Abstract
A general SI (Susceptible-Infected) epidemic system of host-parasite interactions operating under Allee effects, horizontal and/or vertical transmission, and where infected individuals experience pathogen-induced reductions in reproductive ability, is introduced. The initial focus of this study is on the analyses of the dynamics of Density-Dependent and Frequency-Dependent effects on SI models (SI-DD and SI-FD). The analyses identify conditions involving horizontal and vertical transmitted reproductive numbers, namely those used to characterize and contrast SI-FD and SI-DD dynamics. Conditions that lead to disease-driven extinction, or disease-free dynamics, or susceptible-free dynamics, or endemic disease patterns are identified. The SI-DD system supports richer dynamics including limit cycles while the SI-FD model only supports equilibrium dynamics. SI models under “small” horizontal transmission rates may result in disease-free dynamics. SI models under with and inefficient reproductive infectious class may lead to disease-driven extinction scenarios. The SI-DD model supports stable periodic solutions that emerge from an unstable equilibrium provided that either the Allee threshold and/or the disease transmission rate is large; or when the disease has limited influence on the infectives growth rate; and/or when disease-induced mortality is low. Host-parasite systems where diffusion or migration of local populations manage to destabilize them are examples of what is known as diffusive instability. The exploration of SI-dynamics in the presence of dispersal brings up the question of whether or not diffusive instability is a possible outcome. Here, we briefly look at such possibility within two-patch coupled SI-DD and SI-FD systems. It is shown that relative high levels of asymmetry, two modes of transmission, frequency dependence, and Allee effects are capable of supporting diffusive instability.
Keywords: Allee Effects, Horizontal Transmission, Vertical Transmission, Disease-Driven Extinction, Disease-Free Dynamics, Susceptibles-Free Dynamics, Diffusive Instability
1. Introduction
Parasitism contributes to the selection of future generations of hosts through their impact on factors that lead to reductions in fitness (Hudson et al. 2002) and as a result, wildlife managers must account for emerging and/or re-emerging diseases. Competition for space and resources (finding mates or food) also impact the reproductive ability and likelihood of survival of individuals, particularly those housing pathogens or parasites. Hosts’ dynamics (survival in particular) often depends on the ability of a population to maintain a critical mass (Kang and Castillo-Chavez 2012).The impact of heterogenous transmission factors including multiple transmission modes by altering a population’s dynamics may lessen the plausibility of conservation goals or the economic viability of selected management policies (Potapov et al 2012). Hence, it is not surprising that the pressure which parasites or pathogens place on their hosts and the relation of such interactions to community and/or ecosystem structure has been the subject of continuous empirical and theoretical studies. Some of the theoretical consequences associated to host-pathogen dynamics with factors like: i) multiple modes of disease transmission; (ii) host population density; and (iii) the presence or absence of critical host population thresholds, are addressed in this manuscript.
Modes of disease transmission, like horizontal and vertical, differentially facilitate the colonization of host populations by bacteria, fungi, or viruses. Colonization (horizontal transmission) is sometimes seen as the result of close interactions (contacts) between disease-free host and infected individuals. A contact process that implicitly assumes the sharing of a common, local habitat. The passage of a disease-causing agent from a mother to offspring during the “ birth” process is also sometimes possible (vertical transmission). Feline leukemia (FeLV) and feline immunodeficiency (FIV) viruses are transmitted horizontally and vertically. Leishmaniasis, a disease caused by the protozoan parasite Leishmania infantum, is transmitted horizontally and vertically. Domesticated dog populations are presumed to be a reservoir for Leishmania infantum; a reservoir maintained by the differential contributions of multiple modes of transmission (Santaella et al. 2011). The deadly septicaemia, which manages to kill 80% of septicaemia-infected birds, gets lodged in the ovary of surviving birds; passed later to the birds eggs (vertical transmission); spreading horizontally within the hatcher and brooder.
Traditionally, density-dependent transmission (DDT) and frequency-dependent transmission (FDT) are two extreme forms of disease transmission. DDT refers to parasite transmission in which the rate of contact between susceptible hosts and the source of new infections increases with host density while FDT refers to the rate of contact between susceptible hosts and the source of new infections is independent of host density. Teasing out the roles of density- and frequency-dependent transmission (DDT versus FDT) on the dynamics of host-parasite systems is carried out for theoretical and policy reasons. FDT is the result of density-independent contact rates between susceptible and infected individuals. DDT assumes that infection risks increase with host density. Density-dependent transmission (DDT) may require a minimal number of available susceptible hosts, that is, a threshold density, for transmission to occur. Density-dependent parasitic disease transmission plays a role in regulating host population size (Anderson and May 1978&1991) while frequency-dependent parasitic transmission does not require host density thresholds or regulatory host population constraints on the birth or death rates to ”work” (Getz and Pickering 1983).
In population biology we often lack absolutes. And so, vector- and sexually-transmitted diseases have been seen to thrive in frequency-dependent transmission settings while density-dependent infections that lead to pathogens being shed by infected hosts into common environments may sometimes need a critical mass of susceptible individuals to thrive (Anderson and May 1991; Antonovics et al. 1995). Pathogens can be spread via “direct” contacts (kissing can spread herpes viruses), aerosol (sneezing can spread influenza viruses), or via indirect contacts (ingesting water contaminated with fecal material can cause result in cholera infections), or through vectors (ticks and mosquitoes often spread viruses and bacteria to their hosts), or via some combination of direct and indirect modes, sometimes mediated by a vector. Empirical work on mice, voles, lady bird beetles, frogs, and plants has shown that pathogen transmission often involve DD and FD transmission modes, with one predominant mode (Hudson et al. 2002). The negative impact of deliberate releases of pathogens via aerosol or in water systems tends to increase with host density. On the other hand, sexually transmitted pathogens seem to thrive equally well or bad in small or large population settings while some vector-borne diseases have been shown to support frequency-dependent transmission patterns (Anderson and May 1991; Antonovics and Alexander 1992; Ferrari et al 2011). Antonovics and Alexander (1992) manipulated the density and frequency of infected hosts Silene latifolia and in the process they found out that deposition of the anther smut fungus Microbotryum violaceum by pollinating insects managed to increase with the frequency of infection.
A pathogen may or may not be deleterious enough to regulate the dynamics of host populations and so it is not surprising that the impact of pathogens on hosts is tied in to virulence. Pathogen’s levels of virulence differentially impacts host’s fitness. Often, increases in virulence result in a reduced probability survival or a diminished ability of a host to reproduce successfully, or both (Anderson and May 1978; Hudson et al. 2001; Hilker et al. 2009). Pathogens whose transmission successes increases with host density seem to have managed to select for variants capable of regulating a host population. Dwyer et al (1990) studied a host-pathogen system where a detailed account of virus titer on infected hosts could be estimated. Their study focused on studying the ability of the Myxoma virus to control an exploding rabbit populations over a long window in time. Empirical evidence from systems involving conjunctivitis in house finches or parasitic nematodes in red grouse and feral Soay sheep provide an example of a system where disease regulates population size (Gulland 1992; Hudson et al. 1998; Hochachka and Dhondt 2000). Pathogen infections are contributors to the decline or the extinction of some species (Dwyer et al 1990; Daszak et al. 1999; Smith et al. 2006; Thieme et al. 2009). The deleterious role of chytridiomycosis in amphibians, chestnut blight in American chestnuts, avian malaria in Hawaiian birds, devil facial tumour disease in Tasmanian devils, or sudden oak death in Californian trees provide classical examples of the role of disease in regulating a population. Theory suggests that density-dependent specialist pathogens (i.e., those infecting a single host) alone rarely drive their hosts’ extinction but can lead to extinction of the pathogen while frequency-dependent transmission may be capable of supporting significant decreases, including the potential extinction of host and parasite populations in the presence of moderately lethal pathogens (de Castro and Bolker 2005; Ferrari et al 2011; Kilpatrick and Altizer 2012).
The impact of disease outbreaks can be devastating and their dynamics must be particularly monitored within populations near extinction; that is, those with population levels near established Allee effects thresholds (Allee 1938; Stephens and Sutherland 1999; Stephens et al 1999; Courchamp et al 2009; Kang and Lanchier 2011). The relevance of threshold effects has been identified within a wide array of taxa (Courchamp et al. 2008; Kramer et al. 2009). Populations under Allee effects or facing extinction or both must be effectively managed (Drake 2004; Hilker 2009). The fragility of these populations means that limiting the transmission of highly deleterious diseases is critical (Deredec and Courchamp 2007; Hilker 2009). Recurrent infectious disease outbreaks tend to enhance the deleterious role of Allee effects within diseases capable of inducing reductions in host fitness (Hilker et al. 2005; Deredec and Courchamp 2006; Yakubu 2007; Hilker et al. 2009; Thieme et al. 2009; Hilker 2010; Friedman and Yakubu 2011; Kang and Castillo-Chavez 2013 a&b). The results of this manuscript seem to be in sync with the overall conclusions reached the study of predator-prey systems (e.g., Cushing 1994; Emmert and Allen 2004; Drew et al 2006; Jang and Diamond 2007; Berezovskaya et al. 2010; Kang and Armbruster 2011; Kang and Castillo-Chavez 2012).
