Abstract
Juzen-taiho-to (JTT) is an immunostimulatory herbal formulation that is clinically used in East Asia for cancer patients undergoing chemotherapy and radiation. The formulation stimulates various leukocytes, including T, B, and NK cells and macrophages (MΦ). Although JTT is known to contain numerous compounds with various pharmacological activities, it is not clear which compounds are responsible for the stimulation of individual cell types. Here, we conducted what we call, “biomarker-guided screening,” to purify compounds responsible for the MΦ stimulatory activity. To this end, gene expression was analyzed by a DNA array for MΦ treated with JTT and DMSO (vehicle control), which identified intercellular adhesion molecule 1 (ICAM-1) as a biomarker of MΦ-stimulation by JTT. A qRT-PCR assay of ICAM-1 was then used to guide the purification of active compounds. The screening resulted in the purification of a glycolipid mixture, containing β-glucosylceramides. The glycolipid mixture potently stimulated ICAM-1 expression in primary dendritic cells (DC) as well as in primary CD14+ (MΦ) cells. Identification of this glycolipid mixture opens an opportunity for further studies to understand how plant-derived glycolipids stimulate MΦ and DC in a safe and effective manner as demonstrated by JTT.
Keywords: biomarker-guided screening, glycolipid, immunostimulation, β-glucosylceramides
Introduction
Juzen-taiho-to (JTT; Shi-Quan-Da-Bu-Tang in Chinese) is an immunostimulatory herbal formulation with an ideal balance of safety and efficacy [1,2]. In East Asia, the formulation has been used clinically for immunocompromised individuals, such as cancer patients undergoing chemotherapy and radiation [1-4]. JTT stimulates various leukocytes, including T, B, and NK cells and macrophages (MΦ), which result in the anti-tumor and anti-metastatic effects as well as alleviation of anemia and leukopenia [5-8]. Although biological effects of JTT have been well documented, its chemical characterization has been hampered by the enormous complexity of this formulation, which is a decoction of ten different herbs (Astragalus membranaceus, Cinnamomum cassia, Rehmannia glutinosa, Paeonia lactiflora, Ligusticum wallichii, Angelica sinensis, Panax ginseng, Poria cocos, Atractylodes macrocephala, Glycyrrhiza uralensis) (Table S1). Each of these herbs contains numerous compounds with known pharmacological activities [9]. However, it is not clear which of those compounds are playing key roles in the observed therapeutic effects. This is an important problem because, without the knowledge of key therapeutic agents, it would not be possible to understand the molecular mechanism underlying the safe and effective immunostimulatory effects exhibited by JTT.
In order to identify key therapeutic agents in JTT, we conducted what we call, “biomarker-guided screening [10].” In biomarker-guided screening, genomic tools are used to identify a biomarker associated with a therapeutic effect of herbal medicine. The identified biomarker is then used to guide the purification of therapeutically relevant compounds. In the current study, DNA arrays were used to identify a biomarker of MΦ-stimulation by JTT. Purification of MΦ-stimulatory compounds was guided by the qRT-PCR assay of the identified biomarker, which led to the identification of a glycolipid mixture that collectively exhibited potent immunostimulatory activity toward primary dendritic cells (DC) as well as primary CD14+ (MΦ) cells.
Results
As the first step of biomarker-guided screening [10], a biomarker of MΦ-stimulation by JTT was identified by gene expression profiling. For this screening a human monocytic leukemia cell-line (THP-1) was used, because this cell-line is known as a good model of monocytes and MΦ [11-13]. THP-1 was treated with JTT and a vehicle control (DMSO). Genes regulated by JTT were identified by Affymetrix Human Genome U133 Plus 2.0 array (Table 1). Many observed genes turned out to be the targets of the NF-κB signaling [14]. Among them was ICAM-1, a well-known biomarker of activated MΦ [14,15]. ICAM-1 was an ideal gene for our screening because its expression could be readily analyzed at both mRNA and protein levels by qRT-PCR and FACS, respectively. We, therefore, decided to use ICAM-1 to guide the purification of MΦ-stimulants from JTT.
Table 1.
