Abstract
Neuroimaging studies of episodic memory retrieval have revealed activations in the human frontal, parietal, and medial-temporal lobes that are associated with memory strength. However, it remains unclear whether these brain responses are veritable signals of memory strength or are instead regulated by concomitant subcomponents of retrieval such as retrieval effort or mental search. This study used event-related fMRI during cued recall of previously memorized word-pair associates to dissociate brain responses modulated by memory search from those modulated by the strength of a recalled memory.
Search-related deactivations, dissociated from activity due to memory strength, were observed in regions of the default network, whereas distinctly strength-dependent activations were present in superior and inferior parietal and dorsolateral PFC. Both search and strength regulated activity in dorsal anterior cingulate and anterior insula. These findings suggest that, although highly correlated and partially subserved by overlapping cognitive control mechanisms, search and memory strength engage dissociable regions of frontoparietal attention and default networks.
INTRODUCTION
Experimental electroencephalographic and fMRI paradigms that manipulate encoding depth, acquire subjective recognition confidence ratings, or compare recollection with familiarity have revealed distinct neural correlates of memory strength (Wais, Squire, & Wixted, 2010; Kirwan, Wixted, & Squire, 2008; Eichenbaum, Yonelinas, & Ranganath, 2007; Montaldi, Spencer, Roberts, & Mayes, 2006; Staresina & Davachi, 2006; Yonelinas, Otten, Shaw, & Rugg, 2005; Henson, Rugg, Shallice, & Dolan, 2000; Buckner, Koutstaal, Schacter, Wagner, & Rosen, 1998; Smith, 1993). However, procedures that effectively modulate memory strength will also influence concomitant processes that covary with strength but that are only indirectly related to the retrieval event itself (Tulving, 1984). Identifying such concomitant processes may be a particular challenge for fMRI studies where brain blood flow responses are recorded while participants retrieve and evaluate memories. Both retrieval and its evaluation involve subprocesses that contribute to the recorded aggregate brain activity, and each may be differentially influenced by memory strength. Nevertheless, attempts to isolate neural responses related to memory will benefit from improved fractionation of these additional correlated elements. In particular, our current understanding of the mechanisms of recollection is limited by an inability to fully differentiate effects related to retrieval success from those sensitive to retrieval attempt or effort.
Memory retrieval efforts, in addition to recruiting brain regions that are highly specialized to perform memory operations, may also recruit regions with broad functional overlap across cognitive domains. For example, cognitive control and attention critically contribute to episodic memory retrieval efforts and success (Ciaramelli, Grady, & Moscovitch, 2008; Moscovitch, 1992). A variety of attention-dependent processes might be sensitive to retrieval strength, including directing attention toward a spontaneously recalled memory representation (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008), activation of retrieval mode (Buckner, 2003), or guided memory search efforts (Reas, Gimbel, Hales, & Brewer, 2011). For instance, access to a stronger memory may elicit enhanced bottom–up attention to a salient internal stimulus representation (Cabeza et al., 2008; Ciaramelli et al., 2008). In contrast, during directed retrieval, the strength of a target memory may inversely correlate with cognitive control demands, as such demands may be elevated to serve the more difficult retrieval of weaker memories. As opposed to recognition, cued recall attempts may rely more heavily on sequential search processes (Nobel & Shiffrin, 2001) and thus demand increased top–down attention.
Brain regions sensitive to the strength of the retrieved memory include areas of the medial temporal lobe (Wais, 2011; Kirwan et al., 2008) that human lesion and neuro-imaging studies have shown are important for episodic memory encoding and retrieval (Squire, Wixted, & Clark, 2007; Henson, 2005; Gabrieli, Brewer, Desmond, & Glover, 1997; Squire & Zola-Morgan, 1991; Scoville & Milner, 1957) as well as additional regions with functional and anatomical connections to core medial temporal memory structures (Vincent et al., 2006; Greicius, Srivastava, Reiss, & Menon, 2004; Greicius, Krasnow, Reiss, & Menon, 2003). For example, both task-positive activations in frontal and parietal cortex and task-negative responses in the default network can be regulated by retrieval effort, success, or memory strength (Seibert, Gimbel, Hagler, & Brewer, 2011; Kim, 2010; Daselaar et al., 2009; Cabeza, 2008; Moritz, Glascher, Sommer, Buchel, & Braus, 2006; Henson, Hornberger, & Rugg, 2005; Kapur et al., 1995). These areas comprise multiple interacting networks that integrate cognitive control and attention systems with memory regions (Kim, 2010; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). Thus, guided retrieval efforts that directly modulate search and control processes might account for some strength-related responses in regions serving supportive attention functions.
