Abstract
27 inbred strains of mice were tested for spike-wave discharge activity by video-electroencephalographic recordings over a 24-hour recording period. 8 strains had reproducible, frequent spike-wave discharges, including 5 strains (C57BLKS/J, CBA/J, DBA/1J, NOR/LtJ, SM/J) previously undiagnosed for this distinctive phenotype. 18 other strains exhibited no such activity. Spike-wave discharges usually occurred while the subject was motionless, and in a significant number of annotated instances coincided with an arrest of the subject’s relatively unrestrained locomotor activity, which resumed immediately after the discharge ended. In all 5 new strains, spike-wave discharges were suppressed by ethosuximide administration. From the genealogy of inbred strains, we suggest that two ancestors, A and DBA, transmitted genotypes required for spike-wave discharge in all positive strains. Together these strains with spike-wave discharges provide new opportunities to understand the genetic core susceptibility of this distinctive electroencephalographic activity and to explore its relationship to absence epilepsy, a human disorder for which few genes are known.
Keywords: genetic inheritance, rodent, strain survey, mouse EEGs, absence epilepsy
Introduction
Absence seizures are characterized by brief, spontaneous losses of consciousness, occurring together with generalized, rhythmic spike-wave discharges (SWDs) of 2.5–4 Hz. SWDs are typically detected by cortical EEG and are believed to arise primarily from excitatory and inhibitory imbalance of the corticothalamic loop encompassing cerebral cortex (layer V), and thalamic and reticular thalamic nuclei (Crunelli et al. 2011; Paz et al. 2011). From familial studies, the most common absence epilepsies – childhood and juvenile – show strong heritability but not as single gene, Mendelian traits (Ottman 2005), making causal genetic variants difficult to identify.
Laboratory rodents offer tractable models for gene discovery and follow-up functional studies, with the vast majority being in mice. To date over 20 mouse mutants with single gene defects associated with spontaneous SWD have been reported; most were detected as secondary to overt abnormal behaviors such as cerebellar ataxia (Ap3d1mh2J (Kantheti et al. 2003), BS/Ori (Gigout et al. 2013), Cacna1atg, Cacna2d2ducky, Cacnb4lh, Cacng2stg (Noebels 1999), Scn8a8J (Papale et al. 2009), Slc9a1swe (Cox et al. 1997), Snap25Cm/+ (Zhang et al. 2004), Celf4Ff/Ff (Yang et al. 2007). More recently, SWD-causing genes have been targeted or made transgenic in mice including FTDP-17 tau (García-Cabrero et al. 2013), Gababr1 (Wang et al. 2009), Gabrg2 (Nicolazzo et al. 2010), Hcn2 (Ludwig et al. 2003), Plcb4 (Cheong et al. 2009), Ssadh (Cortez et al. 2004), and Cacna1g (as an over-expressed wild-type transgene) (Ernst et al. 2009).
In addition to these examples, three common (so-called “wild-type”) inbred mouse strains are known to have occasional or frequent SWDs: DBA/2, C3H/He and A/J (Marrosu et al. 2006; Strohl et al. 2007; Beyer et al. 2008). To date only one causal gene has been described – Gria4 in the HeJ substrain of C3H (Beyer et al. 2008); genetic causes of SWD in the other strains appears to be multigenic.
Laboratory mouse strains, such as DBA/2J, C3H/HeJ and A/J were inbred in the early twentieth century in efforts to establish that cancer is an inherited disorder. It was clear to these early researchers that the complications of using the mouse as a test model were exacerbated by unknown variables within the donor and recipient outbred mice. An inbred strain was considered to be fully inbred after 20 successive brother-sister matings. Many inbred lines have been successfully constructed by selecting for the hardiest mice in sufficient numbers in each generation, to overcome potentially disastrous sterility problems and debilitating recessive traits.
Here we describe an EEG screen of 27 common inbred strains, including DBA/2J, C3H/HeJ and A/J as positive controls, to determine whether additional strains display SWDs. Although mice are typically motionless during a SWD episode, EEG recordings are essential because lack of movement is not necessarily a harbinger of SWD activity. In rats and mice, SWD appear as discrete episodes, almost always 5–8 Hz and are suppressed by the pharmaceutical reagent, ethosuximide. The identification of five new SWD-positive strains has implications for the complex genetic basis of this distinctive brain activity in mice.
Materials and Methods
Animal Care
All mice were housed with approval of Institutional Animal Care and Use Committee (IACUC). The inbred mice were obtained from The Jackson Laboratory, and maintained in a room with a 14h hour light on/10h light off cycle. The mice were housed in pairs and given free access to Lab Chow meal and water.
EEG
Adult mice aged between 8 and 16 weeks were anesthetized with tribromoethanol (400 mg/kg i.p.). Small burr holes were drilled (1 mm anterior to the bregma and 2 mm posterior to the bregma) on both sides of the skull 2 mm lateral to the midline. Four teflon-coated silver wires were soldered onto the pins of a microconnector (Mouser electronics, Texas). The wires were placed between the dura and the brain and a dental cap was then applied. The mice were given a post-operative analgesic of carprofen (5 mg/kg subcutaneous) and allowed a 72 h recovery period before recordings were taken. The mice were connected to the EEG Stellate Lamont Pro-36 programmable amplifier (Lamont Medical Instruments, Madison, WI) for a 24 h period and the EEG data was analyzed with the software program Stellate Harmonie (Stellate Systems, Inc., Montreal, Canada). Differential amplification recordings were recorded pair-wise between all four electrodes, providing a montage of 6 channels for each mouse. Mouse activity was captured simultaneously by video monitoring using a Panasonic WV-CP484 model camera, SDIII, with an infrared attachment to allow recordings in the dark. For ethosuximide treatment, mice were recorded for two hours and injected interperitoneally with 200 mg/kg ethosuximide (Sigma-Aldrich, St. Louis, MO). They were recorded for a minimum of one additional hour. Control experiments were performed on the same mice with saline injections at approximately the same time of day. All mouse procedures conformed to IACUC standards.
