (20.6) |
| 5–12 | 971 (41.1) | 1,261 (44.1) | 1,846 (43.4) | 2,058 (38.4) | 3,056 (40.2) |
| 13–18 | 814 (38.6) | 1,044 (37.6) | 1,645 (37.9) | 2,111 (38.7) | 2,786 (36.3) |
| Gender | |||||
| Male | 1,286 (55.0) | 1,586 (55.9) | 2,312 (54.5) | 2,864 (53.5) | 4,094 (53.7) |
| Female | 972 (45.0) | 1,259 (44.1) | 1,940 (45.5) | 2,491 (46.5) | 3,536 (46.3) |
| Race/ethnicity | |||||
| White | 42 (1.5) | 56 (2.0) | 61 (1.5) | 81 (1.5) | 142 (1.8) |
| Black | 1,716 (74.1) | 2,324 (82.8) | 3,161 (73.9) | 3,833 (71.2) | 5,841 (76.2) |
| Hispanic | 196 (6.3) | 168 (5.2) | 258 (5.9) | 338 (6.5) | 414 (5.2) |
| Other | 74 (2.1) | 73 (2.0) | 94 (2.2) | 112 (2.1) | 193 (2.4) |
| Unknown | 230 (16.0) | 224 (8.0) | 729 (16.5) | 1,040 (18.7) | 1,061 (14.4) |
| Insurance type | |||||
| Private | 640 (26.2) | 832 (28.3) | 1,198 (27.9) | 1,355 (25.5) | 1,971 (25.5) |
| Public | 1,573 (72.3) | 1,935 (69.4) | 3,007 (70.2) | 3,903 (71.9) | 5,439 (71.6) |
| Other | 44 (1.5) | 70 (2.3) | 83 (1.9) | 141 (2.6) | 231 (2.9) |
| Household income | |||||
| Quartile 1 | 1,010 (52.5) | 590 (20.3) | 1,861 (44.0) | 2,499 (47.7) | 3,519 (47.5) |
| Quartile 2 | 407 (19.4) | 944 (34.0) | 1,112 (26.0) | 1,190 (22.7) | 1,723 (23.2) |
| Quartile 3 | 295 (12.0) | 623 (22.6) | 818 (19.5) | 912 (17.6) | 1,315 (17.6) |
| Quartile 4 | 460 (16.1) | 639 (23.1) | 444 (10.5) | 619 (12.0) | 888 (11.7) |
| Hospital bedsize | |||||
| Small | 315 (14.5) | 402 (16.6) | 656 (16.7) | 634 (13.1) | 597 (10.1) |
| Medium | 701 (28.6) | 688 (25.4) | 1,094 (26.9) | 981 (19.2) | 1,151 (18.4) |
| Large | 1,242 (56.9) | 1,708 (58.0) | 2,433 (56.4) | 3,583 (67.7) | 4,605 (71.5) |
| Hospital region | |||||
| Northeast | 980 (29.9) | 851 (24.5) | 909 (21.2) | 1,193 (23.7) | 1,868 (22.5) |
| Midwest | 117 (8.0) | 53 (6.3) | 667 (14.4) | 1,060 (18.6) | 1,504 (19.9) |
| South | 617 (45.4) | 1,402 (51.6) | 1,999 (48.3) | 2,513 (46.3) | 3,644 (49.3) |
| West | 544 (16.7) | 539 (17.6) | 728 (16.1) | 638 (11.4) | 637 (8.3) |
| Hospital type | |||||
| Not Children’s Hospital | 946 (34.4) | 899 (32.0) | 1,327 (31.2) | 1,360 (26.1) | 1,566 (24.8) |
| Children’s GH | 352 (17.3) | 641 (28.8) | 1,057 (28.9) | 1,184 (25.9) | 1,397 (24.7) |
| Children’s unit in GH | 960 (48.3) | 1,192 (39.2) | 1,657 (39.9) | 2,499 (48.0) | 3,189 (50.5) |
| Hospital location | |||||
| Rural | 38 (3.4) | 82 (3.6) | 109 (3.1) | 115 (2.5) | 118 (2.1) |
| Urban | 2,220 (96.6) | 2,716 (96.4) | 4,074 (96.9) | 5,083 (97.5) | 6,235 (97.9) |
| Teaching status | |||||
| Non-teaching | 489 (23.6) | 348 (13.0) | 476 (11.3) | 530 (9.9) | 677 (10.9) |
| Teaching | 1,769 (76.4) | 2,450 (87.0) | 3,707 (88.7) | 4,668 (90.1) | 5,676 (89.1) |
| Elective surgery | |||||
| No | 2,148 (94.5) | 2,719 (95.8) | 4,087 (95.8) | 4,948 (96.1) | 6,958 (95.7) |
| Yes | 110 (5.5) | 126 (4.2) | 172 (4.2) | 185 (3.9) | 290 (4.3) |
| Pneumonia/ACS | |||||
| No | 2,103 (92.3) | 2,707 (95.2) | 3,980 (93.5) | 4,917 (96.0) | 6,846 (94.6) |
| Yes | 155 (7.7) | 138 (4.8) | 279 (6.5) | 216 (4.0) | 402 (5.4) |
| VOC | |||||
| No | 1,212 (54.3) | 1,458 (49.9) | 2,210 (51.6) | 1,809 (34.4) | 2,842 (38.4) |
| Yes | 1,046 (45.7) | 1,387 (50.1) | 2,049 (48.4) | 3,324 (65.6) | 4,406 (61.6) |
GH, general hospital.
