Abstract
Background
Krüppel-like factor 4 (KLF4) is a zinc finger transcription factor expressed in the differentiated epithelial cells lining of the intestine. Under physiological conditions, KLF4 inhibits cell proliferation. Conversely, KLF4 mediates proinflammatory signaling in macrophages and its overexpression in the esophageal epithelium activates cytokines, leading to inflammation-mediated esophageal squamous cell cancer formation in mice. Here, we tested whether KLF4 has a proinflammatory activity in experimental colitis in mice.
Methods
Villin-Cre;Klf4fl/fl mice with intestine-specific Klf4 deletion (Klf4ΔIS) and control mice with floxed Klf4 gene (Klf4fl/fl) were treated or not with 3% dextran sodium sulfate (DSS) for 7 days to induce colitis. Additionally, WT mice were administered or not, nanoparticles loaded with scrambled or Klf4-siRNA, and concomitantly given DSS.
Results
Compared with DSS-treated Klf4fl/fl mice, DSS-treated Klf4ΔIS mice were significantly less sensitive to DSS-induced colitis. DSS treatment of Klf4fl/fl mice induced Klf4 expression in the crypt zone of the colonic epithelium. DSS-treated Klf4ΔIS mice had increased proliferation relative to DSS-treated control mice. DSS treatment induced NF-κB signaling pathway in Klf4fl/fl mice colon but not Klf4ΔIS mice. Additionally, WT mice given DSS and nanoparticle/Klf4-siRNA were less sensitive to colitis and had reduced Klf4 expression and while maintaining the proliferative response in the colonic epithelium.
Conclusions
Our results indicate that Klf4 is an important mediator of DSS-induced colonic inflammation by modulating NF-κB signaling pathway and could be involved in the pathogenesis and/or propagation of inflammatory bowel disease. Thus, Klf4 may represent a novel therapeutic target in inflammatory bowel disease.
Keywords: Klf4, DSS, NF-κB
Inflammatory bowel disease (IBD) is a complex multifactorial disease1–3 and is characterized by severe inflammation of the small and/or large intestine leading to recurrent diarrhea and abdominal pain.4 Crohn’s disease and ulcerative colitis are the 2 chronic inflammatory conditions of the gastrointestinal tract that collectively identify IBD.5
Dextran sodium sulfate (DSS)–induced colitis is a well-established animal model of mucosal inflammation that has been used in the study of ulcerative colitis pathogenesis and preclinical studies.6,7 DSS is known to be directly cytotoxic to the cells at multiple levels resulting in the induction of colonic epithelium breakdown.8–13 The intestinal epithelium is a physical and immunological barrier that prevents direct contact of the intestinal mucosa with the luminal microbiota. The breakdown of the epithelium leads to enhanced uptake of toxic antigens from the gut lumen inducing mucosal and systemic inflammatory processes that then promotes colitis.14 The exposure to the gut flora leads to a significant increase in the expression of several proinflammatory cytokines, chemokines, nitric oxide, and inducible nitric oxide synthase.15–19 However, the susceptibility to DSS-induced colitis model is dependent on several factors, individually or combined: (1) the colonic epithelium and its ability to tolerate damage from DSS itself and/or from inflammatory response, (2) the ability of the intestinal mucosa to limit the inflammatory response, and (3) the response of the mucosal immune cells.14,20
The zinc finger transcription factor, Krüppel-like factor 4 (KLF4), is normally expressed in the differentiated epithelial cells of the intestine, and it may function in the switch from proliferation to differentiation.21,22 In vitro, KLF4 inhibits cell proliferation by functioning as a cell cycle checkpoint protein.21,23 In vivo, KLF4 exhibits a tumor suppressive effect on intestinal tumorigenesis24 and was found to be downregulated in a variety of human cancers.25–29 In contrast, KLF4 can also promote tumorigenesis in a context-dependent manner, such as, in the absence of p21CIP1.30,31 Additionally, Klf4 have been shown to be a proinflammatory factor because it activates epithelial cytokines in the esophageal squamous epithelium32 and mediates proinflammatory signaling in macrophages.32,33
Studying the physiological function of KLF4 in the intestinal epithelium has been impeded by the early lethality of mice with whole body deletion of Klf4.34,35 Mice with targeted deletion of Klf4 from the intestine have been previously described.36 They have altered differentiation, proliferation, migration, and positioning of intestinal epithelial cells, demonstrating an essential role for KLF4 in maintaining normal intestinal epithelial homeostasis.36 In this study, we provide the first evidence that Klf4 in the colonic epithelium plays a crucial role in promoting DSS-induced colitis by modulating NF-κB pathway inflammatory response.
MATERIALS AND METHODS
Generation of Mice with Intestine-specific Deletion of the Klf4 Gene
C57BL/6 mice carrying floxed Klf4 gene (Klf4fl/fl) were previously described.35 C57BL/6 mice carrying Cre recombinase gene under the regulation of villin promoter (Vil/Cre) were purchased from The Jackson Laboratory in Bar Harbor, ME.37 Mice lacking Klf4 in their intestinal epithelium were generated by mating Klf4fl/fl mice with Vil/Cre mice followed by backcrossing to produce mice with intestinal specific deletion of Klf4 (Klf4ΔIS). All protocols involving mouse work has been approved by the Institutional Animal Care and Use Committee of Stony Brook University and of Georgia State University, and executed according to the criteria outlined by the Guide for the Care and Use of Laboratory Animals.
DSS Treatment and Clinical Scoring
For acute colitis induction, mice were given or not 3% DSS in water ad libitium for 6 days. Mice were weighed daily and were euthanized when there was a weight loss of > 10% (around day 7). For clinical scoring and histological and immunohistochemical characterization, the large intestines were removed from age-matched littermates of Klf4 mutant mice (Klf4ΔIS) and control (Klf4fl/fl) mice on the last day of the experiment. The colon length of each mouse was measured. Stool content was examined for consistency and for occult blood using Hematocult SENSA kit (Beckman Coulter, Pasadena, CA). Clinical scoring was given after scoring system as described by Cooper et al.38
In Vivo Anti-inflammatory Effect of Encapsulated Klf4-siRNA-loaded Nanoparticles
Scrambled small interfering RNA (siRNA) (SC-siRNA) or Klf4-siRNA (Santa Cruz, Dallas, TX) were loaded into biomaterial nanoparticles (NPs) prepared as detailed before.39 In brief, 0.5 ng of siRNA in 1 mg of polylactic acid NPs that was encapsulated in hydrogel composed of alginate and chitosan, and 100 µL of the hydrogel were administered by gavage per mice a day. Mice were given or not 3% DSS in water ad libitium for 7 days and were gavage fed daily during the 7 days with the encapsulated Klf4-siRNA or SC-siRNA. Mice were weighed daily; and on the last day of treatment, the mice were euthanized and the large intestines were removed. The colon length of each mouse was measured, and clinical scoring was done as explained above.
