Abstract
Embodied cognition offers an approach to word meaning firmly grounded in action and perception. A strong prediction of embodied cognition is that sensorimotor simulation is a necessary component of lexical-semantic representation. One semantic distinction where motor imagery is likely to play a key role involves the representation of manufactured artifacts. Many questions remain with respect to the scope of embodied cognition. One dominant unresolved issue is the extent to which motor enactment is necessary for representing and generating words with high motor salience. We investigated lesion correlates of manipulable relative to non-manipulable name generation (e.g., name a school supply; name a mountain range) in patients with nonfluent aphasia (N=14). Lesion volumes within motor (BA4) and premotor (BA6) cortices were not predictive of category discrepancies. Lesion symptom mapping linked impairment for manipulable objects to polymodal convergence zones and to projections of the left, primary visual cortex specialized for motion perception (MT/V5+). Lesions to motor and premotor cortex were not predictive of manipulability impairment. This lesion correlation is incompatible with an embodied perspective premised on necessity of motor cortex for the enactment and subsequent production of motor-related words. These findings instead support a graded or ‘soft’ approach to embodied cognition premised on an ancillary role of modality-specific cortical regions in enriching modality-neutral representations. We discuss a dynamic, hybrid approach to the neurobiology of semantic memory integrating both embodied and disembodied components.
Keywords: Semantic Memory, Aphasia, Lesion Correlation, Category Specificity, Embodied Cognition
Investigations of semantic category structure highlight divergence in the ways that sensory and motor feature knowledge support word and object meaning. For example, pencils tend to evoke gestural motor programs linked to writing, whereas elephants and mountains are more commonly linked to visual attributes (Chao & Martin, 1999; Crutch & Warrington, 2003; Farah & Feinberg, 2000; Farah & McClelland, 1991; McRae, Cree, Seidenberg, & McNorgan, 2005; Samson & Pillon, 2003). One of the longest running questions in neuroscience involves how the brain integrates and cohesively represents the many fragments of information that comprise object concepts (i.e., the binding problem). Two somewhat mutually exclusive theoretical perspectives offer answers. The first holds that knowledge is stored in a propositional form that is largely abstracted from perception. The second perspective, known as embodied cognition, holds that meaning is inextricably grounded in the sensorimotor system.
Proponents of embodied cognition have argued that semantic features are decomposed and stored either within or proximal to brain regions dedicated to perception of the same features. Object knowledge is then recomposed online through mental operations such as sensorimotor simulation whereby the brain enacts perceptuomotor patterns that are most salient for a given concept (Barsalou, 1999, 2008; Barsalou, Simmons, Barbey, & Wilson, 2003; Gallese & Lakoff, 2005; Martin, Ungerleider, & Haxby, 2000). For example, holding and slicing likely contribute to the representation of knives, whereas color is more diagnostic for fruits and vegetables (Binder & Desai, 2011; Buxbaum, Kyle, Grossman, & Coslett, 2007; Chao & Martin, 1999, 2000; Crutch & Warrington, 2003; Samson & Pillon, 2003; Samson, Pillon, & De Wilde, 1998). The ecology of object structure and our own egocentric interactions with objects give rise to distinct patterns of feature salience and weighting of modality-specific knowledge.
One semantic feature of intense recent interest in cognitive neuroscience is motor manipulability (i.e., unique patterns of hand-related grasp and gesture). Embodied semantics theories predict that twisting, pinching, and other fine-motor aspects of manipulation support the meanings of many classes of manufactured artifacts and verbs. The strongest embodied approaches to word meaning hold that such motor simulation processes are essential for meaning in the absence of redundant detail from other modalities (Barsalou, 1999, 2008; Gallese & Lakoff, 2005; Meteyard, Cuadrado, Bahrami, & Vigliocco, 2012). In sharp contrast, theories of semantic memory premised exclusively on conceptual abstraction hold that manipulability is critical for execution of an action but not necessary for the representation of an action-related concept. A fundamental challenge has involved reconciling these views. Vast bodies of empirical evidence now exist in favor of both (Chatterjee, 2010). Consequently, many theorists have merged toward to a centrist position where object concepts share some combination of embodied and disembodied representation (Damasio, 1989; Dove, 2009; Garcea & Mahon, 2012; Mahon & Caramazza, 2008, 2009; Reilly & Peelle, 2008; Reilly, Peelle, Antonucci, & Grossman, 2011). Ongoing refinement of these hybrid approaches has involved specifying what is necessary versus sufficient regarding the contribution of sensorimotor simulation to word and object meaning.
Many hybrid semantic models today share a common ancestor proposed in the late 1980’s by Damasio and colleagues (1989). The Convergence Zone framework was premised on a dynamic interplay between distributed modal regions (vision, olfaction, audition) that ultimately feed a series of local polymodal convergence zones. Damasio hypothesized that feature-based information is stored in primary sensory areas in an analog format that is progressively refined into an abstract set of conjunctions within convergence zones. Thus, one might delineate modal cortex as veridical and embodied, whereas convergence zones are propositional and abstract. Importantly, these embodied and disembodied components of knowledge representation were hypothesized to communicate through reciprocal/bidirectional connectivity for the purpose of conceptual retroactivation.
In the decades following its proposal, the convergence zone framework has undergone significant refinement. Several competing hybrid models of semantic memory exist today (Binder & Desai, 2011; Binder, Desai, Graves, & Conant, 2009; Lambon Ralph, Pobric, & Jefferies, 2008; Lambon Ralph, Sage, Jones, & Mayberry, 2010; Mahon & Caramazza, 2003, 2008, 2009, 2011; Patterson, Nestor, & Rogers, 2007; Rogers & McClelland, 2004). Most of these approaches share similarities with respect to the presence of local amodal hubs coordinating activity with radiating modality-specific spokes. However, model architectures tend to diverge with respect to the nature of the convergence zones (e.g., where they are, how many there are, what they do, how they are controlled) and the degree of flexibility between local and distributed components(Kiefer & Pulvermüller, 2012; Willems & Casasanto, 2011). Recent efforts have focused on specifying the putative role(s) of modal spokes and amodal hubs within the semantic network.
Polymodal Convergence Zones, Hubs, and Spokes in Semantic Networks
Neuroscience generally accepts the notion that the brain has evolved a range of highly modular and distributed subsystems. The retinotopic organization of the primary visual cortex and analogous tonotopic organization of regions of the primary auditory cortex offer prime examples of segregated subsystems of the brain that are dedicated to modality-specific processing. One special property of these early sensory regions is the veridical manner in which ecology is mapped directly onto the cortex. In groundbreaking work, Hubel and Wiesel, for example, demonstrated the presence of specialized neurons (simple, complex, and hypercomplex) in the primary visual cortices of cats and macaques that selectively fire in the context of edge boundaries and specific line orientations within the animal’s external field of view (Hubel & Wiesel, 1962, 1977; Hubel, Wiesel, & Stryker, 1978).
Direct links between the external world and the human brain are less apparent as sensory information is conveyed via efferent pathways to a series of polymodal convergence zones, or hubs. According to Sporns and colleagues (2007), hubs can be classified as one of two subtypes. Provincial hubs act as nodes within a neural network that receives afferent connections from mostly within a particular modular subsystem (e.g., vision). In contrast, connector hubs act as nodes that link anatomically distinct, often remote modules (e.g., vision, audition, olfaction) that otherwise show little or no direct structural connectivity. Cognitive neuropsychology has long been interested in the effects of catastrophic damage to the connector hubs that support semantic memory. Lambon Ralph, Patterson, and colleagues have proposed perhaps the most influential neurobiological theory of semantic memory to date, implicating connector hubs. The Hub and Spoke model of semantic cognition is premised upon the analogy of a wheel with one core hub radiating outward to a series of modality-specific (modular) spokes. Lambon Ralph et al. have argued that this single putative hub is situated bilaterally in a region of high centrality within the anterior temporal lobes (temporal pole, anterior fusiform gyrus) (Binney, Embleton, Jefferies, Parker, & Lambon Ralph, 2010; Lambon Ralph et al., 2008; Lambon Ralph et al., 2010; Pobric, Jefferies, & Lambon Ralph, 2007; Visser, Jefferies, Embleton, & Lambon Ralph, 2012; Visser & Lambon Ralph, 2011). Under this model, a key function of the anterior temporal lobes involves binding information from disparate modalities (e.g., language, vision) into cohesive concepts. Upon this view, the conceptual representations ultimately formed within hubs are thought to be abstract and amodal (Coccia, Bartolini, Luzzi, Provinciali, & Lambon Ralph, 2004; Hoffman, Jones, & Ralph, 2012; Hoffman & Lambon Ralph, 2013; Patterson, 2007; Patterson et al., 2007; Rogers et al., 2006; Rogers, Hodges, Lambon Ralph, & Patterson, 2003; Rogers et al., 2004). Thus, the existing Hub-Spoke model essentially disembodies concepts in favor of abstract, amodal object representations.
Despite the popularity and utility of the hub-spoke approach, the neuroscience of hub organization remains fraught with controversy and alternate views. Numerous approaches to semantic representation are premised upon the absence of a central organizing principle. Fully distributed views of semantic representation, for example, gained sway under Wernicke and Freud in the nineteenth century (Freud, 1891; Gage & Hickok, 2005; Reilly & Martin, in press; Wernicke, 1874). These early distributed semantics approaches, along with contemporary refinements, hold that object concepts reflect the co-activation of a series of distributed multi-regional cell assemblies (Martin, 2007; Martin, Haxby, Lalonde, Wiggs, & et al., 1995; Martin et al., 2000; Martin, Wiggs, Ungerleider, & Haxby, 1996; Warrington & Shallice, 1984). In support of this distributed, no hub perspective, Kandel (2006) criticized hublike theories on philosophical grounds as representing a return to Cartesian mind-body dualism. That is, the concept of a hub places a master control center akin to a homunculus inside of the brain. Kandel quotes vision researcher, Semir Zeki (p. 304):
At first glance the problem of integration may seem quite simple. Logically it demands nothing more than that all the signals from the specialized visual areas be brought together, to ‘report’ the results of their operations to a single master cortical area…. If all the visual areas report to a single master cortical area, who or what does that single area report to?… The problem is not unique to the visual image or the visual cortex. Who, for example, listens to the music provided by the master auditory area, or senses the odour provided by the olfactory cortex? It is in fact pointless pursuing this grand design. For here one comes across an important anatomical fact, which may be less grand but perhaps more illuminating in the end: there is no single cortical area to which all other cortical areas report exclusively, either in the visual or in any other system.
Dissent with respect to the very presence of hubs reflects the inherent methodological challenge posed by elucidating hub structure. As a macroscale behavioral discipline, hubs fit well within many theoretical models advanced within cognitive neuropsychology. Yet, from a microscale neurophysiological perspective, very little remains known about the ways that hubs bind, conjugate, integrate, and repackage information (Ding, Van Hoesen, Cassell, & Poremba, 2009; Olson, Plotzker, & Ezzyat, 2007). Against this backdrop, we outline a theory of semantic representation involving both hubs and spokes informed by macroscale anatomical and patient-based behavioral evidence.