Gene expression profile of THP-1 treated with JTT
| UniGene ID | Gene | Fold Change | Log ratio a | Change p-value b |
|---|---|---|---|---|
| Hs.75703 | CCL4 chemokine | 89.0 | 6.5 | 0.00006 |
| Hs.437322 | TNF-α induced protein 6 | 74.8 | 6.2 | 0.00002 |
| Hs.632586 | CXCL10 chemokine | 54.8 | 5.8 | 0.00002 |
| Hs.450230 | IGF binding protein 3 | 26.9 | 4.8 | 0.00040 |
| Hs.574492 | Nucleoporin | 26.0 | 4.7 | 0.00017 |
| Hs.137459 | Shroom family 3 | 23.4 | 4.6 | 0.00075 |
| Hs.31210 | BCL3 | 17.8 | 4.2 | 0.00009 |
| Hs.514107 | CCL3 chemokine | 17.4 | 4.1 | 0.00003 |
| Hs.126256 | Interleukin 1β (IL1B) | 16.0 | 4.0 | 0.00035 |
| Hs.624 | Interleukin 8 (IL8) | 14.9 | 3.9 | 0.00003 |
| Hs.632592 | CXCL11 chemokine | 14.2 | 3.8 | 0.00070 |
| Hs.241570 | TNF | 13.5 | 3.8 | 0.00007 |
| Hs.643447 | ICAM-1 | 11.9 | 3.6 | 0.00033 |
| Hs.591338 | TNF-α induced protein 3 | 11.5 | 3.5 | 0.00002 |
The expression profiling was carried out with Affymetrix Human Genome U133 Plus 2.0 array.
Log Ratio is log2(signal of JTT-treated cells/signal of control cells).
Change p-values were calculated using the Wilcoxon signed rank test and Tukey Biweight on the Affymetrix Microarray Suite Version 5.0 (MAS5) Software.
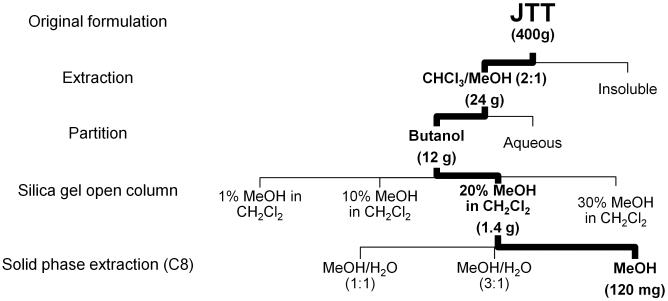
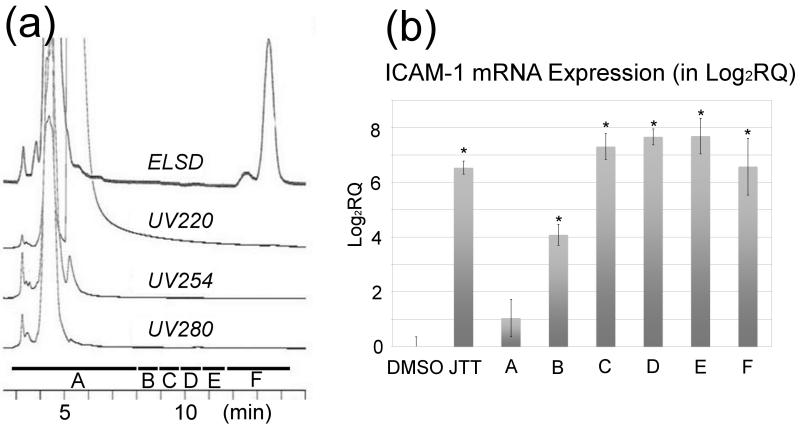
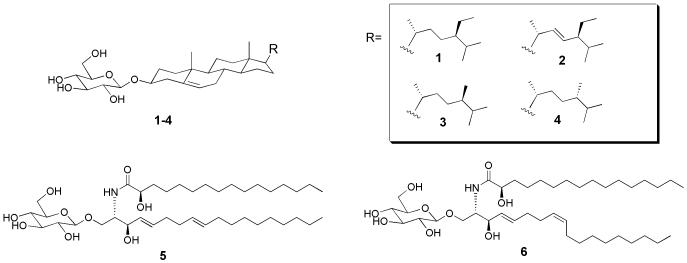
Figure 1 summarizes the fractionation scheme used in this study. At each stage of fractionation, activities of fractions were characterized by the qRT-PCR assay of ICAM-1 using THP-1 cells. The fractionation led to the enrichment of the MΦ-stimulatory activity (that is, the “MeOH” fraction in Fig. 1). Reversed phase HPLC (C18) of this “MeOH” fraction gave hardly any UV-visible peaks (Fig. 2a); the large peak around 5 min was DMSO, which was used to dissolve the “MeOH” fraction. Analysis of the HPLC eluent with evaporative light scattering detector (ELSD), which can detect compounds with weak or no UV-chromophores, resulted in the observation of two peaks around 12-14 min (Fig. 2a). The ELSD peaks were purified and subjected to spectroscopic analyses by NMR and mass spectrometry (MS). The largest ELSD peak turned out to be β-sitosteryl β-d-glucoside (BSSG) 1, whereas the smaller peak contained stigmasteryl β-D-glucoside 2, campesteryl β-d-glucoside 3 and 24-epicampesteryl β-d-glucoside 4 (Fig 3) [16,17]. Since these peaks were the major constituents in the “MeOH” fraction and exhibited MΦ-stimulatory activity, we initially thought these phytosteryl glucosides were responsible for the activity. To our surprise, however, further studies of HPLC fractions revealed that the maximum activity