RNA Extraction and Real-time PCR
Total RNA was extracted from colons collected from mice, RNeasy kit (Qiagen, Valencia, CA) followed by a further purification step using lithium chloride to purify the isolated RNA from all polysaccharides including DSS40 (see Text, Supplemental Digital Content 1, http://links.lww.com/IBD/A441). Real-time PCR was carried out using primers for the following: Klf4 (catalog: QT00104174; Qiagen), Il-1β,41 Il-6,41 and TNFα.41 Samples were run in triplicates on real-time thermal cycler Mastercycler ep realplex machine (Eppendorf, Hauppauge, NY).
Protein Extraction and Western Blot
Epithelial cells from the luminal side of collected colons were scraped off and collected for protein extraction and Western blot analysis. Antibodies against IκBα, Klf4, and β actin for Western blot protein detection (see Text, Supplemental Digital Content 1, http://links.lww.com/IBD/A441).
Histology, Immunohistochemistry, and Immunofluorescence
Following stool analysis, isolated colons were fixed and paraffin embedded and sectioned. Some sections were used for standard H&E staining. For immunohistochemistry (IHC), sections were stained for Klf4, BrdU, Ki67, NF-κB (p65), γH2AX, F4/80, CD11c, Ly-6G and IL-6 (see Text, Supplemental Digital Content 1, http://links.lww.com/IBD/A441).
Heterotypic Cell Adhesion Assay
In vitro assay of Jurkat E6.1 T-cell line as a model of T lymphocytes adhesion to Caco2-BBE monolayers as a model of colonic epithelium was carried out as described before42 (see Text, Supplemental Digital Content 1, http://links.lww.com/IBD/A441). Results are expressed as percent of fluorescence change compared with controls.
5-Bromo-2-deoxyuridine Labeling
Mice were injected intraperitoneally (IP) with Bromo-2-deoxyuridine (BrdU) (Sigma, St. Louis, MO) at 50 µg/g of body weight then euthanized at 4-hour postinjection.
Statistical Analysis
Statistical analysis for significance between treatments was performed by t test and one-way analysis of variance.
RESULTS
Intestine-specific Deletion of Klf4 Renders Mice Less Susceptible to DSS-induced Colitis
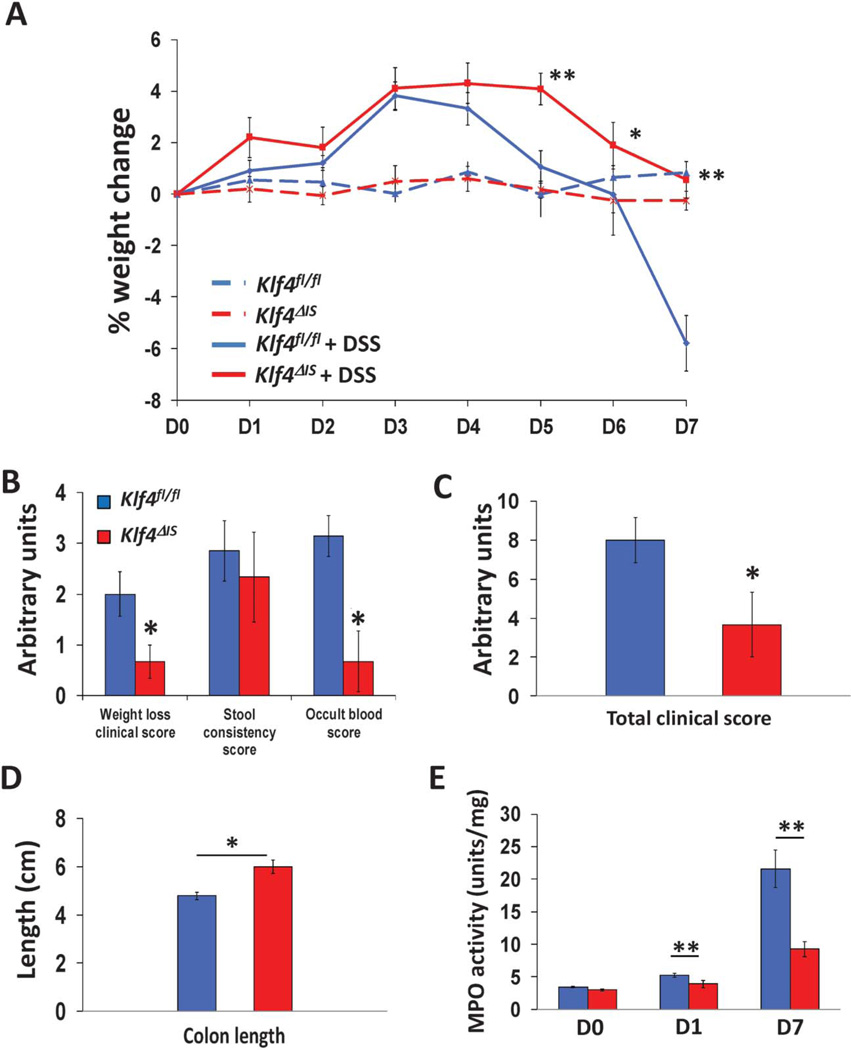
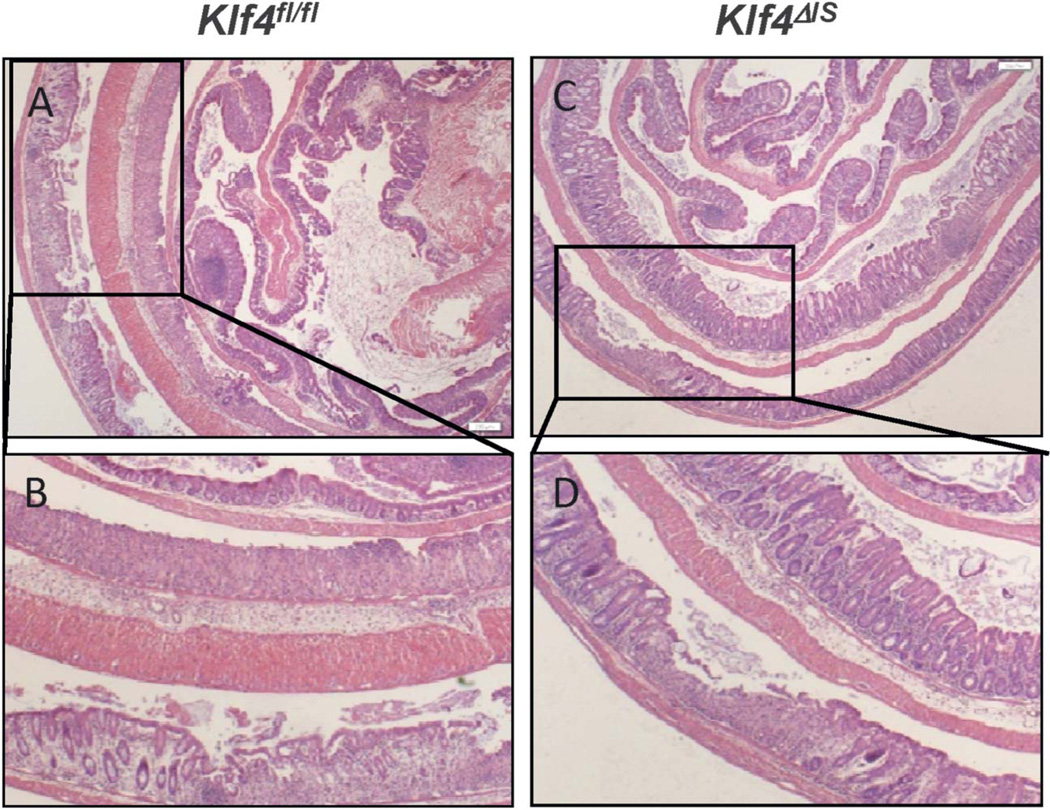
To determine the role of deleting Klf4 in DSS-induced colitis, mice with or without intestine-specific Klf4 deletion, Klf4ΔIS or Klf4fl/fl, respectively, were given regular water (control) or 3% of DSS in drinking water for 7 days. Control Klf4fl/fl and Klf4ΔIS mice had no significant weight change over the experimental period (Fig. 1A). Klf4fl/fl given DSS showed significant weight loss compared with control Klf4fl/fl mice; whereas on the other hand, Klf4ΔIS mice showed significantly less weight loss compared with DSS-treated Klf4fl/fl (Fig. 1A). Compared with DSS-treated Klf4fl/fl mice, Klf4ΔIS mice had overall significantly lower clinical score and MPO activity (Fig. 1B–E). The protection of Klf4ΔIS mice from DSS-induced colitis was further confirmed by examining H&E-stained colon sections form DSS-treated Klf4fl/fl and Klf4ΔIS mice. As shown in Fig. 2, Klf4fl/fl mice had increased loss of colonic epithelium (Fig. 2A, B), whereas Klf4ΔIS mice had minimal colonic epithelium loss and inflammation(s).
FIGURE 1.