Because retrieval effort is expected to negatively correlate with both the strength of a memory and success at recalling the memory, neural activations driven directly by mental search may confound findings attributed to strength or success. Yet, it remains unknown the extent to which the neural circuitries underlying these interdependent components during attempted recollection overlap or diverge. Previous efforts to dissociate retrieval subprocesses have identified frontal and parietal activations differentially mediated by retrieval success and retrieval effort or mode (Kahn, Davachi, & Wagner, 2004; Donaldson, Petersen, Ollinger, & Buckner, 2001). However, memory strength interacts with both success and effort. Recent evidence demonstrates that activations related to memory strength and successful recollection are separable, such that the hippocampus may support strength, whereas prefrontal and inferior parietal cortex support recollection (Wais, 2011). Further research is necessary to fully disentangle responses associated with retrieval effort from those regulated by the strength of a recalled memory.
The current investigation sought to dissociate the contributions of retrieval effort and recollection strength to BOLD signal changes during episodic memory retrieval. Event-related fMRI was performed while participants recalled previously studied word-pair associates or performed a nonmemory classification task. Memory strength was modulated by varying study repetitions; episodic memory search, a postulated component of retrieval effort, was examined by isolating both successful and unsuccessful recall attempts. On the basis of prior evidence, either or both search and strength were predicted to engage medial and lateral prefrontal, medial and lateral parietal, and superior temporal cortices. By segregating conditions demanding memory search from conditions that varied in strength level, this study further sought to distinguish subregions that are differentially activated by search- and strength-dependent components of episodic retrieval.
METHODS
Participants
Participants included 21 volunteers from the University of California, San Diego (UCSD) community and surrounding areas. All participants were healthy, right-handed, English-speaking, and with normal or corrected vision and gave informed written consent in accordance with criteria of the UCSD Institutional Review Board. Recall performance was poor in four participants, including three participants with fewer than 15% remembered trials in the low-study recall condition and one with no successfully recalled words from the postscan cued recall test. Data from the remaining 17 participants (seven men, mean age = 24.7 years, SD = 2.2 years) were included for analysis.
Stimuli
Stimuli were 240 English nouns, pseudorandomly combined into 120 pairs that were screened for obvious semantic associations. Half of the words represented living items, and the other half represented nonliving items. Pairs were divided equally (40 pairs in each condition) into low, medium, and high repetition study conditions.
Experimental Design
During a prescan encoding task, participants studied 120 word pairs presented one at a time on a laptop, and participants were instructed to remember each word-pair association. To avoid task-irrelevant sources of variability associated with subjective confidence ratings (de Zubicaray, McMahon, Dennis, & Dunn, 2010), memory strength was manipulated by varying study repetitions rather than evaluating retrieval confidence during scanning. Paired associates were repeated one, three, or five times (henceforth referred to as low-, medium-, and high-study) over the course of five 288-sec study runs. Each pair was displayed for 3 sec, followed by a fixation cross for 1 sec (Figure 1A).
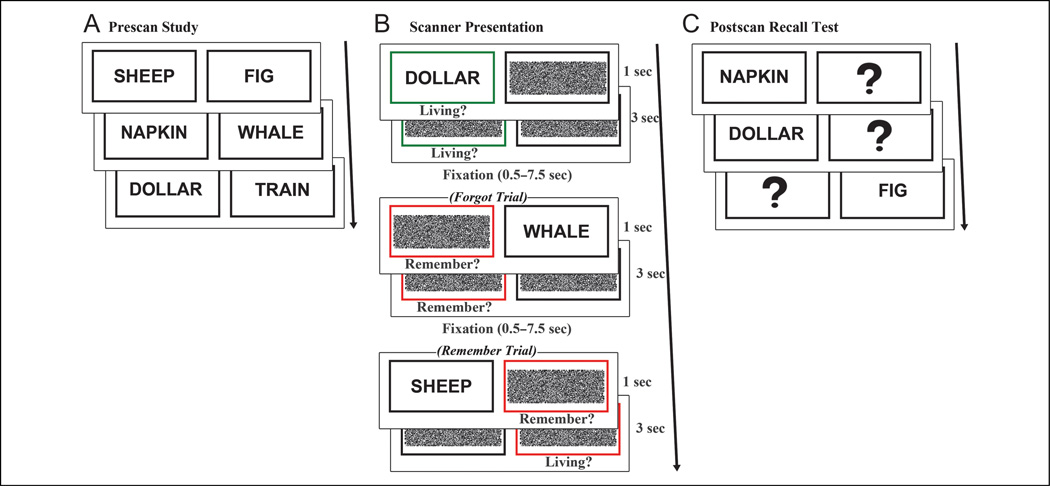
Figure 1.