SWD phenotyping
SWD consist of adjacent, connected spike-wave (or wave-spike) complexes. SWD episodes were scored using the following criteria: the EEG recording showed at least 2 connected spike-wave complexes (typically spanning at least 0.4 seconds) with amplitudes at least two fold higher than background and observed concurrently in the majority of the 6 recording channels per mouse.
Statistical analysis
The effect of ethosuximide versus saline treatment on SWD counts of each strain in the respective 2 hour period was evaluated in a repeated measures type of design by a conservative nonparametric 2 × 2 contingency table analysis (before/after : ethusuximide/saline), using a Fisher Exact test (1-tail) due to the very low or zero number of events in some cells.
To determine the temporal clustering of SWD in SWD-positive strains, the plots were generated using the computer program JMP (SAS Institute, Cary, NC), and the goodness of fit tests were done using the KS and Anderson-Darling stats packages in R.
Pointwise p-values for haplotype mapping using the efficient mixed-model association (EMMA) analysis were calculated on the EMMA server (Kang et al. 2008). False discovery rate (FDR) q-values (Benjamini & Hochberg 1995), were calculated using the FDR add-in in JMP software. For the haplotype association mapping of SWD in 27 inbred strains, the EMMA algorithm (Kang et al. 2008) was used to determine the association between three SWD traits and 233554 SNPs stored and run at the EMMA correction server (http://mouse.cs.ucla.edu/emmaserver/).
Results
We performed EEG recordings from two male mice from each of 27 common inbred strains, all between two to four months of age. Although we have never observed any significant SWD differences by sex in inbred strains that have SWD, for this study males were chosen to retain a consistent testing profile between strains.
The recordings were taken over a 24-hour time period. All but one strain could be assigned either as SWD-positive or SWD-negative (Table 1). The 18 SWD-negative strains had no clear SWD over the 24 hours of recording. The exception was AKR/J where one of the 2 mice displayed SWDs. Further testing of 2 additional male AKR/J mice revealed no SWDs in either mouse. The AKR mouse with SWDs had a relatively low rate – but the events were clear and were suppressed by ethosuximide treatment.
Table 1.
Summary of EEG recordings from 27 inbred strains.
| Mice with SWDs (8) | Mice with no SWDs (18) | Other (1) | |
|---|---|---|---|
| A/J | BALB/cByJ | MRL/LpJ | AKR/J (1 in 4 mice had SWDs) |
| C3H/HeJ | BTBR T<+>tf/J | NOD/ShiLtJ | |
| C57BLKS/J | C57BL/6J | NZB/B1NJ | |
| CBA/J | C57BL/6NJ | NZW/LacJ | |
| DBA/1J | C57BL/10J | P/J | |
| DBA/2J | C57L/J | PL/J | |
| NOR/LtJ | FVB/NJ | SJL/J | |
| SM/J | I/LnJ | SWR/J | |
| LP/J | 129/SvlmJ | ||
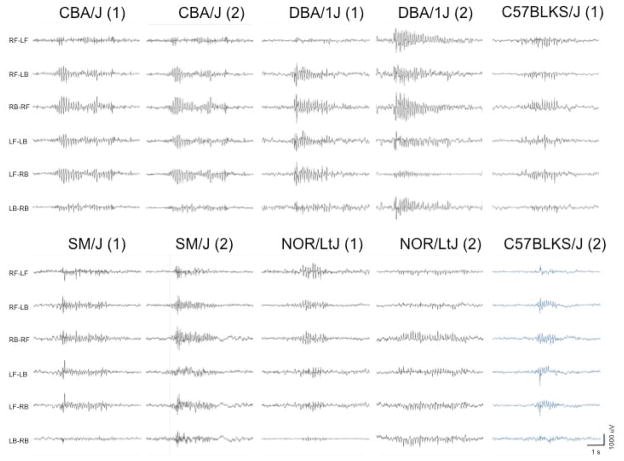
Among the strains with SWD, there was considerable variation in the number of events per hour, even within a strain (Table 2). This may reflect the sporadic nature of spontaneous seizure events. The strains with the highest SWD incidence were CBA/J, C3H/HeJ, DBA/1J and SM/J. Among the strains tested, the highest incidence was from DBA/1J, with one mouse showing as many as 177 seconds of SWD activity over a single hour interval. Three strains, A/J, C3H/HeJ and DBA/1J, had the longest SWD duration, averaging greater than 2 seconds. Typical EEG recordings from the 5 new inbred strains with SWDs (C57BLKS/J, CBA/J, DBA/1J, NOR/LtJ and SM/J) are illustrated in Fig. 1.
Table 2.
Results of 24 h EEG recordings in the 9 inbred strainsa with SWDs.