A Hybrid Approach to Hub-Spoke Interactivity in Semantic Networks
We recently proposed an approach to semantic memory premised upon interactivity between spokes and hubs. More specifically, we hypothesized that one function of a hub is to strip modality-specific features (such as manipulability) of their sensorimotor and linguistic salience, bind information from disparate modalities, and store such information as sparse primitives through a process that is analogous to episodic memory consolidation (Reilly, Peelle, et al., 2011). Damasio argued in a similar vein, remarking that “convergence zones do not represent a refined representation…. They know about neural activity in the feeding [modal] cortices and can promote further cortical activity by feedback/retroactivation.” (p 46, 1989). Under this view, hubs are crucial for object representation, whereas the contribution of spokes will vary by task demands, feature salience, and feature redundancy (for discussions of flexibility in embodied cognition see also Hoenig, Sim, Bochev, Herrnberger, & Kiefer, 2008; Kiefer & Pulvermuller, 2012; Willems & Casasanto, 2011).
Various imaging techniques, behavioral paradigms, and lesion models yield highly disparate results regarding the necessity for embodied cognition in semantic representation (Chouinard & Goodale, 2010; Devlin et al., 2002). A relatively stable finding from fMRI studies, for example, is that exposure to modality-salient words (e.g., thunder) tends to engage corresponding modality-specific regions of cortex (e.g., auditory association cortex) (Binder et al., 2009; Kellenbach, Brett, & Patterson, 2001; Kellenbach, Hovius, & Patterson, 2005; Kemmerer, Rudrauf, Manzel, & Tranel, 2012). One possibility is that such activation is critical for conceptual representation. An alternate account holds that such modal activity instead reflects backpropagation from convergence zones and that such activation may in fact be epiphenomenal and/or related to entirely different processes (e.g., orthosyntactic processing) (de Zubicaray, Arciuli, & McMahon, 2013; Postle, Ashton, McFarland, & de Zubicaray, 2013; Postle, McMahon, Ashton, Meredith, & de Zubicaray, 2008). Constraints imposed by the temporal resolution of fMRI have made this question difficult to answer. Pulvermüller, Hauk and colleagues have investigated the time course of motor activation from linguistic input and shown through EEG/MEG paradigms that word recognition for arm-, leg-, and face-related verbs (e.g., pick, kick, lick) evokes somatotopic activation of motor regions in a temporal window beginning 250 milliseconds after word onset (Hauk & Pulvermüller, 2004; Pulvermüller, Härle, & Hummel, 2001). These studies demonstrate that modal cortex is activated almost simultaneous to word onset, a phenomenon that supports the radically embodied claim that conceptual representations are identical to sensorimotor representations.
Many neuroimaging modalities have been criticized for specificity, signal dropout over key temporal lobe regions, and the use of behavioral paradigms that only nominally engage the semantic system (e.g., lexical decision) (Lambon Ralph et al., 2008; Lambon Ralph et al., 2010; Sabsevitz, Medler, Seidenberg, & Binder, 2005; Simmons, Reddish, Bellgowan, & Martin, 2010). Confidence in the functional imaging literature could be bolstered by converging support from neuropsychological case studies. Yet, such evidence has been sparse, if not contradictory. A compelling test of embodied cognition might involve assessing patients with motor programming impairments that impact both the execution and imagery of skilled, sequential hand movements. Nature offers just such a lesion model in the form of post-stroke motor programming deficits (e.g., ideomotor and ideational apraxia). Patients with apraxia typically experience difficulties in gesturing and/or demonstrating appropriate use of implements that require fine-grained hand movements (e.g., scissors, staplers, hammers) (Buxbaum et al., 2007; Gonzalez Rothi, Ochipa, & Heilman, 1997). Despite the often profound impairments incurred in object use and offline pantomimed gesture of tools, patients with apraxia typically demonstrate preserved knowledge of object function for the implements they cannot gesture (Buxbaum, Schwartz, & Carew, 1997; Garcea & Mahon, 2012). Parallel work on the relation between limb apraxia and language impairment has revealed similar findings. A general finding is that apraxia is not typically associated with category specific impairments for naming of manipulable objects (Rosci, Chiesa, Laiacona, & Capitani, 2003).
In contrast to an isolated limb apraxia, category specific semantic impairments have been reported in patients with various forms of aphasia (Capitani, Laiacona, Mahon, & Caramazza, 2003). A very small minority of patients with aphasia have manifested category specific semantic impairments either for small, manipulable artifacts (Arévalo et al., 2007; Warrington & McCarthy, 1987) or for the broader category of living relative to nonliving entities (Hillis & Caramazza, 1991; Sacchett & Humphreys, 1992; Warrington, 1975). Nonfluent aphasia in particular may offer a compelling lesion model for assessing the effects of a manipulation impairment on language comprehension and expression. It is not uncommon for a left middle cerebral artery stroke to simultaneously impact BA44/45 (Broca’s Area), insular cortex, premotor (BA6) and motor cortices (BA4) (Arévalo et al., 2007; Baldo, Wilkins, Ogar, Willock, & Dronkers, 2011; Dronkers, 1996; Dronkers, Wilkins, van Valin, Redfern, & Jaeger, 2004).
The brain injury that produces nonfluent aphasia also commonly results in co-morbid hemiparesis, hemiplegia, or apraxia of the right (dominant) hand, especially in the context of damage to the left ventral premotor cortex (Kan, Kable, Van Scoyoc, Chatterjee, & Thompson-Schill, 2006). Therefore, nonfluent aphasia might present a sensitive lesion model for assessing the impact of manipulation impairment on lexical-semantic processing. Arévalo and colleagues recently examined this possibility among a heterogeneous group of patients with chronic aphasia using voxel-based lesion symptom mapping (2007). Patients in this study showed advantages for non-manipulable objects across several distinct language tasks. However, the lesion data were inconclusive, and aphasia subtype did not moderate the effect of manipulability. Patients with fluent posterior aphasia were equally likely to show manipulability impairment as their nonfluent counterparts, thus weakening the apparent link between motor and category-specific language impairment (for a similar finding in progressive nonfluent aphasia see Reilly, Rodriguez, Peelle, & Grossman, 2011). At the conclusion of their work, Arévalo and colleagues identified several important follow-up questions and methodological considerations we further consider here.
Aims of the current work
Much remains unclear about the nature of embodied cognition with respect to the dimension of object manipulability. We ask the question of what is necessary versus sufficient regarding the roles of spokes (e.g., motor cortex) and connector hubs (e.g., anterior temporal lobe (ATL), posterior middle temporal gyrus (pMTG) in generating exemplars for manipulable relative to non-manipulable semantic categories. We extend the work of Arévalo and colleagues and focus our contrasts exclusively on patients with chronic nonfluent stroke aphasia. We correlated behavioral performance with regional distributions of brain damage using voxel-based lesion symptom mapping (VLSM) (Bates et al., 2003; Dick et al., 2007; Saygin, Dick, Wilson, Dronkers, & Bates, 2003). This technique can yield powerful group level inference about voxels that are necessary for executing a particular task.
We hypothesize that modal spokes (e.g., premotor cortex) likely serve an ancillary role in conceptual representation by enriching sparse amodal representations (Reilly & Peelle, 2008; Reilly, Peelle, et al., 2011; Reilly, Rodriguez, et al., 2011). More specifically, we hypothesize that modal regions that support enactment and execution of manipulation (e.g., premotor cortex, motor cortex) are not necessary for the durable representation of manipulable objects.1 In contrast, the integrity of convergence zones located primarily in bilateral temporal cortex is crucial for all categories of semantic knowledge.
METHOD
This research was conducted with ethical standards in accord with Insitiutional Review Boards of the University of Florida and the Malcom Randall Veterans Affairs Medical Center in Gainesville, Florida.
Participants
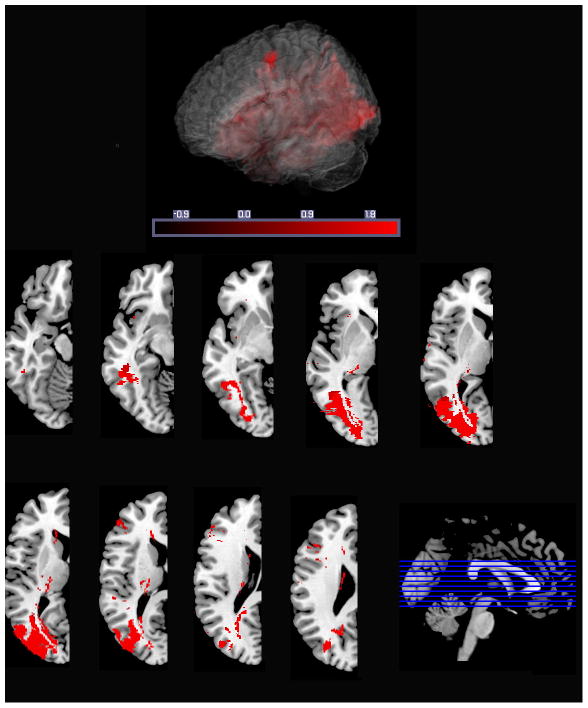
Participants included 14 patients with chronic aphasia (>6 months post) resulting from left hemisphere stroke with radiological confirmation of posterior frontal lobe involvement. Figure 1 represents an aggregate image of the lesions overlaid upon a single normalized brain.
Figure 1.
Lesion summary overlap image
Note: Lesion overlap map of 14 patients on a multi-slice axial brain image. ‘Hotter’ areas of bright white indicate regions of greater lesion overlap.
Participants were right hand dominant, native English speakers with a mean age of 68.8 years (SD=12.6) and mean years of education of 13.7 years (SD=2.0). The sex distribution was 6f/8m. Relevant psycholinguistic, neuropsychological and demographic data appear in Table 1. Patients provided written informed consent at the Brain Rehabilitation Research Center of the Malcolm Randal Veterans Affairs Medical Center in Gainesville, Florida or the Brooks Center for Rehabilitation Studies in Jacksonville, Florida.
Table 1.