Parasites and hosts co-evolve in response to environmental clues and/or selective pressures (Kilpatrick and Altizer 2012). Mammals, birds, fish, and insects generate mobility patterns as they track resources and as it is well known movement and/or dispersal can impact disease dynamics (Altizer et al 2011). In short, mobility has been a key player in the evolution of host-parasite systems. Studies that in addition to disease and mobility (dispersal) also include the impact of Allee effects are not well understood (Rios-Soto et al. 2006; Hilker et al. 2007; Kang and Castillo-Chavez 2013 a&b). Hilker et al. (2007) used a reaction-diffusion SI model within a frequency-dependent transmission framework in their explorations of the impact of disease and mobility on the spatiotemporal patterns of disease transmission. SI models that incorporate disease-reduced fertility have been explored by a number of researchers (see Diekmann and Kretzshmar (1991) and Berezovskaya et al. (2004)). In Kang and Castillo-Chavez (2013b) a two-patch SI model with density-dependent transmission is used to show that the differential movement of susceptible and infected individuals can enhance or suppress the spread of a disease. A SI model that incorporates a horizontally and vertically transmitted disease; infectives giving birth to infectives; susceptibles giving birth to susceptibles; Allee effects within the net reproduction term; disease-induced death rate; and disease reduced reproductive ability, is used in this manuscript to begin to address questions that include: What is the role of multiple modes of transmission? Will density-dependent and frequency-dependent vertical transmission affect host-parasite dynamics differentially? Under what conditions would Allee effects alter disease-free dynamics or facilitate disease-driven extinction? Would Allee thresholds on reproductive fitness become altered (reduced) by disease? What is the role of DDT or FDT in support of diffusive instability?
In Section 2, a general SI (Susceptible-Infected) model with Allee effects built in the reproduction that incorporates horizontal and vertical transmission modes, is formulated. The basic dynamic properties of the model are characterized, in particular, sufficient conditions in support of disease-free and persistence of species results are identified (Theorem 2.1 and its corollary 2.2). In Section 3, the dynamics of SI models under frequency-dependent or density-dependent horizontal transmission are contrasted. Boundary dynamics are characterized (Proposition 3.1) and sufficient conditions for disease- and susceptible-population persistence are provided in Theorem 3.2. A classification of interior dynamics comes in Theorem 3.3. In Section 4, disease-driven extinction, disease-free or susceptible-free dynamics, and endemic persistent dynamics are characterized. The nature of bifurcations supported by SI models is studied with the aid of the reproduction numbers linked to horizontal and vertical transmission modes. In Section 5, sufficient conditions leading to diffusive instability (Theorem 5.1) are identified. The nature of mechanisms potentially capable of supporting diffusive instability in SI-models and prey-predator models, is briefly discussed. The implications of the results in this manuscript are discussed in the Conclusion.
2. An SI model with Allee effects and vertical transmission
The model outlined in this section deals with a population facing a disease that can be effectively captured within a SI framework under assumptions that include the possibility of multiple modes of transmission, that is, horizontal and vertical. It is therefore assumed that infected individuals can give birth to infected hosts; that Allee effects alter the net reproduction term (possibly due to mating limitations or predator saturation); the presence of increases in mortality due to disease-induced deaths; and the fact that infected individuals may experience reductions in reproductive ability.
We let S and I denote the susceptible and infective populations, respectively, with N = S + I denoting the total host population. The approach followed from Deredec and Courchamp (2006) leads to the following set of nonlinear equations after the incorporation of the above assumptions:
| (2.1) |
where r > 0, ρ ∈ [0, 1], d > 0 are respectively the intrinsic growth rate, the reduction of growth rate due to disease, and the excess death rate from the disease. The horizontal transmission term ϕ(N) includes density-dependent transmission, ϕ(N) = βN (the law of mass action) or frequency-dependent transmission, ϕ(N) = β (proportionate mixing or standard incidence). In the absence of disease, the SI Model (2.1) reduces to the following single species growth model:
| (2.2) |
where the per capita growth function rf(N) is subject to strong Allee effects, i.e., there exists an Allee threshold K− and a carrying capacity K+ such that
| (2.3) |
Thus, the population model described by Equation (2.2) converges to 0 if initial conditions are below K− or converges to its carrying capacity K+ whenever the initial conditions are above K−.
Note: The formulation of this SI model (2.1) is similar in approach to that found in Boukal and Berec (2002), Deredec and Chourchamp (2006), Courchamp et al (2009), Hilker et al (2009), Thieme et al (2009), and Kang and Chavez-Castillo (2012), particularly in the way we model the effects of Allee effects and disease. Our models allow for infectives to give birth to infectives with the caveat that their reproductive ability may be reduced; a feat that being captured with the parameter ρ.
The need for biological consistency (well posed model) is addressed with the help of the state-space naturally associated with Model (2.1), namely, with its interior defined as . The state space when ϕ(N) = β is . The assumption that f(N) is differentiable leads to the following theorem for Model (2.1):
Theorem 2.1 (Basic dynamical features of (2.1)). Assume that r > 0, d > 0, ρ ∈ [0, 1] and both f, ϕ are continuous in X with f satisfying Condition (2.3), then the system (2.1) is positively invariant and bounded in X with the following property
In addition, we have the following:
If N(0) ∈ (0, K−), then limt→∞ N(t) = 0.
- If there exists a positive number α > K− such that rρf(α) > d, then
If , then lim supt→∞ I(t) = 0.
Proof. Since both S = 0 and I = 0 are invariant manifold for the system (2.1), then according to the continuity of the system, we can easily show that (2.1) is positively invariant in X. In addition, the system (2.1) gives the following equation:
| (2.4) |
where N = S + I. We have the following three cases
-
If K− ≤ N ≤ K+, we have the following inequalities
which indicates that limt→∞ N(t) = 0 if N(0) < K− since f(N) satisfies the condition (2.3), i.e., f(N) > 0 when K− ≤ N ≤ K+. Similarly, if N < K− or N > K+, then we have the following inequalities(2.5)
which indicates that lim supt→∞ N(t) ≤ K+ if N(0) > K− since f(N) satisfies the condition (2.3), i.e., f(N) < 0 when N < K− or N > K+.(2.6) The discussion above also implies that lim supt→∞ N(t) ≤ K+.
-
If there exists a positive number α > K− such that rρf(α) > d, then according to (2.5), we have
Therefore, lim inft→∞ N(t) ≤ α for any N(0) > α.
- If , then from (2.1) and the fact that lim supt→∞ N(t) ≤ K+, we have
which indicates that lim supt→∞ I(t) = 0.
Notes: Some of the consequences that follow from Theorem 2.1 are:
The size of the initial population is extremely important for persistence regardless of the disease due to Allee effects.
The total population will not be above its carrying capacity K+ in the long run.
Species persistence requires that the initial population α is above the Allee threshold K−, and excess deaths are small enough, smaller than the per capita growth function evaluated at the total population α, i.e., .
-
In the absence of vertical transmission, disease free dynamics requires thatIn the presence of vertical transmission, disease free dynamics requires that
For convenience, we can consider that f(N) has a generic form of (N − K−)(K+−N) and ϕ(N) = β (i.e., frequency-dependent) or βN (i.e., the law of mass action) then scaling and setting
leads to the following two SI models with both horizontal and vertical transmission and Allee effects:
| (2.7) |
| (2.8) |
and
| (2.9) |
| (2.10) |
where f(N) = (N − θ) (1 − N) is the per capita growth in the absence of disease; the parameter represents the Allee threshold; ρ ∈ [0, 1] represents the reduce reproductive ability due to the disease; β represents the disease transmission rate while d denotes the additional death rate coming from infections. The direct application of Theorem 2.1 to the systems (2.7)–(2.8) and (2.9)–(2.10) gives the following corollary:
Corollary 2.2 (Basic dynamic features of (2.7)–(2.8) and (2.9)–(2.10)). The systems (2.7)–(2.8) and (2.9)–(2.10) are positively invariant and bounded in their state space X with the following property
In addition, we have the following:
If N(0) ∈ (0, θ), then limt→∞ N(t) = 0.
- If there exists a positive number α > θ such that ρf(α) > d, then
If , we have lim supt→∞ I(t) = 0.
Proof. The application of Theorem 2.1 is direct by letting r = 1, f(N) = (N − θ)(1 − N) and ϕ(N) = β or βN. We only show the item 3. Since we have
therefore, the system (2.7)–(2.8) has the following property
For the system (2.9)–(2.10), we also have
Therefore, if , we have lim supt→∞ I(t) = 0 for the system (2.7)–(2.8) and the system (2.9)–(2.10).
Notes: The traditional basic reproduction number for SI- Allee effects free- and vertical transmission free-models, namely is naturally no longer relevant. The item 3 of Corollary 2.2 indicates that the additional condition is needed for disease-free dynamics. Since our systems (2.7)–(2.8) and (2.9)–(2.10) involve both horizontal and vertical disease transmissions, their dynamics can be governed by the vertical transmission reproduction number, i.e., and the horizontal transmission reproduction number, i.e., . The remainder of this article focuses on the dynamics of the systems (2.7)–(2.8) and (2.9)–(2.10).