Two mice were tested for 8 of the 9 strains, and 4 mice for the AKR/J strain.
| STRAIN | Total no. of SWD episodes (24h) | SWD episodes/h | Total SWDs (seconds) | average SWD duration (secs) |
|---|---|---|---|---|
| A/J | 258 | 10.8 | 545.3 | 2.1 |
| 52 | 2.2 | 105.7 | 2.0 | |
| AKR/J | 122 | 5.1 | 171.7 | 1.4 |
| 0 | 0.0 | 0.0 | 0.0 | |
| 0 | 0.0 | 0.0 | 0.0 | |
| 0 | 0.0 | 0.0 | 0.0 | |
| C3H/HeJ | 335 | 14.0 | 844.0 | 2.5 |
| 349 | 14.5 | 831.8 | 2.4 | |
| CBA/J | 227 | 9.5 | 347.3 | 1.5 |
| 110 | 4.6 | 116.8 | 1.1 | |
| C57BLKS/J | 410 | 17.1 | 244.7 | 0.6 |
| 210 | 8.8 | 126.7 | 0.6 | |
| DBA/1J | 241 | 10.0 | 567.7 | 2.4 |
| 787 | 32.8 | 2334.2 | 3.0 | |
| DBA/2J | 162 | 6.8 | 236.8 | 1.5 |
| 42 | 1.8 | 48.2 | 1.1 | |
| NOR/LtJ | 84 | 3.5 | 139.2 | 1.7 |
| 62 | 2.6 | 80.1 | 1.3 | |
| SM/J | 600 | 25.0 | 691.0 | 1.2 |
| 357 | 14.9 | 260.9 | 0.7 |
In these initial tests, 2 mice were tested for 8 of the 9 strains, and 4 mice for the AKR/J strain.
Figure 1.
Typical EEG recordings from the 5 new inbred strains of mice with SWDs. EEGs from 2 mice for each strain are represented. The 6 channels show the differential amplification signal between the 4 electrodes; left front (LF), right front (RF), left back (LB) and right back (RB).
We visually inspected the profile of the SWDs to determine if there were any consistent qualitative differences between strains. Most SWDs occurred within the observed range of 6–8 Hz, typical for rodents, as illustrated in Fig. 1 for DBA/1J and DBA/2J, with no obvious differences in profiles between subjects. The exception was the SM/J, with SWDs consistently in the range of 9–10 Hz (Fig. S1).
We reviewed the SWD incidence for all 8 strains over the 24-hour recording period to determine if the SWDs were distributed randomly, by examining deviation from the beta distribution (Molinari et al. 2001) appropriate for testing clustering of events that are not necessarily independent from each other. From this analysis we determined that the incidence of SWDs was not randomly distributed, with a surfeit of shorter inter-SWD intervals, as illustrated in Figure S2. Each distribution deviated significantly from the expected beta distribution (p<0.0001, Anderson-Darling goodness of fit test). Clustering of SWDs is yet a further characteristic feature of absence seizures (Midzyanovskaya et al. 2006).
As five of the 8 SWD-positive strains represent new models of SWD activity, we examined EEGs from 2 further male subjects for each strain to establish that these SWD were reproducible. In every case the mice showed SWD activity accompanied by behavioral arrest. To quantify this measure, we systematically examined the video-EEG of the first 40 SWD events where the entire subject was visible to the camera, from each of the 5 strains to confirm the lack of movement during the SWD and to characterize motor activity immediately before and after each episode. Importantly, in 10% or more of these events we observed that the mouse exhibited normal activity prior to the SWD, paused during the SWD episode and resumed movement immediately following, all within a period of a few seconds. Indeed, in the SM/J strain, perhaps the most novel new model with its higher frequency SWD 9–10 Hz, about 30% of its SWD were flanked by movement of the mouse, (28 such events in 86 total SWD episodes noted). From these observations we conclude that these 5 new SWD-positive strains have the behavioral characteristics of absence seizures.
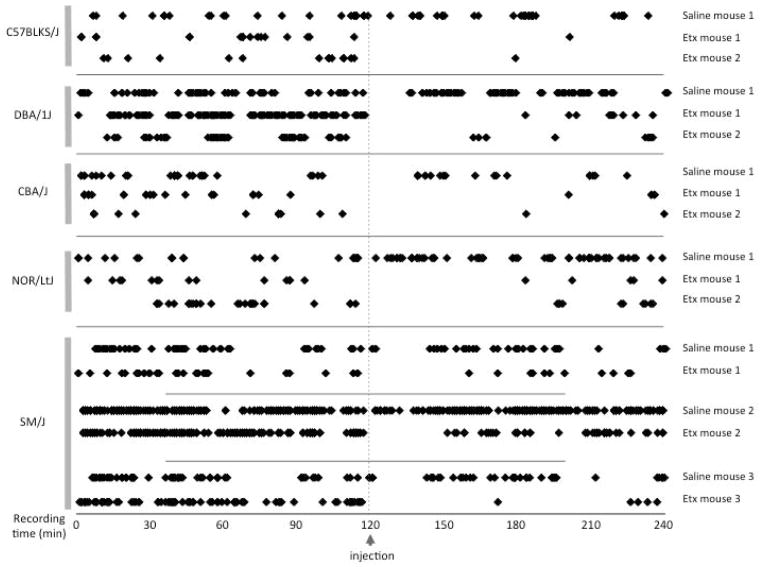
We next examined the 5 new SWD positive strains critically for their sensitivity to treatment using the anti-absence epilepsy drug ethosuximide (Fig 2). In each test, we counted the number of SWD episodes for 2 hours prior to a single acute treatment, and continued to monitor the EEGs for a further 2 hours. In all ethosuximide-treated mice there was an appreciable window where no SWD events occurred, ranging from 31 to 81 minutes post-treatment, and often infrequent SWD thereafter. In the four new SWD-positive strains that exhibited 5–8 Hz SWD burst frequencies that are typical for rodent absence epilepsy models, ethosuximide protection was significant for up to 2 hours post-injection compared with saline control (C57BLKS/J, p<0.001; CBA/J, p=0.028; DBA/1J, p<0.001 and NOR/LtJ, p<0.001). SM/J mice had a 9–10 Hz SWD profile (Fig. 1), atypical for rodent models, but ethosuximide clearly also protected against these, as determined similarly in 3 separate subjects treated first with saline and then with ethosuximide (Fig 2. lower panel: mouse 1, p=0.040; mouse 2, p<0.001 and mouse 3, p<0.001).