Demographic and neuropsychological data
| ID | Age | Edu | Post | Sex | WAB | BNT | PPVTrs | PPVTss | Speech Rate | Speech Productivity | Audible Struggle |
|---|---|---|---|---|---|---|---|---|---|---|---|
| s01 | 79 | 12 | 1.13 | F | 51.2 | 8 | 175 | 85 | 1.57 | 0.55 | 2.00 |
| s02 | 52 | 12 | 8.26 | M | 74.4 | 43 | 186 | 83 | 2.58 | 0.53 | 4.67 |
| s04 | 53 | 12 | 5.14 | M | 65.8 | 27 | 197 | 89 | 2.73 | 0.17 | 4.67 |
| s06 | 62 | 18 | 7.04 | F | 57.8 | 16 | 196 | 89 | 1.85 | 0.50 | 4.00 |
| s07 | 80 | 14 | .975 | F | 78 | 40 | 202 | 96 | 3.56 | 0.55 | 3.67 |
| s08 | 55 | 14 | 0.90 | M | 67.1 | 31 | 215 | 104 | 2.61 | 0.30 | 4.00 |
| s11 | 68 | 14 | 7.25 | M | 67.8 | 28 | 198 | 91 | 2.63 | 0.81 | 4.33 |
| s12 | 63 | 18 | 1.25 | F | 66.6 | 11 | 218 | 111 | 2.22 | 0.86 | 3.67 |
| s13 | 50 | 14 | 1.46 | M | 40.7 | 5 | 199 | 91 | 1.91 | 0.14 | 4.00 |
| s14 | 61 | 12 | 1.12 | M | 75.8 | 30 | 172 | 81 | 2.63 | 0.36 | 5.00 |
| s15 | 68 | 16 | 1.30 | F | 69.4 | 43 | 220 | 115 | 2.26 | 0.30 | 3.00 |
| s16 | 59 | 14 | 3.14 | M | 74.5 | 30 | 186 | 83 | 1.41 | 0.29 | 3.33 |
| s17 | 42 | 12 | 0.92 | F | 82.5 | 29 | 168 | 76 | 3.46 | 0.56 | 4.67 |
| s19 | 64 | 12 | 9.37 | M | 90 | 37 | 196 | 89 | 1.82 | 0.65 | 2.00 |
| MEANS | 61.14 | 13.86 | 3.52 | n/a | 68.69 | 27 | 194 | 92 | 2.38 | 0.47 | 3.79 |
Note: Western Aphasia Battery Aphasia Quotient (WAB AQ) is a composite score of 10 WAB subtests that represents aphasia severity; a score <93.8 is considered aphasic (Kertesz, 1982). BNT represents the total correct of 60 items from the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). PPVTrs represents the total correct of 228 items from the Peabody Picture Vocabulary Test-IV. PPVTss represents patients’ scaled score on the PPVT-IV (M = 100, SD = 15) (Dunn & Dunn, 1997). Speech rate is defined as the number of discrete syllables produced per second as participants completed a narrative speech sample. Speech productivity represents the ratio of total speaking time without silences over total speaking time with silences during a given interval. Audible struggle represents the average rating on articulatory struggle by three independent speech-language pathologists evident during one’s production (1 = most struggle, 5 = least struggle) (Park et al., 2011).
Quantification and classification of language dysfluency
An additional recruitment criterion for this group was that they must have received diagnostic classification as nonfluent by a licensed speech language pathologist upon initial screening. Once patients were initially recruited, we subsequently conducted additional analyses of language fluency through the application of a novel scaling procedure and normative study (see Park et al., 2011). Narrative speech samples were obtained from each patient from a 3–5 minute sample of the Cookie Theft picture description subtest of the Boston Diagnostic Examination of Aphasia (Goodglass & Kaplan, 1983). We then asked three expert, blind raters to evaluate these 14 recordings, along with 47 additional samples from a mixture of patients with temporal lobe pathologies (e.g., Wernicke’s Aphasia, Alzheimer’s Disease) and age-matched controls. Raters assigned a categorical (fluent/nonfluent) judgment to each narrative sample. We then conducted follow-up analyses using logistic regression to determine perceptual attributes (e.g., audible struggle, rate, restarts) that discriminated fluent from nonfluent samples. All of the patients included in the lesion mapping had narrative samples that were rated as nonfluent by consensus. Attributes of the language samples (rate, struggle) appear in Table 1.
Behavioral Measures & Stimulus Norming
For the lesion mapping analyses to follow, we correlated a continuous measure of behavioral performance with a categorical measure of brain pathology. The behavioral measure involved accuracy for naming exemplars drawn from a range of orally presented semantic categories (e.g., name an ice cream flavor, name a school supply). The key variable of interest for this generative naming task was the salience of object manipulability among the category cues. That is, one condition included highly manipulable categories (e.g., name a school supply) and the other involved categories with low or no manipulation component (e.g., name a color). This task required the respondent to generate an appropriate response in the absence of additional perceptual cues (e.g., contextual cues within pictures), engaging sensorimotor and motor imagery relative to prescribed tasks such as picture naming or word-picture matching (for empirical comparison of confrontation naming versus generative fluency naming see Riva, Nichelli, & Devoti, 2000).
We isolated a series of high and low manipulability semantic category cues via a normative study using the online crowdsourcing program Amazon Mechanical Turk (for validity disussions see Buhrmester, Kwang, & Gosling, 2011; Mason & Suri, 2012). We administered surveys to anonymous respondents from the United States who self-identified as native English speakers. Participants (N=55, mean age=30.9 yrs) rated a set of 73 semantic categories using a 7-point Likert scale, guided by the following instructions (adapted from Salmon, McMullen, & Filliter, 2010):
The way we interact with some objects requires us to make skilled hand movements. For example, using chopsticks requires careful grasp and manipulation using our our dominant hand. Read each phrase below. Rate the extent to which each phrase would require the use of skilled hand movements. A rating of VERY HIGH would indicate extensive use of fine, skilled movements of your hands, while NOT AT ALL would require no skilled movement. Of course, there will also be phrases which fall within the two extremes. Some may require a lot of skilled hand movement and would therefore be HIGH or MODERATELY HIGH, while others would require only little and would be LOW or MODERATELY LOW.
We eliminated all respondents who completed the entire survey in under four minutes and/or who did not complete the entire survey. This produced a loss of 26% (19 subjects) of the original participant pool. We then averaged subjects’ ratings to derive a mean manipulability score for each item. Our goal was to isolate a set of high and low manipulability semantic categories and also to eliminate any potentially neutral or ambiguous items. We did this by isolating the tails of the distribution (i.e., high and low manipulability only) and omitting the central region corresponding to manipulability ratings between −0.5>z<.0.5. These trimming procedures resulted in a set of high- (N=19) and low- (N=22) manipulability semantic categories constituting the behavioral measure for the lesion mapping analyses to follow (see Appendix A). (Rorden, 2007).
Experimental control for studies of single word naming typically involve matching words on a variety of lexical, sublexical, and semantic variables (e.g., frequency, age-of-acquisition, imageability, name agreement). The generative naming paradigm we employed involved multiword utterances (carpenter’s tool) prefaced by a carrier phrase (i.e., Name a…). Thus, the nature of presenting multi-word stimuli preclude matching across all variables traditionally controlled in studies of single word processing. Therefore, we assessed item difficulty across the manipulable and non-manipulable categories using two methods. First, we conducted a control study where healthy participants (N=11, mean age=61.2 years) generated a single exemplar using a speeded naming format for each semantic category. We collected reaction times as a proxy measure of difficulty.
We first standardized stimulus delivery by creating digital audio recordings of the category cues in Appendix A. At test, participants were seated in quiet lab at a PC laptop computer running E-Prime Professional software. Participants were then instructed to generate a single spoken response for each category cue as “quickly and accurately as possible”. Trials were structured such that participants first viewed an attention fixation cross for 250ms, followed by a 500ms whitescreen, and then the onset of each auditory stimulus (e.g., “name an ice cream flavor..”). After a brief familiarization sequence composed of five category cues, E-Prime presented the true category cues in a fixed pseudorandom order with a timeout of 2500ms.
In addition to implicit reaction times for the speeded generation task, we also asked controls to explicitly rate the subjective difficulty of each category cue using a 7-point Likert scale guided by the following instructions:
For most of us it is very easy to come up with the name of an animal. However, generating the name of European capitol might be more difficult. Please rate the difficulty of the following categories on the scale below, where 1 represents Very Easy and 7 represents Very Difficult.
Appendix A reflects reaction time and subjective difficulty ratings for each category cue. Reaction times did not differ across the manipulable (RT Mean=1.02s, s.d.=.28) relative to the non-manipulable category cues (RTmean=1.15s, s.d.=.37) [t(39)=1.25, p>.05] Average ratings of subjective difficulty (on a 1–7pt scale) were also similar across the manipulable (rated difficulty mean=1.52, s.d.=.34) relative to the non-manipulable category cues (rated difficulty mean=1.51, s.d.=.40) [t(39)=.04, p>.05]. These matching statistics indicate that among a healthy control sample of comparable age to the aphasia sample, the item pools were roughly matched in difficulty and response latencies.
Behavioral Testing Procedures
Patients with aphasia were first seated in the presence of an examiner in a quiet testing room. After a brief familiarization, each patient was asked to verbally generate a single exemplar corresponding to each category probe. Participants were given two different opportunities to present an exemplar for each probe (i.e., the entire category list was presented twice in random order). The examiner provided no semantic or phonological response cueing. Responses were scored offline (correct/incorrect) by a blinded rater. Phonemic distortions were counted as correct if the production shared at least one syllable overlap with a target (e.g., ‘umbrellug’ was counted as ‘umbrella’). Omissions (e.g., name a river → I don’t know) and semantic errors (e.g., name a river → bank) were scored as incorrect. We converted response accuracy to proportion correct in order to facilitate comparisons across the unbalanced item pools for manipulable and nonmaniplable objects.
Neuroimaging Methods
From each participant we collected a high resolution, 3-dimensional, T1-weighted structural magnetic resonance image on a 3-Tesla scanner (Philips, Inc.) situated at the University of Florida using the following imaging parameters: Voxel Size = 1 mm3 ; Slice Gap=0; Number of slices=160 ; Echo Time (TE)=3.7ms; Repetition Time (TR)=8.1ms; Matrix Size= 240×240mm, Field of View=240×240×160mm; Sagittal Slice Acquisition Plane.
We first manually mapped each participant’s lesion in native space, making a binary judgment (±lesion) at each voxel. The distinction between healthy versus diseased tissue in a post-stroke brain is not always readily apparent, especially for diffuse lesions. Therefore, prior to lesion tracing, we made several methodological decisions. The first issue involved discriminating lesion from ventricle. All patients experienced asymmetric expansion of the left ventricle. We did not trace enlarged ventricles as part of the primary lesion; however, lesions often abutted and/or joined the ventricle. In order to demarcate a lesion-ventricle boundary we imposed a bounding rule such that the disconnected margins of a ventricle formed endpoints of an imaginary border. A second methodological consideration involved treatment of Wallerian degeneration, a phenomenon characterized by the degradation of remote axonal projections (white matter tracts) subsequent to the destruction of cortical cell bodies (gray matter) (Waller, 1850). Since this damage was contiguous with and resulted directly from the primary brain injury, we marked visible degeneration as lesion.2
We employed a multi-step lesion tracing procedure, first manually marking lesions volume-by-volume in a descending axial plane in conjunction with a board certified neuroradiologist while simultaneously referencing the coronal and sagittal views in the ITK-Snap image processing program (Yushkevich et al., 2006). We then conducted a second consensus review with minor adjustments on lesion margins. Upon reaching group consensus on lesion margins, we warped the original brains and their respective lesion masks into stereotaxic space (MNI-space) using the non-linear FNIRT algorithm of FSL (http://www.fmrib.ox.ac.uk/fsl/).
We conducted standard spatial preprocessing by first re-aligning each brain along the axes of its respective anterior-posterior commissures, followed by segmentation into distinct tissue types (i.e., gray matter, white matter, cerebrospinal fluid, skull). We then normalized each brain by first applying each patient’s respective lesion tracing as a mask with the goal of preventing tissue distortion during the warping process. After this initial step, we applied the normalization parameters to the masks themselves. These procedures generated a set of 14 normalized lesions that were amenable to group level comparisons.
Lesion Symptom Correlation Method
We used MRIcron’s nonparametric lesion mapping (NPM) function to correlate a binary categorical measure of brain pathology (±lesion) with a continuous measure of behavioral performance. We added a constant (+100) to ensure that all scores were positive and then correlated each patient’s manipulability score with their lesion distributions.