Resistance of Klf4ΔIS mice to symptoms of DSS-induced colitis. A, Mice with intestinal deletion of Klf4 (Klf4ΔIS) showed significant resistance to weight loss after treatment with DSS compared with DSS-treated mice with intact intestinal Klf4 (Klf4fl/fl). B and C, DSS-treated Klf4ΔIS mice had significantly lower clinical scores compared with DSS-treated Klf4fl/fl mice. D, DSS-treated Klf4ΔIS mice maintained significantly longer colon lengths compared with DSS-treated Klf4fl/fl mice. E, DSS-treated Klf4ΔIS mice had significantly lower myeloperoxidase (MPO) activity compared with DSS-treated Klf4fl/fl mice. N = 8 mice per group. ±SE. *P < 0.05, **P < 0.01.
FIGURE 2.
Minimal colonic epithelium loss and inflammation in Klf4ΔIS mice after DSS treatment. A and B, H&E staining of DSS-treated Klf4fl/fl mice colon showed extensive colonic epithelium loss. C and D, H&E staining of DSS-treated Klf4ΔIS mice showed minimal loss of colonic epithelium and ulceration regions.
Colonic NF-κb Signaling Pathway Is Suppressed After DSS Treatment of Mice with Intestine-specific Deletion of Klf4 (Klf4ΔIS)
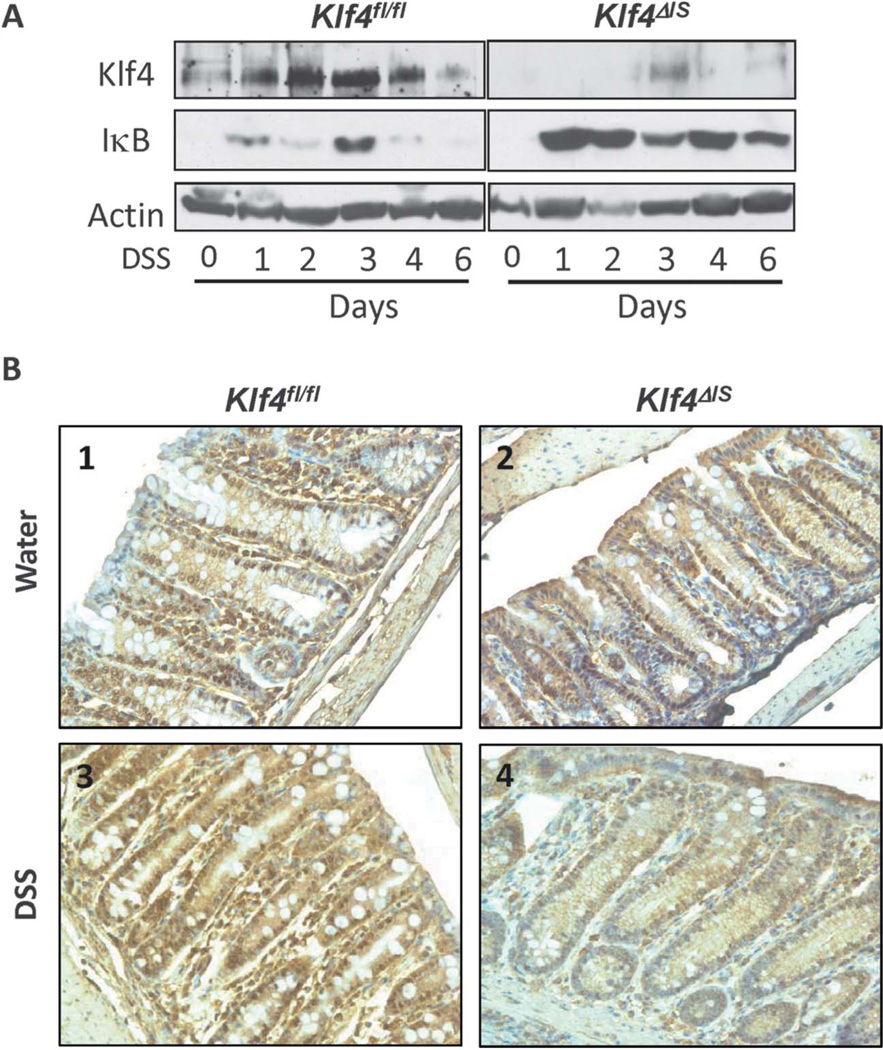
To determine the effects of DSS treatment on Klf4 expression in the colonic epithelial cells and on the inflammatory response in the presence and absence of Klf4, colonic epithelium was harvested every day for 6 days from Klf4fl/fl and Klf4ΔIS mice given DSS or not. As shown in Fig. 3A, western blot analysis of Klf4 protein level in Klf4fl/fl mice was increased in response to DSS treatment, and, as expected, Klf4ΔIS mice had no or very low levels of Klf4, even after DSS treatment. Relative Klf4 mRNA levels mirrored the change in Klf4 expression level shown in Fig. 3A (see Fig. A, Supplemental Digital Content 2, http://links.lww.com/IBD/A442). NF-κB has been shown to be activated by DSS treatment43 and to play an important role in intestinal inflammation.44–46 Additionally, Klf4 has been shown to mediate NF-κB signaling pathway. 32,33 Consistent with the previous findings, Klf4fl/fl mice had low-to-moderate increase of IκB (a suppressor of NF-κB) after DSS treatment, whereas Klf4ΔIS mice had relatively higher levels of IκB after DSS treatment, as compared with DSS-treated Klf4fl/fl mice (Fig. 3A). Staining for NF-κB (p65 subunit) showed basal nuclear localization and comparable staining level of NF-κB in the colonic epithelium in both Klf4fl/fl and Klf4ΔIS mice (Fig. 3B, 1 and 2, respectively). However, after DSS treatment, Klf4fl/fl mice had increased cytoplasmic and nuclear staining of NF-κB (Fig. 3B, 3), as compared with untreated Klf4fl/fl mice. Interestingly, Klf4ΔIS mice showed reduction both in overall staining and in the nuclear localization of NF-κB after DSS treatment (Fig. 3B, 4), as compared with both untreated Klf4fl/fl and Klf4ΔIS mice. On analyzing the mRNA levels of inflammatory cytokines Il-1β, Il-6, and TNFα after DSS treatment, Klf4ΔIS mice had significantly lower levels of these cytokines when compared with Klf4fl/fl mice (see Fig. B–D, Supplemental Digital Content 2, http://links.lww.com/IBD/A442). Additionally, quantitation of inflammatory cell infiltrates: macrophages (F4/80), lymphocytes (CD11c), granulocytes and monocytes (Ly-6G), and for IL-6 positive cells by immunofluorescence staining showed a significant increase