3. Mathematical analysis
Notice that the system (2.7)–(2.8) is not defined at (S, I) = (0, 0) but from Corollary 2.2, we know that
Thus, we artificially define (0, 0) as the extinction equilibrium. Hence, the systems (2.7)–(2.8) and (2.9)–(2.10) have the same boundary dynamics since both of them can be reduced to the system given by
and
Therefore, the systems (2.7)–(2.8) and (2.9)–(2.10) have the following three boundary equilibria
If, in addition, holds, then systems (2.7)–(2.8) and (2.9)–(2.10) support the following additional boundary equilibria on the I-axis:
We have arrived at the following proposition regarding the boundary equilibria of the systems (2.7)–(2.8) and (2.9)–(2.10):
Proposition 3.1 (Boundary equilibria of the systems (2.7)–(2.8) and (2.9)–(2.10)). The systems (2.7)–(2.8) and (2.9)–(2.10) always have boundary equilibria E0,0 = (0, 0), Eθ,0 = (θ,0), E1,0 = (1, 0). If in addition Condition holds then both systems will support two additional boundary equilibria E0,θ = (0, θ2) and E0,1 = (0, K2) where
The nature of the stability of these boundary equilibria is listed in Table 3.1.
Table 3.1.
The local stability of boundary equilibria for the systems (2.7)–(2.8) and (2.9)–(2.10) where , and .
| Boundary Equilibria | Stability Condition |
|---|---|
| E0,0 | Always locally asymptotically stable |
| Eθ,0 | For Model (2.7)–(2.8)- Saddle if ; Source if . For Model (2.9)–(2.10)-Saddle if ; Source if |
| E1,0 | Saddle if ; Locally asymptotically stable if |
| E0,θ | For Model (2.7)–(2.8)- Saddle if ; Source if For Model (2.9)–(2.10)-Saddle if (i.e., ); Source if (i.e., ) |
| E0,1 | For Model (2.7)–(2.8)- Saddle if ; Locally asymptotically stable if ; For Model (2.9)–(2.10)-Saddle if (i.e., ); Locally asymptotically stable if (i.e., ) |
Proof. If S = 0, the systems (2.7)–(2.8) and (2.9)–(2.10) are reduced to the following equation:
Therefore, if , we have
Notice that , therefore,
and
Thus, we have
The stability of the boundary equilibria is obtained from the signs of eigenvalues of the corresponding Jacobian matrices. We omit the details but collect the results in Table 3.1.
Notes: The results in Proposition 3.1 are used to determine the global dynamics in the absence of interior equilibrium (see the proof of Theorem 3.3 for details).
Theorem 3.2 (Persistence of disease or susceptibles). Assume that and the initial condition N(0) ∈ (θ2, K2) then the following statements follow:
For the systems (2.7)–(2.8) and (2.9)–(2.10), a sufficient condition for the persistence of disease is .
For the system (2.7)–(2.8), a sufficient condition for the persistence of susceptibles is while for the system (2.9)–(2.10) is .
The persistence of disease (or susceptibles) means that there exists a positive number ε such that
Proof. Define . Notice that θ2 and K2 are roots of the equation if the condition holds, thus we have
This indicates that h(α) > 0 for any α ∈ (θ2, K2).
Since h(N) = ρf(N) − d then Theorem 2.1 and (its) Corollary 2.2 imply that
The use of Theorem 2.1 and (its) Corollary 2.2 again allows to conclude that the systems (2.7)–(2.8) and (2.9)–(2.10) attract to the compact set 0 ≤ N ≤ 1 and are positively invariant within α ≤ N ≤ 1 for any α ∈ (θ2,K2).
Letting BS = {(S, I) ∈ X : α ≤ S+I ≤ 1}∩{I = 0} and BI = {(S, I) ∈ X : α ≤ S+I ≤ 1}∩{S = 0} leads to the facts that (i) BS and BI are positively invariant and that (ii) the omega limit set of BS is E1,0 while the omega limit set of BI is E0,1.
The results (Theorem 2.5 and its corollary) in Hutson (1984) guarantee that the persistence of disease is determined by the sign of while the persistence of susceptibles is determined by the sign of .
Letting ϕ(N) = β or βN means that the dynamics of I-class are governed by
which gives
and
The results (Theorem 2.5 and its corollary) in Hutson (1984) guarantee that the persistence of disease for the systems (2.7)–(2.8) and (2.9)–(2.10) as long as and the initial condition N(0) ∈ (θ2, K2).
The dynamics of the S-class are governed by
which gives
and
Therefore, applications of the results in Hutson ((Theorem 2.5 and its corollary, 1984) allows us to conclude that:
A sufficient condition for the persistence of susceptibles in the system (2.7)–(2.8) is that as long initial condition are such that N(0) ∈ (θ2, K2).
A sufficient condition for the persistence of susceptibles in the system (2.9)–(2.10) is that as long initial condition are such that N(0) ∈ (θ2, K2).
Note: The system (2.7)–(2.8) or the system (2.9)–(2.10) satisfies the definition of permanence provided that there exists a positive number ε such that for any N(0) ∈ (θ2, K2)
An application of Theorem 3.2 leads to the following permanency results:
We postulate (throughout the rest of this manuscript) that the system (2.7)–(2.8) or the system (2.9)–(2.10) have disease-free dynamics if its attractor is E0,0 ∪ E1,0; or the system (2.7)–(2.8) or (2.9)–(2.10) has susceptibles-free dynamics if its attractor is E0,0 ∪ E0,1; or the system (2.7)–(2.8) or (2.9)–(2.10) has disease-driven extinction if its attractor is E0,0.
3.1. Interior equilibrium
Notice that the equilibria of the system (2.7)–(2.8) satisfy the following equations:
The equilibria of the system (2.9)–(2.10) satisfy the following equations
If we let (S*, I*) be an interior equilibrium of the system (2.7)–(2.8) or the system (2.9)–(2.10) then we have that:
-
The following equation
for the system (2.7)–(2.8) must be satisfied, and so, we see that the system (2.7)–(2.8) has no interior equilibrium if d ≥ β (i.e., ) and(3.1) The Jacobian matrix of Model (2.7)–(2.8) evaluated at the interior equilibrium (S*, I*) can be represented as follows
where N* = S* + I*. Its two eigenvalues λi, i = 1, 2 satisfy the following equalities:(3.2) (3.3) Thus, if an interior equilibrium (S*, I*) exists, it would be locally asymptotically stable provided that , or a saddle if . We conclude that the system (2.7)–(2.8) has either no interior or two interior equilibria , i = 1, 2 and if we happen to have two interior equilibria then we must have that is always a saddle and is always a source.
-
The following equation
for the system (2.9)–(2.10). According to Corollary 2.2, we have N* < 1, thus (3.4) implies that the system (2.9)–(2.10) has no interior equilibrium if and(3.4) On the other hand if then the system (2.9)–(2.10) may have at most one interior equilibrium, namely,The Jacobian matrix of the system (2.9)–(2.10) evaluated at the interior equilibrium (S*, I*) is represented as
where N* = S* + I*. Its two eigenvalues λi, i = 1, 2 satisfy the following equalities:(3.5)
Thus, when the interior (S*, I*) exists, it is locally asymptotically stable as long as while a saddle whenever . It is a source if(3.6) If the system (2.9)–(2.10) has two interior equilibria , i = 1, 2 then using their expressions and the criteria for interior stability allow us to conclude that is always a saddle while can be a sink or source. If, on the other hand, the system (2.9)–(2.10) has only one interior equilibrium, namely , then we see that it can be a sink or source depending on parameter values.
The above discussion can be summarized in the following theorem:
Theorem 3.3 (Interior equilibria of Models). Let and then existence and stability conditions for the interior equilibria of the system (2.7)–(2.8) are listed in Table 3.2. Existence and stability conditions for the interior equilibria of the system (2.9)–(2.10) are listed in Table 3.3.
Table 3.2.
| Interior Equilibrium | Condition for existence | Condition for local asymptotically stable |
|---|---|---|
| Ei1 | or | Always a saddle |
| Ei2 | or | Always locally asymptotically stable. |
Table 3.3.
| Interior Equilibrium |
Condition for existence | Condition for stability |
|---|---|---|
| Ei1 | and (a) or (b) & N1 < θ2 | Always a saddle |
| Ei2 | Case (1): and (a) or (b) & N2 < θ2 or (c) , N2 > K2; Case (2): and (a) or (b) , N2 < θ2 or (c) , N2 > K2 | Locally asymptotically stable if while it’s a source if |
Sufficient conditions leading to no interior equilibrium and the related global dynamics of the systems (2.7)–(2.8) and (2.9)–(2.10) are listed in Table 3.4.
Table 3.4.
No interior equilibrium and the related global dynamics for the systems (2.7)–(2.8) and (2.9)–(2.10)
| Cases | The system (2.7)–(2.8) | The system (2.9)–(2.10) | ||
|---|---|---|---|---|
| No interior equilibrium | Case (1): ; Case (2): ; Case (3): . | Case (1): ; Case (2): ; Case (3): , i = 1, 2; Case (4): , θ2 < N2 < K2 | ||
| Disease-free dynamics | ||||
| Susceptible-free dynamics | . | and Conditions of Case (2) or Case (3) or Case (4) | ||
| Disease-driven extinction | and . | |||
| Permanence | N(0) ∈ (θ2, K2) and . | N(0) ∈ (θ2, K2) and . |
Proof. The above discussion shows that a necessary condition for the systems (2.7)–(2.8) and (2.9)–(2.10) to a have interior equilibrium is that while the existence of interior equilibrium for the systems (2.7)–(2.8) and (2.9)–(2.10) can be classified with the conditions and .