Associations Between Year and Blood Transfusion
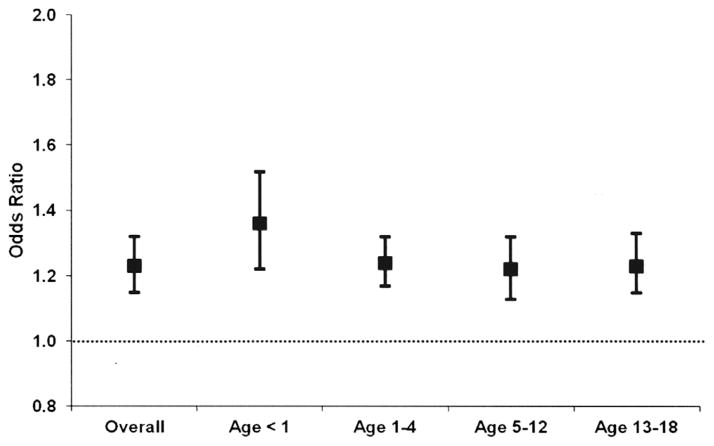
Among all SCD-related hospitalizations, there were higher odds (OR 1.23, 95% CI 1.15–1.32) of blood transfusion for each successive study interval (3 years), after controlling for patient demographics and hospital characteristics. In addition to assessing time trends for overall SCD-related hospitalizations, we also conducted sub-analyses for relevant diagnoses and surgical procedures. The OR and CI for principal diagnoses are graphically depicted using plots (Fig. 1). Dots indicate OR and error bars indicate 95% CI. Results are adjusted for child- and hospital-level factors. Among hospitalizations with VOC as the principal discharge diagnosis, there were higher odds (OR 1.35, 95% CI 1.27–1.43) of transfusion for each successive study interval. Among hospitalizations where ACS/pneumonia was a principal diagnosis, there was a time trend such that the odds of transfusion increased over time (OR 1.24, 95% CI 1.13–1.35). Among surgeries, there was no time trend in transfusions. The odds of transfusion among SCD-related hospitalizations increased over time for all age groups, with OR ranging from 1.22 to 1.36 (Fig. 2). Results are adjusted for child- and hospital-level factors.
Fig. 1.
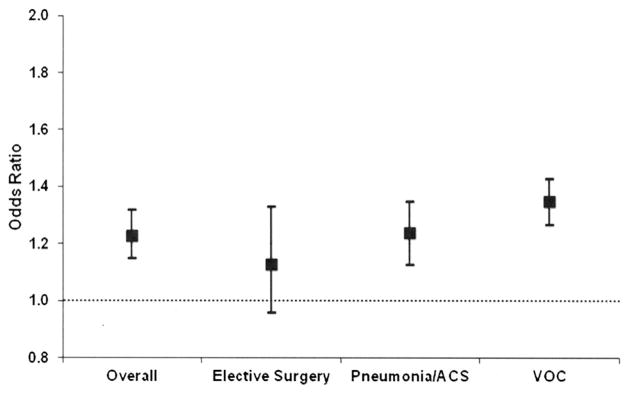
Odds of blood transfusions among children with sickle cell disease (1997–2009) according to diagnosis. Dots indicate odds ratios and error bars indicate 95% confidence intervals for SCD-related hospitalizations overall and specific principal diagnoses (x-axis) for the time frame 1997–2009. Results adjusted for child- and hospital-level factors. ACS, acute chest syndrome; VOC, vaso-occlusive crisis.