In addition to voxel-wise measures, we also computed groupwise volumetric correlations to determine whether lesion size was predictive of category-specific impairment. We first derived lesion volumes in native space using the original lesion tracings. We then derived total intracranial volume (TIV) for each patient by segmenting distinct tissue classes and then summating the volumes of gray matter, white matter, and cerebrospinal fluid using Statistical Parametric Mapping (SPM)-08 software (Wellcome Trust Centre for Neuroimaging, 2009). We then derived a % Lesion measure for each patient by dividing lesion volume by total intracranial volume.
For the lesion mapping analyses to follow, we first obtained accuracies for generative naming of the manipulable and non-manipulable categories independently. We then derived a single absolute difference score for each patient by subtracting their percent accuracy for non-manipulable categories from manipulable categories. Key for the interpretation of a category specific impairment is that it must involve a “disproportionate or even selective impairment for one semantic category compared to other semantic categories” (see pg. 29, Mahon & Caramazza, 2009). Thus, a relative difference in performance across conditions marks the phenomenon. In addition to delineating the magnitude of category discrepancies, difference scores offer several additional advantages. First, when considering a single condition in isolation (e.g., manipulable items, verbs, etc.), it is impossible to know whether performance was tainted by a confounding variable (e.g., pure word deafness). In most cases, difference scores alleviate this concern by assuming that the error variance is spread evenly across conditions (e.g., patients do not typically experience pure word deafness exclusively for one condition) (for the exception see Franklin, Howard, & Patterson, 1994). Another significant advantage of the difference score approach is that it can cope with apparent floor or ceiling effects. Consider, for example, a fictional study with five patients who performed tasks A & B. Task A accuracy was 0%, 0%, 0%, 0%, and 0%. Task B accuracy was 50%, 60%, 70%, 80%, and 90%. The fact that there is no variance posed by task A’s floor effect prohibits any further analysis. Yet, comparison of the two conditions reveals a meaningful difference.
It is important to note that the performance of our patient sample was not compromised by ceiling or floor effects for either the manipulable or non-manipulable conditions. Therefore, it would be feasible to report two distinct VLSM analyses, correlating lesion distribution with manipulable object generation in the first analysis and lesion distribution with non-manipulable object generation in the second analysis. One might then conduct posthoc subtraction analyses of the resultant lesion maps under the assumption that the two conditions are orthogonal and/or linear. Rather than aggregating these two analyses, however, we opted to correlate their difference. Our rationale was multifold. Foremost, the difference score was the best reflection of our construct of interest, i.e., a relative performance difference for manipulable relative to non-manipulable category. Second, difference scores quantify a construct that is fundamentally distinct and conceptually special relative to that gleaned by a posthoc cognitive subtraction.
We submitted difference scores (manip-nomanip) to the lesion mapping analysis using a nonparametric t-test with 2000 permutations. We applied a cluster threshold of 8 contiguous voxels and thresholded to maintain a false discovery rate of p<.05.
Results
Behavioral Performance
Table 4 reflects behavioral performance. With respect to group means, generative naming accuracy was nearly identical for manipulable relative to non-manipulable category probes [paired t(13)=.14. p=.89]. Manipulable category probes elicited correct responses with an average accuracy of 61.09% (s.d.=21.78), whereas non-manipulable category probes elicited an average accuracy of 61.54% (s.d.=17.64).
Table 4.
Generative naming accuracy
| Subject | Raw Manip (of 38) | Raw No-Manip (of 44) | %Acc Manip | %Acc NoManip | % Difference (Manip-NoManip) |
|---|---|---|---|---|---|
| s01 | 8 | 20 | 21.1 | 45.5 | −24.4 |
| s02 | 29 | 31 | 76.3 | 70.5 | 5.9 |
| s04 | 15 | 28 | 39.5 | 63.6 | −24.2 |
| s06 | 30 | 31 | 78.9 | 70.5 | 8.5 |
| s07 | 31 | 36 | 81.6 | 81.8 | −0.2 |
| s08 | 18 | 23 | 47.4 | 52.3 | −4.9 |
| s11 | 29 | 30 | 76.3 | 68.2 | 8.1 |
| s12 | 14 | 17 | 36.8 | 38.6 | −1.8 |
| s13 | 13 | 9 | 34.2 | 20.5 | 13.8 |
| s14 | 28 | 30 | 73.7 | 68.2 | 5.5 |
| s15 | 29 | 35 | 76.3 | 79.5 | −3.2 |
| s16 | 19 | 22 | 50.0 | 50.0 | 0.0 |
| s17 | 33 | 33 | 86.8 | 75.0 | 11.8 |
| s19 | 29 | 34 | 76.3 | 77.3 | −1.0 |
We correlated aphasia severity with task performance to discern whether global language impairment was predictive of a category advantage for either manipulable or non-manipulable object generation. Both the manipulation and no-manipulation categories showed strong correlations with aphasia severity as assessed by WAB AQ [Rmanip=0.66; Rno-manip=0.73] and picture naming ability as assessed by the Boston Naming Test [Rmanip=0.70; Rno-manip=0.80]. In addition, generative naming accuracy for manipulable items strongly correlated with the generation of non-manipulable items across subjects [R=.85]. Table 2 reflects a correlation matrix detailing relations among numerous lesion and neuropsychological characteristics.
Table 2.
Summary of Intercorrelations for Scores on the WAB, BNT, PPVT, Speech Fluency, and Lesion Size
| WAB AQ | BNT | PPVT | Speech Rate | Speech Prod. | Audible Struggle | % Brain Lesion | BA 44 | BA 45 | BA 04 | BA 06 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WAB AQ | --- | .78** | −.15 | .41 | .37 | .02 | −.44 | −.32 | .09 | −.66* | −.60* |
| BNT | --- | .08 | .44 | −.02 | .11 | −.07 | .07 | .34 | −.43 | −.41 | |
| PPVT | --- | −.06 | .02 | −.18 | .50 | .37 | .29 | .16 | .18 | ||
| Speech Rate | --- | .12 | .58* | −.07 | −.24 | .31 | −.35 | −.50 | |||
| Speech Prod. | --- | −.18 | −.47 | −.18 | −.07 | −.53 | −.50 | ||||
| Audible Struggle | --- | .24 | −.27 | .06 | −.04 | −.11 | |||||
| % Brain Lesion | --- | .32 | .40 | .57* | .53 | ||||||
| BA 44 | --- | −.02 | .54* | .56* | |||||||
| BA 45 | --- | −.22 | −.31 | ||||||||
| BA 04 | --- | .96** | |||||||||
| BA 06 | --- |
p < .05
p < .01
In reference to Table 2, several additional bivariate correlations bear mention. Motor speech performance as assessed by speech rate [R=.41, p>.05] and audible struggle [R=.02] were uncorrelated with aphasia severity as measured by the WAB-Aphasia Quotient. In addition, speech rate [R=.18] and struggle [R=.40, p>.05] (markers of speech praxis impairment) were uncorrelated with manipulable object impairment.
Individual Differences in Performance
Previous reports of category specific naming impairment have relied heavily upon single case studies. This approach often involves exhaustive testing and neuropsychological characterization of a small number of often highly idiosyncratic patients. In contrast, lesion mapping studies tend to smooth individual variability in favor of a group solution. One potential pitfall of the lesion mapping approach is that important individual variability is potentially overlooked, inflating the potential for Type 2 error. Inspection of Table 4 reveals two patients who show moderate discrepancies for generating manipulable relative to non-manipulable category exemplars. S01 generated manipulable objects with an average accuracy of 21% relative to an accuracy of 46% for non-manipulable objects (24% difference). S04 generated manipulable objects with an average accuracy of 40% relative to an accuracy of 64% for non-manipulable objects (24% difference). One question with respect to individual differences regards whether S01 and S04 manifest category specific deficits rather than representing the impaired tail of a normal probability distribution. We reviewed previous case studies reporting category specific effects for manipulable relative to non-manipulable objects, as well as a range of additional dissociations to discern whether S01 and S04 constitute category specific outliers.
In some of the most cited case studies of category specific impairment, Warrington, Shallice, and McCarthy reported patients such as V.E.R. who performed in the range of 90% accuracy for naming living things (flowers and animals) relative to 63% correct for non-living things (Warrington & McCarthy, 1983). Since these early reports of double dissociations, advances in statistical methology coupled with increasing rigor have raised the bar for what qualifies as a category specific impairment. To name a few, in the two cases presented by Hillis and Caramazza (1991), patient J.J. was 77 to 100% correct for animals vs. 8–33% correct for non-animal categories across oral naming, written naming, spoken word/picture verification, printed word/picture verification, whereas patient P.S. was more impaired with animal and vegetable categories vs. other categories especially in oral and written naming (35~40% vs. 76~90%). In another often cited case study, patient E.W. was 100% in naming fruits and vegetables (24/24), 92% correct for the artefacts, but only 34% correct for animal pictures (Caramazza & Shelton, 1998). Similarly, patient K.R. named animals with an accuracy of 30% correct relative to near perfect naming for other control categories (Hart & Gordon, 1992). Finally, patient C.W. named man-made artefacts with 35% accuracy relative to 95% naming accuracy for natural kinds (Sacchett & Humphreys, 1992).
In light of the previously described case studies, we conclude that S01 and S04 did not show discrepancies that constitute a classical dissociation. Thus, the 24% difference we observed in S01 and S04 likely falls within the range of normal variability among a sample of patients that showed roughly comparable performance for manipulable relative to non-manipulable category cues.
Lesion-Behavior Correlations
Table 5 reflects correlations of manipulability scores with lesion correlations. Figure 2 depicts a rendering of these statistical maps. Peak lesion distributions were primarily evident in a posterior distribution of brain regions primarily impacting posterior middle temporal gyrus, and angular gyrus. Several recent neuroimaging and neuromodulation studies have demonstrated the supramodal and/or crossmodal response nature of these regions, suggesting that they are viable candidates for large-scale connector hubs (Bonner et al., 2009; Fairhall & Caramazza, 2013; Papeo et al., 2014; Turken & Dronkers, 2011).
Table 5.
