Abstract
Objective
To investigate the roles of hedgehog and Wnt signaling pathways on accumulation of superficial zone protein (SZP) in surface zone articular chondrocytes.
Methods
Chondrocytes were isolated from surface zone of articular cartilage from calf stifle joints and cultured as monolayers in serum-free chemically defined medium. Accumulation of SZP in the culture medium in response to hedgehog proteins (Sonic hedgehog; Shh and Indian hedgehog; Ihh), Wnt proteins (Wnt-3a, -5a and -11), agonists of Wnt/β-catenin pathway (glycogen synthase kinase-3β; GSK-3β inhibitors), and antagonists of Wnt/β-catenin pathway was investigated. Accumulation of β-catenin after treatment with Wnt-3a and GSK-3β inhibitor was also analyzed by immunoblots.
Results
Hedgehog proteins (Shh and Ihh) stimulated SZP accumulation. Activation of Wnt/β-catenin pathway by Wnt-3a and GSK-3β inhibitors led to inhibition of SZP accumulation, but Wnt-5a and -11 which can activate β-catenin-independent Wnt pathways had no influence on SZP accumulation. On the other hand, antagonists of Wnt/β-catenin pathway stimulated SZP accumulation. In addition, there were additive effects on SZP accumulation by combined treatment of transforming growth factor-β1 and hedgehog proteins or antagonists of Wnt/β-catenin pathway.
Conclusion
Sonic and Indian hedgehog signaling pathway has a profound effect on SZP accumulation by surface zone articular chondrocytes. These results provide insights into a better understanding of articular cartilage homeostasis and maintenance.
INTRODUCTION
Lubrication of articular cartilage is critical for normal joint function. Superficial zone protein (SZP), homologous to lubricin (1), is a large proteoglycan that is specifically synthesized and secreted into synovial fluid by chondrocytes in the surface zone of articular cartilage and synovial cells (2–5). SZP is known to function as a boundary lubricant in articular cartilage and reduce the coefficient of friction (6,7). In addition to their function as a boundary lubricant, SZP has been shown to have other biological functions, such as cell proliferation, cytoprotection, and matrix binding (8,9). Both SZP and lubricin are encoded by proteoglycan 4 (Prg4) gene (9) and mutations in the Prg4 gene result in camptodactyly-arthropathy-coxa vara-pericarditis syndrome, an autosomal recessive disease which results in alteration of the articular surface and attendant degradation of articular cartilage, causing early-onset noninflammatory joint damage and failure (10,11). Besides, SZP inhibits synovial cell overgrowth and protects articulating surfaces from protein and cell adhesion and infiltration (12). Loss of SZP influences the functional properties of synovial joints, and a focal decrease in SZP in early osteoarthritis (OA) could have a role in the pathogenesis of cartilage degeneration (12,13). Elsaid et al. demonstrated that there was a strong association between the loss of boundary lubricating abilities of synovial fluid and damage to the articular cartilage after joint injury in an experimental rabbit model of arthritis (14). Taken together, these findings suggest that SZP plays an essential role in maintaining healthy joint function and homeostasis.
It is well established that cytokines play important roles in cartilage homeostasis and that SZP is regulated in part by the cytokines of the joint involved in cartilage homeostasis (1,8). We have previously reported that the level of SZP secreted into the media can be regulated by different cytokines (4,5) including transforming growth factor-β (TGF-β), a critical regulator of SZP accumulation in surface zone articular chondrocytes.
There is growing recognition of several novel signaling pathways in articular cartilage (15–17). Hedgehog and Wnt signaling play key roles in skeletal development including chondrogenesis by regulating cell proliferation, differentiation, survival and migration (15,18–20). A novel role in synovial joint formation has been proposed for hedgehog and Wnt signaling pathway (19,21–23). In addition, hedgehog and Wnt signaling have been implicated in the pathogenesis of OA (17,24). However, the actions of hedgehog and Wnt signaling on surface zone articular chondrocytes have not been investigated. Therefore we hypothesized that hedgehog and Wnt signaling might regulate SZP accumulation in the surface zone of the articular cartilage.
The hedgehog gene was first identified in Drosophila melanogaster and has a role in embryonic segment polarity (25). In mammals, there are three hedgehog orthologs, Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (20). Shh and Ihh have distinct and overlapping roles in embryonic development (20), and Ihh plays a central role in coordinating growth and differentiation of chondrocytes through the formation of negative feedback loop with parathyroid hormone-related protein (PTHrP) in the developing endochondral skelton (26–28). In the absence of hedgehog ligands, Ptch1 represses the activity of Smoothened (Smo) which mediates all vertebrate signaling (29). Binding of hedgehog ligands to the Patched-1 (Ptch1) receptor release its inhibition on Smo, and then, Smo can signal to process the glioma-associated oncogene homolog (Gli) family of transcription family factors, upregulating downstream target genes (20).