Fig. 2.
Odds of blood transfusions among children with sickle cell disease (1997–2009) according to age. Dots indicate odds ratios and error bars indicate 95% confidence intervals for overall study population and across age categories (x-axis) for the time frame 1997–2009. Results adjusted for child- and hospita-level factors.
Patient Demographics and Hospital Characteristics Associated With Transfusion
Associations between patient demographics, hospital characteristics, and transfusion were assessed using the 2009 HCUP KID. In general, age had an inverse relationship with the odds of receiving a transfusion. Compared to infants, the following age categories had higher odds of transfusion: 13–18 years of age (OR 2.70, 95% CI 2.13–3.42), 5–12 years of age (OR 2.90, 95% CI 2.30–3.65), and 1–4 years of age (OR 2.07, 95% CI 1.66–2.60). Females had lower odds (OR 0.89, 95% CI 0.82–0.97) of transfusion compared to males. No other significant associations were found between patient demographics, hospital characteristics, and transfusion.
DISCUSSION
This is the first study to examine the inpatient trend in the use of blood transfusions among children with SCD in a national sample of hospitals. We found that the percentage of SCD-related hospitalizations with transfusions have significantly increased from the years 1997 to 2009, even after controlling for patient- and hospital-level factors. Our findings raise key questions with respect to the inpatient management of pediatric SCD and needs for adequate blood supply.
The rising trend of transfusion among pediatric SCD hospitalizations represents a novel finding. Our results are similar to those of Draser et al. who also noted an increase in the use of blood transfusions for acute sickle-related complications among adult patients in the UK between the years 2000–2001 and 2008–2009 [18]. During the 10-year study period, the percentage of SCD-related hospitalizations with transfusion increased from 17% to 25%. The majority of hospitalizations were attributable to VOC. While the study by Draser et al. provides comprehensive data regarding trends in transfusion for SCD, it was limited to adults within a single institution. Our study complements this prior work by examining trends for children at a national level.
The increasing trend in blood transfusions among hospitalized children with SCD has several potential etiologies. First, transfusions may be rising due to increasing indications for transfusion therapy in the acute management of SCD. The National Acute Chest Syndrome Study Group showed that transfusion prevents the progression of lung injury [20]. The preoperative transfusion in SCD Study Group demonstrated that peri-operative simple transfusions could help minimize surgical complications [13]. Other less evidence-based indications for transfusion (e.g., leg ulcer, priapism) may also be partially driving the increase in transfusion. Second, with improvements in transfusion medicine and reductions in the risk of transfusion [14], clinicians may be more aggressively treating acute complications of SCD with blood transfusions. Third, it is possible that inpatient transfusions are being given as part of outpatient chronic transfusion protocols. Some patients may have received their scheduled chronic transfusions while being hospitalized for an acute event. From our data, it is not possible to discern transfusions given for acute events from those given as part of maintenance of outpatient transfusion regimens. Lastly, it is possible that trends in transfusion among children with SCD may be following secular trends in transfusion among hospitalized children. We found that transfusions increased for all pediatric hospitalizations in addition to SCD. Future studies should utilize medical records to more specifically determine reasons for performance of transfusion and assess differences between specific SCD-related diagnoses.
Although we found that the overall use of blood transfusions increased, we did find a decline in the use of exchange transfusions. Acute ischemic stroke is an indication for the use of exchange transfusion in SCD. A recent study by McCavit et al. [21] found that the incidence of hospitalization for treatment of stroke in the pediatric SCD population has decreased by 45% between the time periods 1998 to 2000–2009. It is possible that the lower incidence of hospitalization for stroke among children with SCD may account for the decline in the use of exchange transfusion in our study population. It is also possible that the concurrent decline in exchange transfusion and increase in simple transfusion may be related to earlier identification of symptoms suggestive of ACS and aggressive treatment with simple transfusion. Newer data have shown that early simple transfusions given to patients showing signs and symptoms of ACS can halt its progression and alleviate the need for exchange transfusions [22,23]. However, the decline in exchange transfusion should be interpreted with caution given the small numbers of exchange transfusions documented for SCD in HCUP KID.