Manipulable minus non-manipulable anatomical correlation
| Antomical Region | N-Vox | % Lesion | Sum>0 | Mean>0 | Max | Max-X | MaxY | MaxZ |
|---|---|---|---|---|---|---|---|---|
| Middle Occipital | 9673 | 37.2 | 23940.1 | 0.92 | 2.22 | −20 | −85 | −4 |
| Superior Parietal | 2035 | 12.3 | 4958.8 | 0.30 | 2.22 | −18 | −70 | 40 |
| Inferior Occipital | 827 | 11.0 | 1903.8 | 0.25 | 2.22 | −25 | −85 | −8 |
| Superior Occipital | 779 | 7.2 | 1769.8 | 0.16 | 2.22 | −18 | −91 | 2 |
| Middle Temporal | 2017 | 5.1 | 17559.5 | 0.45 | 2.22 | −48 | −37 | −13 |
| Angular | 420 | 4.5 | 1875.1 | 0.20 | 2.22 | −50 | −66 | 26 |
| Precuneus | 1068 | 3.8 | 2549.5 | 0.09 | 2.22 | −15 | −53 | 23 |
| Inferior Parietal | 646 | 3.3 | 2629.4 | 0.14 | 2.24 | −61 | −34 | 43 |
| Inferior Temporal | 822 | 3.2 | 3957.4 | 0.15 | 2.22 | −51 | −45 | −15 |
| Pars Triangularis | 613 | 3.0 | 5255.7 | 0.26 | 2.24 | −52 | 35 | 14 |
| Precentral | 841 | 3.0 | 2941.0 | 0.10 | 2.24 | −49 | 14 | 35 |
| Caudate | 216 | 2.8 | 1867.3 | 0.24 | 4.06 | −17 | −10 | 26 |
| Middle Frontal | 906 | 2.3 | 2982.9 | 0.08 | 2.24 | −45 | 39 | 17 |
| Thalamus | 197 | 2.3 | 1239.6 | 0.14 | 3.10 | −18 | −16 | 13 |
| Calcarine | 400 | 2.2 | 912.3 | 0.05 | 2.22 | −17 | −96 | −2 |
| Cuneus | 267 | 2.2 | 677.5 | 0.06 | 4.06 | −19 | −55 | 23 |
| Frontal Operculum | 181 | 2.2 | 1434.6 | 0.17 | 2.24 | −35 | 15 | 27 |
| Lingual | 155 | 0.9 | 372.9 | 0.02 | 2.22 | −20 | −82 | −5 |
Note: This correlation reflects the association of a composite behavioral score [manipulable – non-manipulable] with lesion volumes of interest. Values reflect only left hemisphere regions whose lesion correlation exceeded 1% of the total volume of a particular anatomical area. N Voxels >0 represents the raw number of voxels in a given anatomical region with a statistical score greater than zero; % Lesioned Vox represents the percentage of a specific anatomical region demonstrating a significant correlation with the behavioral measure; Mean Statistic: represents the average statistical value of all of the voxels within a specified region; Max Statistic represents the maximal observed statistical significance for a voxel within a specified region; Peak MNI Coordinates represents the x,y,z coordinate point of the voxel of maximum significance within a region.
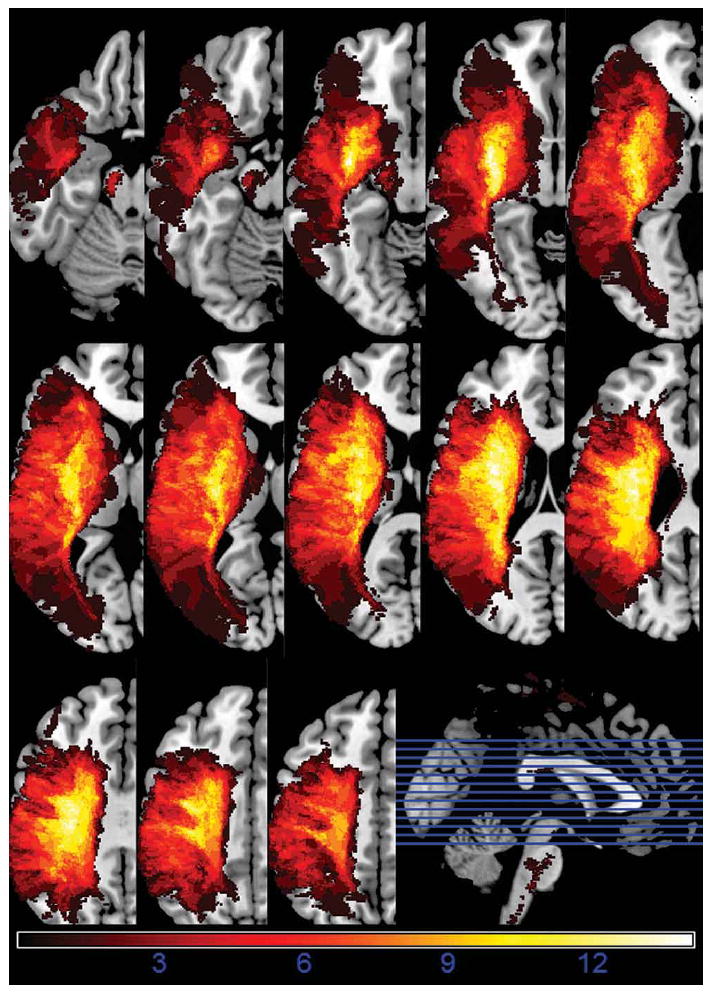
Figure 2.
Lesion correlation rendering
Note: The top view represents the aggregated lesion map overlaid upon the CH2better template brain of MRIcronGL. Slices (below) represent the lesion map overlaid upon a series of ascending left hemisphere axial images. The orthogonal view provides landmarks for these axial slices.
In addition to AG and pMTG, damage to anterior and superior portions of occipital cortex were also predictive of manipulable object generation impairments. Peak lesion correlations were also found in anterior projections of the visual cortex (i.e., BA18, encompassing V4 and V5). Visual inspection of the lesion maps suggested that this damage impacted a portion of the dorsal visual pathway implicated in motion perception (MT/V5). In the absence of functional localizer scans, it is impossible to assert that damage to Area MT/V5 proper was predictive. However, we plotted the peak MNI coordinates for several recent fMRI and postmortem cytoarchitectonic studies that demarcated Area MT/V5 (Bosco, Carrozzo, & Lacquaniti, 2008; Dumoulin et al., 2000; Kolster, Peeters, & Orban, 2010; Malikovic et al., 2007; Orban et al., 2003; Wilms et al., 2005). All of these peaks fell within the results cluster of our lesion map for that region. Therefore, we will proceed with caution in discussing localization and the potential contribution of MT/V5 to the manipulable object naming impairment in our patient sample.3
Small swaths of inferior frontal cortex lesions were predictive of category discrepancies (e.g., pars triangularis). However, we observed no significant correlations with manipulable object generation and motor (BA4) or ventral premotor (BA6) damage.
None of the volumetric measures (i.e., %Lesion, lesion volume, total intracranial volume) significantly correlated with generating either manipulable or non-manipulable category exemplars [p>.05, all bivariate correlations].
GENERAL DISCUSSION
We observed the most robust correlations for generating exemplars of manipulable objects in regions of the brain comprising classical polymodal convergence zones [pMTG and AG]. In addition to hub damage, lesions to a projection of the dorsal visual pathway (Area MT/V5+) were also predictive of category discrepancies for generating manipulable objects. Area MT/V5+ is commonly regarded as a modality specific spoke implicated in the perception of motion and haptic imagery (Beauchamp, Lee, Haxby, & Martin, 2002; Kable & Chatterjee, 2006; Pelphrey, Morris, Michelich, Allison, & McCarthy, 2005; Wu, Morganti, & Chatterjee, 2008). In contrast, lesions of the motor (BA4) and ventral premotor (BA6) cortex were not significant predictors of manipulable object naming impairment.
Our observed lesion correlations are grossly consistent with the dominant hub-spoke perspective on semantic organization but with two important exceptions. First, the primary hubs revealed in our analyses were the angular gyrus (AG) and the posterior middle temporal gryus (pMTG) but not the left anterior temporal lobe. Second, the lack of an observed correlation with motor and premotor regions is inconsistent with a strong embodied approach premised upon a necessary role of motor simulation in the semantic representation of manipulable objects. To follow, we interpret these findings in favor of a multiple hub approach to semantic representation involving dynamic interactivity between a series of hubs with high centrality driving more distally situated modal spokes.
Hubs & the architecture of semantic representation
Prominent models of episodic memory hold that during the process of long-term consolidation, memories are ultimately stripped of non-essential detail and stored within the lateral temporal neocortex in a fragmented manner (McClelland, McNaughton, & O’Reilly, 1995). This sparsification process, initiated during hippocampal encoding, offers the advantage of parsimonious storage of only the most essential details of an event memory. As these sparse fragments of event memories are recycled, we re-experience the original event in a nuanced form. Thus, reliving an event memory involves a fluid reconstructive process, operating from a downsampled version of the original event. One novel possibility is that a similar online reconstructive process occurs in semantic memory. That is, object concepts are stored within hubs in a sparse, unrefined form that are enriched through the retroactivation of a series of modal spokes (see also Reilly & Peelle, 2008; Reilly, Peelle, et al., 2011; Reilly, Rodriguez, et al., 2011). This hypothesis holds that hubs are essential for binding long-term semantic knowledge and that spokes act as a supporting cast (see also Papeo et al., 2014). As stated by Dove (2009), “perceptual representations could be a consequence of thinking rather [than] the vehicles of thinking” (pg. 416).
We have argued that hubs are necessary and that spokes play an ancillary role in enriching sparse representations. This does, however, beg the question of whether spokes are necessary for the integrity of semantic representation. The answer depends upon the object concept in question. Concepts are often inherently endowed with a rich multimodal array of sensory and linguistic features (Rosch, 1973). Such diversity offers redundancy in that the semantic system may compensate for the selective loss of one information channel (e.g., BA6 = enactment of manipulation via grasp) by invoking other preserved spokes (e.g., BA18=perception of motion) (Sartori, Gnoato, Mariani, Prioni, & Lombardi, 2007; Sartori & Lombardi, 2004; Warrington & McCarthy, 1987). This richness in multimodal conceptual structure is not always apparent, however. Some concrete concepts (e.g., thunder) and virtually all abstract concepts (e.g., independence) lack a full range of complementary sensorimotor information. For these exceptions, the spoke system offers less redundancy (e.g., thunder is supported by sound and emotion but not vision). In such cases, damage to hubs may result in more catastrophic semantic loss because there is little or no supporting structure to aid in enactment and enrichment (Bonner, Peelle, Cook, & Grossman, 2013; Breedin, Saffran, & Coslett, 1994).
Although all patients showed extensive posterior frontal lobe damage, none showed a selective impairment for generating manipulable object names as would be predicted by a strict embodied cognition approach. Rather, the lesion maps implicated both a series of candidate hub regions (AG, pMTG), as well as a visual spoke (MT/V5) in generating the names of manipulable objects. Thus, damage to both hub and spoke components were predictive of the relative naming impairments incurred for manipulable objects in this patient sample.
Contextualizing the findings
Pulvermüller (2013) recently employed the metaphor of master and slave to the relation between cognition and action-perception systems in the human brain. Pulvermüller remarked that dominant amodal models are premised upon a’master’ semantics module that subjugates action and perception as ancillary slave modules. In contrast, Action Perception Theory (APT) turns the table on the power disparity, grounding concepts directly in action and perception while subjugating amodal symbolic representations (Pulvermüller, 2005; Pulvermüller, 2013; Pulvermüller, Moseley, Egorova, Shebani, & Boulenger, 2014). Much of the empirical data in favor of this approach hinge on the near simultaneous temporal activation and robust functional connectivity between effector-specific motor regions and polymodal hubs (Boulenger et al., 2006; Hauk, Johnsrude, & Pulvermüller, 2004; Hauk & Pulvermüller, 2004). Thus, this approach can be considered radically embodied in that over time and through repeated experience, word meaning becomes inextricably linked with the sensorimotor system through Hebbian learning.