Experimental protocol. (A) Before scanning, participants studied 120 word-pair associates. Pairs were presented one, three, or five times during the study session. (B) Event-related fMRI was conducted while participants performed classify (green box) or recall (red box) tasks. During recall trials, a classification response was prompted after “remember” responses. (C) After scanning, participants performed a cued recall test on all studied word pairs.
After a delay of approximately 20 min, event-related fMRI data were acquired while participants completed a recall task and a control classify task. In each trial, a black box and a colored box were presented for 1 sec, after which, a previously studied word appeared in one of the boxes for 1 sec. The colored box surrounded the presented word or its missing pair and served as a cue to perform either a classify (green box) or recall (red box) task (Figure 1B). In the classify task, participants were instructed to make a response indicating if the presented word was living or nonliving. In the recall task, they were instructed to first indicate “remember” or “forgot” as soon as they recalled or decided they could not remember the word’s pair and, if recalled, to use a second response to classify the recalled word as living or nonliving. Participants were encouraged to respond as quickly and accurately as possible with their right hand using two buttons of a response box. The cue boxes remained on the screen for 3 sec following word presentation, and trials were jittered with 0.5–7.5 sec of fixation baseline, calculated to optimize the study design for modeling the hemodynamic response to trials (Dale, 1999; Dale & Buckner, 1997). Equal numbers of classify and recall trials (120 trials per condition) were pseudorandomly distributed across five 388-sec runs. The two words composing a pair were assigned to the same condition (classify or recall), and pairs from the three study levels (low-, medium-, and high-study) were distributed evenly across both tasks.
Participants then completed a postscan self-paced cued recall test (Figure 1C) to allow for overt assessment of recall accuracy as compared with covert recall during the scanned recall task. One word from each pair was presented, and participants were instructed to verbally report the word’s pair.
fMRI Parameters
Imaging was performed using a 3.0-T General Electric scanner at the UCSD Keck Center for Functional MRI. Functional data were acquired using a gradient-echo, echo-planar, T2*-weighted pulse sequence (time repetition = 2.5 sec, one shot per repetition, echo time = 30 sec, flip angle = 90°, bandwidth = 31.25 MHz). Each volume contained 40 slices oriented perpendicular to the long axis of the hippocampus with voxels of 3.4 × 3.4 × 4 mm. Field maps were acquired to measure and correct for static field inhomogeneities (Smith et al., 2004). A high-resolution (1 mm3) T1-weighted anatomical scan was acquired using an inversion-recovery-prepared spoiled gradient-recalled sequence providing high gray–white contrast for anatomical delineation. An additional T1-weighted structural scan was acquired in the same plane and of the same voxel size as the functional scans to confirm alignment between the functional and anatomical images.
fMRI Data Analysis
Functional data were corrected for spatial distortions using field maps (Smith et al., 2004), and data from each run were reconstructed using the Analysis of Functional Neuroimages (AFNI) suite of programs (Cox, 1996). Slices were temporally aligned and coregistered using a three-dimensional image alignment algorithm, and a threshold mask of the functional data was applied to remove voxels outside the brain. Each functional run was smoothed with a 4-mm FWHM Gaussian blur, corrected for motion and concatenated. Standard landmarks were manually defined on the anatomical scans before normalizing the anatomical scans and the functional data to Talairach space (Talairach & Tournoux, 1988).