The Wnt proteins are a family of highly conserved secreted glycoproteins that mediate receptor-mediated signaling pathways. There are 19 mammalian homologs of Wnts (23,30). The name “Wnt” is derived from a combination of the Drosophila wingless and mouse Int (31). Wingless was originally identified as a segment polarity gene in Drosophila melanogaster and was shown to be homologous to Int1 gene identified as an oncogene. Wnts are implicated in embryogenesis and adult limb formation during mouse development (32). Signal transduction of Wnts is well scrutinized and the most well-understood pathway is the Wnt/β-catenin pathway, also known as “canonical” pathway. In this pathway, in the absence of Wnt ligands, β-catenin, the main mediator of the signal relay, is bound in a “destruction complex” composed of glycogen synthase kinase-3β (GSK-3β), Axin, adenomatous polyposis coli gene product (APC), and other interacting proteins. As a result, β-catenin is phosphorylated and degraded, resulting in low cytosolic β-catenin levels (16). Some members of the Wnt family, such as Wnt-1 and Wnt-3a, bind to Frizzled receptors and the co-receptors low-density lipoprotein receptor-related receptor 5/6 (LRP5/6), and inhibit GSK-3β-mediated phosphorylation of β-catenin (30,33). Stabilized β-catenin then accumulates in the cytosol and translocates to the nucleus, where it interacts with lymphoid enhancer factor/T cell-specific transcriptional factor to affect transcription (16,34). On the other hand, other Wnts, such as Wnt-5a and -11, can instead activate β-catenin-independent Wnt pathways, generally referred as “non-canonical” pathways, such as the planar cell polarity pathway and Wnt/Ca2+ pathways (35,36).
The aim of this study was to investigate the roles of hedgehog or Wnt signaling in SZP accumulation in surface zone articular chondrocytes using primary cell culture. In this study, we investigated the influence of two hedgehog proteins (Shh, Ihh), PTHrP, PTH(1–34), and three different Wnt proteins (Wnt-3a, -5a and -11) on SZP accumulation. Also, the influences of agonists and antagonists of Wnt/β-catenin pathway on SZP accumulation were determined. We further examined the interactions between hedgehog or Wnt signaling and TGF-β1 which is a critical regulator of SZP accumulation in surface zone articular chondrocytes.
MATERIAL AND METHODS
Cell isolation and culture
Stifle (knee) joints from 3-month-old calves were obtained within 6 hours of slaughter from a local abattoir. The joints were dissected under aseptic conditions, exposing the femoral condyles. Surface zone of articular cartilage was harvested from the anterior half of both femoral condyles (approximately 100 µm thick) using a dermatome. The cartilage slices were divided to small pieces by razor blade and digested with 0.2% collagenase P (Roche, Indianapolis, IN) in Dulbecco’s modified Eagle medium: nutrient mixture F-12 (DMEM/F12, Gibco, Grand Island, NY) containing 50 µg/ml ascorbate-2-phosphate (Sigma Aldrich, St. Louis, MO), 0.1% bovine serum albumin (BSA, Sigma Aldrich) and antibiotics (Medium-A) with 3% fetal bovine serum (FBS, Gibco) for 2 hours at 37°C. The released chondrocytes from the tissues were filtered through a cell strainer (70 µm, Falcon, BD Bioscience) and rinsed with DMEM/F-12. Isolated chondrocytes were plated as monolayers at a density of 1×105 cells/well (approximately 2.5×104 cells/cm2) in 12-well culture plates (Corning, Corning, NY) in Medium-A with 10% FBS and incubated at 37°C in a moist atmosphere of 5% carbon dioxide and 95% air. After 24-hour equilibration in the culture medium, cells were switched to fresh Medium-A with ITS+ Premix (BD Biosciences, Bedford, MA) containing various concentrations of hedgehog proteins, Wnt proteins, other proteins or chemical compounds.
Recombinant proteins of Shh, Ihh, Wnt-11, Sclerostin (SOST), Dickkopf-1 (DKK-1), and TGF-β1 were purchased from R&D systems (Minneapolis, MN). PTHrP, PTH(1–34) and GSK-3β inhibitors; lithium chloride (LiCl), BIO, SB216763 and SB415286 were purchased from Sigma-Aldrich. Wnt-3a and -5a were generous gifts from Dr. R. Nusse (Stanford University, Stanford, CA).
Cyclopamine (Sigma Aldrich), an inhibitor of hedgehog signaling by directly binding to Smo (37), was used as an antagonist of hedgehog signaling. Cells were pretreated with cyclopamine (1 µM or 10 µM) for 3 hours before addition of Shh (1 µg/ml).
Enzyme-linked immunosorbent assay (ELISA) for SZP
Since most SZP is secreted into the culture medium (2), the medium from the cultures was harvested after the 4-day treatment and quantitatively analyzed for SZP by sandwich ELISA using purified SZP as standard (5). Each well of 96-well MaxiSorp plates (Nalge Nunc International, Rochester, NY) was coated with 1 µg/ml peanut lectin (EY Laboratories Laboratories, San Mateo, CA) in 50 mM sodium carbonate buffer (pH 9.5). Then the wells were blocked with 1% BSA in the same buffer. Aliquots of culture medium were incubated in the wells. The wells were incubated with monoclonal antibody S6.79 (1:5000) (a generous gift from Dr. T. Schmid, Rush Medical Collage, Chicago, IL) as the primary antibody and goat anti-mouse IgG conjugated with horseradish peroxidase (1:3000, Bio-Rad, Hercules, CA) as the second antibody. Finally, SuperSignal ELISA Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific Inc., Rockford, IL) was used and quantified by measuring relative light units (RLU) in a luminometer. Wells were washed with phosphate buffered saline containing 0.05% Tween 20 (Sigma) between all steps. SZP levels were calculated using an SZP standard which was purified by affinity chromatography on a peanut lectin column; purity was verified by immunoblot analysis and quantified using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific Inc.).