of inflammatory cell infiltrate in Klf4fl/fl mice after DSS treatment as compared with DSS-treated Klf4ΔIS mice (see Fig. E, Supplemental Digital Content 2, http://links.lww.com/IBD/A442). Our in vitro data did not indicate whether there is direct or indirect interaction between Klf4 and NF-κB; however in a heterotypic cell adhesion assay, there was significant reduction and increase in lymphocytes (Jurkat cells) adhesion to Caco2-BBE colonic epithelial cells after suppression or overexpression of Klf4 in Caco2-BBE cells, respectively (see Fig. A, Supplemental Digital Content 3, http://links.lww.com/IBD/A443).
FIGURE 3.
Suppression of NF-κB signaling in the colonic epithelium of Klf4ΔIS mice after DSS treatment. A, Western blot showing increase in Klf4 and low levels of IκB protein levels in Klf4fl/fl mice in response to DSS treatment, whereas DSS-treated Klf4ΔIS mice showed absence of Klf4 expression and very high levels of IκB protein level. B, IHC staining of NF-κB (p65). Both Klf4fl/fl and Klf4ΔIS mice had similar staining level and localization for NF-κB (p65) at basal level (B1 and B2, respectively). After DSS treatment, Klf4fl/fl showed increased staining of NF-κB (p65) (B3), whereas Klf4ΔIS mice showed much reduced staining (B4).
DSS Treatment in WT Mice Alters the Pattern of Distribution of Klf4 Expression in the Colonic Crypts and Suppresses Proliferation
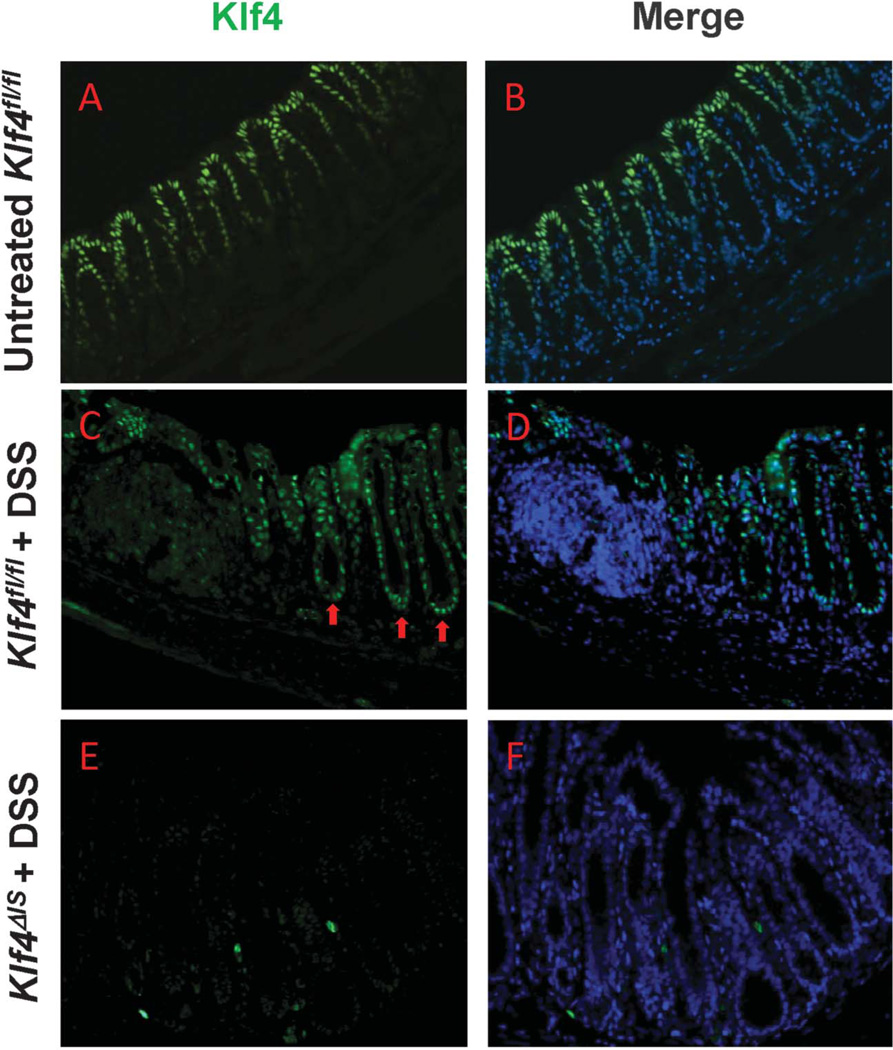
Next, we examined the effects of DSS treatment on Klf4 expression pattern in the colonic crypts. Klf4 is normally expressed in the upper one-half of the colonic epithelium, sparing the proliferating crypt cells in the lower half (Fig. 4A, B). On treatment of Klf4fl/fl mice with DSS, Klf4 expression extended to the proliferating zone of the colonic crypts (Fig. 4C, D). Klf4ΔIS mice showed complete absence of Klf4 in the colonic epithelial cells (Fig. 4E, F). Because Klf4 has been previously shown to suppress proliferation, we examined the effects of DSS treatment on proliferation in the colonic epithelium crypts by staining for both BrdU and the proliferation marker, Ki67. Klf4fl/fl mice had reduced staining for both BrdU and Ki67 (see Fig. B1–B3, Supplemental Digital Content 3, http://links.lww.com/IBD/A443), whereas Klf4ΔIS mice had more proliferation as evidenced by increased number of cells staining positive for both BrdU and Ki67 (see Fig. B4–B6, Supplemental Digital Content 3, http://links.lww.com/IBD/A443). Several stress factors are known to induce Klf4 expression such as DNA damage47,48 and oxidative stress.49,50 DSS is known to cause inflammation and oxidative stress to colonic epithelial cells,9–11,51 and the resulting oxidative stress can induce DNA damage.12,52 To confirm the presence of DNA damage in the colonic epithelium after DSS treatment, we stained for γH2AX (a marker for DNA double-strand breaks53) in Klf4fl/fl and Klf4ΔIS mice given DSS or not. Untreated Klf4fl/fl and Klf4ΔIS mice had low or no staining (see Fig. A–C and D–F, Supplemental Digital Content 4, http://links.lww.com/IBD/A444, respectively), whereas DSS-treated mice of both genotypes showed strong nuclear staining for γH2AX (see Fig. G–I and J–L, Supplemental Digital Content 4, http://links.lww.com/IBD/A444, respectively).