If then the systems (2.7)–(2.8) and (2.9)–(2.10) have no boundary equilibria E0,θ and E0,1 on I-axis and therefore
Necessary conditions for the existence of interior equilibrium for the systems (2.7)–(2.8) and (2.9)–(2.10) are tied in to the existence of solutions of the following equations:
| (3.7) |
and
| (3.8) |
Therefore, if then the systems (2.7)–(2.8) and (2.9)–(2.10) have no interior if or if the following conditions
and
If , then the systems (2.7)–(2.8) and (2.9)–(2.10) have boundary equilibria E0,θ and E0,1 on the I-axis. Additional conditions are needed to guarantee the existence of interior equilibrium for the systems (2.7)–(2.8) and (2.9)–(2.10). The systems (2.7)–(2.8) and (2.9)–(2.10) are discussed separately.
-
For the system (2.7)–(2.8), if Equation (3.7) should have solutions in the interval (0, θ2) or (K2, 1) since
The schematic nullclines for the system (2.7)–(2.8) when are illustrated in Figure 3.1. Two interior equilibria occur whenever with one interior a saddle (i.e., the horizontal line intercepts in the green region of (N − θ)(1 − N)) and the other a sink (i.e., the horizontal line intercepts in the blue region of (N − θ)(1 − N)). There is no interior equilibrium when the horizontal line intercepts (crosses) the black region of (N − θ)(1 − N), i.e., .
-
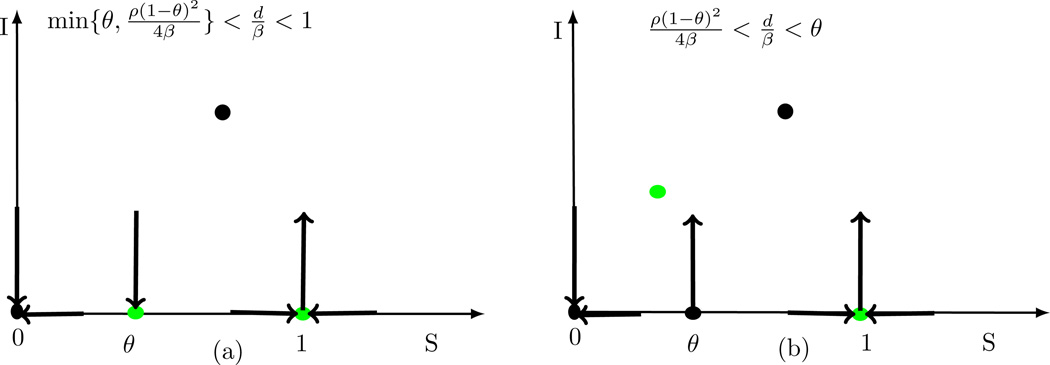
For the system (2.9)–(2.10), whenever Equation (3.8) should have solutions in the interval (0, θ2) or (K2, 1) sinceThe schematic nullclines for the system (2.9)–(2.10) when are found in Figure 3.2. There are two cases depending on the sign of : (1) (see Figure 3.2(a)), two interior equilibria occur whenever
and
where is always a saddle (i.e., the line ) intercepts the green region of (N − θ)(1 − N)) and can be sink (i.e., the line intercepts the red region of (N − θ)(1 − N)) or source (i.e., the line intercepts the blue region of (N − θ)(1 − N)). If Condition or does not hold, i.e., then the system (2.9)–(2.10) has only one interior equilibrium , a saddle. (2) If (see Figure 3.2(b)) then only one interior equilibria occurs whenever
and this interior equilibrium can be a sink or a source, depending on parameters’ values. There is no interior if the line intercepts the black region of (N − θ)(1 − N) when
or
Fig. 3.1.
Schematic nullclines for the system (2.7)–(2.8) when (i.e., ). Potential (two) interior equilibria are the intercepts of the horizontal line and the curve (N − θ)(1 − N) with their stability determined by the location of the intercept. The green region of (N − θ)(1 − N) is a saddle and the blue region is a sink. Two interior equilibria (see the purple horizontal line) occur whenever (i.e., ) where one interior is saddle and the other one a sink.
Fig. 3.2.
Schematic nullclines for the system (2.9)–(2.10) when Potential interior equilibria are intercepts of the line and the curve (N − θ)(1 − N) with their stability determined by the location of the intercept. The green region of (N − θ)(1 − N) is a saddle; the red region is a source and the blue region is a sink. The left graph corresponds to the case when : one (see the black line ) or two interior equilibria (see the purple or dark green line ) are possible. The right graph corresponds to the case when , potentially one interior equilibria (see the dark green or purple line ).
In short, sufficient conditions for the existence of interior equilibria and their stability have been identified and listed in Table 3.2 for the system (2.7)–(2.8) and in Table 3.3 for the system (2.9)–(2.10).
The above analysis has identified conditions (sufficient) that guarantee the absence of interior equilibria for the systems (2.7)–(2.8) and (2.9)–(2.10); listed in Table 3.4. In the absence of interior equilibrium, we can conclude thanks to the Poincaré-Bendixson Theorem (Guckenheimer & Holmes 1983), that a trajectory starting with arbitrary initial conditions in X converge to its locally asymptotically stable boundary equilibria since the systems (2.7)–(2.8) and (2.9)–(2.10) support each a global compact attractor {(S, I) ∈ X : 0 ≤ S + I ≤ 1} in X. The fact that E0,0 is always an attractor results according to Proposition 3.1 in the following three cases:
Disease-free dynamics that corresponds to the case where E0,0 and E1,0 are the only locally asymptotically stable boundary equilibria while other existing boundary equilibria are unstable. This implies that β ≤ d (i.e., ) is a sufficient condition in support of disease-free dynamics within the systems (2.7)–(2.8) and (2.9)–(2.10).
Susceptible-free dynamics that corresponds to the case where E0,0 and E0,1 are the only locally asymptotically stable boundary equilibria while other existing boundary equilibria (including those on the I-axis) are unstable. This implies that and for the systems (2.7)–(2.8) and (2.9)–(2.10), in addition to the conditions of non-existence of interior equilibrium. Our results imply that large value of and may lead to the susceptible-free dynamics
Disease-driven extinction that corresponds to the case where E0,0 is the only locally asymptotically stable boundary equilibria provided that there is no boundary equilibria on the I-axis a result based on Theorem 3.2. This implies that and for the systems (2.7)–(2.8) and (2.9)–(2.10) in conjunction to the conditions that there are no interior equilibrium.
Detailed conditions on the three cases discussed above are listed in Table 3.4.
Notes: Theorem 3.3 implies the following:
Small values of ρ make the systems (2.7)–(2.8) and (2.9)–(2.10) prone to disease-driven extinction since one necessary condition for disease-driven extinction requires that according to Theorem 3.2. This also suggests that vertical transmission may save a species from extinction provided that the reproductive ability of infectives is large enough (some additional conditions must be met).
The system (2.7)–(2.8) has simpler dynamics than the system (2.9)–(2.10). In fact, the system (2.7)–(2.8) has no interior equilibria or two interior equilibria (a saddle and a sink) while the system (2.9)–(2.10) may have one or two interior equilibria.
4. Classifications on dynamics and related bifurcation diagrams
This section focuses on the classification of the dynamics and related bifurcations of the systems (2.7)–(2.8) and (2.9)–(2.10) by using the horizontal transmission reproduction number and the vertical transmission reproduction number .
4.1. The SI model with frequency-dependent horizontal transmission
For the system (2.7)–(2.8), notice that
Thus, if the system (2.7)–(2.8) has boundary equilibria on the I-axis, i.e., , then the no interior equilibria condition says that the boundary equilibrium E0,θ is a source while E0,1 is locally asymptotically stable according to Proposition 3.1 and the fact that
Therefore, the system (2.7)–(2.8) have two interior equilibria provided that
or
Corollary 2.2, Proposition 3.1, and Theorem 3.2–3.3 lead to the study of three cases for the system (2.7)–(2.8):
-
The disease-driven extinction occurs in the situation depicted in Figure 4.1. First, no interior equilibrium, which requires . Within Figure 4.1, we see that the existence and the stability of boundary equilibria requires and . Thus, a sufficient condition that makes Figure 4.1 possible is
The system (2.7)–(2.8) may also support disease-driven extinction whenever it supports an interior equilibrium. In such a case, disease-driven extinction occurs as a result of catastrophic events, that is, when a stable limit cycles merges with the adjacent saddle, leading to the annihilation of the susceptible and infected sub-populations.
- An endemic situation occurs whenever the system (2.7)–(2.8) supports the interior equilibria shown in Figure 4.2. A necessary condition is that and thus we can conclude that the sufficient condition leading to Figure 4.2(a) is
while the sufficient condition leading to Figure 4.2(b) is - Disease-free or susceptible-free dynamics occur when the system (2.7)–(2.8) has no interior equilibrium with either E1,0 or E0,1 locally asymptotically stable, as shown in Figure 4.3. Figure 4.3(a) highlights a disease-free situation for which the condition
is sufficient. Figure 4.3(b) highlights a susceptible-free state for which sufficient the condition is
Fig. 4.1.
Schematic phase plane for the system (2.7)–(2.8) when it experiences the possibility of disease-driven extinction.
Fig. 4.2.
Schematic phase plane for the system (2.7)–(2.8) when it has the endemic occurs.
Fig. 4.3.
Schematic phase plane for the system (2.7)–(2.8) when it has no coexistence of susceptibles and infectives.