Cognitive neuropsychology has traditionally deonstructed semantic organization by examining the performance of patients with selective damage to one or more components of an undisputedly distributed system. Semantic memory is essential for many of our most basic interactions with the world, and the system accordingly offers great redundancy. As human beings, we routinely compensate for impoverished input, retrieving coherent semantic representations from fragments of detail. Patients with focal neurological injuries often show gross compensation for the loss of one information channel by invoking spared homologous regions in the contralateral cerebral hemisphere or by reverting to alternate sensory channels. As a result, semantic deficits are often only apparent in the context of sensitive, targeted, and/or very challenging experimental paradigms. Several recent works have examined the effects of relatively selective deficits of the motor complex on targeted semantic tasks (e.g., verb and/or manufactured artifact matching). Pazzaglia and colleagues (2008), for example, examined lesion correlates of environmental sound recognition for hand or mouth generated actions (e.g., snapping, slurping) in patients with limb and/or buccofacial apraxia. Patients with these forms of apraxia did indeed show deficits in recognizing and matching environmental sounds generated by the impaired effector. Moreover, lesion mapping in the apraxia sample demonstrated associations between damage to left inferior frontal structures in buccofacial apraxia and impaired matching of sounds generated by mouth. Patients with limb apraxia secondary to frontoparietal damage showed the opposite dissociation characterized by impaired matching of limb-related sounds (e.g., finger snapping).
The Pazzaglia et al. (2008) double dissociation results are consistent with a strong embodied perspective on semantic representation. Related work has demonstrated dissociations for action verbs in movement disorders such as Parkinson’s Disease (Boulenger et al., 2008) and Amyotrophic Lateral Sclerosis (Grossman et al., 2008; Libon et al., 2012). These studies link the integrity of motor execution with offline conceptual semantic processing. It would be ideal to jump from these well-characterized associations directly to causal inference (e.g., sensormotor impairment = central semantic impairment). Yet, even the most sophisticated lesion-behavior correlation analyses are subject to the adage that correlation does not imply causation. Many studies (including our own) have failed to find associations between activation or damage to the motor complex and successful performance on an offline semantic task (e.g., verb or artifact naming) (de Zubicaray et al., 2013; Druks et al., 2006; Reilly, Cross, Troiani, & Grossman, 2007; Reilly, Rodriguez, et al., 2011).
Here we found that the integrity of the motor cortex was not predictive of a category discrepancy for generating manipulable objects. In contrast, damage to a series of polymodal hubs (AG/pMTG) and one modality-specific spoke (MT/V5) were predictive of this relative impairment. We interpret these findings as supportive of a hybrid semantics model premised upon interactivity between modal and amodal systems. The approach we advance (i.e., sparse polymodal representations enriched by modality-specific simulation) obviates the metaphor of subjugation in favor of symbiosis between embodied and disembodied components.
Caveats and limitations
Studies of embodied cognition are often rife with methodological complications and theoretical assumptions, and the findings here are no exception. The lack of an observed lesion correlation with motor regions does not preclude contribution of these areas to the task. Many sources of error can potentially obscure results, including issues related to thresholding, deformation of the lesions during spatial normalization, and/or statistical robustness of the lesion correlation method (Rorden, Fridriksson, & Karnath, 2009). Perhaps the most obvious confounding variable is the possibility that virtually all patients could have experienced damage to motor regions. Such a contingency would result in an error of statistical reasoning analogous to correlating Age with Weight for a sample composed exclusively of six-year-olds. The absence of variance in either predictor invalidates the test. Our patient sample was vulnerable to such a contingency in that all participants suffered left hemisphere strokes and experienced nonfluent aphasia. Yet, their lesion distributions demonstrate variability with respect to the extent of motor cortex damage and other variables such as total lesion size. Table 2 reflects variability in the extent of damage incurred in key Brodmann Areas (4/6/44/45).
Perhaps the most serious limitation here involves statistical power. Rorden and colleagues (2009) proposed formal criteria for improving the validity of voxel-based lesion symptom mapping, including implementation of permutation testing and thresholding for voxels that are lesioned with sufficient overlap to control for the potentially spurious effects of outliers. These procedures yield conservative estimates that reduce the likelihood of elicting type 1 errors (i.e., finding spurious effects) in large samples where the central limit theorem applies. However, in a sample size as small as ours (N=14), these stringent thresholding amplified the likelihood of a type 2 error (i.e., failing to discover a true effect). We integrated several conservative measures (e.g., cluster threshold, permutation testing, false discovery rate) but not all of those advocated by Rorden and others (i.e., excluding voxels with <30% patient overlap). We are confident that these methods err toward conservative while balancing the value of running a focused small-n study. However, it is clear that our method will not garner universal agreement. A larger sample and converging multimodal imaging data (e.g., DTI) are essential extensions of this work.
Can a brain be both embodied and disembodied?
The extent to which cognition is embodied remains one of the most fundamental questions in neuroscience and the philosophy of mind. Although the swinging pendulum of consensus has recently favored embodied cognition, a steady body of work supports the idea that links between language and motor systems are often more oblique. Dove (2009) elegantly argued the necessity for both embodied and disembodied cognition but did not specify a mechanism by which these phenomena might co-mingle in the human brain. The hub and spoke approach to semantic memory can potentially accomplish this feat. Hubs may contribute disembodied, sparsified representations. Spokes, in contrast, serve to enrich this fragementary knowledge through sensorimotor enactment or enrichment. From a neurophysiological perspective, much remains to be learned about the nature of hubs and their dynamic interactivity with spokes. The lesion maps we derived here point to pMTG and AG as hub candidates, thus, supporting a multiple convergence zone framework. Crucial to this approach are contributions from both modal and amodal cognitive processes. We conclude by returning to the central question, can a brain be both embodied and disembodied? The answer is yes.
Table 3.
Lesion characteristics
| Subject | ICV/TIV (mL) | Lesion Volume (mL) | % Lesion Brain | % Lesion BA44 | % Lesion BA45 | % Lesion BA04 | % Lesion BA06 |
|---|---|---|---|---|---|---|---|
| s01 | 1669.6 | 23.9 | 1.4 | 7.2 | 3.9 | 2.3 | 1.6 |
| s02 | 1410.5 | 106.5 | 7.6 | 14.8 | 13.6 | 2.3 | 4 |
| s04 | 1680.6 | 122.9 | 7.3 | 1 | 0 | 1.5 | 3 |
| s06 | 1705.0 | 80.6 | 4.7 | 4 | 0 | 5.6 | 8.8 |
| s07 | 1066.3 | 36.0 | 3.4 | 4.9 | 12.6 | 0 | 0 |
| s08 | 1624.2 | 150.4 | 9.3 | 0 | 25 | 1 | 0 |
| s11 | 1595.2 | 75.8 | 4.8 | 0 | 0 | 1 | 0 |
| s12 | 1318.3 | 50.7 | 3.8 | 7.3 | 1.4 | 0 | 3.1 |
| s13 | 1449.8 | 154.7 | 10.7 | 15 | 0 | 39.7 | 26.7 |
| s14 | 1570.7 | 28.7 | 1.8 | 0 | 0 | 0 | 0 |
| s15 | 1780.4 | 85.6 | 4.8 | 21.9 | 1.5 | 10.8 | 9.1 |
| s16 | 1575.9 | 50.0 | 3.2 | 2.2 | 0 | 7 | 10 |
| s17 | 1235.0 | 16.7 | 1.4 | 0 | 0 | 0 | 0 |
| s19 | 1570.1 | 65.8 | 4.2 | 4.5 | 1 | 1.4 | 4.7 |
| MEANS | 1518.0 | 74.9 | 4.9 | 5.9 | 4.2 | 5.2 | 5.1 |
Note: ICV/TIV = intracranial volume reflecting the summed volumes of gray matter, white matter, and cerebrospinal fluid inside the skull casing. Lesion Volume = reflects total volume of traced lesion (including CSF, GM, & WM); %Lesion Whole Brain = Lesion Volume divided by ICV; %Lesion BA44 = Proportion of Brodmann Area 44 (Broca’s Area/Pars Operculum) lesioned for each patient as defined by the MRIcron Brodmann template; %Lesion BA45: Proportion of Brodmann Area 45 (Pars Triangularis) lesioned for each patient; %Lesion BA4: Proportion of Brodmann Area 4 (Primary Motor Cortex) lesioned for each patient; %Lesion BA6: Proportion of Brodmann Area 6 (Premotor Cortex) lesioned for each patient. *The lesion proportions corresponding to the Brodmann Areas above were derived using the Brodmann template in MRIcron. The volumes of subcortical lesions are not reflected in the Brodmann area totals. Patient S17, for example, suffered an extensive subcortical stroke extending from the frontal lobe into the periventricular white matter.
Acknowledgments
This work was supported by US Public Health Service grants R01 DC013063 (JR), K23 DC010197 (JR), and R01 DC007387 (BC). We are grateful to Jeffrey Bennett, Hyejin Park, Alison Paris, Stephen Towler, and Michelle Benjamin for their assistance with anatomical tracing, brain normalization, and patient testing.
APPENDIX
| Count | Category Cue | Manip | Turk-Mean | Z-Turk | RT-mean | Diff-mean |
|---|---|---|---|---|---|---|
| 1 | medical instrument | Yes | 1.62 | −2.80 | 1.21 | 2.18 |
| 2 | musical instrument | Yes | 1.82 | −2.59 | 0.53 | 1.27 |
| 3 | carpenter’s tool | Yes | 2.21 | −2.19 | 1.16 | 1.45 |
| 4 | circus act | Yes | 2.38 | −2.01 | 1.65 | 2.09 |
| 5 | weapon | Yes | 2.44 | −1.95 | 1.11 | 1.27 |
| 6 | sport | Yes | 2.46 | −1.93 | 0.98 | 1.18 |
| 7 | writing utensil | Yes | 2.51 | −1.87 | 0.54 | 1.09 |
| 8 | sewing supply | Yes | 2.64 | −1.74 | 0.69 | 1.18 |
| 9 | vehicle | Yes | 2.97 | −1.39 | 1.26 | 1.27 |
| 10 | gardening tool | Yes | 3.16 | −1.20 | 0.91 | 1.45 |
| 11 | cosmetic | Yes | 3.26 | −1.09 | 1.22 | 1.82 |
| 12 | outdoor activity | Yes | 3.31 | −1.05 | 1.37 | 1.82 |
| 13 | kitchen utensil | Yes | 3.36 | −0.99 | 0.80 | 1.36 |
| 14 | electric appliance | Yes | 3.46 | −0.89 | 1.08 | 1.82 |
| 15 | car (part of a) | Yes | 3.49 | −0.86 | 1.01 | 1.36 |
| 16 | dance (type of) | Yes | 3.54 | −0.81 | 1.16 | 1.82 |
| 17 | jewelry | Yes | 3.59 | −0.76 | 0.83 | 1.18 |
| 18 | bicycle part | Yes | 3.69 | −0.65 | 0.83 | 1.36 |
| 19 | camping supply | Yes | 3.71 | −0.63 | 1.11 | 1.91 |
| 20 | meat (type of) | No | 4.82 | 0.52 | 1.26 | 1.55 |
| 21 | human dwelling | No | 4.85 | 0.55 | 1.37 | 1.82 |
| 22 | money (type of) | No | 4.90 | 0.60 | 1.38 | 1.45 |
| 23 | pizza topping | No | 4.97 | 0.68 | 0.89 | 1.00 |
| 24 | liquor (type of) | No | 4.97 | 0.68 | 1.51 | 1.73 |
| 25 | building (part of a) | No | 5.00 | 0.71 | 1.98 | 2.18 |
| 26 | body of water | No | 5.03 | 0.73 | 1.26 | 1.55 |
| 27 | candy | No | 5.05 | 0.76 | 1.71 | 1.45 |
| 28 | flower | No | 5.08 | 0.79 | 0.88 | 1.27 |
| 29 | furniture | No | 5.08 | 0.79 | 0.88 | 1.45 |
| 30 | frozen food | No | 5.15 | 0.87 | 1.33 | 2.09 |
| 31 | bread (type of) | No | 5.16 | 0.87 | 1.24 | 1.27 |
| 32 | store (type of) | No | 5.23 | 0.95 | 1.41 | 2.36 |
| 33 | shape | No | 5.31 | 1.03 | 1.04 | 1.27 |
| 34 | color | No | 5.38 | 1.11 | 0.57 | 1.00 |
| 35 | dairy product | No | 5.38 | 1.11 | 0.72 | 1.09 |
| 36 | room (type of) | No | 5.41 | 1.13 | 0.91 | 1.36 |
| 37 | tree (part of a) | No | 5.41 | 1.13 | 0.69 | 1.36 |
| 38 | cheese (type of) | No | 5.46 | 1.19 | 0.88 | 1.36 |
| 39 | tree (type of) | No | 5.46 | 1.19 | 1.24 | 1.36 |
| 40 | natural earth formation | No | 5.62 | 1.35 | 1.53 | 2.27 |
| 41 | ice cream flavor | No | 5.64 | 1.37 | 0.70 | 1.09 |
Note: Manip reflects the categorical distinction of manipulable or non-manipulable based on ratings from the Mechanical Turk (see method). Turk Mean reflects the average Likert-scale rating of manipulability from participants in the normative study of the Mechanical Turk. Z-Turk reflects the respective z-score for each category cue based on its rated manipulability salience. RT-Mean reflects the mean reation time across participants (N=11) for the offset of the stimulus until the onset of overt naming. Diff-Mean reflects the average rated difficulty of each category cue from the subset of participants who completed the reaction time experiment.