Protein extraction and immunoblot analysis
After treatment with Wnt-3a (100 µg/ml) or BIO (1 µM) for 6 hours, cultured cells were washed twice with phosphate-buffer saline and lysed in buffer containing, 50 mM Tris HCl, 150 mM NaCl, 1% Na-dexycholate, 1% Triton X-100 and 0.1% Sodium dodecyl sulfate. The cell lysates were sonicated and then centrifuged at 14,000 rpm for 10 minutes. Protein concentration was determined with Micro BCA Protein Assay Kit.
For immunoblot analysis, equal quantities of protein extracts were resolved by SDS-polyaclylamidegel electrophoresis and transferred to a PVDF membrane. The membrane was blocked with 5% nonfat dry milk in TBST (25 mM Tris-HCl, 125 mM NaCl, and 0.1% Tween 20) for 1 hour and incubated overnight at 4°C with rabbit monoclonal antibodies against β-catenin or β-actin (Cell Signaling Technology, Danvers, MA) at a 1:1000 dilution. β-actin was examined as a loading control. The membrane was incubated with a goat anti-rabbit IgG conjugated with horseradish peroxidase (1:3000, Bio-Rad) for 1 hour followed by 1 minute incubation with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc.) and developed.
Statistical analysis
For all experiments, the sample size was 6. Values were presented as the mean ± standard deviations. A one-way analysis of variance and Fisher’s protected least significant difference post hoc test were performed using StatView statistics (SAS Institute, Inc., Cary, NC) to determine the influence of hedgehog proteins, Wnt proteins, SOST, and DKK-1 on SZP accumulation. A paired t-test was performed to determine the difference between the control and treated cells. P values less than 0.05 were considered statistically significant.
RESULTS
Influence of hedgehog proteins on SZP accumulation
The hedgehog proteins were used at the concentration of 0.1, 0.3 and 1 µg/ml. Both Shh and Ihh stimulated SZP accumulation significantly (p<0.01) at all concentrations. Compared to control, 1 µg/ml Shh treatment elicited a maximum increase of 2.4-fold while 1 µg/ml Ihh produced a 1.7-fold increase (Figure 1). On the other hand, PTHrP and PTH(1–34) showed no influence on SZP accumulation (data not shown).
Figure 1.
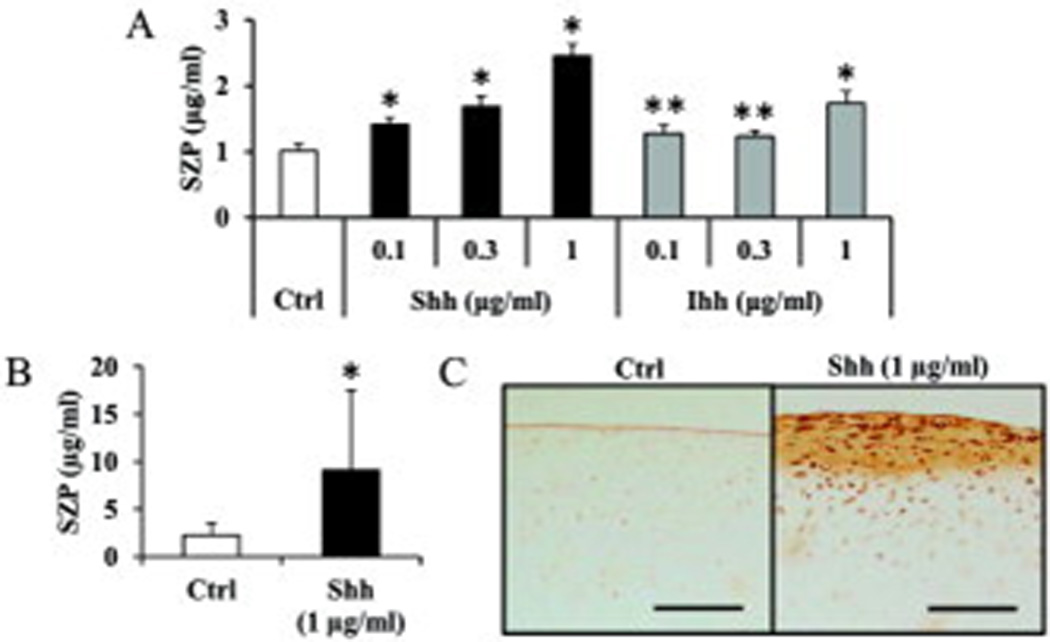
Influence of hedgehog proteins on superficial zone protein (SZP) accumulation in surface zone articular chondrocytes. Primary cells were cultured for 4 days as monolayers in serum-free chemically defined medium with graded levels (0.1, 0.3 and 1µg/ml) of Sonic hedgehog (Shh) and Indian hedgehog (Ihh). SZP accumulation in spent medium was quantified according to enzyme-linked immunosorbent assay. Both Shh and Ihh stimulated the SZP accumulation significantly at all concentration. Values are the mean ± standard deviations, with n=6 (*p<0.01 compared with the value of the control cultures).