The vertical transmission reproduction number, , and the horizontal transmission reproduction number, help, using the above discussions and the analytical results in previous sections, understand the effects of parameters ρ, d, β, θ on the dynamics of the system (2.7)–(2.8). The results can briefly summarized as follows:
A horizontal transmission reproduction number less than 1 supports disease-free dynamics for the system (2.7)–(2.8) (see Theorem 3.3 when combined with the relevant results in Table 3.4).
Both initial condition N(0) = S(0)+I(0) and the value of the vertical transmission reproduction number, , are important in determining global dynamics (see Corollary 2.2 and Theorem 3.3). We can conclude that large values of tend to lead to susceptible-free dynamics; while intermediate values of tends lead to the coexistence of susceptibles and infectives; and small values of tends to lead to disease-driven extinction.
The SI model with frequency-dependent transmissions or the system (2.7)–(2.8) supports relatively simple equilibrium dynamics. It can support no interior or two interior equilibria, with one of the interior equilibrium always stable (see Theorem 3.3, Table 3.3–3.4 and Figure 3.1).
4.2. The SI model with density-dependent horizontal transmissions
In this subsection, the dynamics and potential bifurcations of the SI model, with density-dependent horizontal transmission, given by the system (2.9)–(2.10), are classified. The classification of stability of boundary equilibria for the system (2.9)–(2.10) on the I-axis E0,θ and E0,1 when can be determined from the signs of and . Since
Hence, the signs can be determined by solving from the equations and , i.e.,
and
Letting and leads, making use of Proposition 3.1, to the following (a two dimensional bifurcation diagram example is shown in Figure 4.4 when β = 0.1 and θ = 0.15) results:
- Black area in Figure 4.4: E0,θ is a saddle and E0,1 is locally asymptotically stable if
- Cyan area in Figure 4.4: E0,θ is a source and E0,1 is locally asymptotically stable if
- Green area in Figure 4.4: E0,θ is a source and E0,1 is a saddle if
- White area in Figure 4.4: there is no boundary equilibrium on I-axis, i.e.,
Fig. 4.4.
An example of two dimensional bifurcation diagram (d − ρ) of the system (2.9)–(2.10) when β = 0.1 and θ = 0.15. The black area indicates that the reproduction number of vertical transmission is large, i.e., ; the cyan area indicates that has intermediate values, i.e., ; the green area indicates that has small values, i.e., and the white area indicates that . The green dots indicate that the system (2.9)–(2.10) has only one interior equilibrium which can be a source, saddle or sink while the red dots indicate that the system (2.9)–(2.10) has two interior equilibria where one is a saddle and the other one can be a sink or source.
We therefore identify only four cases for the system (2.9)–(2.10):
Case one: There is no boundary equilibrium E0,θ and E0,1 when the reproduction number of vertical transmission is small enough, i.e., . For a certain range of parameter values, the system (2.9)–(2.10) can have a unique interior attractor, which can be an interior equilibrium (see Figure 4.5; where within the the sub-figure (a) corresponds to the white area with blue dots, i.e., , and (b) corresponds to the white area with red dots, i.e., , below the green area of Figure 4.4) or a stable limit cycle through Hopf-Bifurcation. This is the case when the system (2.9)–(2.10) can support disease-driven extinction as it was the case for the system (2.7)–(2.8).
Case two: There are boundary equilibria E0,θ and E0,1 when the reproduction number of vertical transmission is large enough, i.e., and the reproduction number of horizontal transmission has a large value, i.e., . An example of this case is shown in the black area [whose dynamics is corresponding to the sub-figure (a) of Figure 4.6] and the green area [whose dynamics is corresponding to the sub-figure (b) of Figure 4.6] on the right of the purple vertical line d = 0.015 of Figure 4.4.
Case three: There are boundary equilibria E0,θ and E0,1 and the reproduction number of horizontal transmission has intermediate values, i.e., . An example of this case is shown in the green area [whose dynamics corresponds to the sub-figure (a) of Figure 4.7] and the black area [whose dynamics is corresponding to the sub-figure (b) of Figure 4.7] on the left of the purple vertical line d = 0.015 of Figure 4.4.
Case four: There are boundary equilibria E0,θ and E0,1 and the reproduction number of vertical transmission has intermediate values, i.e., . An example of this case is shown in the cyan area of Figure 4.4 whose dynamics on the right side of the purple vertical line d = 0.015, i.e., the reproduction number of horizontal transmission has large values, i.e., . Dynamics represented by the sub-figure (a) of Figure 4.8 and the dynamics on the left side of the purple vertical line d = 0.015, i.e., , represented by the sub-figure (b) of Figure 4.8.
Fig. 4.5.
Schematic phase plane of the system (2.9)–(2.10) when I-class is not able to persist in the absence of S-class, i.e., .
Fig. 4.6.
Schematic phase plane of the system (2.9)–(2.10) when I-class is able to persist in the absence of S-class, i.e., .
Fig. 4.7.
Schematic phase plane of the system (2.9)–(2.10) when I-class is able to persist in the absence of S-class, i.e., .
Fig. 4.8.
Schematic phase plane of the system (2.9)–(2.10) when I-class is able to persist in the absence of S-class, i.e., .
The above discussion and the associated analytical results, including Proposition 3.1, Theorem 2.1, 3.2, 3.3 lead us to conclude that the effects of parameters ρ, d, β, θ on the dynamics of the system (2.9)–(2.10) can be summarized as follows:
The large values of ρ, θ, β and the small values of d can destabilize the system (2.9)–(2.10) (see Figure 4.9).
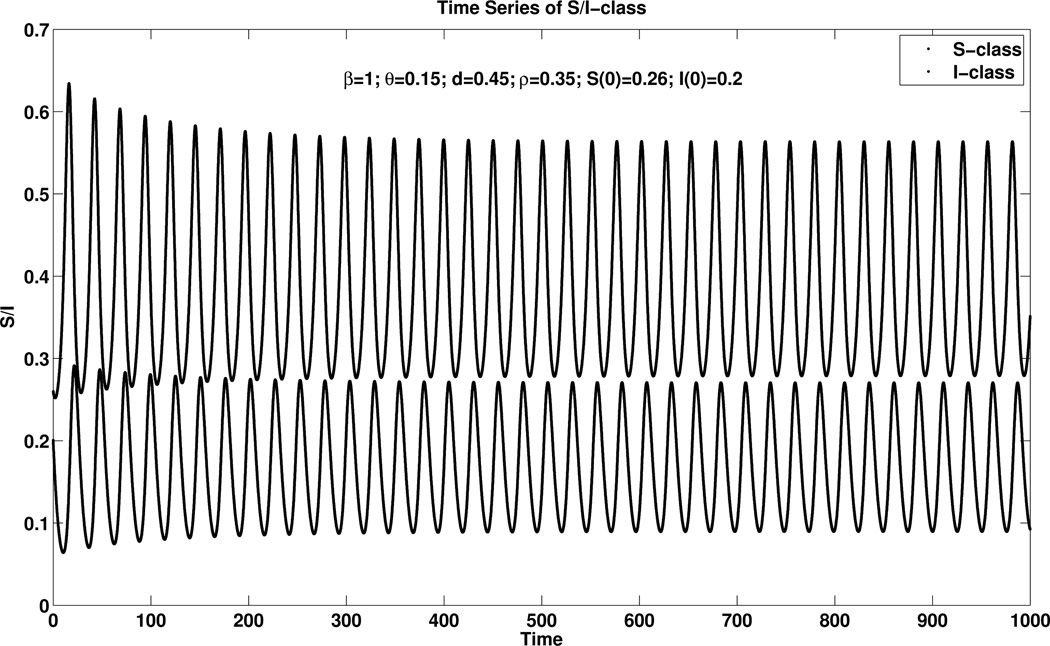
The system (2.9)–(2.10) can have a stable limit cycle; an example is included in Figure 4.10.
Fig. 4.9.
Bifurcation Diagrams of ρ, β, d, θ for the system (2.9)–(2.10) where the blue dots in the left figure means the interior equilibrium is locally asymptotically stable while the red dots means source.
Fig. 4.10.
An example of a stable limit cycle when β = 1, θ = 0.15, d = 0.45, ρ = 0.35, S(0) = 0.26, I(0) = 0.2 where the blue dots in the right figure means the population of I-class while the red dots means the population of S-class.
5. Diffusive instability
The ecological and evolutionary dynamics of host-pathogen or host-parasite systems are theoretically challenging for factors that include the impact of recurrent disease invasion, the ability of a parasite or pathogen to modify a host’s mobility-tied fitness, or reducing life span, or limiting/eliminating reproductive ability. Dispersal is capable of shaping the boundaries of habitats through increases or reductions on the size of the sphere-of-influence of infectious hosts, a cumulative process possibly altering infection rates (reductions or increases in effective contact rates), or its ability to generate clusters, or disease-driven selection of particular behavioral types (Altizer et al 2011; Diaz 2010; Levin 1974; Murray 2003).
Diffusive instability arises when diffusion or migration destabilizes stable situations (Segel and Jackson 1972; Levin 1974; Segel and Levin 1976). It may emerge as a result of the dynamics of metapopulation systems when coupled by dispersal or reaction-diffusion (Diaz 2010; Levin 1974; Murray 2003). The possible emergence of diffusive instability from two-patch systems, coupled by dispersal, when the local dynamics are governed by variants of the general SI-FD or SI-DD systems, is briefly addressed in this section.