While hydroxyurea has emerged as a major therapeutic agent for pediatric SCD, it is not clear what role, if any, it has played in the rise of blood transfusions. It can be anticipated that hydroxyurea may ultimately decrease transfusion use in some clinical situations [14]. Studies of hydroxyurea among children with SCD have shown reductions in VOC, ACS, and hospitalizations [4]. Improved clinical outcomes secondary to hydroxyurea use may lead to fewer hospitalizations for SCD which may in turn decrease overall number of transfusions. At the same time, children responsive to hydroxyurea may increasingly account for a smaller proportion of hospitalizations thus effectively increasing the concentration of those with severe disease being admitted for SCD-related hospitalizations. Therefore the percentage of hospitalizations with transfusion may artificially rise due to an elevated concentration of children with severe disease receiving inpatient care. HCUP KID does not contain data on patient medications or disease severity. Therefore, we could not determine the impact of hydroxyurea or disease severity on inpatient transfusion.
According to evidence-based guidelines, transfusions are a mainstay of therapy for ACS [1]. Interestingly, we noted a lower frequency of transfusions among patients hospitalized for ACS/pneumonia as the principal diagnosis. Several reasons may account for this finding. First, the frequency of transfusions among principal diagnosis ACS/pneumonia may be underestimated due to variation in coding for ACS. The ICD-9 code for ACS was introduced in 2003 and therefore only reflected in 2006 and 2009 datasets. Prior to 2003, ACS may have been coded as pneumonia or sickle cell (with or without crisis). Assessing trends in transfusion for ACS may be more accurate in the future as it is more routinely used as an ICD-9 code. It is also possible that ACS/pneumonia may be the principal indication for a transfusion but not coded as the principal diagnosis. Alternatively, our findings may reflect variable management of ACS among clinicians in diverse hospital settings. It is possible that clinicians in hospitals may not be consistently using blood transfusion in the setting of ACS.
The high proportion of transfusions among hospitalizations in which VOC was the primary diagnosis was surprising given that transfusions are not indicated as routine treatment for VOC. This finding is most likely due to coding errors within HCUP KID. It may be that another condition (e.g., severe anemia or ACS) was the clinical indication for the transfusion. However, that condition was not listed as a co-diagnosis or principal diagnosis. Alternatively, it is also possible that the high proportion of transfusions among VOC hospitalizations may reflect response to recent clinical trials demonstrating improved outcomes in pre-operative settings, abnormal Transcranial Doppler findings, and aggressive management of ACS [5,7,11–13,22,23].
From a policy perspective, the increase in transfusion use among hospitalized children with SCD has several implications. First, strategies must be devised to expand the pool of blood donors of African and Hispanic descent [7]. It is well-known that African-American and Hispanics comprise a small number of total blood donors [24,25]. Patterns of RBC antigens among African-Americans differ from the predominantly Caucasian blood donor base [7]. Among individuals with SCD, the receipt of blood products from those who are not racial/ethnic minorities can lead to antigenic disparities resulting in significant complications such alloimmunization and hyperhemolytic transfusion reactions [7,24,26]. These transfusion related complications add to the rising health care costs associated with periodic and chronic transfusions [27,28]. For many children with SCD, public programs such as Medicaid bear the costs attributable to transfusion complications [29]. Therefore, expansion of the racial/ethnic minority donor pool will be critical if performance of transfusion continues to rise. Recruitment of ethnic or phenotypically matched donors may require collaborations between the American Red Cross, regional blood donation centers, and hospitals as well as active recruitment of donors from civic, religious institutions, and historically black colleges and universities [30–33]. Second, monitoring must occur to prevent iron load and associated complications from increased transfusion [34–36]. Despite the increasing availability of chelating agents [37,38], iron overload remains a serious complication of transfusion [7,39].
Our study had several methodological limitations. Although the KID is considered to provide a representative sample of pediatric hospitalizations, discharge information originates from a limited number of states and is only released every 3 years. Data is not available for interval years. The KID data set does not contain unique patient identifiers or record linkages, thereby preventing analysis of utilization according to detailed patient characteristics, assessment of multiple hospitalizations of the same patient, and controlling for illness or disease severity. Therefore, we were unable to determine if the increase in blood transfusions usage was a result of an increase in illness severity among our population. It is also possible that a small number of patients accounted for a large proportion of the increase in blood transfusion therapy as demonstrated in prior studies [18]. As an administrative database, KID is subject to coding errors and misclassifications. Therefore, we could not validate that primary diagnoses were the actual indications for transfusion. KID does not contain specific data on medications taken or infusions given during hospitalization. Therefore, we could not assess the impact of the hydroxyurea on transfusion or quantify units of blood given during hospitalizations.