FIGURE 4.
DSS treatment induces the expression of Klf4 in the colonic crypts proliferative zone. Immunofluoroscence staining of Klf4 in the colonic epithelium. A and B, Expression of Klf4 in the normal colonic epithelium of untreated Klf4fl/fl mice is limited to the nonproliferating differentiated cells in the upper half of the colonic crypts. C and D, Klf4 expression is extended into the proliferative zone (arrows) of Klf4fl/fl mice after DSS treatment. E and F, Absence of Klf4 staining in the colonic epithelium of Klf4ΔIS mice.
WT Mice Treated with NP/Klf4-siRNA Are Protected from Symptoms of DSS-induced Colitis
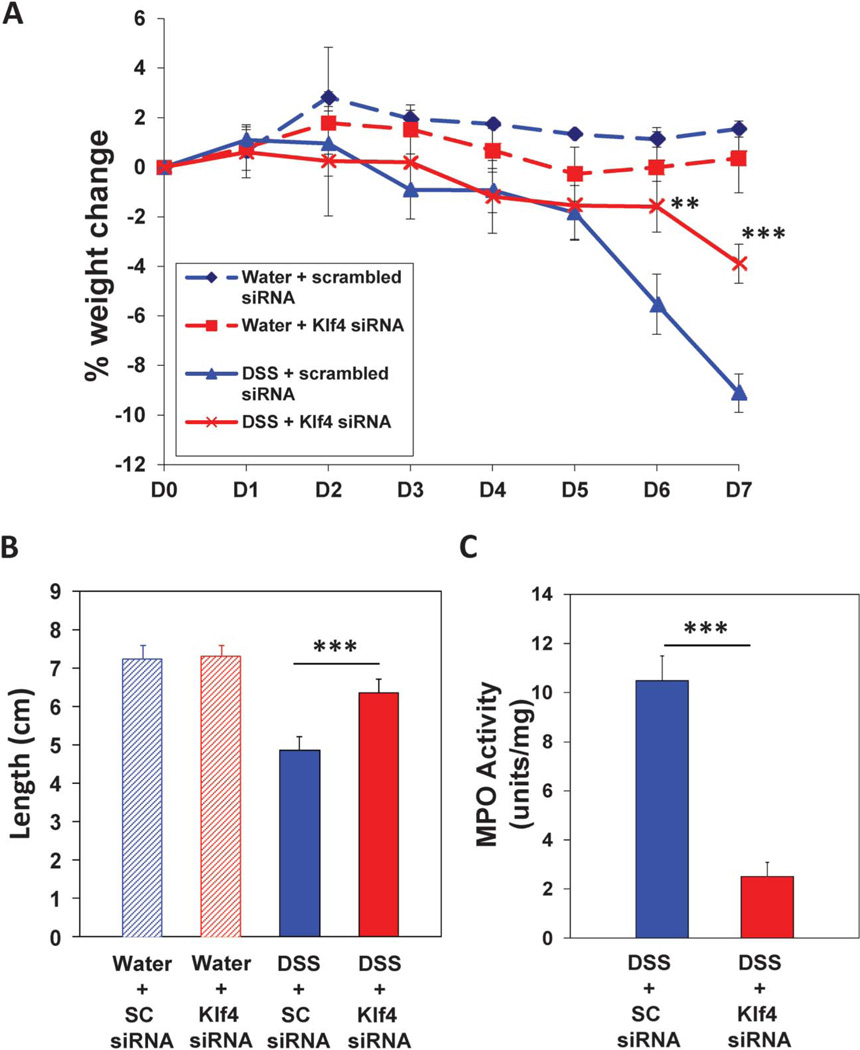
To investigate whether temporary inhibition of Klf4 expression in the colonic epithelium during DSS treatment will render the mice resistant to DSS-induced colitis, WT mice were administered, or not, biodegradable nontoxic NPs loaded with SC-siRNA (NP/SC-siRNA) or Klf4-siRNA (NP/Klf4-siRNA). First, we tested the effectiveness of Klf4-siRNA in suppressing Klf4 expression in WT mouse embryonic fibroblasts. As shown in Figure A, Supplemental Digital Content 5, http://links.lww.com/IBD/A445, Klf4 protein level was efficiently suppressed as determined by Western blot. Next, WT mice were administered DSS, or not, together with NP/SC-siRNA or NP/Klf4-siRNA. Control mice administered water plus NP/SC-siRNA or NP/Klf4-siRNA had no significant weight change over the experimental period (Fig. 5A). Mice given DSS plus NP/SC-siRNA showed significant weight loss compared with control mice given water plus NP/SC-siRNA. However, mice given DSS plus NP/Klf4-siRNA showed significant protection from weight loss compared with mice given DSS plus NP/SC-siRNA (Fig. 5A). Compared with mice treated with DSS plus NP/SC-siRNA, mice given DSS plus NP/Klf4-siRNA had significantly reduced clinical score (as indicated by longer colon length) and MPO activity (Fig. 5B, C). The protection of mice given DSS plus NP/Klf4-siRNA from DSS-induced colitis was further confirmed by examining H&Estained colon sections. Mice given DSS plus NP/SC-siRNA had increased loss of colonic epithelium and infiltration of inflammatory cells (see Fig. B(1), Supplemental Digital Content 5, http://links.lww.com/IBD/A445), whereas mice given DSS plus NP/Klf4-siRNA had minimal colonic epithelium loss and minimal inflammation (see Fig. B(2), Supplemental Digital Content 5, http://links.lww.com/IBD/A445).