Dependence of SZP accumulation on Smoothened (Smo) in hedgehog signaling
We investigated the mechanism underlying the action of hedgehog protein on SZP accumulation. As Smo is critical signal transducer of hedgehog signaling pathway, we used an inhibitor of Smo, cyclopamine, to determine the mechanism. The up-regulation of SZP accumulation by 1 µg/ml Shh was reduced by cyclopamine in part by 1 µM cyclopamine and completely abolished by 10 µM cyclopamine (Figure 2). However, cyclopamine alone showed no influence on SZP accumulation.
Figure 2.
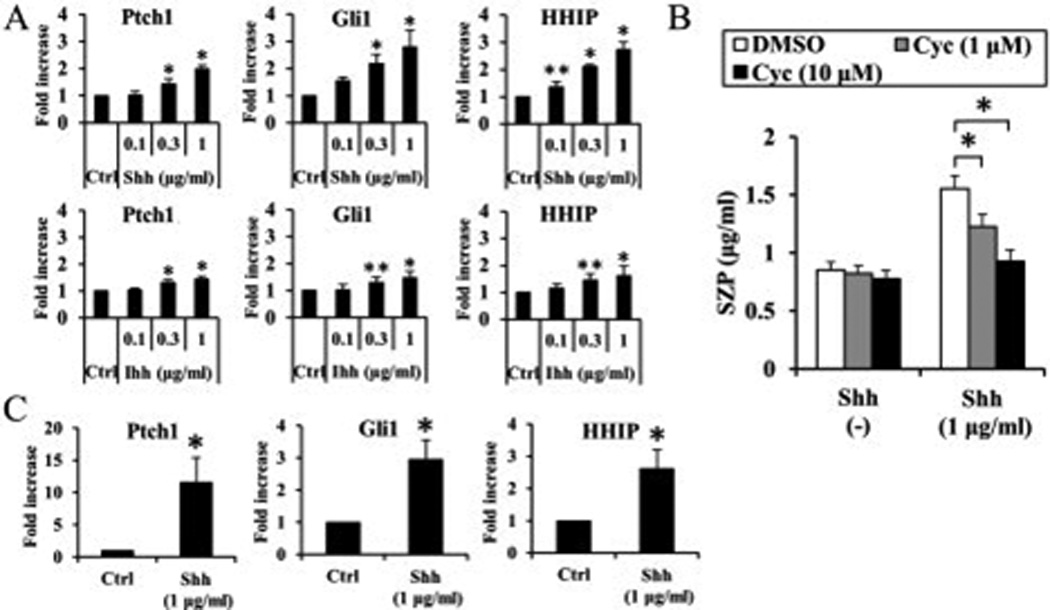
Significant decline of superficial zone protein (SZP) accumulation upon blocking of hedgehog signaling with cyclopamine (Cyc), an inhibitor of Smoothened. Primary cells were pretreated with cyclopamine (1 µM or 10 µM) for 3 hours before addition of Sonic hedgehog (Shh) (1 µg/ml) and cultured for 4 days as monolayers in serum-free chemically defined medium. SZP accumulation in spent medium was quantified according to enzyme-linked immunosorbent assay. The up-regulation of SZP accumulation by 1 µg/ml Shh was reduced by cyclopamine in part by 1µM cyclopamine and completely abolished by 10 µM cyclopamine. However, cyclopmaine alone showed no influence on SZP accumulation (Figure 5). Values are the mean ± standard deviations, with n=6 (*p<0.01).
Influence of Wnt proteins on SZP accumulation
The cells were treated with different concentrations of Wnt proteins (10, 30 and 100 ng/ml). Wnt-3a, which can activate Wnt/β-catenin pathway, suppressed SZP accumulation in a dose-dependent manner with significant difference (p<0.01). On the other hand, Wnt-5a and -11 had no influence on SZP accumulation (Figure 3A).
Figure 3.