eluted between 9 and 12 minutes (Fractions C, D, and E) before these phytosteryl glucosides (Fig. 2b). This observation indicated that minor constituents eluted around 9-12 min were the main MΦ-stimulatory factors. Preliminary NMR and MS analyses suggested that they were glycolipids. Isolation of individual glycolipids, however, was hampered by their similar chromatographic behaviors as well as by their minute quantities. In addition, the activity disappeared whenever we attempted to isolate each glycolipid. We, therefore, shifted our focus on the identification of glycolipids. Per-acetylation of the glycolipid mixture enabled the separation of some products. This led to the purification and identification of β-glucosylceramide isomers (“soya cerebrosides”), 5 and 6 [18] (Supporting Information). In addition, high resolution ESI MS analyses showed the presence of compounds with molecular formula, C40H77NO9, and C49H88O15 (Supporting Information). Given the fact that glucosylceramides, 5 and 6, were observed in the glycolipid mixture, C40H77NO9 was likely to be another monoglycosylated ceramide. On the other hand, C49H88O15 was consistent with a digalactosyldiacylglycerol (DGDG), which is a common glycolipid in plants. ESI MS also indicated the presence of other DGDGs with different acyl groups.
Fig. 1.
Fractionation of JTT guided by the qRT-PCR assay of ICAM-1.
Fig. 2.
Analysis of the fraction (“MeOH” fraction) enriched with the MΦ-stimulatory activity. (a) HPLC (C18) chromatogram of the “MeOH” fraction obtained from the solid phase extraction (C8) in Fig. 1. The HPLC eluent was monitored by PDA detector (at 220, 254, 280 nm) as well as by ELSD. (b) qRT-PCR analysis of THP-1 cells treated with the HPLC fractions. DMSO (vehicle control), JTT (100 μg/mL), LPS (Lipopolysaccharides, positive control, 0.5 μg/mL), all fractions (5 μg/mL). The maximum MΦ-stimulatory activity was eluted between 9 and 12 minutes (Fractions C, D, and E) before the ELSD visible peaks. At least triplicate experiments (n=3) were carried out for each sample. **P < 0.001, compared with the DMSO control.
Fig. 3.
Structures of compounds identified in the “MeOH” fraction.
In order to examine the reproducibility of our purification scheme, we repeated experiments using two different lots of JTT. In all experiments, our purification scheme reproducibly enriched the potent MΦ-stimulatory activity in the same 9-12 min region on HPLC (Fig. 2). At this point, we also confirmed that the observed activity was not due to Endotoxin contamination (Supporting Information), which often plagues studies of immunostimulatory factors [19-21].
