Abstract
Background
The 5-HT1A receptor subtype has been postulated to modulate aggressive behavior particularly when it is excessive. F15599 is a high affinity and selective 5-HT1A receptor agonist that exhibits biased agonism for postsynaptic receptors that are preferentially coupled to Gαi3 protein subunits, with more potent action in the cortex, and with potential for selectively reducing aggression.
Objectives and methods
The aims of the current study were to investigate the anti-aggressive effects of the novel 5-HT1A receptor agonist, F15599, microinjected into the ventral orbital prefrontal cortex (VO PFC) and into the infralimbic cortex (ILC) of CF-1 male mice that had been previously socially provoked and to confirm the specific action at this receptor by blocking its effects using the 5-HT1A receptor antagonist, WAY100,635.
Results
Microinjection of the lower doses of F15599 (0.03 and 0.1 μg) into the VO PFC, but not into the ILC, significantly reduced the frequency of attack bites and sideways threats, without affecting other elements of the behavioral repertoire related to aggression such as pursuing and sniffing the intruder and tail rattle. There were also no changes observed in the duration of walking and rearing. Pretreatment with WAY100,635 prevented the anti-aggressive effects of F15599 when microinjected into VO PFC.
Conclusions
The present results demonstrated that F15599 is effective in reducing the most intense behavioral elements of aggressive behavior in male mice, when microinjected into the VO PFC, but not into the ILC, without affecting nonaggressive behavior, and confirmed the critical role of this cortical region and specifically the 5-HT1A heteroreceptors in the modulation of escalated aggressive behavior.
Keywords: Aggressive behavior, Social instigation, Serotonin, 5-HT1A receptors, Ventral orbital prefrontal cortex, Infralimbic cortex, F15599
Introduction
The prefrontal cortex (PFC) is one of the critical brain regions responsible for inhibitory control of behavior in general, and the orbitofrontal region is particularly important in inhibitory control of impulsive behavior, including escalated aggression (Blair 2001; Seguin 2004; Cardinal et al. 2004; Spinella 2004; Kheramin et al. 2005; de Almeida et al. 2006; Centenaro et al. 2008). In humans, violent aggressive behavior and violent suicide attempts have been hypothesized to result from impaired impulse control (Plutchik and Van Praag 1989, 1995; Horn et al. 2003). The ILC has connections to the amygdala, but its relationship with aggressive behavior remains unclear. For example, Faccidomo et al. (2008; 2012) found that alcohol-heightened aggression can be both significantly attenuated or accentuated after manipulation of the serotonergic receptors in this cortical region. Other studies did not find a relationship between the ILC and escalated aggressive behavior (Halász et al. 2006; Toth et al. 2010). According to Wall and Messier (2002) and Dalley and colleagues (2004), the ILC has been more involved in anxiety-like effects and cognitive functions such as attention and memory rather than in aggressive behavior.
For several decades the brain serotonin (5-HT) system has been postulated to be essential in the control of aggressive behavior (de Boer and Koolhaas 2005; Takahashi et al. 2011). The soma of 5-HT neurons are located in the raphe nuclei (RN) which project to different brain regions, including the striatum, amygdala, hippocampus, and cerebral cortex (Parent et al. 1981; Steinbusch 1981; Azmitia and Segal 1978; Vertes and Linley 2007). Among the seven families of known 5-HT receptors, there is clear evidence of the involvement of 5-HT1 and 5-HT2 receptors in aggressive behavior (Miczek et al. 2002; Olivier 2004) and some data also point to a role of 5-HT3 receptors and transporter molecules (McKenzie-Quirk et al. 2005; Ricci et al. 2004).
The PFC, including both VO and ILC, is characterized by a considerable density of 5-HT receptors, in particular 5-HT1A and 5-HT1B subtypes (Gould et al. 2011; Blair 2004; de Almeida et al. 2005; Best et al. 2002). The 5-HT1A receptors are inhibitory receptors and are found both presynaptically on 5-HT neurons in the RN, where they act as somatodendritic autoreceptors and in striatal and cortical regions were they act as postsynaptic heteroreceptors (Boschert et al. 1994; Riad et al. 2000; de Boer and Koolhaas 2005). In preclinical studies, systemic administration of 5-HT1A receptor agonists engenders anti-aggressive effects in several species (Bell and Hobson 1994; de Boer and Koolhaas 2005; Sperry et al. 2003; Ten Eyck 2008; Takahashi et al. 2011; Miczek et al. 1998). However, these anti-aggressive effects in vertebrates are often accompanied by nonspecific effects such as sedation, slow motor activity, stereotyped behaviors and reduction of social interest (de Boer and Koolhaas 2005; Olivier et al. 1995). Previous studies with rats also demonstrated that activation of the 5-HT1A receptors in post-synaptic sites, including the PFC, effectively reduce species-typical aggressive behavior (Sijbesma et al. 1991; Mos et al. 1992). However, de Almeida and Lucion (1997) found that activation of postsynaptic 5-HT1A receptors in the medial septal area can increase maternal aggressive behavior in rats. What remains to be clarified are which receptor subtype populations in which cortical regions are critical in the modulation of high levels of aggressive behavior in different species, mainly in mice. In this present study, we targeted two cortical regions (VO PFC and ILC) that are similar in connections sites and presence of 5-HT receptors.
A promising novel tool is F15599 which has high affinity (pKi~8.5) selectively for the 5-HT1A receptor acting as a biased agonist preferentially in postsynaptic sites, not observed with other 5-HT1A receptor agonists (Newman-Tancredi 2011; Newman-Tancredi et al. 2009; Depoortère et al. 2010; Assié et al. 2010). In tests of signal transduction, electrophysiological and neurochemical data show that F15599 preferentially activates postsynaptic 5-HT1A receptors that are coupled to Gαi3 protein subunits (Lemoine et al. 2010; Newman-Tancredi et al. 2009; Assié et al. 2010). F15599 is also able to bind to 5-HT1A receptors located in the RN, but this interaction does not readily result in the activation of autoreceptors, as suggested by the fact that high doses of this agonist are needed to reduce the electrical activity in this region (Newman-Tancredi et al. 2009). Considering the unique properties of this compound, to act in a specific receptor subpopulation, we expect that substances like F15599 may become useful tools in the study of aggressive behavior, particularly after microinjection into the PFC.