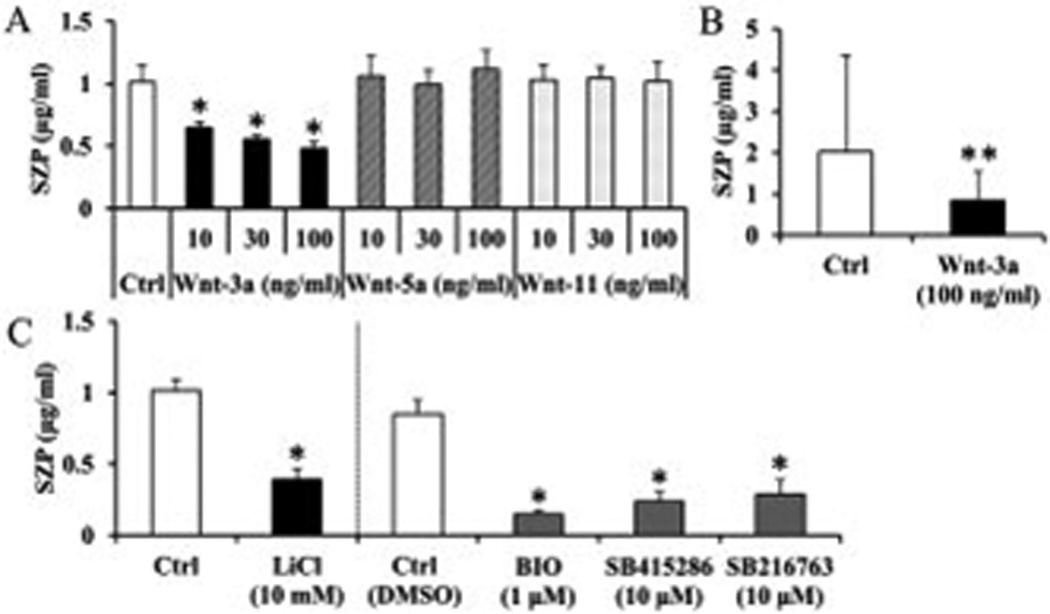
Influence of Wnt proteins (A) and GSK-3β inhibitors (B) on superficial zone protein (SZP) accumulation in surface zone articular chondrocytes. Primary cells were cultured for 4 days as monolayers in serum-free chemically defined medium with graded levels (10, 30, and 100 ng/ml) of Wnt proteins (Wnt-3a, -5a, and -11) or indicated concentration of GSK-3β inhibitors (Lithium chloride (LiCl), BIO, SB415286 and SB216763). SZP accumulation in spent medium was quantified according to enzyme-linked immunosorbent assay. Wnt-3a significantly suppressed SZP accumulation, but Wnt-5a and -11 had no influence on SZP accumulation (A). All GSK-3β inhibitors significantly suppressed SZP accumulation compared to untreated and vehicle control (B). Values are the mean ± standard deviations, with n=6 (*p<0.01 compared with the value of the control cultures).
Inhibitory effect of Wnt/β-catenin pathway on SZP accumulation
Next, we examined the inhibitory effect of Wnt/β-catenin pathway on SZP accumulation. For this experiment, we utilized GSK-3β inhibitors, Lithium chloride (LiCl), BIO, SB415286, and SB216763, which lead to the accumulation of β-catenin and activate Wnt/β-catenin pathway. All GSK-3β inhibitors significantly suppressed SZP accumulation compared to untreated and vehicle control (p<0.01) (Figure 3B). We also showed that treatment with Wnt-3a (100 ng/ml) or BIO (1 µM) resulted in accumulation of cytosolic β-catenin protein (Figure 4). These results indicated that activation of Wnt/β-catenin pathway lead to suppression of SZP accumulation.
Figure 4.
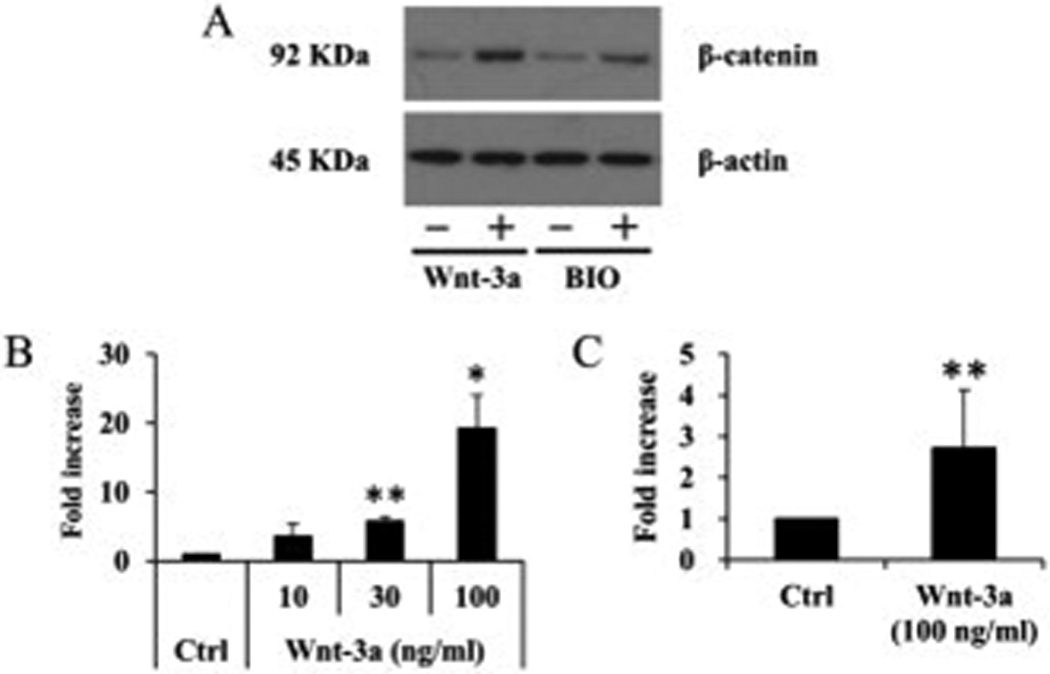
Accumulation of β-catenin by Wnt-3a or BIO. After treatment with Wnt-3a (100 ng/ml) or BIO (1 µM) for 6 hours, whole cell lysates were collected and analyzed by immunoblot. Treatment with Wnt-3a (100 ng/ml) or BIO (1 µM) resulted in accumulation of cytosolic β-catenin protein. Results shown are representative of 3 individual experiments.