Figure 2.
Ethosuximide treatment of the 5 new SWD-positive inbred strains. The symbols represent each SWD episode for the 2 hours before and after injection of ethosuximide (Etx) or saline. For C57BLKS/J, DBA/1J, CBA/J and NOR/LtJ, mouse 1 was treated with Etx, and a saline control. For SM/J, all 3 mice were treated with both Etx and saline.
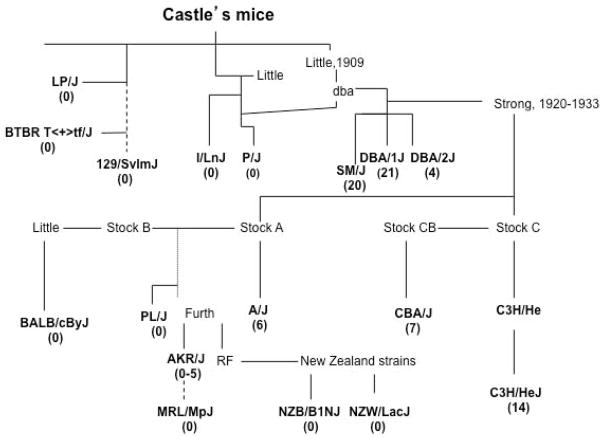
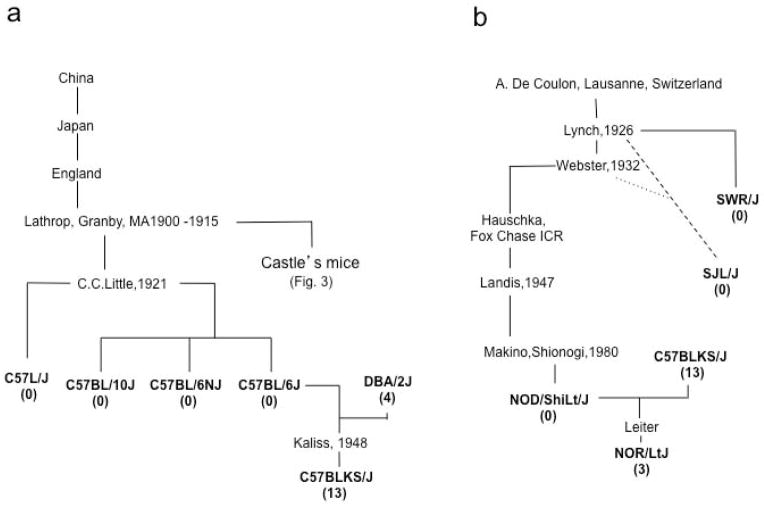
The strains examined here represent three genealogical hierarchies from the early days of mouse genetics; William Castle’s mice, C57-related strains and Swiss mice (Figs. 3 and 4), allowing us to follow SWD activity through three major laboratory mouse “family trees.”
Figure 3.
Genealogical tree of Castle’s mice. The average number of SWD episodes/hour is listed below each inbred line in parenthesis. The dotted line indicates an inferred or indirect descent.
Figure 4.
Hierarchy of C57 and Swiss mice. (a): C57 related strains. (b): Swiss derived mice. The average number of SWD episodes/hour is listed below each inbred line in parenthesis. The dotted line indicates an inferred or indirect descent.
Many of the strains tested here originally derive from Abbie Lathrop, who established a breeding farm in the early 1900s in Granby, MA (Russell 1978; Beck et al. 2000). Lathrop’s mice contributed to the majority of William Castle’s original stocks. Many strains that show SWD appear to have genealogical ties to “dba” mice (Figure 3). The present day DBA/1 and DBA/2 strains were originally inbred from a “dba” stock in 1909 by Clarence C. Little, a former student with Castle (Russell 1978), and both exhibit significant SWD activity. The closely related SM/J strain was inbred by Chen K. Chai (1954) and maintained for its small body size. It too had SWD activity (Table 2 and Fig. 3).
DBA heritage was clearly not sufficient to pass on the SWD phenotype to all descendant stocks. For example, DBA was an early and significant contributor to the SWD-negative P/J strain. Most other Castle mice were also negative, including I/LnJ, LP/J, 129/SvlmJ and BTBR T<+>tf/J (Fig. 3). Interestingly, despite having no detectable SWD both I/LnJ and P/J, like DBA, have a very low electroconvulsive thresholds (Frankel 2009). Together these observations are consistent with a shared, genetically complex heritability of seizure susceptibility in inbred mouse strains.
Leonell C. Strong continued to inbreed the Stocks A, B and C (Fig. 3). Stock C came from Halsey J. Bagg’s albino strain crossed to a DBA mouse from C.C. Little (Strong 1978). One line, C3H, was retained as it was particularly susceptible to mammary tumors, and the CB line was noticeably long-lived and maintained as a cancer resistant control line. As previously described, all the C3H substrains tested, including HeJ, HeOuJ, eB/FeJ and HeSnJ, exhibit SWDs, although to varying degrees (Tokuda et al. 2009). In the present study we found that the CBA/J strain also had SWDs.
Stock A was generated by Strong from Bagg’s albino crossed to Little’s albino male, and led to the inbred A/J line that also had SWDs, both from our data and others (Strohl et al. 2007). This line appears to be unrelated to the DBA stock and thus represents a second genealogical line of SWD inheritance.