Segel and Jackson (1972) using a simple predator-prey model studied the possibility of diffusive instability in predator-prey systems. Hence, first, some classical results addressing the emergence of diffusive instability in predator-prey systems, are revisited. Segel and Jackson (1972) showed that the addition of random dispersal was enough to generate instability from an otherwise initially stable uniform steady-state distribution. Diffusive instability, as shown by Levin (1974), also arises from the effects of dispersal on predator-prey interactions under the pressure of Allee effects. Segel and Levin (1976) used approximate methods and a multiple-time scale theoretical approach in their development of a small amplitude nonlinear theory of prey-predator interactions under random dispersal; a process modeled via diffusion-like terms in discrete and continuous settings. Segel and Levin (1976) showed that dispersal can destabilize spatially uniform states; diffusive instability moving the system to new nonuniform steady states. Levin and Segel (1976 & 1985) noted that the emergence of diffusive instabilities may explain some of the spatial irregularities observed in nature. The possibility of diffusive instability in general SI models is briefly discussed since identifying conditions that lead to diffusive instability on systems where disease and dispersal play a non-independent role are explored. The discussion below, we hope, will instigate further research on the study of diffusive instability in the settings introduced in this manuscript.
A general SI-model can be represented abstractly via the following set of equations
| (5.1) |
operating under the assumption that the system (5.1) has a local asymptotically stable interior equilibrium (S*, I*), an assumption formulated using the inequalities
| (5.2) |
where .
The inclusion of dispersal leads, for example, to the study of symmetric two-patch models. An example of such a system is given by the following set of equations:
| (5.3) |
where lS is the dispersal rate of the S-class and lI is the dispersal rate of I-class. A typical pseudo diffusion model analog, involving constant diffusion coefficients, is given by the following system:
| (5.4) |
where Δ is the Laplacian; DS, DI are the constant diffusion coefficients for susceptibles and infectives, respectively. We say the SI Model (5.1) supports diffusive instability (or Turing Effects) if (S*, I*) is a locally asymptotically stable interior equilibrium of the system (5.1) but (S*, I*, S*, I*) becomes unstable when embedded in the symmetric two-patch model given by the system (5.3) for certain values of lI, lS. We can achieve similar results as long as the (S*, I*) equilibrium is unstable for the Diffusion the system (5.4) at least for some values of DS, DI. The following theorem provides conditions that support the diffusive instability of the systems (2.7)–(2.8) and (2.9)–(2.10):
Theorem 5.1 (Diffusive instability). The general SI model (5.1) can have diffusive instability only if fSgI < 0. In particular, the system (2.7)–(2.8) can support diffusive instability provided that the following inequalities hold
The system (2.9)–(2.10) does not support diffusive instability.
Proof. Recall that the general SI model (5.1) has locally asymptotically stable interior equilibrium (S*, I*) if
Since the two-patch model (5.3) is symmetric, thus, (S*, I*, S*, I*) is its interior equilibrium. The Jacobian matrix of two-patch model (5.3) evaluated at (S*, I*, S*, I*) can be represented as follows:
which has four eigenvalues λi, i = 1, 2, 3, 4. These four eigenvalues satisfy the following two conditions:
λ1 and λ2 are eigenvalues of the Jacobian matrix of the SI model (5.1) evaluated at (S*, I*), thus they are both negatives.
- λ3 and λ4 have the following properties:
and
This implies that (S*, I*, S*, I*) is an interior equilibrium of its two-patch model (5.3) with its stability being determined by the sign of Λ. Thus, diffusive instability can occur only if Λ < 0 which indicates that fSgI < 0, that is, if either fS or gI, is positive then (fSlI + gIlS) > 0 can be made positive and large enough with the right combination of lS, lI; in other words, we conclude that for these parameter values, we have that Λ < 0. For example, if fS > 0 then we can select the dispersal rate of the I-class lI large enough and the dispersal rate of S-class lS small so that the condition Λ < 0 is met. Now, under gI > 0 diffusive instability may be possible as long as lS is large and lI is small.
Relying on the discussion in Section 8.9 of Brauer and Castillo-Chavez (2012), we conclude that (S*, I*) is a steady state of its reaction-diffusion model, namely Model (5.4), where the necessary and sufficient conditions for diffusive instability are given by
which also implies that fSgI < 0.
From Theorem 3.3, we know that if an interior equilibrium (S*, I*) is locally asymptotically stable then for the system (2.7)–(2.8) or the system (2.9)–(2.10).
Thus, for the system (2.7)–(2.8), its Jacobian matrix (3.2) evaluated at the interior equilibrium (S*, I*) gives
which implies that gI < 0 since . Therefore the possibility of diffusive instability in the system (2.7)–(2.8) requires that fS > 0. Since
we have
Since
therefore fS > 0 requires that for the existence of N* based on Theorem 3.3 and
For the system (2.9)–(2.10), its Jacobian matrix (3.5) evaluated at (S*, I*) gives
which implies that fS < 0 and gI < 0 since . Therefore, we conclude that the system (2.9)–(2.10) does not support diffusive instability.
Remark: A direct application of the proof for Theorem 5.1 leads to the following statements:
If fS > 0 and gI < 0, then diffusive instability for the patchy model (5.3) requires that is large enough and while diffusive instability for the reaction-diffusion model (5.4) requires that is large enough.
If fS < 0 and gI > 0, then diffusive instability for the patchy model (5.3) requires to be large enough and while diffusive instability for the reaction-diffusion model (5.4) requires to be large enough.
In addition, Theorem 5.1 indicates that the SI system (2.7)–(2.8) with frequency-dependent horizontal transmission can support diffusive instability under certain conditions. For example, when the system (2.7)–(2.8) has β = 1, d = 0.85, ρ = 0.05, θ = 0.2, then it has two interior equilibria
where Ei1 is a saddle and Ei2 is locally asymptotically stable with
Thus if we choose large enough and then diffusive instability occurs.
These results agree with the study of predator-prey systems by Timm and Okubo (1992), which suggest that the existence of diffusive instability in such systems may require density effects on intra-specific coefficients and on a predator’s diffusivity that must be sufficiently larger when compared to the prey’s. There is a critical value involving the ratio of the prey/predator diffusivities that must be crossed before diffusive instability sets in. An alternative SI model (5.5)–(5.6) with Allee effects and horizontal and vertical transmission disease that can also support diffusive instability is given by the system
| (5.5) |
| (5.6) |
with a locally asymptotically stable interior equilibrium (S*, I*) given by
| (5.7) |
For example, when β = .1, θ = .2, d = 0.095, ρ = 0.001, the system (5.5)–(5.6) has a unique locally asymptotically stable interior equilibrium (S*, I*) = (0.95, 0.044) with
Thus, if we choose large enough and , then diffusive instability occurs. This suggests that the existence of diffusive instability in (5.5)–(5.6) requires that susceptible’s diffusivity is sufficiently larger than that of infectives with a critical value involving the ratio of the susceptible/infectives diffusivities moving beyond a threshold after which diffusive instability sets in.
The SI system (5.8)–(5.9) with Allee effects and disease modified fitness studied by Kang and Castillo-Chavez (2013a) is given by
| (5.8) |
| (5.9) |
where the assumptions of the model and the detailed biological meaning of parameters can be found in Kang and Castillo-Chavez (2013a), cannot support diffusive instability. The model can have a locally asymptotically stable interior equilibrium (S*, I*) with
| (5.10) |
However, if we replace density-dependent transmission with frequency-dependent transmission in the SI the system (5.8)–(5.9) then we obtain the following SI the system (5.11)–(5.12) by letting ρ = 0, α1 = α2 = 1:
| (5.11) |
| (5.12) |
who supports the unique locally asymptotically stable interior equilibrium
and
| (5.13) |
Thus, the system (5.11)–(5.12) can have diffusive instability if
Therefore, according to the proof of Theorem 5.1 and the discussion on the system (2.7)–(2.8), we can conclude that sufficient conditions leading to diffusive instability are that is large enough and the following inequalities hold
Since SI-disease and prey-predator interaction models share structural similarities, we first look at the following two patch prey-predator model (5.14) with differential migration coefficients μ, ν introduced by Levin (1974):
| (5.14) |
which supports the equilibrium:
and
| (5.15) |
According to Theorem 5.1, we conclude that diffusive instability arises if is large enough and the following equalities hold
Notice that the positivity of gI comes from the assumption that yi is able to survive without xi, i.e., yi → ∞ if yi(0) > L/d. If there is no Allee effects, i.e., d = 0, then the prey-predator Model (5.14) does not have diffusive instability. However, if we replace a Holling-Type I functional response with a Beddington-DeAngelis type functional response in the Prey-predator Model (5.14) with d = 0, then we obtain the following two-patch prey predator model that can have diffusive instability:
| (5.16) |
which supports a unique locally asymptotically stable interior equilibrium (x*, y*) whenever
and
| (5.17) |
For example, if K = .5, L = 0.01, c = 2.5, a = .1, h1 = 1.5, h2 = 1., b = 1, μ = ν = 0, then Prey-predator Model (5.14) has a unique locally asymptotically stable interior equilibrium (x*, y*, x*, y*) = (0.008084, 1.008, 0.008084, 1.008) with fS = 0.0022 > 0, gI = −0.005.