FIGURE 5.
Resistance of mice treated with NP and Klf4-siRNA to symptoms of DSS-induced colitis. A, Mice treated with NP/Klf4-siRNA showed significant resistance to weight loss after treatment with DSS compared with DSS-treated mice given NP/scrambled RNA. B, DSS-treated mice given NP/Klf4-siRNA had significantly lower clinical scores compared with DSS-treated mice given NP/scrambled RNA. C, DSS-treated mice given NP/Klf4-siRNA had significantly lower MPO activity compared with DSS-treated mice given NP/scrambled RNA. N = 8 mice per group. ±SE. **P < 0.01.
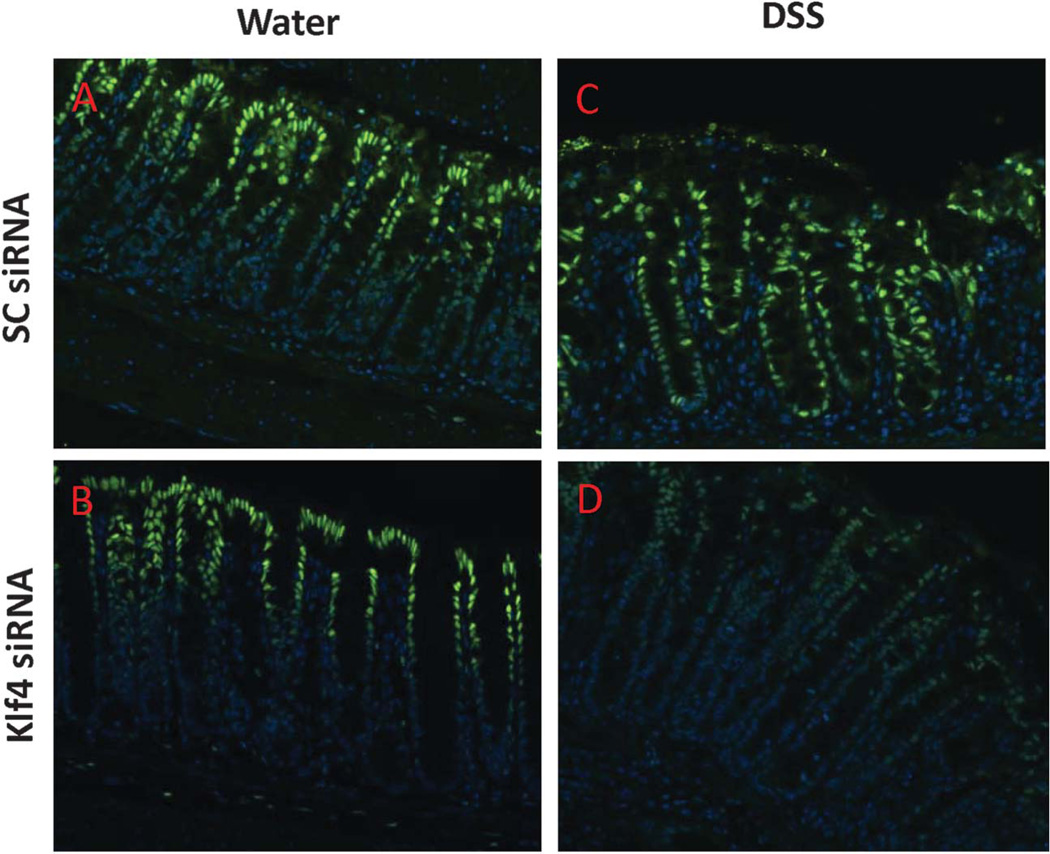
Administration of NP/Klf4-siRNA to WT Mice During DSS Treatment Results in Suppression of Klf4 Expression in the Crypt Proliferative Zone and in Sustained Proliferation
To determine the extent of Klf4 suppression in the colonic epithelium in mice given NP/Klf4-siRNA, colons from mice were administered DSS or not together with NP/SC-siRNA or NP/Klf4-siRNA were stained for Klf4. NP/SC-siRNA had no effect on Klf4 expression (Fig. 6A). In mice given NP/Klf4-siRNA, there was no visible difference in the level or the extent of Klf4 expression as compared with the mice given NP/SC-siRNA (Fig. 6B). In mice given both DSS and NP/SC-siRNA, the expression of Klf4 was increased and extended to the proliferative zone of the colonic crypt (Fig. 6C). However, in mice given both DSS and NP/Klf4-siRNA, there was an overall decrease in Klf4 expression; and importantly, the expression of Klf4 did not extend to the proliferative zone of the colonic crypt (Fig. 6D). Additionally, Western blot analysis for Klf4 from colonic protein extracts confirmed the reduction of Klf4 level in mice given both DSS and NP/Klf4-siRNA compared with mice given NP/Klf4-siRNA alone or to those given both DSS and NP/SC-siRNA (see Fig. A, Supplemental Digital Content 6, http://links.lww.com/IBD/A446). The effect of treating mice with NP/Klf4-siRNA on proliferation in the colonic crypts was also examined. Mice given water and either NP/SC-siRNA or NP/Klf4-siRNA had no differences in proliferation as indicated by Ki67 staining (see Fig. B(1) and B(2), Supplemental Digital Content, respectively). As expected, proliferation was reduced in mice given DSS and NP/SC-siRNA (see Fig. B(3), Supplemental Digital Content 6, http://links.lww.com/IBD/A446). However, proliferation was sustained in mice given DSS and NP/Klf4-siRNA (see Fig. B(4), Supplemental Digital Content 6, http://links.lww.com/IBD/A446).
FIGURE 6.
Reduced Klf4 expression in the colonic epithelium of mice treated with DSS and given NP and Klf4-siRNA. Immunoflouroscence staining for Klf4 (Klf4 [green] and Hoechst [blue]) in the colonic epithelium of mice given NP/scrambled RNA (A), NP/Klf4-siRNA (B), DSS and NP/scrambled RNA (C), or DSS and NP/Klf4-siRNA (D).