Stock B also arose from Bagg’s albino strain. BALB was derived from this stock and showed no seizure activity. From this point it appears that SWDs are generally lost on this side of the family tree. Stock B may have given rise indirectly to PL/J and AKR/J. PL/J had no SWD activity, although they do have epilepsy in the form of convulsive seizures (Kitami et al. 2004). Jacob Furth established the AKR inbred strain for its high susceptibility to lymphoid leukemia. The AKR/J strain was the only strain in our survey that gave mixed results, with 3 out of 4 mice having no SWDs and one mouse with a low incidence of SWDs. It is possible this represents a new mutation not yet fixed in the AKR/J line.
The New Zealand strains, NZB and NZW, arose from the RF mice, also originally established by J. Furth. Neither strain showed any SWD activity. The final inbred line in this hierarchy, MRL/MpJ, arose from 4 lines (LG, AKR, C3H and C57BL/6) (Beck et al. 2000) and also had no SWD activity. 12% of the MRL genome is C3H-like and from our results it appears that sufficient C3H seizure-susceptibility alleles were not inherited by MRL.
C.C. Little established the line C (Fig. 4a) and the descendants of female 57 became the C57 inbred line, including C57L, C57BL/6 and C57BL/10, none of which showed any SWD activity. The C57BLKS/J line was inbred by Nathan Kaliss and was shown to be derived from a mix of inbred strains; mostly C57BL/6, and DBA/2, with lesser contributions from 129, C57BL/10 and a further unknown strain (Mao et al. 2006). Simple sequence length DNA polymorphisms (SSLPs) revealed that C57BLK/J retained about 16% DBA/2J (Naggert et al. 1995). Despite this relatively small contribution from the seizure-susceptible DBA strain, the C57BLKS/J mice had SWDs.
The Swiss line was inbred by Clara J. Lynch to give rise to SWR and SJL, and beyond to the NOD strains (Fig. 4b). None of these strains had SWD activity. However Edward H. Leiter ‘s NOR strain, selected as insulitis-resistant and diabetes-free (Reifsnyder et al. 2005), had a low incidence of SWDs. This strain was derived from NOD and C57BLKS/J, possibly reflecting the continuing influence of seizure-susceptibility originating from the DBA/2 strain.
Given the genealogical ties between SWD-positive mouse strains, we examined their genetic relatedness by comparing their identity of single nucleotide polymorphisms (SNP). Not surprisingly, each SWD-positive strain showed more identity (average 82%) with the other SWD-positive strains as a group, than to the SWD-negative strains (average 79%; Fig. S3), with exceptions in both directions. We then sought preliminary insight into chromosomal locations of putative SWD susceptibility loci by performing a genome-wide association analysis, using the efficient mixed-model association (EMMA) algorithm to correct the results for bias in population structure (Kang et al. 2008). Given the large number of SWD-negative strains, to be conservative we focused on binary SWD susceptibility, although SWD length and incidence were also examined. After a false discovery rate correction, 2000 SNPs were significant at q<0.05, comprising approximately 25–30 clusters (Fig. S4). For the binary trait, the two peak regions were a single SNP on Chr 17, and multiple SNPs in two closely linked clusters (7.1 and 7.2) on Chr 7 (Figs. S4 and S5). We examined allele distributions for these, as well as the peak SNP for SWD length on Chr 1, in the strain set (Fig. S5). Although these results are preliminary, together they suggest there is no single region that accounts for the SWD susceptibility of all SWD-positive strains. Assuming that the regions detected can be validated, it appears that the inheritance of SWD susceptibility among these related strains exhibits locus heterogeneity, a common source of genetic complexity.
Discussion
We screened a number of widely used inbred strains for the presence of spike-wave discharges (SWDs). We find that two distinct mouse strain lineages contributed to SWDs; DBA and A. Dr. C.C. Little’s DBA mice were the progenitors for several further SWD-positive lines including the C3H, CBA, C57BLKS, NOR and SM mice. A/J arose from an albino stock, a genealogically distinct line to DBA (Morse 1978). However the SWD phenotype was not observed in the BALB/J strain, a close relative to A/J. The C57 and Swiss mouse hierarchies did not exhibit any SWD except for when DBA/2J was accidentally introduced, as with C57BLKS and subsequently NOR.
These and prior studies of the C3H strain family illustrate that inheritance of SWDs may either be caused by a single, recent gene mutation, or by inheritance of multiple strain variants that arose in the past. In the C3H/HeJ substrain, frequent SWD are caused predominantly by a single gene mutation, identified as an intracisternal A particle (IAP) insertion that had stably integrated into the Gria4 gene, disrupting the expression of the excitatory neuronal AMPA receptor subunit 4 (Beyer et al. 2008). As none of the other C3H substrains retained this mutation, it was deduced that the IAP insertion happened after the substrains diverged. Nevertheless, EEG analysis of two other C3H/He substrains lacking the Gria4 IAP allele also revealed frequent SWDs. Attempts to determine the genes underlying this phenotype in one subline, C3H/HeOuJ, failed to identify any significant loci (Tokuda et al. 2009). These results indicate that there are probably multiple genes underlying this trait, each individually making a minor but, as yet undetected contribution.