We employed an experimental protocol that induces high levels of aggressive behavior in mice, even when they are fitted with cannula implants. Social provocation consists of previous exposure of the resident animal to an “instigator” opponent (Potegal 1991; Fish et al. 1999; de Almeida and Miczek 2002; Centenaro et al. 2008). The main objectives of this study were: first, to examine the possible anti-aggressive effects of F15599, when microinjected into the VO PFC or into the ILC of male mice previously subjected to social provocation, to analyze the role of both cortical regions on aggressive behavior and, second, to confirm the specificity of the receptor involved in the modulation of this behavior, using the 5-HT1A receptor antagonist, WAY100,635, in order to block the effects of F15599.
Material and methods
Animals
The subjects used for this experiment, were adult CF-1 male mice (Mus musculus), from the animal house of FEPPS (Fundação Estadual de Produção e Pesquisa em Saúde, Porto Alegre, RS, Brazil), kept at the laboratory of UNISINOS (Universidade do Vale do Rio dos Sinos, São Leopoldo, RS, Brazil), weighing 30 to 40 g each. The mice were assigned to serve as “residents” (N=79), “instigators” (N=40) and “intruders” (N=85), as specified below. Each resident male was kept in a clear polycarbonate cage (28×17×14 cm) with a female (N=79) of the same strain. The other animals, intruders and instigators, were kept in groups of 10, in polypropylene cages (46×24×15 cm). All cages were lined with pine shavings. The animals were kept in the same environment, with temperature 23 ± 2 °C, relative humidity ranging from 50 to 60%and under a light/dark cycle of 12/12 h (light cycle starting at 06:00 a.m.), controlled automatically. Water and food (specific laboratory rodent diet—NUVILAB CR1) were available to the animals ad libitum. The experiments were performed during the light phase of the photoperiod from 09:00 to 16:00, in order to compare the results with those from earlier studies using the same procedure (Centenaro et al. 2008).
The experimental procedures followed the rules of SBCAL/COBEA (Sociedade Brasileira de Ciência em Animais de Laboratório/Colégio Brasileiro de Experimentação Animal) (www.cobea.org.br) and were in accordance with the Federal Law No. 11.794, of October 8, 2008, which “establishes procedures for the scientific use of animals”. The project was duly submitted to the Ethics Committee on Animal Use (CEUA), Federal University of Rio Grande do Sul, UFRGS, and approved under number 19758.
“Resident versus intruder” behavioral test
Upon arrival in the laboratory, resident males were housed in pairs with their respective females for 21 days. After this period, each resident was subjected to successive tests for aggressive behavior three times per week with a minimum interval of 48 h, to establish the baseline of this behavior. During testing, the female and pups were removed, and an intruder male was placed into the resident’s cage. Each test lasted 5 min, starting with the first attack bite by the resident. If the resident did not attack the intruder, the test was terminated after 5 min (Miczek and O’Donnell 1978). Resident mice that attacked the intruder more than ten times in the last three baseline tests and had stable number of bites (i.e., maximum variation of ten bites considering the three last tests) proceeded to the next experimental phase. If the resident was attacked by the intruder, the latter was immediately replaced. Typically, outbred resident mice achieved a stable number of attacks after six or seven tests with the same intruder (Winslow and Miczek 1984). There was a loss of about 15 % of resident male mice as a result of inconsistent patterns of aggressive behavior.
Social instigation
The social instigation procedure was used in order to increase the levels of aggression of the resident male, particularly in preparation for intracranial implant surgery. In this procedure, female and pups were removed from the home cage and, for 5 min, a male mouse of the same strain (instigator) was introduced into the resident’s cage, protected inside a transparent acrylic perforated tube (18×6 cm.) so that the resident male had visual, auditory and olfactory contact, but no tactile access (Fish et al. 1999).
Five minutes after the social instigation, an intruder male was introduced into the cage of the resident mouse, without any protection, allowing direct confrontation between the two animals. This behavioral test lasted for 5 min and was video-recorded. Mice with at least three tests of social aggression after provocation, and with stable number of bites, were implanted with an intracerebral guide cannula.
Surgery
Immediately after displaying stable aggressive behavior, the resident mice were anesthetized with ketamine anesthetic (100 mg/kg body weight) and xylazine muscle relaxant and analgesic (10 mg/kg body weight) intramuscularly and positioned in a stereotaxic frame to implant a guide cannula (26- gauge guide, Plastics One Inc., USA) into the VO or IL region of the left hemisphere of the PFC, according to the stereotaxic atlas for mice (Paxinos and Franklin 2001), using the coordinates: 2.3mm anterior to bregma, 0.6mm lateral to the sagittal midline and 1.0 mm below the dura for VO region, and 1.9 mm anterior to the bregma, 0.4 mm lateral to the sagittal midline and 1.2 mm bellow the dura for IL region. After surgery, an obdurator (33-gauge, Plastics One Inc., USA) was inserted in the guide cannula in order to prevent blockage, and the mice were allowed to recover for 5 days.
Microinjections and behavioral analysis
After recovery from surgery, the animals were microinjected with F15599, a 5-HT1A receptor agonist or with WAY100,635, a 5-HT1A receptor antagonist, or vehicle (distilled water). For microinjection, the obdurator was removed, and an injector (33-gauge, Plastics One Inc., USA) was inserted into the guide cannula, 1.0 mm longer than the guide, allowing injection of the drug 2.0 mm below the dura for VO region and 2.2 mm below the dura for IL region, in the target area. The solution was slowly injected over a period of 2 min with the injector connected through a polyethylene tube (P20) to a 1.0 μl Hamilton syringe. After microinjection, the injector was maintained in the site for 30 s, allowing the complete diffusion of the drug and preventing reflux.
Vehicle (distilled water, 0.2 μl) or F15599 at different doses (0.03, 0.1, 0.3 and 1.0 μg/0.2 μl for VO region and 0.1 and 1.0 μg/0.2 μl for IL region) were administered 15 min before the behavioral testing to separate groups of resident mice so that each subject received only one dose. Similarly to testing prior to surgery, an instigator protected within a perforated cylindrical acrylic tube was introduced inside the resident’s cage, 5 min after microinjection, for a period of 5 min. After 5 min of social provocation, followed by a 5-min interval, an intruder male was introduced into the resident’s cage, without any protection, in order to allow direct confrontation between the two subjects.