DISCUSSION
Under physiologic conditions, KLF4 is mainly expressed in the postmitotic, differentiated epithelial cells in the small intestine and colon.21,54 We have previously demonstrated that Klf4 has a crucial role in regulating intestinal epithelial cell homeostasis in vivo.36
Here, we tested the role of Klf4 in the colonic epithelium during DSS-induced colitis. Deletion of Klf4 from the colonic epithelium rendered the mice resistant to DSS-induced colitis (Figs. 1 and 2). The reduced inflammatory response obsereved in Klf4ΔIS mice (Fig. 1) indicates that Klf4 plays a role in mediating the proinflammatory signaling in response to DSS treatment. Several lines of work suggest that NF-κB activation actively contributes to the development and maintenance of intestinal inflammation. NF-κB was found to be activated in mucosal cells of patients with inflammatory bowel disease44 and to play an important role in intestinal inflammation.44–46 It has been previously shown that NF-κB is activated by DSS treatment and plays a role in mediating DSS-induced colitis,43 and the inhibition of NF-κB activity ameliorated intestinal inflammation in mouse models of colitis.45,55,56 These studies also suggest that NF-κB inhibition can have therapeutic effects. However in these studies, it was not clear whether the pathogenic effect of NF-κB was due to NF-κB activation in epithelial or in mucosal immune cells.20 Additionally, Klf4 has been shown to be a crucial mediator of NF-κB signaling pathway.32,33 Our results indicate that under normal conditions, Klf4 might not play a role in the NF-κB signaling pathway since both the level of IκB protein and the level and localization of NF-κB p65 were similar between untreated Klf4fl/fl and Klf4ΔIS mice groups (Fig. 3). However, compared with DSS-treated Klf4fl/fl mice, the increase in IκB level and the reduction in NF-κB p65 staining in DSS-treated Klf4ΔIS mice (Fig. 3) suggest that in the colonic epithelium the role of Klf4 in mediating NF-κB signaling is limited to the inflammatory response. The results also suggest that Klf4 might play a role in the regulation of IκB expression during the inflammatory response because the IκB level was greatly increased in DSS-treated Klf4ΔIS mice compared with DSS-treated Klf4fl/fl mice. Taken together, our results strongly suggest a central role for Klf4 in mediating the proinflammatory response through NF-κB pathway, at the intestinal epithelium level, in response to DSS treatment, and that it is required for NF-κB to maintain its nuclear localization in response to inflammatory signals. It is not yet clear whether the Klf4 mediation of NF-κB pathway in the colonic epithelium is by a direct33 or an indirect interaction32 of the 2 factors.
The increased proliferative response observed in DSS-treated Klf4ΔIS mice (see Fig., Supplemental Digital Content 3, http://links.lww.com/IBD/A443) compared with suppressed proliferation in DSS-treated Klf4fl/fl mice and given the role of Klf4 as antiproliferation, highlights the importance of colonic epithelium regeneration as a factor in reducing the sensitivity to DSS-induced colitis. Klf4 expression is known to be induced by oxidative stress50,57 and in response to DNA damage.47,48 DSS has been shown to cause oxidative stress to colonic epithelial cells9–11,51 that can induce DNA damage.12,52 Here, we have demonstrated the occurrence of DNA double-strand breaks in the colonic epithelial cells after DSS treatment in both Klf4fl/fl and Klf4ΔIS mice (see Fig., Supplemental Digital Content 4, http://links.lww.com/IBD/A444). Our data suggest that the increased expression of Klf4 and the extension of its expression to the proliferative zone of the colonic crypts are at least in part because of oxidative stress-induced DNA damage in the colonic epithelium after DSS treatment.
For clinical relevance, the effect of temporarily suppressing Klf4 in the colonic epithelium during the course of DSS-induced colitis was then tested. The siRNA knockdown of Klf4 at the mRNA level has been chosen for this study because of the lack of drugs known to specifically suppress Klf4 expression. In vitro, Klf4-siRNA showed high efficiency in suppressing Klf4 expression in mouse embryonic fibroblasts as shown in Figure A, Supplemental Digital Content 5, http://links.lww.com/IBD/A445. For in vivo use, to overcome the low penetration of naked siRNA across cell membranes,58 we resorted to NPs that have previously shown high potential in binding and delivering siRNA.39,41,59–61 Here, we used biodegradable noncytotoxic NPs for targeting of Klf4-siRNA with the aim to inhibit Klf4 expression in the colonic epithelium. This method has been used successfully before to target the colonic epithelial cells for load delivery.39,61,62 There was no significant difference in clinical scoring between WT mice given water and NP/SC-siRNA or NP/Klf4-siRNA (Fig. 5) indicating that treatment with NP/Klf4-siRNA has no negative side effects on the mice. The susceptibility of WT mice to DSS-induced colitis when given DSS plus NP/SC-siRNA (see Fig. 5 and Fig. B(1), Supplemental Digital Content 5, http://links.lww.com/IBD/A445) indicates that giving NP/SC-siRNA confers no protection to the mice. However, the resistance of WT mice to DSS-induced colitis when given DSS plus NPs/Klf4-siRNA, (see Fig. 5 and Fig. B(2), Supplemental Digital Content 5, http://links.lww.com/IBD/A445) is in line with the findings observed with DSS-treated Klf4ΔIS mice and strongly confirming a role for Klf4 in mediating colonic inflammation.
It is worth noting that there was a marked reduction in Klf4 expression in mice given DSS plus NP/Klf4-siRNA when compared with WT given NP/Klf4-siRNA only (see Fig. 6 and Fig. A, Supplemental Digital Content 6, http://links.lww.com/IBD/A446), in particular in the nonproliferating zone of the colonic epithelium (Fig. 6). This could be attributed to an enhanced uptake of the NP/Klf4-siRNA by the colonic epithelium as a result of DSS treatment because DSS has been shown to cause nano-lipocomplexes in colonic epithelial cells and affect cell membrane permeability13; thus, possibly enhancing NPs uptake by colonic epithelial cells. Also when compared with WT mice given DSS plus NP/SC-siRNA, mice given DSS plus NP/Klf4-siRNA had no Klf4 expression extending to the proliferative zone (Fig. 6), indicating a more robust suppression of Klf4 expression in the proliferative zone than in the nonproliferative zone. This could be attributed, in addition to the DSS-effect mentioned above, to the fact that proliferating cells uptake NPs much more efficiently than nonproliferating cells.63,64 Additionally, mice given DSS plus NP/Klf4-siRNA also had sustained proliferation (see Fig. B, Supplemental Digital Content 6, http://links.lww.com/IBD/A446) consistent with previous findings that Klf4 is antiproliferative.
In conclusion, results from our study indicate a role for Klf4 in the colonic epithelium in promoting colonic inflammation and strongly suggest that it does so by modulating the activity of NF-κB pathway.