In this section, we have discussed diffusive instability in the context of five different SI-models and three different Prey-predator models. So, what are the criterions and mechanisms leading to diffusive instabilities? Comparisons between models supporting diffusive instability [SI-Models (2.7)–(2.8), (5.5)–(5.6), (5.11)–(5.12); Prey-predator models (5.14), (5.16)] and models not supporting diffusive instability [SI-models (2.9)–(2.10), (5.8)–(5.9); the prey-predator model (5.14) with d = 0] are summarized in Table 5.1.
6. Conclusion
Parasites and pathogens are sometimes effective “regulators of natural populations” (Anderson and May 1978; Dwyer et al 1990). Hence, it is of theoretical and empirical interest to study when multiple transmission modes are preferred; or whether pathogen/disease transmission depends on either host population density or its frequency; or the role of small populations (Allee effects) on populations living under the selection pressures placed by pathogens or parasites. Answers to such questions are needed to assess the role and impact of selection on populations, communities and ecosystems. In this manuscript, we explore the contributions of some of these factors on the dynamics of host-parasite interactions within a controlled setting, namely a general SI model that includes: (a) Horizontally and vertically-transmitted disease modes, (b) Net reproduction terms that account for the limitations posed by Allee effects, (c) Disease induced death rates, and (d) Disease-driven reductions in reproduction ability. The analyses carried out in the prior sections leads to the following conclusions and observations:
Density- versus frequency-dependent horizontal transmission: From Theorem 3.3, we know that the system (2.7)–(2.8) can have two interior equilibria, one a saddle and one a locally asymptotically stable equilibrium. the system (2.9)–(2.10) can support stable limit cycles that emerge via Hopf-Bifurcation (see Figure 4.4 and 4.10). In other words, the SI model with density-dependent horizontal transmission turns out to support more complicated outcomes than its frequency-dependent counterpart.
- Effects of ρ, β, d and θ: is identified as the horizontal-transmission reproduction number and as the vertical-transmission reproduction number.
- The SI-DD system (2.9)–(2.10) supports the following outcomes: (i) No boundary equilibrium on the I-axis and possibly disease-driven extinction for a range of parameter values whenever and . (ii) Susceptible-free dynamics for -intermediate values; values that satisfy the inequality . (iii) An unique interior equilibrium whenever and possibly two interior equilibria if . (iv) Large values of ρ, θ, β and small values of d can destabilize the system (bifurcation diagrams; Figure 4.9).
-
Horizontal versus vertical modes of transmission in the SI systems: Small values of the horizontal transmission rate can lead to the disease-free dynamics for both the systems (2.7)–(2.8) and (2.9)–(2.10)) with low reproductive rates for infectives leading, under certain conditions, to disease-driven extinction.
The systems (2.7)–(2.8) and (2.9)–(2.10) have similar dynamics to those of the SI-model
whenever the horizontal-transmission reproductive number is not greater than 1 and the vertical-transmission reproduction number is dominant. In this case, the system (6.1) has E0,0 ∪ E1,0 as its global attractor (disease-free dynamics).(6.1) If horizontal transmission is dominant, that is, the vertical transmission rate is small due to the low reproductive ability (ρ) of those infected then the systems (2.7)–(2.8) and (2.9)–(2.10) have similar dynamics to those supported by the SI-Models (6.2) and (6.3), respectively:
and(6.2) (6.3) Model (6.2) and (6.3) can in fact have the disease-driven extinction, under some conditions.
- Diffusive instability: Sufficient conditions leading to diffusive instability (Theorem 5.1 in Section 5) require that SI/Prey-Predator models support a locally asymptotically stable interior equilibrium with the product of the diagonal entries of the Jacobian matrix (evaluated at this interior equilibrium) being negative. In this manuscript, we have investigated possible ways for diffusive instability to emerge in five different SI-models while contrasting their behavior with those of three different prey-predator models. The results of these comparisons have been summarized (Table 5.1). From Table 5.1 we conclude that:
- In the context of our SI models, asymmetricity and nonlinearity that emerge as a result of frequency-dependent horizontal transmission or some forms of vertical transmission in populations under Allee effects, can generate diffusive instability.
-
In the context of Prey-Predator models, asymmetricity and nonlinearity arising from certain forms of functional responses such as Beddington-DeAngelis type functional response or Allee effects in the predator population, can generate diffusive instability.In conclusion, the presence of asymmetricity and nonlinearity such as nonlinear density dependent factors including Allee effects, could be critical for the generation of diffusive instabilities in both SI models and Prey-Predator models.
Table 5.1.
Summary of diffusive instability for SI models and Prey-Predator models
| Models | fS | fI |
Diffusive Instability |
Potential Mechanisms | ||
|---|---|---|---|---|---|---|
| SI-Model (2.7)–(2.8) | Yes | Asymmetricity and nonlinearity arise from frequency-dependent horizontal transmission | ||||
| SI-Model (2.9)–(2.10) | fS = S* (1 + θ − 2N*) < 0 | gI = ρI* (1 + θ − 2N*) < 0 | No | Symmetricity arises from certain forms of vertical transmission with Allee effects; Linearity arises from density-dependent vertical transmission | ||
| SI-Model (5.5)–(5.6) | fS = S* (1 − N* − S* + θ) < 0 | gI = ρI* (1 − N* − I* + θ) > 0 | Yes | Asymmetricity and nonlinearity of arise from certain forms of vertical transmission with Allee effects | ||
| SI-Model (5.8)–(5.9) | fS = [2S* + (α1 + ρ)I* − θ] (1 − S* − α2I*) − (S* + ρI*)(S* + α1I* − θ) − βI* < 0 | gI = 0 | No | Linearity arises from density-dependent horizontal transmission | ||
| SI-Model (5.11)–(5.12) | Yes | Asymmetricity and nonlinearity arise from frequency-dependent horizontal transmission | ||||
| PP-Model (5.14) | fS = −ax* < 0 | gI = dy* > 0 | Yes | Nonlinearity arises from Allee effects in predator | ||
| PP-Model (5.14) with d = 0 | fS = −ax* < 0 | gI = 0 | No | Linearity arises from a Holling Type I functional response | ||
| PP-Model (5.16) | Yes | Asymmetricity and nonlinearity arise from a Beddington-DeAngelis type functional response |
We study SI models with Allee effects and different modes of disease transmissions.
We identify sufficient conditions of disease-free and endemic disease patterns.
The SI Density-Dependent system can support rich dynamics such as limit cycles.
The SI Frequency-Dependent model can only support equilibrium dynamics.
High levels of asymmetry and Allee effects can generate diffusive instability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research of CCC is partially supported by the grant number 1R01GM100471-01 from the National Institute of General Medical Sciences (NIGMS) at the National Institutes of Health. The research of Y.K. is partially supported by Simons Collaboration Grants for Mathematicians (208902) and the research scholarship from School of Letters and Sciences. The authors would also like to thank Simon Levin for suggesting that we connect with the concepts of diffusive instability.