In another group of animals, WAY100,635 (1.0 μg/0.2 μl) was microinjected 30 min before F15599 (0.1 μg/0.2 μl) or vehicle, only in the VO region. In this experiment, animals received two microinjections: first, vehicle or antagonist, and second, agonist or another vehicle.
The behavioral test after microinjections was recorded with a video camera and subsequently analyzed by a trained researcher using the program “The Observer”, version 3.0 (Noldus, The Netherlands). The behavioral repertoire was analyzed as previously defined (Miczek and O’Donnell 1978), which included latency and frequency of aggressive elements (biting, sideways threat, chasing, sniffing the intruder and tail rattle) and duration of nonaggressive elements (walking, grooming, and rearing).
Drugs
F15599 (Pierre Fabre, France), 5-HT1A receptor agonist, and WAY100,635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-N-(2 pyridinyl) cyclohexane carboxamide trihydrochloride) Wyeth-Ayerst Laboratories, Princeton, NJ, USA, 5-HT1A receptor antagonist, were dissolved in distilled water.
Histological analysis
After the experiments, all resident male mice were sacrificed in a GSCO2 (BEIRAMAR Indústria e Comércio Ltda) CO2 chamber and were perfused transcardially with 0.9 % saline and buffered 4 % paraformaldehyde. After perfusion, the brains of these animals were removed and immersed in buffered 4 % paraformaldehyde for at least 7 days. Subsequently, the brains were cut into 80-μm thick coronal sections on a vibratome. The resulting tissue slices were mounted on gelatinized slides and stained with hematoxylin and eosin, and covered.
The location of the guide cannula and the exact location of the microinjection were verified with a digital camera coupled to a microscope and stereotactic atlas of the mouse brain (Paxinos and Franklin 2001). Only animals whose microinjections were confirmed by histological analysis in the VO PFC or IL region were considered in the statistical analysis. The animals whose microinjection occurred outside VO PFC or IL served as anatomical controls (N=51).
Statistical analysis
All data were homogeneously distributed and expressed as means ± SEM. The effect of different doses of the agonist (F15599) and antagonist (WAY100,635) were separately analyzed using a one-way analysis of variance (ANOVA). When there were statistically significant F values (p≤0.05), Dunnett’s post hoc tests were conducted comparing drug treatments with the corresponding vehicle group. Regarding nonaggressive behaviors, data of all agonist and antagonist groups were compared to those of their respective control groups using ANOVA. When there were statistically significant F values (p≤0.05), Dunnett’s post hoc tests were conducted comparing drug treatments with the corresponding vehicle group. All statistical analyses were performed using SPSS 18 program.
Results
Histological analysis
Histological analysis of mouse brains which received microinjections showed that, among all subjects tested, 63 had correct placements in the VO region (Fig. 1) and 16 in the IL region (Fig. 2). Data from other animals microinjected into regions adjacent to VO and IL (N=51) were not considered in the statistical analysis.
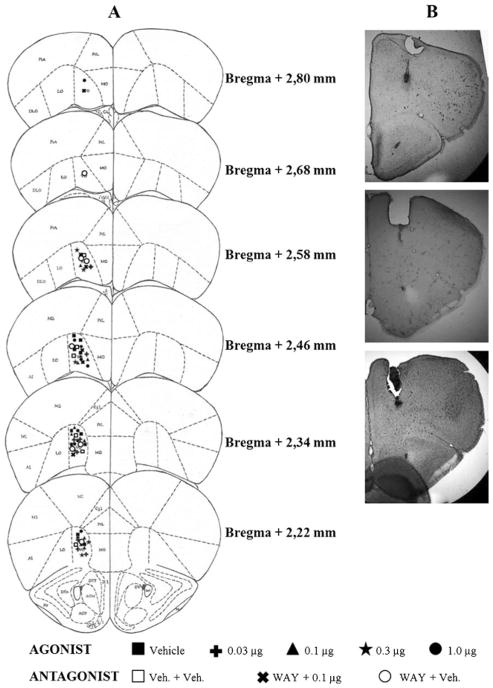
Fig. 1.
a Schematic representation of successive coronal sections of the mouse brain showing the histological verification of injection placement into the VO PFC (n=63) (rostral to caudal: 2.80, 2.68, 2.58, 2.46, 2.34, and 2.22 mm anterior to the bregma). VO ventral orbital cortex; Cgl cingulate cortex, area 1; PrL prelimbic cortex; MO medial orbital cortex; LO lateral orbital cortex; M1 primary motor cortex; M2 secondary motor cortex. All images are adapted from Paxinos and Franklin (2001). b Photomicrographs showing correct placement of guide cannula and microinjection at VO PFC region
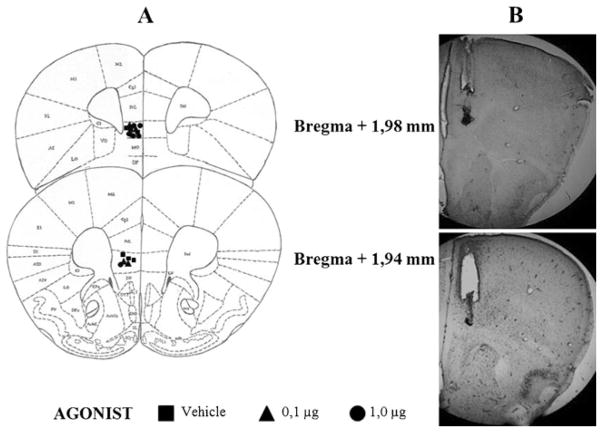
Fig. 2.
a Schematic representation of successive coronal sections of the mouse brain showing the histological verification of injection placement into the IL PFC (n=16) (rostral to caudal: 1.98 and 1.94 mm anterior to the bregma). IL infralimbic cortex; VO ventral orbital cortex; Cgl cingulate cortex, area 1; PrL prelimbic cortex; MO medial orbital cortex; LO lateral orbital cortex; M1 primary motor cortex; M2 secondary motor cortex; DP dorsal peduncular cortex. All images are adapted from Paxinos and Franklin (2001). b Photomicrographs showing correct placement of guide cannula and microinjection at IL PFC region
Effect of microinjection of different doses of F15599 into VO PFC on aggressive behavior after social instigation
Microinjection of F15599 into VO PFC of CF-1 male mice did not cause significant changes in the latency for the first bite, comparing treated groups with the control group (vehicle) (Fig. 3). However, it significantly reduced attack bite frequency at 0.03 μg (F(4,39)=4.49, p=0.041; Fig. 4) and 0.1 μg (F(4,39)=4.49, p=0.010; Fig. 4) doses, when compared to the control group. For animals from groups that received microinjections of the remaining doses (0.3 and 1.0 μg) of the agonist, this behavior remained unchanged (Fig. 4). In addition to attack bites, F15599 significantly reduced sideways threats at the 0.1 μg (F(3,39)=3.01, p=0.031; Fig. 5) dose, when microinjected into VO PFC. The frequency of pursuit and sniffing the intruder and tail rattling remained unchanged after microinjection with any of the F15599 doses compared to the control group (Table 1).