Increased SZP accumulation by inhibition of Wnt/β-catenin pathway
As activation of Wnt/β-catenin pathway suppressed SZP accumulation, we used the antagonists of LRP5/6 co-receptor, SOST and DKK-1 which inhibit Wnt/β-catenin pathway (38,39). Both SOST (1 and 3 µg/ml) and DKK-1 (0.1, 0.3 and 1 µg/ml) stimulated SZP accumulation significantly (p<0.01 or 0.05). Compared with control, the maximum increase was 2-fold with 3 µg/ml SOST and 1.5-fold with 0.3 µg/ml DKK-1 (Figure 5).
Figure 5.
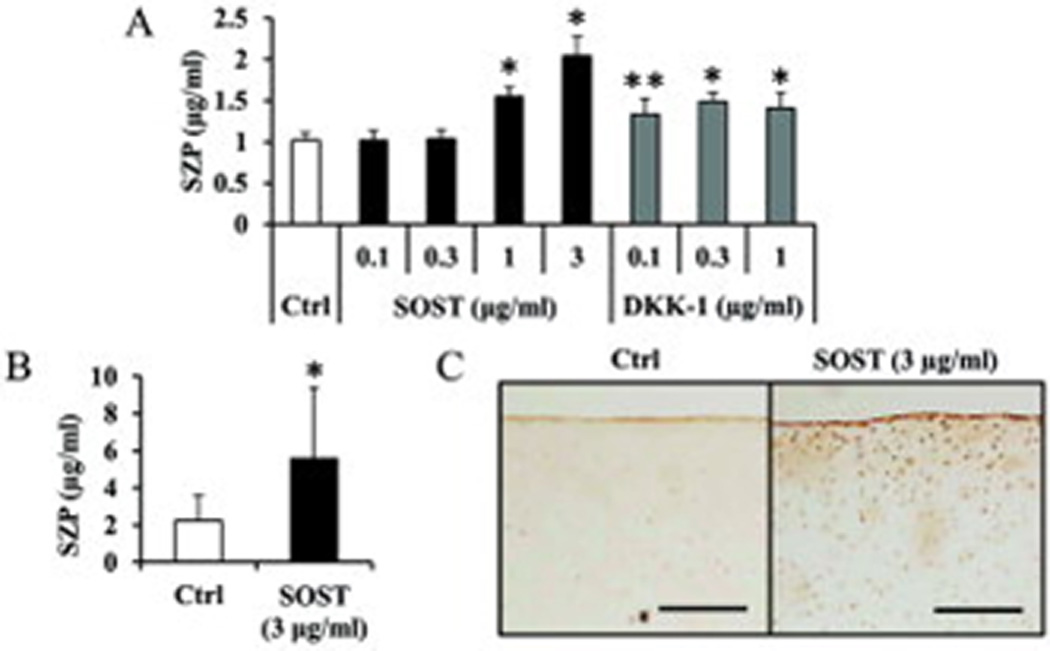
Increased superficial zone protein (SZP) accumulation by inhibition of Wnt/β-catenin pathway. Primary cells were cultured for 4 days as monolayers in serum-free chemically defined medium with antagonist of LRP5/6 co-receptor; Sclerostin (SOST) or Dickkopf-1 (DKK-1). SZP accumulation in spent medium was quantified according to enzyme-linked immunosorbent assay. Both SOST (1 and 3 µg/ml) and DKK-1 (0.1, 0.3 and 1 µg/ml) significantly stimulated SZP accumulation. Values are the mean ± standard deviations, with n=6 (*p<0.01 and **p<0.05, compared with the value of the control cultures).
Additive effects of TGF-β1 and hedgehog proteins or antagonists of Wnt/β-catenin pathway
Next we examined the interaction between TGF-β1 (1, 3 and 10 ng/ml) and Shh (1 µg/ml), Ihh (1 µg/ml), SOST (1 µg/ml), or DKK-1 (0.3 µg/ml). Treatment with 1, 3 and 10 ng/ml of TGF-β1 stimulated SZP accumulation. Additional treatment with Shh (1 µg/ml), Ihh (1 µg/ml), SOST (1 µg/ml), and DKK-1 (0.3 µg/ml) stimulated further accumulation of SZP over and above TGF-β1 alone (Figures 6A and 6B).
Figure 6.