Studies of SWD activity in the DBA/2J mice indicate that more than one genetic locus also underscores susceptibility in this strain (Ferraro et al. 2010). The Szs1 locus to which some SWD activity maps, is on distal Chromosome 1, within which the Kcnj10 gene was shown to be a major contributor to convulsive seizure susceptibility in DBA/2J caused by kainic acid, pentylenetetrazol or maximal electroshock (Ferraro et al. 2011). More recent EEG analysis suggested that this same genetic interval may partly explain the SWD phenotype in DBA/2J, although the Kcnj10 variant was not yet proven to be causal (Bessaïh et al. 2012). The C57BLKS/J strain also has SWDs and 16% of its genotype is DBA-like (Naggert et al. 1995), thus it is possible that C57BLKS/J has retained SWD susceptible loci from DBA/2J. Significantly DBA haplotype blocks are retained on Chromosomes (Chr) 1, 3, 4, 7, 11, 14 and 17 - but notably not around the Szs1 locus. The NOR/LtJ inbred strain also had SWDs and was derived from a cross between NOD and C57BLKS/J. Examining this strain for DBA/2J genomic loci revealed even fewer DBA blocks located on Chr 1, 4, 7 and 11 (E. Leiter, personal communication). Whether these loci contribute to the SWD phenotype in DBA/2J has still to be ascertained, but they at least represent regions of interest for initial studies. However, in a separate preliminary haplotype association analysis, using the entire panel of SWD-positive vs. SWD-negative strains including NOR/Lt and C57BLKS/J, for the top four associations, only the distal cluster on Chr 7 (Chr 7-2) carried a SWD sensitive allele in these strains, whereas two other SWD-positive strains carried a resistance allele (SM/J, A/J). Together these results imply complex inheritance of SWD susceptibility – likely locus heterogeneity - among the strains.
Laboratory mice and rat strains often serve as “normal” controls for neurological and other studies, but as shown for many phenotypes, they are not necessarily wild-type, as they segregate natural genetic variants that either predispose to disease or cause it outright. Because the mice here are common inbred strains, including the previously validated SWD-positive C3H/HeJ, one may question whether their SWDs represent true absence seizures as opposed to a kind of merely related, precursor or primordial activity. Indeed, in both normal and epileptic rats a non-seizure 7–12 Hz thalamocortical rhythm has been observed that can be interrupted by tactile stimuli (Pinault et al. 2001; Wiest & Nicolelis 2003). But this 7–12 Hz activity is focal (not bilaterally synchronous or generalized) and does not arrest behavior in the same manner as we observe in mice, or as has been observed recently in a subset of “normal” Sprague-Dawley rats (Pearce et al. 2014). Re-assortment of non-pathogenic variants leading to a more obvious seizure type was shown previously for the recombinant inbred strain SWXL-4, which has recurrent, electrographically-verified partial complex seizures with secondary generalizations (Frankel et al. 1994). SWXL-4 was derived originally from an intercross between non-seizing SWR and C57L inbred strains. While its recurrent seizures might have been explained by de novo mutation(s), in a mere 2-generation new cross between the same parent strains, the seizure phenotype was replicated (Frankel et al. 1994). This striking demonstration of how disease phenotypes are already “hiding” in resident non-pathogenic form supports the notion that genetically complex diseases are threshold traits at the edge of a normal phenotype distribution, and when the genetic milieu is right (as with the genealogically-related SWD-positive mouse strains we describe) it does not take much to push it over the edge into a disease state. Discriminating between genetic factors that define susceptibility from those few that push the phenotype past threshold may well be one of the difficulties in identifying human genes for idiopathic generalized epilepsy (Ottman 2005).
Although it has not yet been assessed whether and how SWDs will affect the overall mouse performance, it should be noted that patients with childhood absence epilepsy have shown elevated rates of adverse behavioral, psychiatric, language and cognitive comorbidities. They may also have difficulties in visual attention and visuospatial skills, verbal learning and memory (Tenney & Glauser 2013). In view of our findings, investigators may wish to be accordingly wary of SWD status when selecting inbred mouse strains for future studies. We have yet to discover whether the SWDs in these strains accompany any significant effects on other neurological behaviors that are not outwardly discernable, but this study does provide five new animal disease models that based on their shared genealogy, offer new opportunities to understand the genetic basis and consequences of this distinctive phenotype.
Supplementary Material
Figure S1. EEG recordings from the 2 test mice for (A) SM/J, (B) DBA/1J and (C) DBA/2J inbred strains illustrating how the burst frequencies were determined. 9–10 Hz SWDs are observed in both SM/J mice compared to the 6–8 Hz SWDs in the closely related DBA strains. The arrow indicates the onset of the SWD episode.
Figure S2. Temporal clustering of SWD in SWD-positive strains. Distribution of inter-SWD intervals in minutes (X-axis) against the respective beta quantile (Y-axis) for two previously known SWD strains (panel A) and for the five new SWD-positive strains (panel B), taken from 24 hour EEG data. The straight red line in each plot shows the expected distribution fit if SWD were randomly spaced, and 95% confidence limits shown as dashed lines. A significant number of SWD intervals were much shorter than expected (density of black dots to the left of the red line), indicating clustering.
Figure S3. Pairwise SNP identity between SWD+ and SWD- mouse strains. Shown is a matrix of SNP identity between strains used in this study, from SNP genetic variation query tools at the Mouse Phenome Database (http://phenome.jax.org) using a combination of Sanger Centre and Center for Genome Dynamics imputed SNPs. Strains were grouped by SWD-positive (red shading) or SWD-negative (blue shading), and sorted by decreasing identity within each group. At the right the results of Student’s |t|-test are shown, comparing % identities for a test strain with each group – statistically significant or suggestive p-values are shaded in red.
Figure S4. Haplotype association mapping of SWD in 27 inbred strains. The EMMA algorithm was used to examine association between SWD traits (SWD binary - positive or negative for SWD; SWD length; SWD incidence) and 233554 SNPs from the EMMA correction server (http://mouse.cs.ucla.edu/emmaserver/).