Fig. 3.
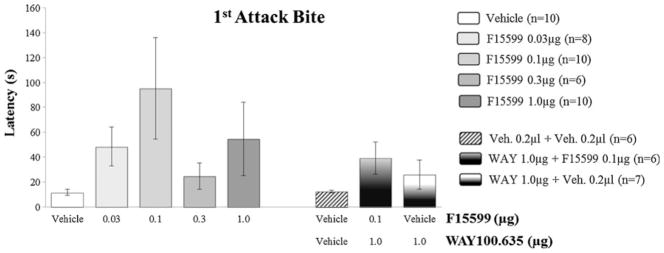
Effect of the microinjection of different doses (0.03, 0.1, 0.3, and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, and after pretreatment with WAY100,635 (1.0 μg/0.2 μl)+F15599 (0.1 μg/0.2 μl) or vehicle, into VO PFC, on first bite latency, after social instigation. Data expressed in mean ± SEM
Fig. 4.
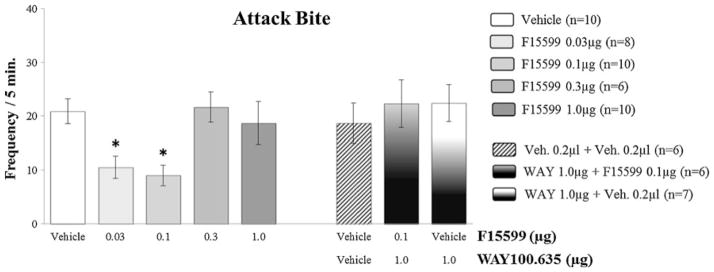
Effect of the microinjection of different doses (0.03, 0.1, 0.3, and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, and after pretreatment with WAY100,635 (1.0 μg/0.2 μl)+F15599 (0.1 μg/0.2 μl) or vehicle, into VO PFC, on attack bite frequency, after social instigation. Data expressed in mean ± SEM. *p ≤ 0.05
Fig. 5.
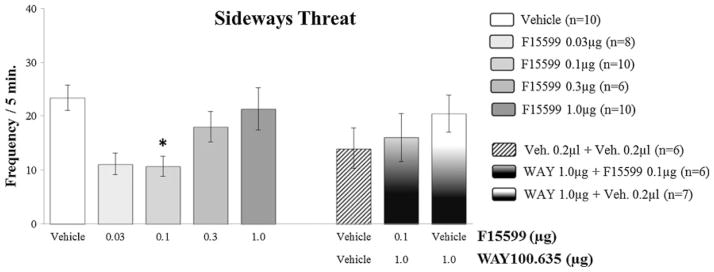
Effect of the microinjection of different doses (0.03, 0.1, 0.3 and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, and after pretreatment with WAY100,635 (1.0 μg/0.2 μl)+F15599 (0.1 μg/0.2 μl) or vehicle, into VO PFC, on sideways threat frequency, after social instigation. Data expressed in mean ± SEM. *p≤0.05
Table 1.
Frequency of aggressive behaviors after microinjection of vehicle, F15599 (5-HT1A receptor agonist, in different doses) and WAY100, 635 (5-HT1A receptor antagonist (before F15599 or vehicle)) in the ventral orbital (VO) prefrontal cortex (PFC)
| Vehicle |
F15599 doses (μg/0.2 μl)
|
Vehicle + | WAY100,635 1.0 μg +
|
|||||
|---|---|---|---|---|---|---|---|---|
| Behavior | 0.2 μl (n=10) | 0.03 (n=8) | 0.1 (n=10) | 0.3 (n=6) | 1.0 (n=10) | Vehicle 0.2 μl (n=6) | F15599 0.1 μg (n=6) | Vehicle 0.2 μl (n=7) |
| Frequency of aggressive elements | ||||||||
| Pursuit | 0.4 ± 0.2 | 0.6 ± 0.3 | 0.1 ± 0.1 | 0.7 ± 0.7 | 1.0 ± 0.7 | 1.3 ± 0.6 | 0.5 ± 0.3 | 1.0 ± 0.5 |
| Sniff | 5.4 ± 1.2 | 6.4 ± 1.5 | 9.5 ± 2.3 | 11.2 ± 2.1 | 8.1 ± 1.8 | 11.0 ± 2.2 | 13.5 ± 1.6 | 12.1 ± 1.7 |
| Tail rattle | 25.0 ± 3.5 | 13.5 ± 2.0 | 15.8 ± 4.2 | 17.2 ± 3.0 | 24.0 ± 6.3 | 15.2 ± 2.4 | 12.7 ± 4.0 | 17.6 ± 2.9 |
Data expressed in mean ± SEM
Effect of microinjection of different doses of F15599 into VO PFC on nonaggressive behavior after social instigation
Regarding nonaggressive behavioral elements, F15599 microinjections at different doses (0.03; 0.1; 0.3 and 1.0 μg/0.2 μl) into VO PFC, did not significantly affect the duration of walking (Fig. 6) and rearing (Fig. 8). As for grooming, an increase in this behavior was observed for the 0.03 μg dose group (F(4,39)=2.96, p=0.010; Fig. 7), when compared to control.
Fig. 6.