Footnotes
This hypothesis applies to an intact semantic system. In the context of damage to convergence zones (e.g., Alzheimer’s Disease), fragmentary support from afferent modal cortices may become a necessary compensatory mechanism (Rorden, 2007).
Wallerian degeneration in the central nervous system can occur slowly, producing structural changes that are often only visible on structural MRI months after the primary injury (but see Druks et al., 2006). We observed Wallerian degeneration in the motor tracts of several patients, extending through the brainstem. This degeneration was visible as a circumscribed “hole” on the T1 structural image that ran continuous with the original lesion in the descending axial plane. We know of no VLSM studies to date that have explicitly addressed the anatomical quandary posed by this phenomenon. Our rationale for marking this damage is that it there is no principled way of discerning the margins of the primary infarct relative to the chronic changes inflicted by secondary metabolic and inflammatory processes. Note, this phenomenon contrasts sharply with that of diffuse cerebral atrophy where causal relations between the primary injury and nonspecific atrophy are unclear.
The VLSM results lack specifity to determine whether behavioral deficits are associated with damage to cortical nodes themselves or their afferent/efferent pathways. This is a serious inferential limitation imposed by VLSM. A better approach would involve integrating VLSM with diffusion tensor imaging to understand the role of disconnection. In the VLSM analyses using the AAL anatomical atlas, for example, descriptive statistics include white matter as one undifferentiated structure. Although numerous atlas resources exist for specifying white matter pathways, the sensitivity of non-diffusion weighted imaging (e.g., T1-3d MRI) is limited.
References
- Arévalo A, Perani D, Cappa SF, Butler A, Bates E, Dronkers N. Action and object processing in aphasia: From nouns and verbs to the effect of manipulability. Brain and Language. 2007;100(1):79–94. doi: 10.1016/j.bandl.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47(7):800–807. doi: 10.1016/j.cortex.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behavioral and Brain Sciences. 1999;22(4):577–660. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34(1):149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and Semantic Dementia. Cerebral Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Bonner MF, Peelle JE, Cook PA, Grossman M. Heteromodal conceptual processing in the angular gyrus. Neuroimage. 2013;71:175–186. doi: 10.1016/j.neuroimage.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Vesely L, Price C, Anderson C, Richmond L, Farag C, Grossman M. Reversal of the concreteness effect in semantic dementia. Cognitive Neuropsychology. 2009;26(6):568–579. doi: 10.1080/02643290903512305. 919094196 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F. Contributions of the human temporoparietal junction and MT/V5+ to the timing of interception revealed by transcranial magnetic stimulation. Journal of Neuroscience. 2008;28(46):12071–12084. doi: 10.1523/jneurosci.2869-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir TA. Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46(2):743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. S0028-3932(07)00357-0 [pii] [DOI] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA. Cross-talk between language processes and overt motor behavior in the first 200 msec of processing. Journal of Cognitive Neuroscience. 2006;18(10):1607–1615. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of the concreteness effect in a patient with semantic dementia. Cognitive Neuropsychology. 1994;11(6):617–660. [Google Scholar]
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: A new source of inexpensive, yet high-quality, data? Perspectives on Psychological Science. 2011;6(1):3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex. 2007;43(3):411–423. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Schwartz MF, Carew TG. The role of semantic memory in object use. Cognitive Neuropsychology. 1997;14(2):219–254. [Google Scholar]
- Capitani E, Laiacona M, Mahon BZ, Caramazza A. What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cognitive Neuropsychology. 2003;20(3–6):213–261. doi: 10.1080/02643290244000266. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain: The animate-inanimate distinction. Journal of Cognitive Neuroscience. 1998;10(1):1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Cortical regions associated with perceiving, naming, and knowing about colors. Journal of Cognitive Neuroscience. 1999;11(1):25–35. doi: 10.1162/089892999563229. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12(4):478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Disembodying cognition. Language and Cognition. 2010;2(1):79–116. doi: 10.1515/LANGCOG.2010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Goodale MA. Category-specific neural processing for naming pictures of animals and naming pictures of tools: an ALE meta-analysis. Neuropsychologia. 2010;48(2):409–418. doi: 10.1016/j.neuropsychologia.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA. Semantic memory is an amodal, dynamic system: Evidence from the interaction of naming and object use in semantic dementia. Cognitive Neuropsychology. 2004;21(5):513–527. doi: 10.1080/02643290342000113. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. The selective impairment of fruit and vegetable knowledge: A multiple processing channels account of fine-grain category specificity. Cognitive Neuropsychology. 2003;20(3–6):355–372. doi: 10.1080/02643290244000220. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- de Zubicaray G, Arciuli J, McMahon K. Putting an "end" to the motor cortex representations of action words. Journal of Cognitive Neuroscience. 2013;25(11):1957–1974. doi: 10.1162/jocn_a_00437. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, Tyler LK. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40(1):54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Dick F, Saygin AP, Galati G, Pitzalis S, Bentrovato S, D’Amico S, Pizzamiglio L. What is involved and what is necessary for complex linguistic and nonlinguistic auditory processing: Evidence from functional magnetic resonance imaging and lesion data. Journal of Cognitive Neuroscience. 2007;19(5):799–816. doi: 10.1162/jocn.2007.19.5.799. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. Journal of Comparative Neurology. 2009;514(6):595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove G. Beyond perceptual symbols: A call for representational pluralism. Cognition. 2009;110(3):412–431. doi: 10.1016/j.cognition.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins D, van Valin RD, Redfern B, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Druks J, Masterson J, Kopelman M, Clare L, Rose A, Rai G. Is action naming better preserved (than object naming) in Alzheimer’s disease and why should we ask? Brain and Language. 2006;98(3):332–340. doi: 10.1016/j.bandl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cerebral Cortex. 2000;10(5):454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test (PPVT-III) Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Fairhall SL, Caramazza A. Brain regions that represent amodal conceptual knowledge. Journal of Neuroscience. 2013;33(25):10552–10558. doi: 10.1523/jneurosci.0051-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Feinberg TE. Visual object agnosia. Farah, Martha J 2000 [Google Scholar]
- Farah MJ, McClelland JL. A computational model of semantic memory impairment: Modality specificity and emergent category specificity. Journal of Experimental Psychology: General. 1991;120(4):339–357. [PubMed] [Google Scholar]
- Franklin S, Howard D, Patterson K. Abstract word meaning deafness. Cognitive Neuropsychology. 1994;11(1):1–34. [Google Scholar]
- Freud S. In: On Aphasia. Stengel E, translator. New York: International University Press; 1891. [Google Scholar]
- Gage N, Hickok G. Multiregional cell assemblies, temporal binding and the representation of conceptual knowledge in cortex: A modern theory by a ‘classical’ neurologist, Carl Wernicke. Cortex. 2005;41(6):823–832. doi: 10.1016/s0010-9452(08)70301-0. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22(3):455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Garcea FE, Mahon BZ. What is in a tool concept? Dissociating manipulation knowledge from function knowledge. Memory and Cognition. 2012 doi: 10.3758/s13421-012-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis and apraxia. In: Gonzaelz Rothi LJ, Heilman KM, editors. Apraxia: The neuropsychology of action. Hove England: Psychology Press/Erlbaum (UK) Taylor & Francis; 1997. pp. 29–49. [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2. Philadelphia: Lea & Febiger Philadelphia; 1983. [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71(18):1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Neural subsystems for object knowledge. Nature. 1992;359(6390):60–64. doi: 10.1038/359060a0. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–207. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermüller F. Neurophysiological Distinction of Action Words in the Fronto-Central Cortex. Human Brain Mapping. 2004;21(3):191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Category-specific naming and comprehension impairment: a double dissociation. Brain. 1991;114(Pt 5):2081–2094. doi: 10.1093/brain/114.5.2081. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Sim EJ, Bochev V, Herrnberger B, Kiefer M. Conceptual flexibility in the human brain: dynamic recruitment of semantic maps from visual, motor, and motion-related areas. Journal of Cognitive Neuroscience. 2008;20(10):1799–1814. doi: 10.1162/jocn.2008.20123. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, Ralph MA. The degraded concept representation system in semantic dementia: damage to pan-modal hub, then visual spoke. Brain. 2012;135(Pt 12):3770–3780. doi: 10.1093/brain/aws282. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Lambon Ralph MA. Shapes, scents and sounds: quantifying the full multi-sensory basis of conceptual knowledge. Neuropsychologia. 2013;51(1):14–25. doi: 10.1016/j.neuropsychologia.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proceedings of the Royal Society of London B Biological Sciences. 1977;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, Stryker MP. Anatomical demonstration of orientation columns in macaque monkey. Journal of Computational Neurology. 1978;177(3):361–380. doi: 10.1002/cne.901770302. [DOI] [PubMed] [Google Scholar]
- Kable JW, Chatterjee A. Specificity of Action Representations in the Lateral Occipitotemporal Cortex. Journal of Cognitive Neuroscience. 2006;18(9):1498–1517. doi: 10.1162/jocn.2006.18.9.1498. [DOI] [PubMed] [Google Scholar]
- Kan IP, Kable JW, Van Scoyoc A, Chatterjee A, Thompson-Schill SL. Fractionating the left frontal response to tools: Dissociable effects of motor experience and lexical competition. Journal of Cognitive Neuroscience. 2006;18(2):267–277. doi: 10.1162/089892906775783723. [DOI] [PubMed] [Google Scholar]
- Kandel E. In Search of Memory. New York, New York: W.W. Norton and Company, Inc; 2006. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Large, colorful, or noisy? Attribute- and modality-specific activations during retrieval of perceptual attribute knowledge. Cogn Affect Behav Neurosci. 2001;1(3):207–221. doi: 10.3758/cabn.1.3.207. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Hovius M, Patterson K. A pet study of visual and semantic knowledge about objects. Cortex. 2005;41(2):121–132. doi: 10.1016/s0010-9452(08)70887-6. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, Tranel D. Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex. 2012;48(7):826–848. doi: 10.1016/j.cortex.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery Revised (WAB-R) New York, New York: Pro-Ed; 1982. [Google Scholar]