The ROI large deformation diffeomorphic metric mapping (ROI-LDDMM) alignment technique was applied to improve alignment of the medial temporal lobe between participants (Miller, Beg, Ceritoglu, & Stark, 2005). Previously described landmarks were used to define perirhinal and entorhinal cortices (Insausti et al., 1998), parahippo-campal cortex (Stark & Okado, 2003), and hippocampus (Chera, Amdur, Patel, & Mendenhall, 2009) for each participant on Talairach-transformed images. These anatomical ROIs for each participant were normalized using ROI-LDDMM to a modified model of a previously created template segmentation (Kirwan, Jones, Miller, & Stark, 2007). Functional imaging data underwent the same ROI-LDDMM transformation as was applied to the anatomical data.
Amplitude modulated regression was performed to examine how BOLD signal was modulated by trial-by-trial response times or by task conditions independent of response time. The general linear model included regressors for task conditions of interest, including remembered low-study recall, remembered medium-study recall, remembered high-study recall, forgotten recall, and classify trials. Trials were weighted by response times, and two regressors were included for each task condition: one for the magnitude of modulation by response time and one corresponding to the BOLD response for the mean response time (controlling for response time). The model additionally included six motion regressors obtained from the registration process. Signal deconvolution with TENT basis functions (Cox, 1996) was used to estimate the hemodynamic response for 15 sec following the stimulus onset.
To identify activity more strongly correlated with response time in the recall task than in the classify task, parameter estimates of the modulation by response time were contrasted between all recall trials (remembered and forgotten) and classify trials. Because contrasting correlations between conditions leads to ambiguous information about the direction of correlation in each condition (i.e., more positively correlated in the recall task vs. more negatively correlated in the classify task), a mask of positive recall response-time correlations was applied to positive activations and a mask of negative recall response-time correlations was applied to negative activations from the recall versus classify contrast.
To examine task-dependent activity independent of time-on-task, the following comparisons were performed on parameter estimates controlling for response time: (1) remembered versus classify, contrasting a condition where episodic and semantic memory search processes and retrieval are present against a condition where only semantic search is present but episodic search and retrieval are absent; (2) forgotten versus classify, contrasting a condition where episodic memory search processes are high and retrieval is absent against a condition where episodic search and retrieval are absent; and (3) recalled trials from the low-, medium-, and high-study conditions (henceforth referred to as the study-level effect), contrasting variable degrees of memory strength under the condition of successful retrieval (Table 1).
Table 1.
Relative Levels of Search, Strength Differences, and Retrieval Success Presented for Each of the Three Comparisons: Remembered versus Classify, Forgotten versus Classify, and Study Level
| Remembered vs. Classify | Forgotten vs. Classify | Study Level | |
|---|---|---|---|
| Search | ++ vs. absent | +++ vs. absent | Some decrease with strength |
| Strength | ++ vs. absent | + vs. absent | + vs. ++ vs. +++ |
| Retrieval success | +++ vs. absent | Absent | Equal |
The overlap of all three comparisons involves varying degrees of search and strength. Search is engaged during remembered and forgotten recall trials, relative to a baseline classification task, and can be isolated from strength by excluding effects of study level. Differences in memory strength are highlighted by comparing successful recall of low-, medium-, and high-study word pairs, and effects of search can be minimized by excluding the forgotten-versus-classify contrast. +++ = high; ++ = medium; + = low.
Conjunctions of these contrasts were performed to identify voxels in which BOLD signal was modulated (1) by both memory search and strength, (2) by memory search but not by strength, and (3) by memory strength but not by search (Table 1).
-
Search and strength
The search and strength analysis inclusively masked activations or deactivations from all three comparisons (i.e., examining the overlap across the following conditions: remembered > classify, forgotten > classify, and study-level effect [either low > medium > high study or low < medium < high study, confirmed by examining impulse response plots]; and separately, the overlap across the following conditions: remembered < classify, forgotten < classify, and study-level effect). As such, these regions were modulated positively or negatively by both search and strength.
-
Search only
The search-only analysis inclusively masked activations or deactivations from recall conditions identified by memory performance (i.e., highlighting regions where the retrieval event was not necessary to yield modulation of activity as demonstrated by overlap between remembered > classify and forgotten > classify or an overlap of remembered < classify and forgotten < classify) with an exclusion mask of the study-level comparison. Thus, these regions were modulated by retrieval conditions in a way that neither depended on retreival being present nor on memory strength.
-
Strength only
The strength-only analysis identified effects of study level during successful recall and applied an exclusion mask of search-based activity (i.e., excluding forgotten-vs.-classify activations and deactivations). Although search processes would also be engaged during remembered trials, the remembered-versus-classify contrast was not added as an exclusion mask because regions showing strength-driven responses may overlap with those activated during successful recall.