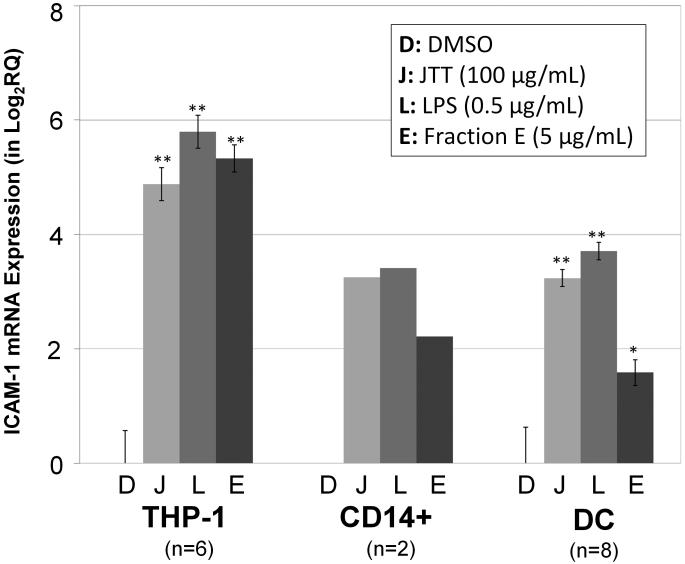
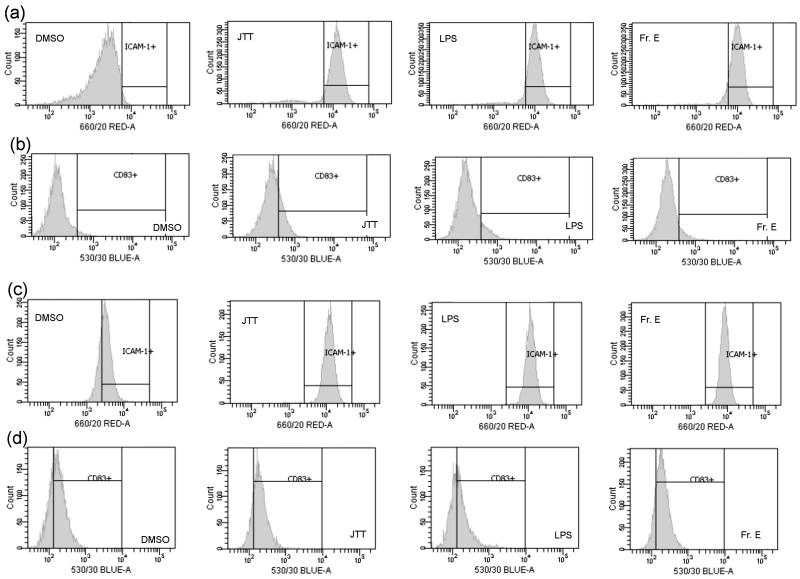
Next, we conducted further biological characterization of the glycolipid mixture using primary MΦ (CD14+ cells) and DC. This was necessary because, up to this point, MΦ-stimulatory activity was examined with an immortalized cell line (THP-1 cells). For this study, Fraction E (Fr. E) on Fig. 2, which exhibited the maximum activity, was examined. The qRT-PCR analysis showed that Fr. E induced ICAM-1 mRNA in both primary MΦ and DC (Fig. 4). At the mRNA level, the potency of Fr. E appeared somewhat weaker than the positive controls (LPS and JTT). However, a more striking effect was observed when CD54 (ICAM-1 protein) expression was examined by FACS. Fr. E potently stimulated the induction of CD54 at the level comparable to LPS and JTT (Fig. 5). These results demonstrated that the glycolipid mixture from JTT could potently stimulate various leukocytes in the innate immune system.
Fig. 4.
qRT-PCR analysis of ICAM-1 mRNA in THP-1, primary macrophages (CD14+) and dendritic cells (DC). Cells were treated with the sample for 4 hours and subjected to RNA isolation and qRT-PCR analysis. DMSO (vehicle control), JTT (100 μg/mL), LPS (Lipopolysaccharides, positive control, 0.5 μg/mL), Fraction E (Fr. E) (5 μg/mL). Number of biological replicates: THP-1 (n=6); CD14+ (n=2); DC (n=8). **P < 0.001, *P < 0.01, compared with the DMSO control.
Fig. 5.
FACS analysis of CD54 (ICAM-1 protein) and CD83 in primary macrophages (CD14+) and dendritic cells (DC). CD83, which is not induced by JTT, was used as the control to monitor non-specific binding of IgG. (a) CD54 in CD14+ cells. (b) CD83 in CD14+ cells. (c) CD54 in DC. (d) CD83 in DC. Cells were treated with the sample for 24 hours and subjected to FACS analysis. DMSO (vehicle control), JTT (100 μg/mL), LPS (Lipopolysaccharides, positive control, 0.5 μg/mL), Fraction E (Fr. E) (5 μg/mL).
Discussion
The current study established a reproducible method to purify potent MΦ/DC-stimulants from JTT. Phytosteryl glucosides 1-4 were the most conspicuous group of compounds identified in the study. Phytosteryl glucosides, especially, BSSG 1, have been linked to immunomodulatory activity in various clinical studies [22-24]. For example, a plant formulation enriched with β-sitosterol and BSSG has been shown to swing the Th1/Th2 balance from Th2 responses to Th1 responses in HIV-infected patients [25]. The same formulation also prevented immunosuppression associated with excessive physical stress [26]. Although β-sitosterol and BSSG may have important immunomodulatory activities, the current study indicates that the key MΦ/DC-stimulatory factor in JTT is the glycolipid mixture that is purified together with BSSG. The glycolipid mixture contained at least two classes of compounds, namely, β-glucosylceramides and DGDGs. β-Glucosylceramides have been shown to serve as immunoadjuvants for HBV and cancer vaccination in preclinical studies [27,28]. In addition, DGDGs are known to possess immunomodulatory effects [29]. Here, it is important to note that the compounds identified in the current study are major constituents in the glycolipid mixture. Minute constituents in the mixture, which were not characterized in the current study, may also contribute to the observed potent immunostimulatory activity.