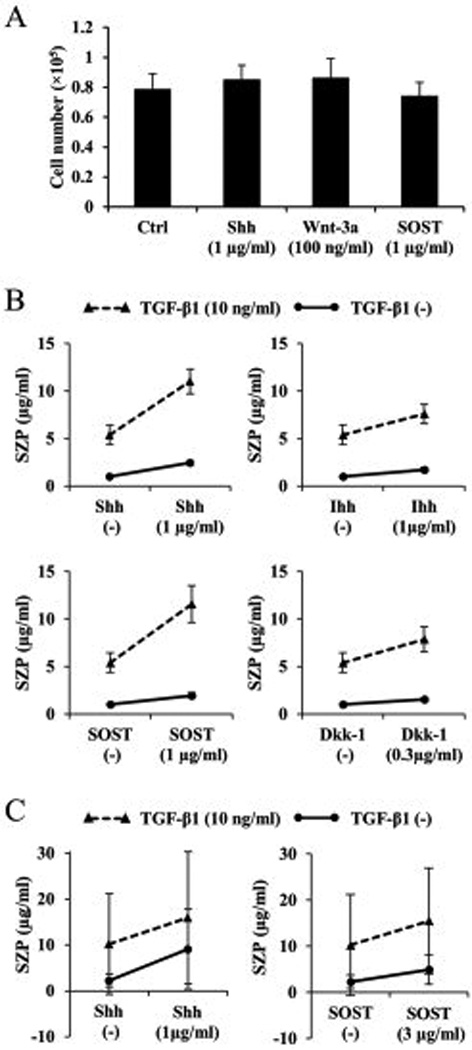
Additive effects of transforming growth factor-β1 (TGF-β1) and hedgehog proteins or antagonists of Wnt/β-catenin pathway. Primary cells were cultured for 4 days as monolayers in serum-free chemically defined medium with combined treatment of TGF-β1 and Sonic hedgehog (Shh), Indian hedgehog (Ihh), Sclerostin (SOST), or Dickkopf-1 (DKK-1). SZP accumulation in spent medium was quantified according to enzyme-linked immunosorbent assay. Treatment with 1, 3 and 10 ng/ml of TGF-β1 stimulated SZP accumulation. Additional treatment with Shh (1 µg/ml), Ihh (1 µg/ml), SOST (1 µg/ml), and DKK-1 (0.3 µg/ml) stimulated further accumulation of SZP over and above TGF-β1 alone (Figures 6). Values are the mean ± standard deviations, with n=6 (*p<0.01 compared with the value of the control cultures (C)).
DISCUSSION
The present investigation demonstrated the actions of hedgehog or Wnt signaling pathway on surface zone articular chondrocytes. Hedgehog proteins (Shh and Ihh) stimulated SZP accumulation. Activation of Wnt/β-catenin pathway by Wnt-3a and GSK-3β inhibitors led to inhibition of SZP accumulation. It is noteworthy that Wnt-5a and -11 showed no influence on SZP accumulation. On the other hand, inhibitors of Wnt/β-catenin pathway stimulated SZP accumulation.
Hedgehog signaling pathway is involved in the initiation of joint induction, endochondral ossification, articular cartilage differentiation, and maitenance and pathogenesis of OA (17,19,21,40). Ihh plays a role in skeletal development, particularly in the growth plate (26), and closely related to Shh which is the main regulator of limb outgrowth (41). Previously, Lin et al., reported that hedehog signaling is activated in OA and higher levels of hedgehog signaling in articular chondrocytes cause a more severe osteoarthritic phenotype (17). However, little is known about the detailed action of hedgehog proteins on normal articular chondrocyte. In the present study, both Shh and Ihh unexpectedly stimulated SZP accumulation in articular chondrocytes from the surface zone of the articular cartilage. This finding indicates that SZ articular cartilage may be in part regulated by hedgehog signaling. As Ihh and PTHrP form a negative feedback loop in the growth plate, in which Ihh stimulates the production of PTHrP by periarticular chondrocytes, we also investigated the influence of PTHrP and PTH(1–34) on SZP accumulation. However, both PTHrP and PTH (1–34) demonstrated no influence on the surface zone chondrocytes.
Wnt signaling pathways also play key roles in synovial joint formation and have implicated in not only cartilage homeostasis, but also in pathogenesis of OA (22–24). It is reported that several proteins have catabolic or anabolic effects on chondrocytes. Wnt-3a has catabolic effect on articular chondrocytes by activating Wnt/β-catenin pathway (42), and Wnt-5a has catabolic effect by activating β-catenin-independent pathway (43,44). On the other hand, Wnt-11 has anabolic effect on articular cartilage by activating β-catenin-independent pathway (44). Suppression of SZP accumulation by activating Wnt/β-catenin pathway by Wnt-3a and GSK-3β inhibitors in the present study is consistent with previous reports. However, both Wnt-5a and -11 had no influence on SZP accumulation in the present study.
Mechanical loading is critical for articular cartilage homeostasis, especially for the surface zone. It is noteworthy that mechanical shear stimulates SZP accumulation in surface zone of articular cartilage (6). Recently, the novel role of Wnt/β-catenin signaling on mechanical loading in cartilage homeostasis was reported. Activation of Wnt/β-catenin signaling by Wnt-3a repressed mechanical loading induced-up-regulation of chondrocyte phenotype markers such as aggrecan and SOX9 (45). In the present investigation, Wnt-3a repressed SZP accumulation in surface zone articular chondrocyte.