Comparisons were performed on parameter estimates from the period of 7.5–12.5 sec of each condition, when the hemodynamic response was expected to be most deflected from baseline based on a previous study using a similar task in a different set of participants (Reas et al., 2011). Group-level two-tailed voxelwise t tests were computed on each contrast, and ANOVA was conducted to examine effects of study level (all analyses: p < .05 and corrected for multiple comparisons). Significant clusters, including at least 13 contiguous voxels, were displayed on a statistical map overlaid onto an across-subject averaged structural image. Correction for multiple comparisons was performed before conjunction analyses using a Monte Carlo simulation on a whole-brain functional volume in AFNI (afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to determine the minimum cluster size necessary to achieve a family-wise error rate of p < .05. The hemodynamic response function was then extracted for each cluster of interest and averaged across participants to examine the signal time course in an impulse-response plot.
RESULTS
Behavior
Participants correctly classified 98 ± 1% (mean ± SE) of classify trials, responded “remember” to 64 ± 3% of recall trials, and correctly classified 86 ± 2% of remembered recall trials. Although accuracy did not differ according to study level in the classify task (p = .78), effects of study level on both recall (F(2, 32) = 97.73,p < .001) and classification (F(2, 32) = 11.44, p < .001) accuracy were observed in the recall task. Pairwise comparisons revealed better recall with increasing study repetitions (36 ± 3%, 73 ± 4%, and 84 ± 4%; ps < .001) and more accurate classification for the high- than low-study recall conditions (90 ± 2% vs. 78 ± 4%,p < .001).
Response times were 1229 ± 76,2205 ± 104, and 2840 ± 87 msec for the classify, recall, and recall plus classification responses, respectively. Recall responses were faster for remembered than forgotten pairs (2027 ± 104 vs. 2725 ± 142 msec; t(16) = 5.17,p < .001). Correct recall responses showed an effect of study level (F(2, 32) = 28.03,p < .001), reflecting faster response times with increasing study repetitions (2399 ± 118,2045 ± 114, and 1866 ± 107 msec; ps < .001). Classify response times did not differ according to study level (p = .75).