The glycolipid mixture provides the new basis to understand the origin of potent MΦ/DC-stimulatory activity. At this point, it is not clear whether the activity arises from a particular glycolipid in the mixture or from synergism [30]. Our current efforts are directed toward obtaining individual glycolipids either synthetically or from each component herb of JTT. Once those compounds are available, it would become possible to conduct systematic studies to understand the molecular basis of the potent MΦ/DC-stimulation. Such studies would also improve the standardization of JTT formulation, which is currently carried out with major chemical constituents, such as glycyrrhizin, paeoniflorin, and cinnamic acid, although these compounds may not be involved in the therapeutic effects of JTT.
Understanding of molecular mechanism of JTT is also important with regard to the development of new immunoadjuvants. Immunoadjuvants are vaccine components to boost desired immune responses [31]. There is an unmet medical need to develop safe and effective immunoadjuvants. The existing immunoadjuvants, such as alum (aluminum salts) and Freund’s complete adjuvant (that is, oil-water emulsion with inactivated bacterial products), suffer from serious side-effects, such as toxicity and severe allergic reactions. It is our expectation that further studies on the plant-derived glycolipids will enable us to find new approaches to stimulate the immune system safely and effectively as demonstrated by JTT.
Materials and Methods
Materials
The solvents for extraction/purification were HPLC grade and were purchased from VWR and Fisher Scientific. Two different lots of Juzen-taiho-to (JTT) formulation (Lot # E24801 and 05F088) were purchased from TCM Zone/Honso Pharmaceutical Co. (Nagoya, Japan) as a dry water extract. Unless specified otherwise, all other chemicals and reagents were obtained from Fisher Scientific and VWR and used without further purification. Stock solutions of JTT and all fractions were prepared in DMSO.
Cell culture (THP-1)
THP-1 cells were purchased from American Type Culture Collection (ATCC). Cells were propagated in RPMI-1640 media containing 25 mM HEPES and l-glutamine (HyClone), 10%(v/v) fetal bovine serum (Fisher), 1%(v/v) penicillin, streptomycin and amphotericin B mixture (VWR) and 0.005 mM β-mercaptoethanol. Cells were maintained in a 37 °C humidified 5% CO2 incubator.
Gene expression profiling with Affymetrix GeneChip®
On the day before the gene expression profiling, 2 million THP-1 cells were plated on a T-75 flask. After 24 hr cells were treated with JTT (100 μg/mL) or DMSO vehicle control for 4 hr in a 37 °C humidified 5% CO2 incubator. Total RNA was purified with a Qiagen RNeasy mini kit. Expression profiling was carried out by following the Affymetrix GeneChip Expression Profiling manual. Briefly, mRNA was reverse-transcribed to cDNA using a T7-Oligo(dT) primers and SuperScriptII reverse transcriptase (Invitrogen). Synthesis of biotinylated cRNA via double-strand cDNA with T7 promoter was carried out with Affymetrix One-Cycle Target Labeling and Control Reagent kit. Biotinylated cRNA samples were fragmented and submitted to the Rockefeller University Genomics Center for hybridization to Human Genome U133 Plus 2.0 array, staining, washing, and scanning. The resulting data were analyzed with Affymetrix GeneChip® Operating Software and ArrayAssist Lite (Stratagene). Two independent experiments were carried out for each treatment condition: JTT-treated and DMSO (vehicle control)-treated THP1 cells. This enabled four comparisons between JTT-treated and control groups. Genes that were called “I (increase)” or “D (decrease)” by Affymetrix GeneChip® Operating Software in at least three out of four comparisons were selected. In order to obtain the most reliable genes for the subsequent real-time PCR guided fractionation, stringent criteria (Change p-value smaller than 0.001 or larger than 0.999) were used to further narrow down the gene list.
qRT-PCR
THP-1 cells were split one day before the treatment at the concentration of 0.2-0.5 × 106 in 2 mL of media in 6- or 12-well plates. After 24 hr, cells were treated with fractions, JTT (positive control, 100 μg/mL), LPS (positive control, 0.5 μg/mL) or DMSO vehicle control for 4 hours in a 37 °C humidified 5% CO2 incubator. RNA purification, cDNA synthesis, and qRT-PCR on an Applied Biosystems 7500 Real-Time PCR system were carried out as described previously [32]. In the qRT-PCR experiment, pre-optimized assay for ICAM-1 (Life Technologies Assay Id: Hs00277001_m1) and GAPDH endogenous control (Life Technologies Catalog Number: 4352934E) were used. The ΔΔCT method was employed to quantify the differential expression of ICAM-1. The raw data were first normalized by the endogenous control (GAPDH) for individual samples. Then the relative quantification (RQ) values, i.e. the ratio, were obtained by comparing the normalized data against the DMSO vehicle control.