We also demonstrated that inhibition of Wnt/β-catenin pathway by antagonists of LRP5/6 co-receptor, SOST and DKK-1, led to the stimulation of SZP accumulation. Articular chondrocytes express low levels of β-catenin, a key mediator of the Wnt/β-catenin signaling pathway. β-catenin levels were significantly increased during de-differentiation of articular chondrocytes by serial monolayer cultures (46). Therefore, inhibition of this low level of endogenous Wnt/β-catenin pathway in surface zone articular chondrocytes leads to the stimulation of SZP accumulation.
The finding that there were additive effects of TGF-β1 and hedgehog proteins or antagonists of Wnt/β-catenin signaling pathway reveal the importance of these signals in the regulation of SZP accumulation. Previously, the interaction between TGF-β and Wnt signaling, or between hedgehog and Wnt signaling was reported in skeletal development including endochondral bone and synovial joint formation (19,47). So, further investigations of the interactions among these signals in accumulation of SZP in surface zone articular chondrocytes will be needed.
The results of this investigation have clinical implications. Several signaling pathways have been implicated in not only homeostasis, but also degradation of articular cartilage in osteoarthritis. Moreover, tissue engineering approaches to articular cartilage regeneration for restoration of joint function may depend on the optimal synthesis of SZP and functional assembly in the surface of articular cartilage.
In conclusion, the present investigation provided novel insights into the role of hedgehog and Wnt signaling pathways in accumulation of SZP in the surface zone of articular cartilage. The results of this investigation have direct implications for the potential utility of hedgehog and Wnt signaling in regeneration of articular cartilage.
ACKNOWLEDEMENTS
We thank Dr. R. Nusse (Stanford University, Stanford, CA) for his generous gifts of Wnt-3a and -5a, and Dr. T. Schmid (Department of Biochemistry, Rush Medical College, Chicago, IL) for his generous gift of monoclonal antibody S6.79.
Dr. Reddi is supported in part by NIH grant R01-AR-061496-01. This research is also supported by Lawrence J. Ellison Endowed Chair in Molecular Biology at the University of California, Davis.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Reddi had full access to all of the data in the study and taked responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Iwakura, Reddi.
Acquisition of data. Iwakura, Inui.
Analysis and interpretation of data. Iwakura, Reddi.
Manuscript preparation. Iwakura, Reddi.
Statistical analysis. Iwakura.
REFERENCES
- 1.Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:677–687. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 4.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 5.Niikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312–2321. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 6.Neu CP, Khalafi A, Komvopoulos K, Schmid TM, Reddi AH. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56:3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 7.DuRaine G, Neu CP, Chan SM, Komvopoulos K, June RK, Reddi AH. Regulation of the friction coefficient of articular cartilage by TGF-beta1 and IL-1beta. J Orthop Res. 2009;27:249–256. doi: 10.1002/jor.20713. [DOI] [PubMed] [Google Scholar]
- 8.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 9.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90:291–297. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 10.Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998;41:730–735. doi: 10.1002/1529-0131(199804)41:4<730::AID-ART22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 12.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 15.Yates KE, Shortkroff S, Reish RG. Wnt influence on chondrocyte differentiation and cartilage function. DNA Cell Biol. 2005;24:446–457. doi: 10.1089/dna.2005.24.446. [DOI] [PubMed] [Google Scholar]
- 16.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 17.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 18.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 19.Mak KK, Chen MH, Day TF, Chuang PT, Yang Y. Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development. 2006;133:3695–3707. doi: 10.1242/dev.02546. [DOI] [PubMed] [Google Scholar]
- 20.Ehlen HW, Buelens LA, Vortkamp A. Hedgehog signaling in skeletal development. Birth Defects Res C Embryo Today. 2006;78:267–279. doi: 10.1002/bdrc.20076. [DOI] [PubMed] [Google Scholar]
- 21.Spater D, Hill TP, O'Sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun JS, Oh H, Yang S, Park M. Wnt signaling in cartilage development and degeneration. BMB Rep. 2008;41:485–494. doi: 10.5483/bmbrep.2008.41.7.485. [DOI] [PubMed] [Google Scholar]
- 25.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 26.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 27.Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 28.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]
- 30.Hinck L, Nelson WJ, Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, et al. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- 32.Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 34.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 35.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 36.Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 39.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423:319–325. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- 42.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88:264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 43.Ge X, Ma X, Meng J, Zhang C, Ma K, Zhou C. Role of Wnt-5A in interleukin-1beta-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheum. 2009;60:2714–2722. doi: 10.1002/art.24779. [DOI] [PubMed] [Google Scholar]
- 44.Ryu JH, Chun JS. Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem. 2006;281:22039–22047. doi: 10.1074/jbc.M601804200. [DOI] [PubMed] [Google Scholar]
- 45.Thomas RS, Clarke AR, Duance VC, Blain EJ. Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther. 2011;9(13):R203. doi: 10.1186/ar3536. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG, Chun CH, et al. Regulation of the chondrocyte phenotype by beta-catenin. Development. 2002;129:5541–5550. doi: 10.1242/dev.129.23.5541. [DOI] [PubMed] [Google Scholar]
- 47.Dong Y, Drissi H, Chen M, Chen D, Zuscik MJ, Schwarz EM, et al. Wnt-mediated regulation of chondrocyte maturation: modulation by TGF-beta. J Cell Biochem. 2005;95:1057–1068. doi: 10.1002/jcb.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]