Figure S5. Inferred alleles for the top four chromosomal locations containing peak SNPs. Shown is a chart of inferred SWD susceptibility alleles recoded to reference their origin as being from SWD-positive strain “S” (susceptible – red shading), or SWD-negative strain “R” (resistant – blue shading). Blank green shaded cells reflect either alternate or indeterminate allele for that respective marker. The SWD phenotypes are shown on the right.
Acknowledgments
Dr. Frankel was supported by the U.S. National Institutes of Health (NIH) (NS031348). We thank Dr. Rebecca Boumil for her helpful discussion, and we are appreciative of Dr. Robyn Ball for consultation regarding statistical analysis.
References
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal Royal Stat Soc B. 1995;57:289–300. [Google Scholar]
- Bessaïh T, de Yebenes EG, Kirkland K, Higley MJ, Buono RJ, Ferraro TN, Contreras D. Quantitative trait locus on distal chromosome 1 regulates the occurrence of spontaneous spike-wave discharges in DBA/2 mice. Epilepsia. 2012;53:1429–1435. doi: 10.1111/j.1528-1167.2012.03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer B, Deleuze C, Letts VA, et al. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Hum Mol Genet. 2008;17:1738–1749. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong E, Zheng Y, Lee K, et al. Deletion of phospholipase C beta4 in thalamocortical relay nucleus leads to absence seizures. Proc Natl Acad Sci U S A. 2009;106:21912–21917. doi: 10.1073/pnas.0912204106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Wu Y, Gibson KM, Snead OC. Absence seizures in succinic semialdehyde dehydrogenase deficient mice: a model of juvenile absence epilepsy. Pharmacol Biochem Behav. 2004;79:547–553. doi: 10.1016/j.pbb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Cox GA, Lutz CM, Yang CL, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:1–20. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Cope DW, Terry JR. Transition to absence seizures and the role of GABA(A) receptors. Epilepsy Res. 2011;97:283–289. doi: 10.1016/j.eplepsyres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29:1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro TN, Smith GG, Ballard D, et al. Quantitative trait loci for electrical seizure threshold mapped in C57BLKS/J and C57BL/10SnJ mice. Genes Brain Behav. 2011;10:309–315. doi: 10.1111/j.1601-183X.2010.00668.x. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Smith GG, Schwebel CL, et al. Confirmation of multiple seizure susceptibility QTLs on chromosome 15 in C57BL/6J and DBA/2J inbred mice. Physiol Genom. 2010;42A:1–7. doi: 10.1152/physiolgenomics.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel WN. Genetics of complex neurological disease: challenges and opportunities for modeling epilepsy in mice and rats. Trends Genet. 2009;25:361–367. doi: 10.1016/j.tig.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel WN, Taylor BA, Noebels JL, Lutz CM. Genetic epilepsy model derived from common inbred mouse strains. Genetics. 1994;138:481–489. doi: 10.1093/genetics/138.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabrero AM, Guerrero-López R, Giráldez BG, Llorens-Martín M, Avila J, Serratosa JM, Sánchez MP. Hyperexcitability and epileptic seizures in a model of frontotemporal dementia. Neurobiol Dis. 2013;58C:200–208. doi: 10.1016/j.nbd.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Gigout S, Louvel J, Rinaldi D, Martin B, Pumain R. Thalamocortical relationships and network synchronization in a new genetic model “in mirror” for absence epilepsy. Brain Res. 2013;1525:39–52. doi: 10.1016/j.brainres.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics. 2008;178:1709–1723. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantheti P, Diaz ME, Peden AE, et al. Genetic and phenotypic analysis of the mouse mutant mh2J, an Ap3d allele caused by IAP element insertion. Mamm Genome. 2003;14:157–167. doi: 10.1007/s00335-002-2238-8. [DOI] [PubMed] [Google Scholar]
- Kitami T, Ernest S, Gallaugher L, Friedman L, Frankel WN, Nadeau JH. Genetic and phenotypic analysis of seizure susceptibility in PL/J mice. Mamm Genome. 2004;15:698–703. doi: 10.1007/s00335-004-3007-7. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. Embo J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao HZ, Roussos ET, Péterfy M. Genetic analysis of the diabetes-prone C57BLKS/J mouse strain reveals genetic contribution from multiple strains. Biochim Biophys Acta. 2006;1762:440–446. doi: 10.1016/j.bbadis.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Santoni F, Fà M, Puligheddu M, Barberini L, Genugu F, Frau R, Manunta M, Mereu G. Beta and gamma range EEG power-spectrum correlation with spiking discharges in DBA/2J mice absence model: role of GABA receptors. Epilepsia. 2006;47:489–494. doi: 10.1111/j.1528-1167.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- Midzyanovskaya I, Strelkov V, Rijn C, Budziszewska B, van Luijtelaar E, Kuznetsova G. Measuring clusters of spontaneous spike-wave discharges in absence epileptic rats. J Neurosci Methods. 2006;154:183–189. doi: 10.1016/j.jneumeth.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Molinari N, Bonaldi C, Daures JP. Multiple temporal cluster detection. Biometrics. 2001;57:577–583. doi: 10.1111/j.0006-341x.2001.00577.x. [DOI] [PubMed] [Google Scholar]
- Morse HC. Historical prespective on the development of inbred mice. In: Morse HC, editor. Origins of Inbred Mice. New York: Acedemic Press, Inc; 1978. pp. 3–21. [Google Scholar]
- Naggert JK, Mu JL, Frankel W, Bailey DW, Paigen B. Genomic analysis of the C57BL/Ks mouse strain. Mamm Genome. 1995;6:131–133. doi: 10.1007/BF00303258. [DOI] [PubMed] [Google Scholar]