CONCLUSIONS
The increase in the use of blood transfusions during SCD-related hospitalizations among a national sample of pediatric hospitalizations, even after controlling for patient and hospital-level factors, indicates that more inquiry is needed regarding the indications for transfusion that may explain the trends found in the study. Due to a lack of validation of primary diagnoses as indications for transfusion, the results regarding specific indications for transfusion must be interpreted with caution. Updated National Heart and Lung and Blood Institute guidelines on SCD management may help to further elucidate current and emerging indications for blood transfusion in SCD. Future studies of clinical databases should elucidate how and why transfusions are being used in inpatient settings and the impact of apparently more aggressive use of transfusions on morbidity, mortality, and other health-related outcomes. In the face of rising numbers of blood transfusions in this population, more effective strategies are needed to expand the pool of blood donors, especially among African-Americans and Hispanics.
Acknowledgments
Grant sponsor: NIH; Grant number: 1K23 HL105568
This study was funded by a grant to Dr. Raphael, NIH grant number 1K23 HL105568.
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.The Management of Sickle Cell Disease. National Institutes of Health: National Heart, Lung, and Blood Institute; 2002. Report. NIH Publication No. 02-2117. [Google Scholar]
- 2.Frank NC, Allison SM, Cant MEC. Sickle cell disease. In: Brown RT, editor. Cognitive aspects of chronic illness in children. New York: Guilford Press; 1999. pp. 172–189. [Google Scholar]
- 3.Smith JA. The natural history of sickle cell disease. Ann N Y Acad Sci. 1989;565:104–108. doi: 10.1111/j.1749-6632.1989.tb24156.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephson CD, Su LL, Hillyer KL, et al. Transfusion in the patient with sickle cell disease: A critical review of the literature and transfusion guidelines. Transfus Med Rev. 2007;21:118–133. doi: 10.1016/j.tmrv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Vichinsky EP, Ohene-Frempong K, Thein SL, et al. Transfusion and chelation practices in sickle cell disease: A regional perspective. Pediatr Hematol Oncol. 2011;28:124–133. doi: 10.3109/08880018.2010.505506. [DOI] [PubMed] [Google Scholar]
- 7.Smith-Whitley K, Thompson AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59:358–364. doi: 10.1002/pbc.24179. [DOI] [PubMed] [Google Scholar]
- 8.Reed W, Vichinsky EP. New considerations in the treatment of sickle cell disease. Annu Rev Med. 1998;49:461–474. doi: 10.1146/annurev.med.49.1.461. [DOI] [PubMed] [Google Scholar]
- 9.Nifong TP, Domen RE. Oxygen saturation and hemoglobin A content in patients with sickle cell disease undergoing erythrocytapheresis. Ther Apher. 2002;6:390–393. doi: 10.1046/j.1526-0968.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 10.Thurston GB, Henderson NM, Jeng M. Effects of erythrocytapheresis transfusion on the viscoelasticity of sickle cell blood. Clin Hemorheol Microcirc. 2004;30:61–75. [PubMed] [Google Scholar]
- 11.Rogers ZR. Review: Clinical transfusion management in sickle cell disease. Immunohematology. 2006;22:126–131. [PubMed] [Google Scholar]
- 12.Ohene-Frempong K. Indications for red cell transfusion in sickle cell disease. Semin Hematol. 2001;38:5–13. doi: 10.1053/shem.2001.20139. [DOI] [PubMed] [Google Scholar]
- 13.Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med. 1995;333:206–213. doi: 10.1056/NEJM199507273330402. [DOI] [PubMed] [Google Scholar]
- 14.Wahl S, Quirolo KC. Current issues in blood transfusion for sickle cell disease. Curr Opin Pediatr. 2009;21:15–21. doi: 10.1097/MOP.0b013e328321882e. [DOI] [PubMed] [Google Scholar]
- 15.Chou ST, Liem RI, Thompson AA. Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol. 2012;159:394–404. doi: 10.1111/bjh.12061. [DOI] [PubMed] [Google Scholar]
- 16.Aygun B, Padmanabhan S, Paley C, et al. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 17.Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Semin Hematol. 2001;38:30–36. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- 18.Drasar E, Igbineweka N, Vasavda N, et al. Blood transfusion usage among adults with sickle cell disease —A single institution experience over ten years. Br J Haematol. 2011;152:766–770. doi: 10.1111/j.1365-2141.2010.08451.x. [DOI] [PubMed] [Google Scholar]