- Kiefer M, Pulvermuller F. Conceptual representations in mind and brain: Theoretical developments, current evidence and future directions. Cortex. 2012 doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Pulvermüller F. Conceptual representations in mind and brain: theoretical developments, current evidence and future directions. Cortex. 2012;48(7):805–825. doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kolster H, Peeters R, Orban GA. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. Journal of Neuroscience. 2010;30(29):9801–9820. doi: 10.1523/jneurosci.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge Is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cerebral Cortex. 2008;19(4):832. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proceedings of the National Academy of Sciences USA. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Avants B, Boller A, Morgan B, Burkholder L, Grossman M. Deficits in concept formation in amyotrophic lateral sclerosis. Neuropsychology. 2012;26(4):422–429. doi: 10.1037/a0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. Constraining questions about the organisation and representation of conceptual knowledge. Cognitive Neuropsychology. 2003;20(3–6):433–450. doi: 10.1080/02643290342000014. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology: Paris. 2008;102(1–3):59–70. doi: 10.1016/j.jphysparis.2008.03.004. S0928-4257(08)00019-3 [pii] [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. Concepts and categories: A cognitive neuropsychological perspective. Annual Review of Psychology. 2009;60:27–51. doi: 10.1146/annurev.psych.60.110707.163532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. What drives the organization of object knowledge in the brain? Trends in Cognitive Sciences. 2011;15(3):97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17(3):562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, et al. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270(5233):102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV. Category specificity and the brain: The sensory/motor model of semantic representations of objects. In: Gazanniga MS, editor. The New Cognitive Neurosciences. 2. Cambridge, MA: MIT Press; 2000. pp. 1023–1036. [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mason W, Suri S. Conducting behavioral research on Amazon’s mechanical Turk. Behavior Research Methods. 2012;44(1):1–23. doi: 10.3758/s13428-011-0124-6. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McRae K, Cree GS, Seidenberg MS, McNorgan C. Semantic feature production norms for a large set of living and nonliving things. Behavior Research Methods. 2005;37(4):547–559. doi: 10.3758/bf03192726. [DOI] [PubMed] [Google Scholar]
- Meteyard L, Cuadrado SR, Bahrami B, Vigliocco G. Coming of age: A review of embodiment and the neuroscience of semantics. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(7):788–804. doi: 10.1016/j.cortex.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain: A Journal of Neurology. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Vanduffel W. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia. 2003;41(13):1757–1768. doi: 10.1016/s0028-3932(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Papeo L, Lingnau A, Agosta S, Pascual-Leone A, Battelli L, Caramazza A. The Origin of Word-related Motor Activity. Cerebral Cortex. 2014 doi: 10.1093/cercor/bht423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Rogalski Y, Rodriguez AD, Zlatar Z, Benjamin M, Harnish S, Reilly J. Perceptual cues used by listeners to discriminate fluent from nonfluent narrative discourse. Aphasiology. 2011;25(9):998–1015. doi: 10.1080/02687038.2011.570770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K. The reign of typicality in semantic memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362(1481):813–821. doi: 10.1098/rstb.2007.2090. 1MW426N6VV32M223 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Pizzamiglio L, Pes E, Aglioti SM. The sound of actions in apraxia. Current Biology. 2008;18(22):1766–1772. doi: 10.1016/j.cub.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: An fMRI study of eye, mouth and hand movements. Cerebral Cortex. 2005;15(12):1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences, U S A. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. 0707383104 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle N, Ashton R, McFarland K, de Zubicaray GI. No specific role for the manual motor system in processing the meanings of words related to the hand. Frontiers in Human Neuroscience. 2013;7:11. doi: 10.3389/fnhum.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI. Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. Neuroimage. 2008;43(3):634–644. doi: 10.1016/j.neuroimage.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6(7):576–582. doi: 10.1038/nrn1706. nrn1706 [pii] [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Semantic embodiment, disembodiment or misembodiment? In search of meaning in modules and neuron circuits. Brain and Language. 2013;127(1):86–103. doi: 10.1016/j.bandl.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Härle M, Hummel F. Walking or talking?: Behavioral and neurophysiological correlates of action verb processing. Brain and Language. 2001;78(2):143–168. doi: 10.1006/brln.2000.2390. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Moseley RL, Egorova N, Shebani Z, Boulenger V. Motor cognition-motor semantics: Action perception theory of cognition and communication. Neuropsychologia. 2014;55:71–84. doi: 10.1016/j.neuropsychologia.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Reilly J, Cross K, Troiani V, Grossman M. Single word semantic judgments in Semantic Dementia: Do phonology and grammatical class count? Aphasiology. 2007;21(6/7/8):558–569. [Google Scholar]
- Reilly J, Martin N. Transcortical sensory aphasia and semantic processing. In: Gonzalez Rothi L, Raymer AM, editors. The Oxford Handbook of Aphasia and Language Disorders. Oxford, UK: Oxford University Press; in press. [Google Scholar]
- Reilly J, Peelle JE. Effects of semantic impairment on language processing in semantic dementia. Seminars in Speech and Language. 2008;29:32–43. doi: 10.1055/s-2008-1061623. [DOI] [PubMed] [Google Scholar]
- Reilly J, Peelle JE, Antonucci SM, Grossman M. Anomia as a marker of distinct semantic memory impairments in Alzheimer’s disease and semantic dementia. Neuropsychology. 2011;25(4):413–426. doi: 10.1037/a0022738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Rodriguez A, Peelle JE, Grossman M. Frontal lobe damage impairs process and content in semantic memory: Evidence from category specific effects in progressive nonfluent aphasia. Cortex. 2011;47:645–658. doi: 10.1016/j.cortex.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Nichelli F, Devoti M. Developmental aspects of verbal fluency and confrontation naming in children. Brain and Language. 2000;71(2):267–284. doi: 10.1006/brln.1999.2166. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, Price CJ. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cognitive, Affective & Behavioral Neuroscience. 2006;6(3):201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hodges JR, Lambon Ralph MA, Patterson K. Object recognition under semantic impairment: The effects of conceptual regularities on perceptual decisions. Language and Cognitive Processes. 2003;18(5–6):625–662. [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and Deterioration of Semantic Memory: A Neuropsychological and Computational Investigation. Psychological Review. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Semantic cognition: A parallel distributed processing approach. Cambridge, MA US: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- Rorden C. MRIcron (Version Beta 7) 2007 Retrieved from http://www.cabiatl.com/mricro/mricron/index.html.
- Rorden C, Fridriksson J, Karnath HO. An evaluation of traditional and novel tools for lesion behavior mapping. Neuroimage. 2009;44(4):1355–1362. doi: 10.1016/j.neuroimage.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch EH. Natural categories. Cogn Psychol. 1973;4(3):328–350. doi: 10.1016/0010-0285(73)90017-0. [DOI] [Google Scholar]
- Rosci C, Chiesa V, Laiacona M, Capitani E. Apraxia is not associated to a disproportionate naming impairment for manipulable objects. Brain and Cognition. 2003;53(2):412–415. doi: 10.1016/s0278-2626(03)00156-8. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27(1):188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Sacchett C, Humphreys GW. Calling a squirrel a squirrel but a canoe a wigwam: A category-specific deficit for artefactual objects and body parts. Cognitive Neuropsychology. 1992;9(1):73–86. [Google Scholar]
- Salmon JP, McMullen PA, Filliter JH. Norms for two types of manipulability (graspability and functional usage), familiarity, and age of acquisition for 320 photographs of objects. Behavior Research Methods. 2010;42(1):82–95. doi: 10.3758/brm.42.1.82. [DOI] [PubMed] [Google Scholar]
- Samson D, Pillon A. A case of impaired knowledge for fruit and vegetables. Cognitive Neuropsychology. 2003;20(3–6):373–400. doi: 10.1080/02643290244000329. [DOI] [PubMed] [Google Scholar]
- Samson D, Pillon A, De Wilde V. Impaired knowledge of visual and non-visual attributes in a patient with a semantic impairment for living entities: A case of a true category-specific deficit. Neurocase. 1998;4(4–5):273–290. [Google Scholar]
- Sartori G, Gnoato F, Mariani I, Prioni S, Lombardi L. Semantic relevance, domain specificity and the sensory/functional theory of category-specificity. Neuropsychologia. 2007;45(5):966–976. doi: 10.1016/j.neuropsychologia.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Sartori G, Lombardi L. Semantic relevance and semantic disorders. Journal of Cognitive Neuroscience. 2004;16(3):439–452. doi: 10.1162/089892904322926773. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Dick F, Wilson SM, Dronkers NF, Bates E. Neural resources for processing language and environmental sounds: evidence from aphasia. Brain. 2003;126(Pt 4):928–945. doi: 10.1093/brain/awg082. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cerebral Cortex. 2010;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2(10):e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Embleton KV, Lambon Ralph MA. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. Journal of Cognitive Neuroscience. 2012;24(8):1766–1778. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- Visser M, Lambon Ralph MA. Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. Journal of Cognitive Neuroscience. 2011;23(10):3121–3131. doi: 10.1162/jocn_a_00007. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philosophical Transactions of the Royal Society of London. 1850;140:423–429. [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27(4):635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy R. Category specific access dysphasia. Brain. 1983;106(Pt 4):859–878. doi: 10.1093/brain/106.4.859. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge. Further fractionations and an attempted integration. Brain. 1987;110:1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(3):829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische symptomemkomplex: Eine psychologische Studie auf anatomischer basis. Breslau: Cohn und Weigert; 1874. [Google Scholar]
- Willems RM, Casasanto D. Flexibility in embodied language understanding. Frontiers in Psychology. 2011;2:116. doi: 10.3389/fpsyg.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms M, Eickhoff SB, Specht K, Amunts K, Shah NJ, Malikovic A, Fink GR. Human V5/MT+: comparison of functional and cytoarchitectonic data. Anat Embryol (Berl) 2005;210(5–6):485–495. doi: 10.1007/s00429-005-0064-y. [DOI] [PubMed] [Google Scholar]
- Wu DH, Morganti A, Chatterjee A. Neural substrates of processing path and manner information of a moving event. Neuropsychologia. 2008;46(2):704–713. doi: 10.1016/j.neuropsychologia.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven JH, HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]