- Nicolazzo JA, Steuten JA, Charman SA, Taylor N, Davies PJ, Petrou S. Brain uptake of diazepam and phenytoin in a genetic animal model of absence epilepsy. Clin Exp Pharmacol Physiol. 2010;37:647–649. doi: 10.1111/j.1440-1681.2010.05362.x. [DOI] [PubMed] [Google Scholar]
- Noebels JL. Single-gene models of epilepsy. Adv Neurol. 1999;79:227–238. [PubMed] [Google Scholar]
- Ottman R. Analysis of genetically complex epilepsies. Epilepsia. 2005;46(Suppl 10):7–14. doi: 10.1111/j.1528-1167.2005.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale LA, Beyer B, Jones JM, et al. Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Hum Mol Genet. 2009;18:1633–1641. doi: 10.1093/hmg/ddp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Bryant AS, Peng K, et al. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat Neurosci. 2011;14:1167–1173. doi: 10.1038/nn.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce PS, Friedman D, LaFrancois JJ, Iyengar SS, Fenton AA, MacLusky NJ, Scharfman HE. Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 2014;32:121–131. doi: 10.1016/j.yebeh.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Vergnes M, Marescaux C. Medium-voltage 5–9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience. 2001;105:181–201. doi: 10.1016/s0306-4522(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Reifsnyder PC, Li R, Silveira PA, Churchill G, Serreze DV, Leiter EH. Conditioning the genome identifies additional diabetes resistance loci in Type I diabetes resistant NOR/Lt mice. Genes Immun. 2005;6:528–538. doi: 10.1038/sj.gene.6364241. [DOI] [PubMed] [Google Scholar]
- Russell ES. Origins and history of mouse inbred strains: Contributions of Clarence Cook Little. In: Morse HC, editor. Origins of inbred mice. New York: Academic Press, Inc; 1978. pp. 33–45. [Google Scholar]
- Strohl KP, Gallaugher L, Lynn A, et al. Sleep-related epilepsy in the A/J mouse. Sleep. 2007;30:169–176. doi: 10.1093/sleep/30.2.169. [DOI] [PubMed] [Google Scholar]
- Strong LC. Inbred mice in science. In: Morse HC, editor. Origins of inbred mice. New York: Academic Press, Inc; 1978. pp. 45–67. [Google Scholar]
- Tenney JR, Glauser TA. The Current State of Absence Epilepsy: Can We Have Your Attention? Epilepsy Currents. 2013;13:135–140. doi: 10.5698/1535-7511-13.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda S, Beyer BJ, Frankel WN. Genetic complexity of absence seizures in substrains of C3H mice. Genes Brain and Behav. 2009;8:283–289. doi: 10.1111/j.1601-183X.2008.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Stewart L, Cortez MA, et al. The circuitry of atypical absence seizures in GABA(B)R1a transgenic mice. Pharmacol Biochem Behav. 2009;94:124–130. doi: 10.1016/j.pbb.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Wiest MC, Nicolelis MA. Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat Neurosci. 2003;6:913–914. doi: 10.1038/nn1107. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mahaffey CL, Bérubé N, Maddatu TP, Cox GA, Frankel WN. Complex seizure disorder caused by Brunol4 deficiency in mice. PLoS Genet. 2007;3:e124. doi: 10.1371/journal.pgen.0030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vilaythong AP, Yoshor D, Noebels JL. Elevated thalamic low-voltage-activated currents precede the onset of absence epilepsy in the SNAP25-deficient mouse mutant coloboma. J Neurosci. 2004;24:5239–5248. doi: 10.1523/JNEUROSCI.0992-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. EEG recordings from the 2 test mice for (A) SM/J, (B) DBA/1J and (C) DBA/2J inbred strains illustrating how the burst frequencies were determined. 9–10 Hz SWDs are observed in both SM/J mice compared to the 6–8 Hz SWDs in the closely related DBA strains. The arrow indicates the onset of the SWD episode.
Figure S2. Temporal clustering of SWD in SWD-positive strains. Distribution of inter-SWD intervals in minutes (X-axis) against the respective beta quantile (Y-axis) for two previously known SWD strains (panel A) and for the five new SWD-positive strains (panel B), taken from 24 hour EEG data. The straight red line in each plot shows the expected distribution fit if SWD were randomly spaced, and 95% confidence limits shown as dashed lines. A significant number of SWD intervals were much shorter than expected (density of black dots to the left of the red line), indicating clustering.
Figure S3. Pairwise SNP identity between SWD+ and SWD- mouse strains. Shown is a matrix of SNP identity between strains used in this study, from SNP genetic variation query tools at the Mouse Phenome Database (http://phenome.jax.org) using a combination of Sanger Centre and Center for Genome Dynamics imputed SNPs. Strains were grouped by SWD-positive (red shading) or SWD-negative (blue shading), and sorted by decreasing identity within each group. At the right the results of Student’s |t|-test are shown, comparing % identities for a test strain with each group – statistically significant or suggestive p-values are shaded in red.
Figure S4. Haplotype association mapping of SWD in 27 inbred strains. The EMMA algorithm was used to examine association between SWD traits (SWD binary - positive or negative for SWD; SWD length; SWD incidence) and 233554 SNPs from the EMMA correction server (http://mouse.cs.ucla.edu/emmaserver/).
Figure S5. Inferred alleles for the top four chromosomal locations containing peak SNPs. Shown is a chart of inferred SWD susceptibility alleles recoded to reference their origin as being from SWD-positive strain “S” (susceptible – red shading), or SWD-negative strain “R” (resistant – blue shading). Blank green shaded cells reflect either alternate or indeterminate allele for that respective marker. The SWD phenotypes are shown on the right.