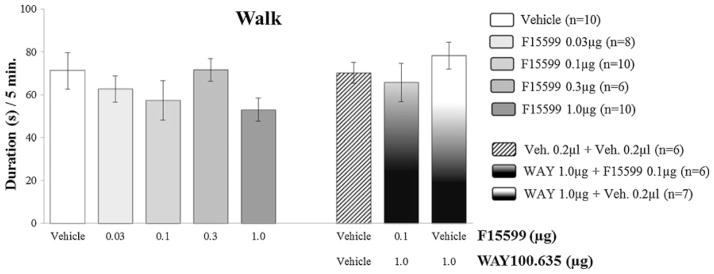
Effect of the microinjection of different doses (0.03, 0.1, 0.3, and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, and after pretreatment with WAY100,635 (1.0 μg/0.2 μl)+F15599 (0.1 μg/0.2 μl) or vehicle, into VO PFC, on walking duration, after social instigation. Data expressed in mean ± SEM
Fig. 8.
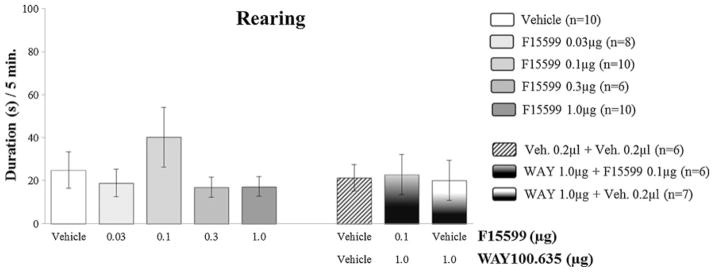
Effect of the microinjection of different doses (0.03, 0.1, 0.3, and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, and after pretreatment with WAY100,635 (1.0 μg/0.2 μl)+F15599 (0.1 μg/0.2 μl) or vehicle, into VO PFC, on rearing duration, after social instigation. Data expressed in mean ± SEM
Fig. 7.
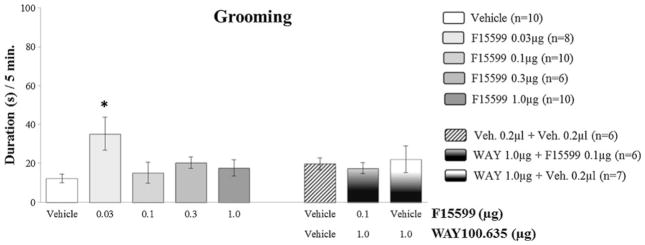
Effect of the microinjection of different doses (0.03, 0.1, 0.3, and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, and after pretreatment with WAY100,635 (1.0 μg/0.2 μl)+F15599 (0.1 μg/0.2 μl) or vehicle, into VO PFC, on grooming duration, after social instigation. Data expressed in mean ± SEM. *p≤0.05
Effect of microinjection of different doses of F15599 into IL PFC on aggressive behavior after social instigation
Microinjection of F15599 into IL PFC of CF-1 male mice, at different doses (0.1 and 1.0 μg/0.2 μl), did not cause significant changes in aggression, for all behavioral elements analyzed, comparing treated groups with the respective vehicle control group (Fig. 9, Table 2).
Fig. 9.
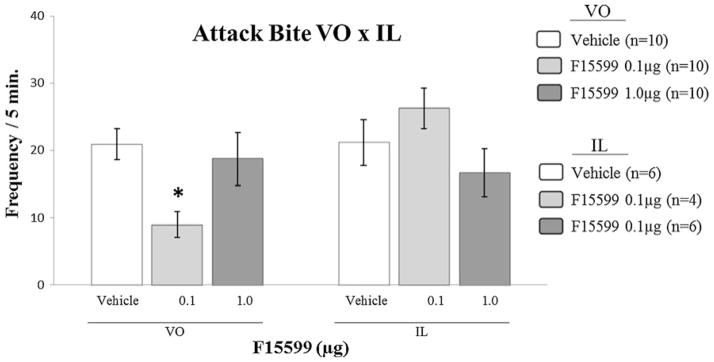
Effect of the microinjection of different doses (0.1 and 1.0 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, into IL PFC, comparing with the same doses microinjected into VO PFC, on attack bite frequency, after social instigation. Data expressed in mean ± SEM. *p≤0.05
Table 2.
Latency (in seconds) to attack bite, frequency of aggressive and duration (in seconds) of nonaggressive behaviors after microinjection of vehicle or F15599 (5-HT1A receptor agonist, in different doses), in the infralimbic (IL) cortex
| Behavior | Vehicle |
F15599 doses (μg/0.2 μl)
|
|
|---|---|---|---|
| 0.2 μl (n=6) | 0.1 (n=4) | 1.0 (n=6) | |
| Latency | |||
| First attack bite (s) | 12.2+1.3 | 7.5+1.7 | 13.0+2.3 |
| Frequency of aggressive elements | |||
| Attack bites | 21.2+3.4 | 26.3+3.0 | 16.7+3.6 |
| Sideways threat | 18.8+1.4 | 25.4+4.1 | 15.5+3.8 |
| Pursuit | 2.2+1.4 | 1.3+0.8 | 0.8+0.5 |
| Sniff | 6.3+1.6 | 8.3+1.4 | 6.0+1.1 |
| Tail rattle | 16.5+2.9 | 18.8+3.1 | 19.5+2.7 |
| Duration of nonaggressive elements | |||
| Walk (s) | 61.5+11.4 | 77.5+14.8 | 59.7+3.3 |
| Groom (s) | 20.2+6.7 | 27.5+14.7 | 23.8+7.9 |
| Rear (s) | 15.0+4.9 | 11.5+3.5 | 10.0+3.3 |
Data expressed in mean ± SEM
Effect of microinjection of different doses of F15599 into IL PFC on nonaggressive behavior after social instigation
Regarding nonaggressive behavioral acts, F15599 microinjections at different doses (0.1 and 1.0 μg/0.2 μl) into IL PFC, did not significantly alter the duration of walking, grooming and rearing (Table 2), when compared to the respective control group.
Antagonism
Pretreatment with the 5-HT1A receptor antagonist WAY100,635 (1.0 μg), prevented the reduction in the number of attack bites (F( 2,16)=0.30, p=0.74; Fig. 4) and sideways threats (F(2,16)=0.80, p=0.47; Fig. 5) produced by F15599 microinjections into VO PFC. Pursuing and sniffing the intruder and tail rattling were not significantly modified when compared to values from the control group (Table 1). No significant effects on aggressive and nonaggressive behaviors were found after microinjections of WAY100,635 or vehicle plus vehicle into VO PFC (Figs. 3, 4, 5, 6, 7, and 8 and Table 1). The duration of walking, grooming, and rearing remained unaltered by pretreatment with WAY100,635 followed by F15599, when compared to control (Table 1).