Fractionation of JTT guided by qRT-PCR of ICAM-1
Every 400 grams of JTT sample was extracted with 3 L of chloroform/methanol (2:1). The mixture was stirred for 24 hr at room temperature, in a covered 5 L round bottom flask. The insoluble material was filtered and the solvent was evaporated using a rotary evaporator. The dried fraction (24 g) was partitioned between n-butanol and water (1:1, total 2,000 mL). The 1-butanol layer, which contained the activity, was separated from the aqueous layer, and evaporated to obtain dark oil (12 g). The 1-butanol fraction (6 g each) was loaded onto an open silica gel column (Silica gel = 120 g; column i.d. 3.5 cm) and eluted with 1%, 10%, 20%, and 30% methanol in dichloromethane (480 mL each). The 20% methanol in dichloromethane, which exhibited the most potent activity, was evaporated under vacuum to obtain a dried material (0.7 g; total 1.4 g after processing all 1-butanol fraction). The dried sample (120 mg each) was dissolved in 50% methanol in water (500 μL) and loaded onto a solid phase extraction C8 column (Alltech SPE C-8). The loaded sample was eluted with 50% MeOHaq (4 mL), 75% MeOHaq (4 mL) and 100% MeOH (8 mL). The “100% MeOH” fraction, which showed the activity, was dried to give ~10 mg of purified material (total ~120 mg after processing all material).
HPLC
The reversed-phase HPLC analysis and purification were carried on an Agilent 1100 series using a semi-preparative C18 column (Alltech®). The “100% MeOH” fraction were dissolved in DMSO and filtered through a 0.22-μm PTFE membrane filter (Whatman). The reversed-phase purification was carried out on an Alltech Econosil semi-preparative C18 10×250 mm column (Alltech) with isocratic elution using 100% methanol at an operating temperature 26°C. Isocratic elution was carried out over a period of 30 min with a flow rate of 3 mL/min. HPLC eluent was monitored by an Agilent 1100 photodiode array detector and an evaporative light scattering detector (ELSD) model 800 (Alltech®). UV signals were collected at 220 nm, 254 nm, and 280 nm. The data were recorded and processed using Agilent ChemStation software. ELSD analysis was performed with nitrogen in a drift temperature of 43°C and nebulizer pressure of 4.4 bar.
NMR
Samples were dissolved in methanol-d4, CDCl3, or a mixture of methanol-d4/CDCl3. NMR spectra were measured by Bruker Avance 500-MHz spectrometer equipped with a dual [13C, 1H] CryoProbe. Data were acquired and processed with the Bruker XWIN-NMR software package.
Purification of primary CD14+ cells and DCs from human blood
Peripheral blood mononuclear cells (PBMCs) were from Leukopaks (New York Blood Center, New York, NY) by Ficoll–Paque Plus (GE Healthcare Life Sciences, Piscataway, NJ) density centrifugation. CD14+ cells were first isolated from PBMCs by using anti-CD14 antibody coupled magnetic microbeads (Miltenyi Biotec, Auburn, CA). Immature DCs were prepared by culturing CD14+ cells for 5 days in the presence of 20 ng/mL of recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN) and 25 ng/mL of recombinant human IL-4 (R&sD Systems).
Determination of CD54 (ICAM-1 protein) on primary CD14+ cells and DCs by FACS
Cells were plated in 96-well flat-bottomed culture plates at a concentration of 3 × 105 cells/well. Cells were treated with 5 μg/mL of Fraction E, 0.5 μg/mL of lipopolysaccharide (LPS) (Sigma), 100 μg/mL of JTT, or DMSO (vehicle control). Allophycocyanin labeled anti-CD54 antibody (Biolegend, San Diego, USA) was used to determine the expression of CD54 (ICAM-1 protein). Allophycocyanin labeled antibody against CD86, which is not induced by JTT, was used to monitor non-specific IgG binding to cells. Acquisition was performed on a LSR II (Becton Dickinson), alive cells are gated by forward scatter and side scatter, and data were analyzed using DIVA software (Becton Dickinson). The mean fluorescent intensities (MFI) of gated cells were determined by DIVA software (BD Biosciences).