REFERENCES
- 1.Allee WC. The social life of animals. Norton, New York: 1938. [Google Scholar]
- 2.Altizer S, Bartel R, Han BA. Animal Migration and Infectious Disease Risk. Science. 2011;331:296–302. doi: 10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RM, May RM. Regulation and stability of host-parasite population interactions I: Regulatory processes; II: Destabilizing processes. J. Anita. Ecol. 1978;47:219–247. 249–267. [Google Scholar]
- 4.Anderson RM, May RM. Infectious diseases of humans: Dynamics and control. London: Oxford University Press; 1991. [Google Scholar]
- 5.Antonovics J, Alexander HM. Epidemiology of Anther-Smut infection of Silene-alba (= S-Latifolia) caused by Ustilago-Violacea-patterns of spore deposition in experimental populations. Proceedings of the Royal Society of London B. 1992;250:157–163. [Google Scholar]
- 6.Boukal DS, Berec L. Single-species models of the Allee effect: extinction boundaries, sex ratios and mate encounters. Journal of Theoretical Biology. 2002;218:375–394. doi: 10.1006/jtbi.2002.3084. [DOI] [PubMed] [Google Scholar]
- 7.Berezovskaya FS, Karev G, Song B, Castillo-Chavez C. A simple epidemic model with surprising dynamics. Mathematical Biosciences and Engineering. 2004;1:1–20. doi: 10.3934/mbe.2005.2.133. [DOI] [PubMed] [Google Scholar]
- 8.Berezovskaya FS, Song B, Castillo-Chavez C. Role of Prey dispersal and refuges on predator-prey dynamics. SIAM J. APPL. MATH. 2010;70:1821–1839. [Google Scholar]
- 9.Brauer F, Castillo-Chavez C. Mathematical Models in Population Biology and Epidemiology. 2nd Edition. Vol. 40. Springer-Verlag: Texts in Applied Mathematics; 2012. p. 530. [Google Scholar]
- 10.de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005;8:117–126. [Google Scholar]
- 11.Deredec A, Courchamp F. Combined impacts of Allee effects and parasitism. OIKOS. 2006;112:667–679. [Google Scholar]
- 12.Courchamp F, Berec L, Gascoigne J. Allee effects in ecology and conservation. Oxford University Press; 2009. [Google Scholar]
- 13.Cushing JM. Oscillations in age-structured population models with an Allee effect. Oscillations in nonlinear systems: applications and numerical aspects. J. Comput. Appl. Math. 1994;52:71–80. [Google Scholar]
- 14.Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases. 1999;5:735–748. doi: 10.3201/eid0506.990601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deredec A, Courchamp F. Combined impacts of Allee effects and parasitism. OIKOS. 2006;112:667–679. [Google Scholar]
- 16.Diaz E. Dissertation: Diffusive Instability And Aggregation In Epidemiology. Arizona State University; 2010. [Google Scholar]
- 17.Drake JM. Allee effects and the risk of biological invasion. Risk Analysis. 2004;24:795–802. doi: 10.1111/j.0272-4332.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 18.Diekmann O, Kretzshmar M. Patterns in the effects of infectious diseases on population growth. Journal of Mathematical Biology. 1991;29:539–570. doi: 10.1007/BF00164051. [DOI] [PubMed] [Google Scholar]
- 19.Drew A, Allen EJ, Allen LJS. Analysis of climate and geographic factors affecting the presence of chytridiomycosis in Australia. Dis. Aquat. Org. 2006;68:245–250. doi: 10.3354/dao068245. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer G, Levin SA, Buttel L. A simulation model of the population dynamics and evolution of myxomatosis. Ecological Monographs. 1990;60:423–447. [Google Scholar]
- 21.Emmert KE, Allen LJS. Population persistence and extinction in a discrete-time stage-structured epidemic model. J. Differ. Eqn Appl. 2004;10:1177–1199. [Google Scholar]
- 22.Ferrari MJ, Perkins SE, Pomeroy LW, Bjornstad ON. Pathogens, social networks, and the paradox of transmission scaling. Interdisciplinary Perspectives on Infectious Diseases. 2011;10:10. doi: 10.1155/2011/267049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman A, Yakubu A-A. Fatal disease and demographic Allee effect: population persistence and extinction. Journal of Biological Dynamics. 2011 doi: 10.1080/17513758.2011.630489. [DOI] [PubMed] [Google Scholar]
- 24.Guckenheimer J, Holmes P. Nonlinear Oscillations, Dynamical Systems, and Bifurcations of Vector Fields. Springer-Verlag: 1983. [Google Scholar]
- 25.Gulland FMD. The Role of Nematode Parasites in Soay Sheep (Ovis-Aries L) Mortality During a Population Crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- 26.Hilker FM, Lewis MA, Seno H, Langlais M, Malchow H. Pathogens can slow down or reverse invasion fronts of their hosts. Biol. Invasions. 2005;7:817–832. [Google Scholar]
- 27.Hilker FM, Langlais M, Petrovskii SV, Malchow H. A diffusive SI model with Allee effect and application to FIV. Mathematical Biosciences. 2007;206:61–80. doi: 10.1016/j.mbs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Hilker FM, Langlais Ml, Malchow H. The Allee Effect and Infectious Diseases: Extinction, Multistability, and the (Dis-)Appearance of Oscillations. The American Naturalist. 2009;173:72–88. doi: 10.1086/593357. [DOI] [PubMed] [Google Scholar]
- 29.Hilker FM. Population collapse to extinction: the catastrophic combination of parasitism and Allee effect. Journal of Biological Dynamics. 2010;4:86–101. doi: 10.1080/17513750903026429. [DOI] [PubMed] [Google Scholar]
- 30.Hochachka WM, Dhondt AA. Density-dependent decline of host abundance resulting from a new infectious disease. Proceedings of the National Academy of Sciences, USA. 2000;97:5303–5306. doi: 10.1073/pnas.080551197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson PJ, Dobson AP, Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- 32.Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP. The Ecology of Wildlife Diseases. Oxford, UK: Oxford Press; 2002. [Google Scholar]
- 33.Hutson V. A theorem on average Liapunov functions. Monatshefte für Mathematik. 1984;98:267–275. [Google Scholar]
- 34.Jang SR-J, Diamond SL. A host-parasitoid interaction with Allee effects on the host. Comp. Math. Appl. 2007;53:89–103. [Google Scholar]
- 35.Kang Y, Armbruster D. Dispersal effects on a two-patch discrete model for plant-herbivore interactions. Journal of Theoretical Biology. 2011;268:84–97. doi: 10.1016/j.jtbi.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 36.Kang Y, Lanchier N. Expansion or extinction: deterministic and stochastic two-patch models with Allee effects. Journal of Mathematical Biology. 2011;62:925–973. doi: 10.1007/s00285-010-0359-3. [DOI] [PubMed] [Google Scholar]
- 37.Kang Y, Castillo-Chavez C. Multiscale analysis of compartment models with dispersal. Journal of Biological Dynamics. 2012;6(2):50–79. doi: 10.1080/17513758.2012.713125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang Y, Castillo-Chavez C. A simple epidemiological model for populations in the wild with Allee effects and disease-modified fitness. Journal of Discrete and Continuous Dynamical Systems-B. 2013a doi: 10.3934/dcdsb.2014.19.89. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang Y, Castillo-Chavez C. A simple two-patch epidemiological model with Allee effects and diseasemodified fitness. The special AMS Contemporary Math Series in honor of Ronald Mickens’ 70th birthday. 2013b In press. [Google Scholar]
- 40.Kilpatrick AM, Altizer S. Disease Ecology. Nature Education Knowledge. 2012;3(10):55. [Google Scholar]
- 41.Kramer AM, Dennis B, Liebhold AM, Drake JM. The evidence for Allee effects. Population Ecology. 2009;51:341–354. [Google Scholar]
- 42.Levin SA. Dispersion and population interactions. The American Naturalist. 1974;108:207–228. [Google Scholar]
- 43.Levin SA, Segel LA. Pattern generation in space and aspect. SIAM Review. 1985;27:45–66. [Google Scholar]
- 44.Murray JD. Mathematical biology, I & II. New York, NY: Springer; 2003. [Google Scholar]
- 45.Potapov A, Merrill E, Lewis M. Wildlife disease elimination and density dependence. Proc. R. Soc. B. 2012 May 16; doi: 10.1098/rspb.2012.0520. Published online, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rios-Soto KR, Castillo-Chavez C, Neubert M, Titi ES, Yakubu A-A. Epidemic Spread in Populations at Demographic Equilibrium. In: Gumel A, Castillo-Chavez C, Clemence DP, Mickens RE, editors. Mathematical Studies on Human Disease Dynamics: Emerging Paradigms and Challenges. Vol. 410. American Mathematical Society; 2006. pp. 297–310. [Google Scholar]
- 47.Santaella Jn, Ocampo CB, Saravia NG, Mndez F, Gngora R, Gomez MA, Munstermann LE, Quinnell RJ. Leishmania (Viannia) Infection in the Domestic Dog in Chaparral, Colombia. Am J Trop Med Hyg. 2011;84(5):674–680. doi: 10.4269/ajtmh.2011.10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scalia-Tomba P. The effects of structural behavior change on the spread of HIV in one sex populations. Math. Biosci. 1991;91:547–555. doi: 10.1016/0025-5564(91)90022-b. [DOI] [PubMed] [Google Scholar]
- 49.Segel LA, Jackson JL. Dissipative structure: an explanation and an ecological example. J. Theor. Biol. 1972;37:545–559. doi: 10.1016/0022-5193(72)90090-2. [DOI] [PubMed] [Google Scholar]
- 50.Segel LA, Levin SA. Application of nonlinear stability theory to the study of the effects of diffusion on predator-prey interactions. In: Piccirelli RA, editor. Topics in Statistical Mechanics in Biophysics: A memorial to Julius L. Jackson; AIP Conference Proceedings; 1976. pp. 123–152. [Google Scholar]
- 51.Skerrat LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 52.Smith KF, Sax DF, Lafferty KD. Evidence for the role of infectious disease in species extinction and endangerment. Conservation Biology. 2006;20:1349–1357. doi: 10.1111/j.1523-1739.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 53.Stephens PA, Sutherland WJ. Consequences of the Allee effect for behaviour, ecology and conservation. Trends in Ecology & Evolution. 1999;14:401–405. doi: 10.1016/s0169-5347(99)01684-5. [DOI] [PubMed] [Google Scholar]
- 54.Stephens PA, Sutherland WJ, Freckleton RP. What is the Allee effect? Oikos. 1999;87:185–190. [Google Scholar]
- 55.Thieme HR, Dhirasakdanon T. Persistence of vertically transmitted parasite strains which protect against more virulent horizontally transmitted strains. In: Ma Z, Zhou Y, Wu J, editors. Modeling and Dynamics of Infectious Diseases. Singapore: World Scientific; 2009. pp. 187–215. [Google Scholar]
- 56.Thieme HR, Dhirasakdanon T, Han Z, Trevino R. Species decline and extinction: synergy of infectious disease and Allee effect? Journal of Biological Dynamics. 2009;3:305–323. doi: 10.1080/17513750802376313. [DOI] [PubMed] [Google Scholar]
- 57.Timm U, Okubo A. Diffusion-driven instability in a predator-prey system with time-varying diffusivities. J. Math. Biol. 1992;30:307–320. [Google Scholar]
- 58.Wang X. Ph.D. Thesis. Purdue University; 2005. Backward bifurcation in a mathematical model for Tuberculosis with loss of immunity. [Google Scholar]
- 59.Wang J, Shi J, Wei J. Predator-prey system with strong Allee effect in prey. Journal of Mathematical Biology. 2011;49:291–331. doi: 10.1007/s00285-010-0332-1. [DOI] [PubMed] [Google Scholar]
- 60.Yakubu A-A. Allee effects in a discrete-time SIS epidemic model with infected newborns. Journal of Difference Equations and Applications. 2007;13:341–356. [Google Scholar]