Discussion
This is the first study to investigate the anti-aggressive effects of F15599, a 5-HT1A receptor agonist with unusually high affinity and selectivity and preferential action at postsynaptic receptors that are coupled to Gαi3 protein subunits, with more potent action in the cortex, than in hippocampus and in dorsal raphe. The present study revealed remarkable behaviorally specific anti-aggressive effects of F15599 in that microinjection of the lower doses of F15599 (0.03 and 0.1 μg), into the VO PFC of male mice, but not into the ILC, significantly reduced the frequency of attack bites and sideways threats. It is also noteworthy that F15599, in contrast to other 5-HT1A receptor agonists, only reduced the most intense elements of aggression, attack bites and sideways threats, but not other elements of the behavioral repertoire related to aggression. Similarly, none of the other behavioral acts and postures was altered by F15599 in the dose range that reduced attacks and threats, indicating remarkable behavioral specificity of the anti-aggressive effects. Pretreatment with WAY100,635 prevented the anti-aggressive effects of F15599 when microinjected into VO PFC, pointing to the high receptor selectivity. Microinjections of F15599 into the ILC, in contrast to the VO, left aggressive and nonaggressive behavior intact, suggesting regional selectivity and no involvement of ILC in escalated aggressive behavior. The induction of higher levels of aggression with the use of social instigation protocol, as used in earlier studies (Fish et al. 1999; Olivier and van Oorschot 2005; Centenaro et al. 2008) proved to be effective in conducting this type of microinjection study.
When interpreting the anti-aggressive effects of F15599, it is clear that there are regional differences in the coupling of 5-HT1A receptors to G proteins (Mannoury la Cour et al. 2006). F15599 is a “biased agonist” at 5-HT1A receptors that are coupled to Gαi protein subunits (Newman-Tancredi et al. 2009), and Gαi protein subunits are greatly present in the cortex (Mannoury la Cour et al. 2006). Biased agonists are compounds that have functional selectivity for certain signal transduction pathways, for example, in receptors that are coupled to different G proteins. These agonists can target brain regions of interest and offer a strategy to identify more effective and better-tolerated drugs and may be therapeutically advantageous (Newman-Tancredi 2011). As mentioned earlier (Depoortère et al. 2010), the selectivity for subpopulations of receptors which are linked to therapeutic activities, is important to develop specific drugs for various diseases involving localized deficiencies, such as schizophrenia and depression.
F15599 has some unique feature: one is high affinity for rat and human 5-HT1A receptors (Newman-Tancredi et al. 2009), and no affinity for other 5-HT receptors (as opposed to 8-OH-DPAT, that also binds to 5-HT7) and dopamine receptors (as opposed to buspirone, that also binds to D2). The other is high efficacy for activation of 5-HT1A receptors with a remarkable bias for receptors with Gαi3 subunits. These features differentiate F15599 from other 5-HT1A receptor agonists (5-HT, 8-OH-DPAT and F13714) (Newman-Tancredi et al. 2009; Newman-Tancredi 2011) and render it a useful tool. Imaging studies with in vivo positron emission tomography revealed that F15599 labeled rat cortical 5-HT1A receptors rather than hippocampal sites. The present study aimed to activate a specific receptor subpopulation in the PFC with F15599, and the results are interpreted in the context of the receptor selectivity and preferentially postsynaptic action that is not found with other 5-HT1A receptor agonists (Lladó-Pelfort et al. 2010; Newman-Tancredi 2011).
5-HT1A receptor agonists can have dose-dependent biphasic effects. When locally administered into the medial prefrontal cortex, F15599 produced an intriguing biphasic dose–response curve, suggesting an interaction with discrete receptor subpopulations. In all likelihood this is due to the fact that the drug differentially acts on 5-HT1A receptors expressed on GABAergic interneurons and 5-HT1A receptors expressed on glutamatergic pyramidal neurons (Lladó-Pelfort et al. 2012). Thus, a 5-HT1A agonist may increase the activity of pyramidal neurons in the PFC at low doses. Our understanding is that F15999 is acting in a similar way like 8-OH-DPAT, activating 5-HT1A receptors in GABAergic interneurons and causing a decrease of the inhibition of pyramidal neurons.
The presently observed reduction of aggression with microinjection of lower doses of F15599 is also in concordance with dose–response pattern seen in previous studies. Upon acute treatment, F15599 at low doses is potently active in the forced swim test. It also potently reduced the duration of ultrasonic vocalization emitted upon presentation of a conditioned stimulus previously associated with unavoidable mild electric foot shocks consistent with anti-stress/anxiolytic properties (Assié et al. 2010).
It has been estimated that a single interneuron can contact more than 200 pyramidal neurons (Cobb et al. 1995), therefore magnifying the effects on single GABAergic interneurons. The activation off 5-HT1A in a limited population off GABA cells in a cortical GABA network can result in the hyperpolarization of the whole network and the subsequent disinhibition of many pyramidal neurons, and this may explain the fact that only at low doses occurs disinhibition of pyramidal neurons and its increased electrical activity (Lladó-Pelfort et al. 2012).
Previous studies have demonstrated that activation of 5-HT1A receptors in post-synaptic sites, including the PFC, is effective in reducing species-typical aggressive behavior in male rats, but not escalated aggression (Sijbesma et al. 1991; Mos et al. 1992). Agonists of 5-HT1A receptors also reduce the aggressive behavior of female rats that were socially instigated (Veiga et al. 2007). However, when microinjected into DRN, this receptor subtype agonists promoted an increase in aggressive behavior in postpartum females (Veiga et al. 2011). These data demonstrate the importance of the PFC and 5-HT1A receptors in the modulation of aggressive behavior and the need of more studies to elucidate modulation of escalated aggression.