- 19.Introduction to the HCUP KIDS Inpatient Database. Agency for Healthcare Research and Quality; 2008. Report. http://www.hcup-us.ahrq.gov/db/nation/kid/kid_2006_introduction.jsp. [Google Scholar]
- 20.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 21.McCavit TL, Xuan L, Zhang S, et al. National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatr Blood Cancer. 2012;60:823–827. doi: 10.1002/pbc.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller ST. How I treat acute chest syndrome in children with sickle cell disease. Blood. 2011;117:5297–5305. doi: 10.1182/blood-2010-11-261834. [DOI] [PubMed] [Google Scholar]
- 23.Abbas HA, Kahale M, Hosn MA, et al. A review of acute chest syndrome in pediatric sickle cell disease. Pediatr Ann. 2013;42:115–120. doi: 10.3928/00904481-20130222-11. [DOI] [PubMed] [Google Scholar]
- 24.Shaz BH, Zimring JC, Demmons DG, et al. Blood donation and blood transfusion: Special considerations for African Americans. Transfus Med Rev. 2008;22:202–214. doi: 10.1016/j.tmrv.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.James AB, Demmons DG, Schreiber GB, et al. Contribution of attitudinal factors to blood donation in African American church attendees. Transfusion. 2011;51:158–165. doi: 10.1111/j.1537-2995.2010.02775.x. [DOI] [PubMed] [Google Scholar]
- 26.Vichinsky EP, Earles A, Johnson RA, et al. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 27.Shander A, Hofmann A, Gombotz H, et al. Estimating the cost of blood: Past, present, and future directions. Best Pract Res Clin Anaesthesiol. 2007;21:271–289. doi: 10.1016/j.bpa.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Wayne AS, Schoenike SE, Pegelow CH. Financial analysis of chronic transfusion for stroke prevention in sickle cell disease. Blood. 2000;96:2369–2372. [PubMed] [Google Scholar]
- 29.Steiner CA, Miller JL. HCUP statistical brief #21. Rockville, MD: Agency for Healthcare Research and Quality; Dec, 2006. Sickle cell disease patients in U.S. hospitals. 2004. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb21.pdf. [PubMed] [Google Scholar]
- 30.Sesok-Pizzini DA, Friedman DF, Smith-Whitley K, et al. Transfusion support of patients with sickle cell disease at the Children’s Hospital of Philadelphia. Immunohematology. 2006;22:121–125. [PubMed] [Google Scholar]
- 31.Hillyer KL, Hare VW, Josephson CD, et al. Partners for life: The transfusion program for patients with sickle cell disease offered at the American Red Cross Blood Services, Southern Region, Atlanta, Georgia. Immunohematology. 2006;22:108–111. [PubMed] [Google Scholar]
- 32.Price CL, Johnson MT, Lindsay T, et al. The Sickle Cell Sabbath: A community program increases first-time blood donors in the African American faith community. Transfusion. 2009;49:519–523. doi: 10.1111/j.1537-2995.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 33.Shaz BH, Hillyer CD. Minority donation in the United States: Challenges and needs. Curr Opin Hematol. 2010;17:544–549. doi: 10.1097/MOH.0b013e32833e5ac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007;82:255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 35.Adamkiewicz TV, Abboud MR, Paley C, et al. Serum ferritin level changes in children with sickle cell disease on chronic blood transfusion are nonlinear and are associated with iron load and liver injury. Blood. 2009;114:4632–4638. doi: 10.1182/blood-2009-02-203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harmatz P, Butensky E, Quirolo K, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76–79. [PubMed] [Google Scholar]
- 37.Raphael JL, Bernhardt MB, Mahoney DH, et al. Oral iron chelation and the treatment of iron overload in a pediatric hematology center. Pediatr Blood Cancer. 2009;52:616–620. doi: 10.1002/pbc.21929. [DOI] [PubMed] [Google Scholar]
- 38.Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136:501–508. doi: 10.1111/j.1365-2141.2006.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364:146–156. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]