Acknowledgments
Supported in part by grants from the National Institutes of Health DK052230 and CA084197 (V.W.Y.), K01-DK-097192 (H.L.) and R01-DK-064711 (D.M.).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ibdjournal.org).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself. 2010;1:299–309. doi: 10.4161/self.1.4.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen Z, Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol. 2004;11:195–204. doi: 10.1080/17402520400004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 7.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ni J, Chen SF, Hollander D. Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996;39:234–241. doi: 10.1136/gut.39.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardieu D, Jaeg JP, Cadet J, et al. Dextran sulfate enhances the level of an oxidative DNA damage biomarker, 8-oxo-7,8-dihydro-2’-deoxyguanosine, in rat colonic mucosa. Cancer Lett. 1998;134:1–5. doi: 10.1016/s0304-3835(98)00228-6. [DOI] [PubMed] [Google Scholar]
- 10.Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 11.Damiani CR, Benetton CA, Stoffel C, et al. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol. 2007;22:1846–1851. doi: 10.1111/j.1440-1746.2007.04890.x. [DOI] [PubMed] [Google Scholar]
- 12.Westbrook AM, Schiestl RH. Atm-deficient mice exhibit increased sensitivity to dextran sulfate sodium-induced colitis characterized by elevated DNA damage and persistent immune activation. Cancer Res. 2010;70:1875–1884. doi: 10.1158/0008-5472.CAN-09-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laroui H, Ingersoll SA, Liu HC, et al. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7:e32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger B, Bajaj-Elliott M, MacDonald TT, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 16.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck PL, Xavier R, Wong J, et al. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137–G147. doi: 10.1152/ajpgi.00309.2003. [DOI] [PubMed] [Google Scholar]
- 18.Beck PL, Li Y, Wong J, et al. Inducible nitric oxide synthase from bone marrow-derived cells plays a critical role in regulating colonic inflammation. Gastroenterology. 2007;132:1778–1790. doi: 10.1053/j.gastro.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Naito Y, Takagi T, Handa O, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 20.Wullaert A, Bonnet MC, Pasparakis M. NF-kappa B in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrett-Sinha LA, Eberspaecher H, Seldin MF, et al. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Johns DC, Geiman DE, et al. Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaleb AM, McConnell BB, Nandan MO, et al. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi S, Ohnami S, Laub F, et al. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308:251–256. doi: 10.1016/s0006-291x(03)01356-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang N, Liu ZH, Ding F, et al. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966–970. doi: 10.3748/wjg.v8.i6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao W, Hisamuddin IM, Nandan MO, et al. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23:395–402. doi: 10.1038/sj.onc.1207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanai M, Wei D, Li Q, et al. Loss of Kruppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12:6395–6402. doi: 10.1158/1078-0432.CCR-06-1034. [DOI] [PubMed] [Google Scholar]
- 29.Wei D, Gong W, Kanai M, et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 30.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- 31.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 32.Tetreault MP, Wang ML, Yang Y, et al. Klf4 overexpression activates epithelial cytokines and inflammation-mediated esophageal squamous cell cancer in mice. Gastroenterology. 2010;139:2124–2134. e9. doi: 10.1053/j.gastro.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feinberg MW, Cao Z, Wara AK, et al. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 34.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 35.Katz JP, Perreault N, Goldstein BG, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghaleb AM, McConnell BB, Kaestner KH, et al. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol. 2011;349:310–320. doi: 10.1016/j.ydbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 38.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 39.Laroui H, Theiss AL, Yan Y, et al. Functional TNF alpha gene silencing mediated by polyethyleneimine/TNF alpha siRNA nanocomplexes in inflamed colon. Biomaterials. 2011;32:1218–1228. doi: 10.1016/j.biomaterials.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viennois E, Chen F, Laroui H, et al. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes. 2013;6:360. doi: 10.1186/1756-0500-6-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson DS, Dalmasso G, Wang L, et al. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charrier L, Yan Y, Nguyen HT, et al. ADAM-15/metargidin mediates homotypic aggregation of human T lymphocytes and heterotypic interactions of T lymphocytes with intestinal epithelial cells. J Biol Chem. 2007;282:16948–16958. doi: 10.1074/jbc.M700158200. [DOI] [PubMed] [Google Scholar]
- 43.Marrero JA, Matkowskyj KA, Yung K, et al. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am J Physiol Gastrointest Liver Physiol. 2000;278:G797–G804. doi: 10.1152/ajpgi.2000.278.5.G797. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, et al. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 46.Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 47.Cullingford TE, Butler MJ, Marshall AK, et al. Differential regulation of Kruppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: effects of endothelin-1, oxidative stress and cytokines. Biochim Biophys Acta. 2008;1783:1229–1236. doi: 10.1016/j.bbamcr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nickenig G, Baudler S, Muller C, et al. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. FASEB J. 2002;16:1077–1086. doi: 10.1096/fj.01-0570com. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Yang JJ, Kim YS, et al. An 8-gene signature, including methylated and down-regulated glutathione peroxidase 3, of gastric cancer. Int J Oncol. 2010;36:405–414. [PubMed] [Google Scholar]
- 50.Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka T, Kohno H, Suzuki R, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong MY, Turner ND, Carroll RJ, et al. Differential response to DNA damage may explain different cancer susceptibility between small and large intestine. Exp Biol Med (Maywood) 2005;230:464–471. doi: 10.1177/153537020523000704. [DOI] [PubMed] [Google Scholar]
- 53.Bassing CH, Chua KF, Sekiguchi J, et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci U S A. 2002;99:8173–8178. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McConnell BB, Ghaleb AM, Nandan MO, et al. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibata W, Maeda S, Hikiba Y, et al. Cutting edge: the IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 56.Dave SH, Tilstra JS, Matsuoka K, et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the Ikappa B kinase inhibitor NEMO binding domain peptide. J Immunol. 2007;179:7852–7859. doi: 10.4049/jimmunol.179.11.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W, Geiman DE, Shields JM, et al. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliv Rev. 2007;59:134–140. doi: 10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Toub N, Bertrand JR, Tamaddon A, et al. Efficacy of siRNA nanocapsules targeted against the EWS-Fli1 oncogene in Ewing sarcoma. Pharm Res. 2006;23:892–900. doi: 10.1007/s11095-006-9901-9. [DOI] [PubMed] [Google Scholar]
- 60.Fattal E, Vauthier C, Aynie I, et al. Biodegradable polyalkylcyanoacrylate nanoparticles for the delivery of oligonucleotides. J Control Release. 1998;53:137–143. doi: 10.1016/s0168-3659(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 61.Laroui H, Geem D, Xiao B, et al. Targeting intestinal inflammation with CD98 siRNA/PEI-loaded nanoparticles. Mol Ther. 2014;22:69–80. doi: 10.1038/mt.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laroui H, Dalmasso G, Nguyen HT, et al. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138:843–853. e1–e2. doi: 10.1053/j.gastro.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt A, Bunte A, Buddecke E. Proliferation-dependent changes of proteoglycan metabolism in arterial smooth muscle cells. Biol Chem Hoppe Seyler. 1987;368:277–284. doi: 10.1515/bchm3.1987.368.1.277. [DOI] [PubMed] [Google Scholar]
- 64.Xia T, Rome L, Nel A. Nanobiology: particles slip cell security. Nat Mater. 2008;7:519–520. doi: 10.1038/nmat2213. [DOI] [PubMed] [Google Scholar]