During the postscan test, participants correctly recalled 78 ± 4% of pairs reported remembered during the recall task and forgot 75 ± 3% of pairs reported forgotten or to which participants did not respond during the recall task, confirming relative consistency between subjective reports and overt assessment of recall. Postscan recall was better for pairs that had appeared in the recall task than in the classify task (60 ± 4% vs. 51 ± 5%; F(1, 16) = 17.24, p < .001), and a main effect of study level (F(2, 32) = 163.92, p < .001) reflected better postscan recall with increasing study repetitions (23 ± 5%, 64 ± 5%, and 79 ± 5%; ps < .001).
fMRI
Response Time Correlations
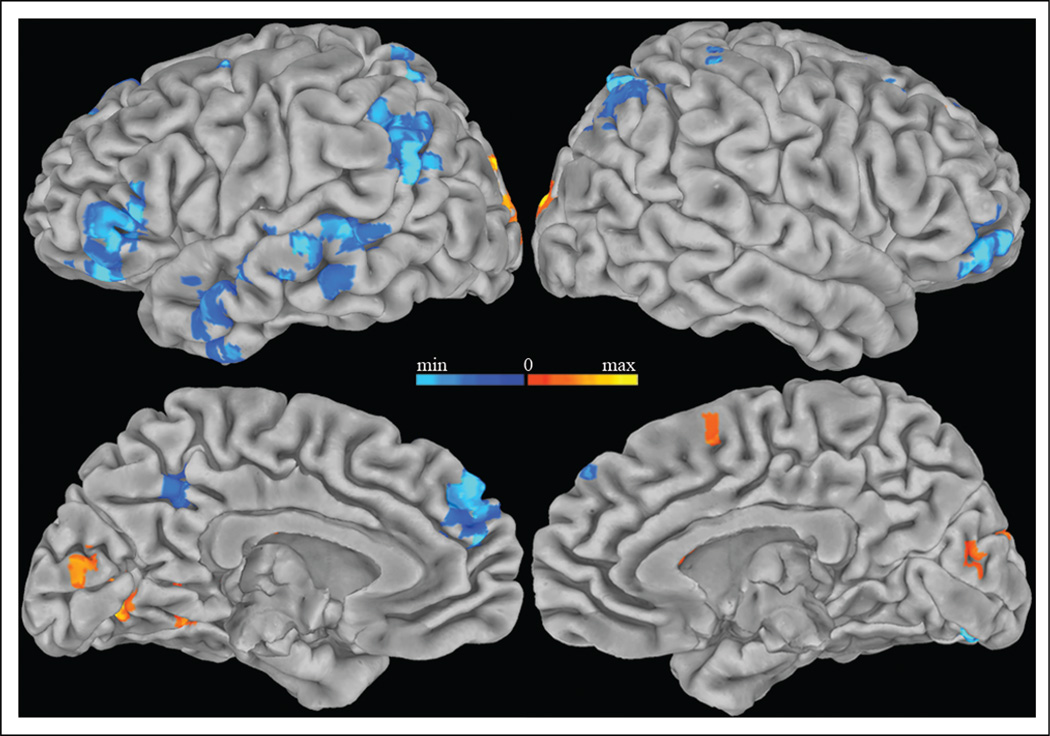
Episodic memory search may involve distinct components that depend either on the duration of the search process or on general engagement in search independent of the search duration. Using response times to approximate the duration of search, amplitude modulated regression was performed to identify voxels in which the hemo-dynamic response magnitude correlated with the response time of recall responses. The response time correlation for the recall task was contrasted with the correlation for the classify task to distinguish response modulation related to episodic memory search from modulation related to semantic memory search. Regions in which BOLD signal showed a greater negative correlation with recall than classify response times (p < .05, two-tailed and corrected for multiple comparisons) included bilateral dorso-medial PFC (DMPFC), inferior parietal and inferior frontal cortex, and left precuneus and middle temporal cortex (Figure 2). Activity in these regions was more deactivated with longer response times during the recall task than the classify task.
Figure 2.
Regions correlated with response time. Areas more positively (warm colors) and negatively (cool colors) correlated with response times during the recall task than the classify task (p < .05, corrected for multiple comparisons) are displayed on the Talairach and Tournoux N27 average pial surface. Longer response times were associated with less activity in bilateral dorsomedial prefrontal, inferior frontal and inferior parietal cortex, and left precuneus and middle temporal cortex.
Search and Strength
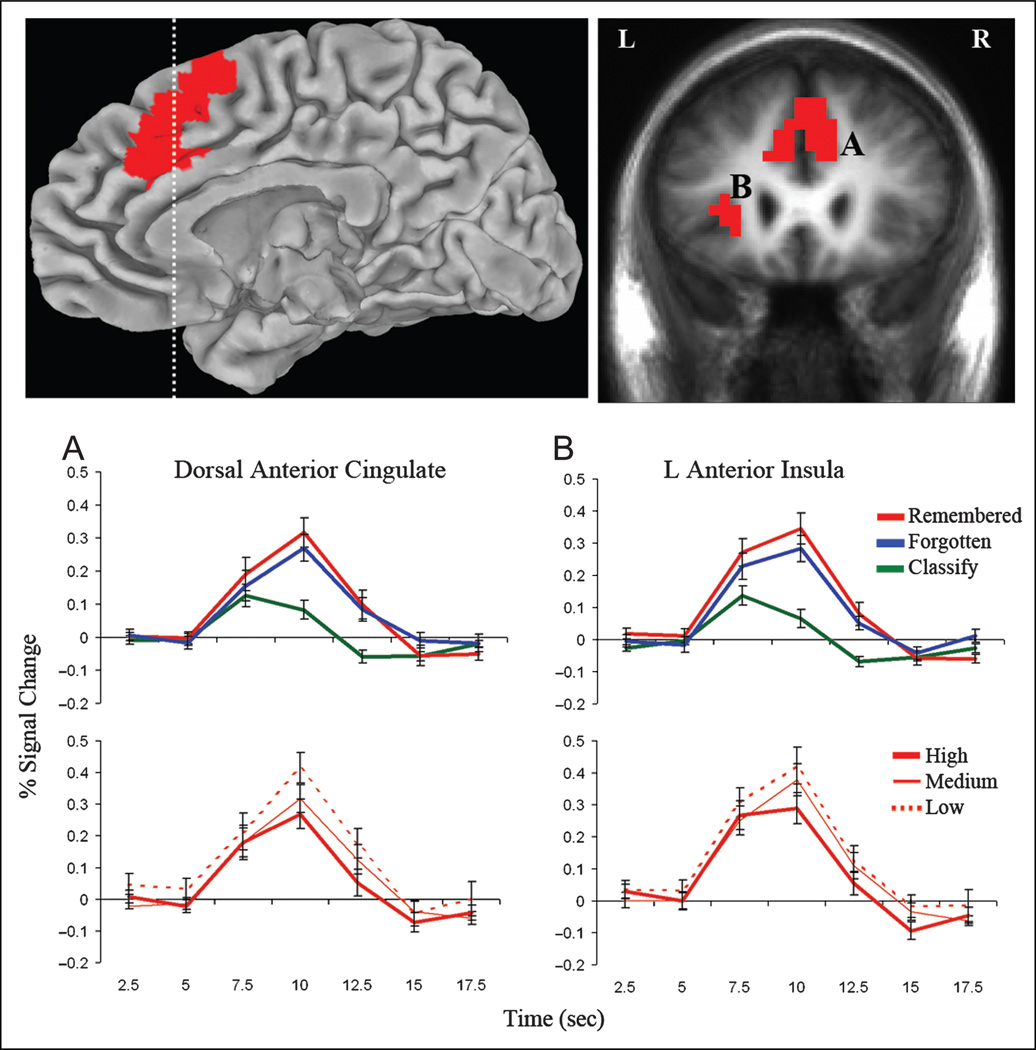
To identify activity related with both episodic memory search and strength, independent of time-on-task, the overlap for the remembered versus classify, forgotten versus classify, and study-level comparisons was examined, controlling for response time in each comparison. Regions identified as responsive to both search and strength (Methods, Analysis 1: Search and Strength; p < .05, two-tailed and corrected for multiple