Human subjects
Human PBMCs from anonymous blood donors were obtained from leukopacks provided by the New York Blood Center (NYBC). The NYBC does not select donors on the basis of gender or race but ensures that all donors are above 18 years of age. Therefore, the work we performed did not require approval from the Institutional Review Board.
Supplementary Material
Acknowledgements
This study was supported by NIGMS/NIH SC3 GM094070 (AK). NIMHD/NIH G12 MD007599-27, which supports the research infrastructure at Hunter College, is also acknowledged. We thank Drs. Clifford E. Soll and Matthew Devany for their help in MS and NMR measurements. We thank Ms. Kriti Kalpana for technical assistance.
Abbreviations
- JTT
Juzen-taiho-to
- MΦ
macrophages
- NF-κB
nuclear factor-kappaB
- mRNA
messenger RNA
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- ICAM-1
intercellular adhesion molecule
- 1C
dendritic cells
- FACS
fluorescence-activated cell sorting
- ELSD
evaporative light scattering detector
- ESI
electrospray ionization
- DGDG
digalactosyldiacylglycerols
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Experimental procedures and data for the identification of β-glucosylceramides (soya cerebrosides), ESI MS data, FACS data table (Table S2), Certificates of Analysis for the two lots of JTT, Endotoxin assay results are provided as Supporting Information.
References
- 1.Yamada H, Saiki I. In: Juzen-taiho-to (shi-quan-da-bu-tang): scientific evaluation and clinical applications. Haruki Yamada, Ikuo Saiki., editors. Taylor & Francis; Boca Raton, Fla.; London: 2005. [Google Scholar]
- 2.Zee-Cheng RK. Shi-quan-da-bu-tang (ten significant tonic decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find Exp Clin Pharmacol. 1992;14:725–736. [PubMed] [Google Scholar]
- 3.Huang SM, Chien LY, Tai CJ, Chiou JF, Chen CS. Effectiveness of 3-week intervention of Shi Quan Da Bu Tang for alleviating hematotoxicity among patients with breast carcinoma receiving chemotherapy. Integr Cancer Ther. 2013;12:136–144. doi: 10.1177/1534735412450513. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa K, Omatsu T, Matsumoto C, Tsuchiya N, Yamamoto M, Naito Y, Yoshikawa T. Protective effect of the Japanese traditional medicine juzentaihoto on myelosuppression induced by the anticancer drug TS-1 and identification of a potential biomarker of this effect. BMC Complement Altern Med. 2012;12:118. doi: 10.1186/1472-6882-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnishi Y, Fujii H, Hayakawa Y, Sakukawa R, Yamaura T, Sakamoto T, Tsukada K, Fujimaki M, Nunome S, Komatsu Y, Saiki I. Oral administration of a Kampo (Japanese herbal) medicine Juzen-taiho-to inhibits liver metastasis of colon 26-L5 carcinoma cells. Jpn J Cancer Res. 1998;89:206–213. doi: 10.1111/j.1349-7006.1998.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hisha H, Yamada H, Sakurai MH, Kiyohara H, Li Y, Yu C, Takemoto N, Kawamura H, Yamaura K, Shinohara S, Komatsu Y, Aburada M, Ikehara S. Isolation and identification of hematopoietic stem cell-stimulating substances from Kampo (Japanese herbal) medicine, Juzen-taiho-to. Blood. 1997;90:1022–1030. [PubMed] [Google Scholar]
- 7.Hisha H, Kohdera U, Hirayama M, Yamada H, Iguchi-Uehira T, Fan TX, Cui YZ, Yang GX, Li Y, Sugiura K, Inaba M, Kobayashi Y, Ikehara S. Treatment of Shwachman syndrome by Japanese herbal medicine (Juzen-taiho-to): stimulatory effects of its fatty acids on hemopoiesis in patients. Stem Cells. 2002;20:311–319. doi: 10.1634/stemcells.20-4-311. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Kato M, Takeda K, Kawamoto Y, Akhand AA, Hossain K, Suzuki H, Nakashima I. T-cell-immunity-based inhibitory effects of orally administered herbal medicine juzentaiho-to on the growth of primarily developed melanocytic tumors in RET-transgenic mice. J Invest Dermatol. 2001;117:694–701. doi: 10.1046/j.0022-202x.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki M, Harada Y, Yamada K, Isobe R, Higuchi R, Matsuura H, Itakura Y. Isolation and structure determination of cerebrosides from garlic, the bulbs of Allium sativum L. Chem Pharm Bull. 1998;46:1153–1156. [Google Scholar]
- 10.Kawamura A. Uncovering the therapeutic potential of natural products with biomarker-guided screening. IDrugs. 2010;13:321–324. [PubMed] [Google Scholar]