There are conflicting data about the involvement of the ILC in the modulation of aggressive behavior. Microinjection of CP- 93,253, a 5-HT1B receptor agonist, into the ILC, but not into de VO PFC, increased aggressive behavior in mice after moderate doses of alcohol intake (Faccidomo et al. 2008). In contrast, using the same animal model, Faccidomo et al. (2012) found that the ILC appears to be critical for the attenuation of species-typical levels of aggression, using CP-93,129, another 5-HT1B receptor agonist. Other studies did not find a relationship between the IL and escalated aggressive behavior (Halász et al. 2006; Toth et al. 2010). Wall and Messier (2002) and Dalley et al. (2004) showed that the ILC has been involved more in anxiety-like effects and cognitive functions such as attention and memory than in aggressive behavior. In the present study, microinjection of F15599 into the ILC left aggressive and nonaggressive behaviors intact, pointing to behavioral specific anti-aggressive effects of this compound and no involvement of 5-HT1A receptors in ILC in modulating aggression.
The action of F15599 indicates a relationship with aggressive behavior and these data are consistent with several other preclinical studies which have demonstrated that 5-HT1A agonists can decrease aggression, for example, 8-OH-DPAT and flesinoxan (Bell and Hobson 1994; de Boer and Koolhaas 2005; Sperry et al. 2003; Ten Eyck 2008). In a previous study from our group (Centenaro et al. 2008), 8-OH-DPAT microinjected at the same brain region has also been shown to reduce aggressive behavior in male mice, but with less behavioral selectivity than F15599. In this present work, we have compared the results of both studies (Fig. 10), which show clear differences in potency between these drugs. 8-OH-DPAT only reduces aggressive behavior at high doses and F15599 only at low doses.
Fig. 10.
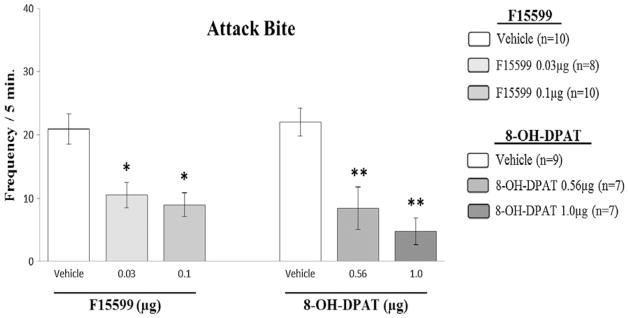
Effect of the microinjection of different doses (0.03 and 0.1 μg/0.2 μl) of the 5-HT1A receptor agonist, F15599, in comparison with previous results (Centenaro et al. 2008) of another 5-HT1A receptor agonist (8-OH-DPAT) in different doses (0.56 and 1.0 μg/0.2 μl) microinjected into VO PFC of male mice, on attack bite frequency, after social instigation. Data expressed in mean ± SEM. *p≤0.05. **p≤0.001
Although it can be expected that administration of the 5-HT1A receptor antagonist WAY100,635 should increase aggression by blocking 5-HT1A heteroreceptors in regions that are involved in aggressive behavior, in the case of this work by microinjection in VO PFC, the observed absence of effects may reflect a ceiling effect. The mechanism of action by which WAY100,635 can increase aggressive behavior, maybe dependent on the receptor pool in a specific brain region. As in our work, other studies failed to see reliable increases in aggressive behavior after WAY100,635 administration, both systemic and local (Miczek et al. 1998). If the action of F15599 in VO PFC is primarily on GABAergic interneurons, then WAY100,635 at the current dose would be expected to be neither inhibitory nor disinhibitory, but silent.
In conclusion, the results of this study confirmed the critical role of the VO PFC, but not the ILC, specifically the 5-HT1A heteroreceptor subtype, in the modulation of escalated aggressive behavior in socially provoked male mice. Furthermore, F15599 is effective in reducing the most intense components of aggressive behavior, but not low intensity social interaction. Complementary studies with systemic administration of this compound and local administration into the raphe promise to provide key data about the utility of F15599 in the modulation of aggressive behavior.
Acknowledgments
The authors thank Dr. A. Newman-Tancredi for arranging the donation of F15599.
Contributor Information
Dirson João Stein, Programa de Pós-Graduação em Neurociências, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
Klaus A. Miczek, Department of Psychology, Tufts University, Medford, MA, USA. Department of Pharmacology, Tufts University, Boston, MA, USA. Department of Neuroscience, Tufts University, Boston, MA, USA. Department of Psychiatry, Tufts University, Boston, MA, USA
Aldo Bolten Lucion, Departamento de Fisiologia, Programa de Pós-Graduação em Neurociências, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil.
Rosa Maria Martins de Almeida, Email: rosa.almeida@ufrgs.br, Laboratório de Psicologia Experimental, Neurociências e Comportamento, Instituto de Psicologia do Desenvolvimento e da Personalidade, Universidade Federal do Rio Grande do Sul (UFRGS), CEP 90035-003, 2600, Bairro Santa Cecília, Porto Alegre, RS, Brazil.