- 11.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 12.Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221:2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 14.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 15.Michielsen AJ, Hogan AE, Marry J, Tosetto M, Cox F, Hyland JM, Sheahan KD, O’Donoghue DP, Mulcahy HE, Ryan EJ, O’Sullivan JN. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One. 2011;6:e27944. doi: 10.1371/journal.pone.0027944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright J, McInnes A, Shimizu S, Smith D, Walter J, Idler D, Khalil W. Identification of C-24 alkyl epimers of marine sterols by C −13 nuclear magnetic resonance spectroscopy. Can J Chem. 1978;56:1898–1903. [Google Scholar]
- 17.Kojima H, Sato N, Hatano A, Ogura H. Sterol glucosides from Prunella vulgaris. Phytochemistry. 1990;29:2351–2355. [Google Scholar]
- 18.Shibuya H, Kawashima K, Sakagami M, Kawanishi H, Shimomura M, Ohashi K, Kitagawa I. Sphingolipids and glycerolipids. I. Chemical structures and ionophoretic activities of soya-cerebrosides I and II from soybean. Chem Pharm Bull (Tokyo) 1990;38:2933–2938. doi: 10.1248/cpb.38.2933. [DOI] [PubMed] [Google Scholar]
- 19.Brix S, Bovetto L, Fritsché R, Barkholt V, Frøkiaer H. Immunostimulatory potential of beta-lactoglobulin preparations: effects caused by endotoxin contamination. J Allergy Clin Immunol. 2003;112:1216–1222. doi: 10.1016/j.jaci.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 20.Marincek BC, Kühnle MC, Srokowski C, Schild H, Hämmerling G, Momburg F. Heat shock protein-antigen fusions lose their enhanced immunostimulatory capacity after endotoxin depletion. Mol Immunol. 2008;46:181–191. doi: 10.1016/j.molimm.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circulation research. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouic PJ, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev. 1999;4:170–177. [PubMed] [Google Scholar]
- 23.Bouic PJ. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care. 2001;4:471–475. doi: 10.1097/00075197-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Bouic PJ. Sterols and sterolins: new drugs for the immune system? Drug discovery today. 2002;7:775–778. doi: 10.1016/s1359-6446(02)02343-7. [DOI] [PubMed] [Google Scholar]
- 25.Breytenbach U, Clark A, Lamprecht J, Bouic P. Flow cytometric analysis of the Th1-Th2 balance in healthy individuals and patients infected with the human immunodeficiency virus (HIV) receiving a plant sterol/sterolin mixture. Cell Biol Int. 2001;25:43–49. doi: 10.1006/cbir.2000.0676. [DOI] [PubMed] [Google Scholar]
- 26.Bouic PJ, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, Van Jaarsveld PP. beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int J Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 27.Long J, Zhou B, Li H, Dai Q, Zhang B, Xing S, Zeng Z, Chen W, Yang J. Improvement of HBsAg gene-modified dendritic cell-based vaccine efficacy by optimizing immunization method or the application of β-glucosylceramide. Immunol Invest. 2013;42:137–155. doi: 10.3109/08820139.2012.744418. [DOI] [PubMed] [Google Scholar]
- 28.Mizrahi M, Lalazar G, Ben Ya’acov A, Livovsky DM, Horowitz Y, Zolotarov L, Adler R, Shouval D, Ilan Y. Beta-glycoglycosphingolipid-induced augmentation of the anti-HBV immune response is associated with altered CD8 and NKT lymphocyte distribution: a novel adjuvant for HBV vaccination. Vaccine. 2008;26:2589–2595. doi: 10.1016/j.vaccine.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Lee IA, Popov AM, Sanina NM, Kostetsky EY, Novikova OD, Reunov AV, Nagorskayaand VP, Shnyrov VL. Morphological and immunological characterization of immunostimulatory complexes based on glycoglycerolipids from Laminaria japonica. Acta Biochim Pol. 2004;51:263–272. [PubMed] [Google Scholar]
- 30.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Clements CJ, Griffiths E. The global impact of vaccines containing aluminium adjuvants. Vaccine. 2002;20(Suppl 3):S24–33. doi: 10.1016/s0264-410x(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura A, Iacovidou M, Takaoka A, Soll CE, Blumenstein M. A polyacetylene compound from herbal medicine regulates genes associated with thrombosis in endothelial cells. Bioorg Med Chem Lett. 2007;17:6879–6882. doi: 10.1016/j.bmcl.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.