References
- Assié MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortère R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A. F15599, a highly selective post-synaptic 5-HT1A receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Intern J Neuropsychopharmacol. 2010;13:1285–1298. doi: 10.1017/S1461145709991222. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bell R, Hobson H. 5-HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neurosci Biobehav Rev. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Best M, Williams M, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J Neurol Neurosurg Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine 1B receptor is localized predominantly on axon terminals. Neurosci. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Cardinal RM, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann NY Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Centenaro LA, Vieira K, Zimmermann N, Miczek KA, Lucion AB, de Almeida RMM. Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacol. 2008;201:237–248. doi: 10.1007/s00213-008-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- De Almeida RMM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacol. 1997;134:392– 400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- De Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) inmice: inhibition by anpirtoline—a 5-HT1B receptor agonist. Neuropsychopharmacol. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- De Almeida RMM, Giovenardi M, Silva SP, Oliveira VP, Stein DJ. Maternal aggression in wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Braz J Med Biol Res. 2005;38:597–602. doi: 10.1590/S0100-879X2005000400014. [DOI] [PubMed] [Google Scholar]
- De Almeida RMM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT1B receptors, ventral orbitofrontal cortex and aggressive behavior in mice. Psychopharmacol. 2006;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Depoortère R, Auclair A, Bardin L, Colpaert FC, Vacher B, Newman- Tancredi A. F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity inmodels of cognition in comparision with reference 5-HT1A receptor agonists. Eur Neuropsychopharmacol. 2010;20:641–654. doi: 10.1016/j.euroneuro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek KA. Escalated aggression after alcohol drinking in male mice: dorsal raphe and prefrontal cortex serotonin and 5-HT1B receptors. Neuropsychopharmacol. 2008;33:2888–2899. doi: 10.1038/npp.2008.7. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Quadros IMH, Takahashi A, Fish EW, Miczek KA. Infralimbic and dorsal raphé microinjection of the 5-HT1B receptor agonist CP-93,129: attenuation of aggressive behavior in CFW male mice. Psychopharmacol. 2012;222:117–128. doi: 10.1007/s00213-011-2629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacol. 1999;146:391–399. doi: 10.1007/PL00005484. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) tansporters, 5-HT1A and 5-HT1B receptors, and effects of their targeting on BTBR T + tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.11/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász J, Tóth M, Kalló I, Liposits Z, Haller J. The activation of prefrontal cortical neurons in aggression—a double labeling study. Behav Brain Res. 2006;175:166–175. doi: 10.1016/j.bbr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliot R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychol. 2003;41:1959–1966. doi: 10.1016/S0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bras D, Newman- Tancredi A, Zimmer L. [18F]F15599, a novel 5-HT1A receptor agonist as a radioligand for PET neuroimaging. Eur J of Nucl Med and Mol Imaging. 2010;37:594–605. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Assié MB, Newman-Tancredi A, Artigas F, Celada P. Preferential in vivo action of F15599, a novel 5-HT1A receptor agonist, at postsynaptic 5-HT1A receptors. Br J Pharmacol. 2010;160:1929–1940. doi: 10.1111/j.1476-5381.2010.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Santana N, Ghisi V, Artigas F, Celada P. 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex. 2012;22:1487–1497. doi: 10.1093/cercor/bhr220. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol. 2006;70:1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Girasa KA, Allan AM, Miczek KA. 5-HT3 receptors, alcohol and aggressive behavior in mice. Behav Pharmacol. 2005;16:163–169. doi: 10.1097/00008877-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacol. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacol. 1998;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, de Bold JF, de Almeida RMM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and g-aminobutyric acid systems. Psychopharmacol. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, van Aken H. The effects of intraventricular administration of eltoprazine, 1-(3-trifluoromethylphenyl), piperazine, hydrochloride and 8-OH-DPAT on resident-intruder aggression in the rat. Eur J Pharmacol. 1992;212:295–298. doi: 10.1016/0014-2999(92)90348-8. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi Biased agonism at serotonin 5-HT1A receptors: preferential postsynaptic activity for improved therapy of CNS disorders. Neuropsychiatry. 2011;1:149–164. doi: 10.2217/npy.11.12. [DOI] [Google Scholar]
- Newman-Tancredi A, Martel JC, Assié MB, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, Cussac D. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol. 2009;156:338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. Serotonin and aggression. Ann NY Acad Sci. 2004;1036:382–392. doi: 10.1196/annals.1330.022. [DOI] [PubMed] [Google Scholar]
- Olivier B, van Oorschot R. 5-HT1B receptors and aggression: a review. Eur J Pharmacol. 2005;526:207–217. doi: 10.1016/j.ejphar.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatr. 1995;28:80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neurosci. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. Academic, N.Y: 2001. CD-Rom. [Google Scholar]
- Plutchik R, Van Praag H. The measurement of suicidality, aggressivity and impulsivity. Prog Neuro-Psychopharmacol Biol Psychiatry. 1989;13:23–34. doi: 10.1016/0278-5846(89)90107-3. [DOI] [PubMed] [Google Scholar]
- Plutchik R, Van Praag H. The nature of impulsivity: definitions, ontology, genetics and relations to aggression. In: Hollander E, Stein D, editors. Impulsivity and Aggression. Oxford, Eng: 1995. pp. 7–24.pp. 372 [Google Scholar]
- Potegal M. Attack priming and satiation in female golden hamsters: tests of some alternatives to the aggression arousal interpretation. Aggress Behav. 1991;17:327–335. doi: 10.1002/1098-2337(1991)17:6<327:AID-AB2480170604>3.0.CO;2-J. [DOI] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somato dendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. doi: 10.1002/(SICI)1096-9861(20000207)417:2<181::AID-CNE4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr Serotonin type 3 receptors modulate the aggression stimulating effects of adolescent cocaine exposure in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2004;118:1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- Seguin JR. Neurocognitive elements of antisocial behavior: relevance of an orbitofrontal cortex account. Brain and Cogn. 2004;55:185–197. doi: 10.1016/S0278-2626(03)00273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-L. [DOI] [PubMed] [Google Scholar]
- Sperry TS, Thompson CK, Wingfield JC. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behavior in male song sparrows (Melospiza melodia morphna) J Neuroendocrinol. 2003;15:150– 160. doi: 10.1046/j.1365-2826.2003.00968.x. [DOI] [PubMed] [Google Scholar]
- Spinella M. Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Intern J Neurosci. 2004;114:95–104. doi: 10.1080/00207450490249347. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neurosci. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RMM, Miczek KA. Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacol. 2011;213:183–212. doi: 10.1007/s00213-010-2000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Eyck GR. Serotonin modulates vocalizations and territorial behavior in an amphibian. Behav Brain Res. 2008;193:144–147. doi: 10.1016/j.bbr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Toth M, Fuzesi T, Halász J, Tulogdi A, Haller J. Neural inputs of the hypothalamic “aggression area” in the rat. Behav Brain Res. 2010;215:7–20. doi: 10.1016/j.bbr.2010.05.050. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res. 2007;40:825–830. doi: 10.1590/S0100-879X2006005000113. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Social instigation and aggression in postpartum female rats: role of 5-HT1A and 5-HT1B receptors in the dorsal raphé nucleus and prefrontal cortex. Psychopharmacol. 2011;213:475–487. doi: 10.1007/s00213-010-2083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB. Comparison of projections of the dorsal and median raphe nuclei, with some functional considerations. Intern Congr Ser. 2007;1304:98–120. doi: 10.1016/j.ics.2007.07.046. [DOI] [Google Scholar]
- Wall PM, Messier C. Infralimbic kappa opioid and muscarinic M1 receptor interactions in the concurrent modulation of anxiety and memory. Psychopharmacol. 2002;160:233–244. doi: 10.1007/s00213-001-0979-9. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Habituation of aggressive behavior in mice: a parametric study. Aggress Behav. 1984;10:103–113. doi: 10.1002/1098-2337(1984)10:2<103::AID-AB2480100294>3.0.CO;2-V. [DOI] [Google Scholar]