comparisons) included dorsal ACC (DACC) and left anterior insula (Figure 3; Table 2A). Impulse response curves in these regions confirmed greater activation during both remembered and forgotten trials than classify trials and increasing activity from high- to medium- to low-study recall conditions. A main effect of task was observed in these regions (F(2, 32) = 26.83, p < .001), and pairwise comparisons revealed greater activation for remembered and forgotten trials than classify trials (ps < .001), with no difference between remembered and forgotten trials (p = .18). A main effect of study level (F(2, 32) = 11.74, p < .001) confirmed greater activation for low- than high-study recall (p < .001) and a stepwise increase in activation from the high- to medium- (p < .01) and medium- to low-study (p < .05) recall conditions. No regions in this conjunction analysis showed the opposite study-level effect, with increasing activity with greater memory strength. Thus, regions activated by attempted memory retrieval, if modulated by strength, were always more activated by retrieval of weaker memories.
Figure 3.
Activity in DACC (A) and left anterior insula (B) increased during search and was modulated by memory strength. Statistical activation maps show the conjunction of regions with greater activity during remembered and forgotten recall trials than classify trials and increasing activity from the high- to medium- to low-study recall conditions (p < .05, corrected for multiple comparisons). Clusters are overlaid on the right medial pial surface of the Talairach and Tournoux N27 average brain and a coronal cross-section (indicated with dashed line) of the mean anatomical image of all participants. Impulse–response plots display the time course of the percent signal change (± SE) in these clusters for the remembered, forgotten, and classify trials and high-, medium-, and low-study recall conditions.
Table 2.
Significant Clusters (p < .05) for the Search and Strength (A), Search-only (B), and Strength-only (C) Analyses
| Region | BA | Volume (mm3) |
x | y | z | Remembered > Classify, Max t |
Forgotten > Classify, Max t |
Study Level, Max F |
|---|---|---|---|---|---|---|---|---|
| (A) Search and Strength | ||||||||
| DACC | 32 | 8704 | 1 | 21.8 | 41 | 6.98 | 6.48 | 16.18 |
| L DLPFC | 9 | 1088 | −45.5 | 13.5 | 32.5 | 7.86 | 3.55 | 11.76 |
| L anterior insula | 13 | 1024 | −29.4 | 22.8 | 6.5 | 7.74 | 6.79 | 14.64 |
| (B) Search-only | ||||||||
| L occipital | 18 | 9408 | −5.8 | −76.2 | 2.7 | 6.47 | 4.18 | 2.80 |
| R superior temporal | 42 | 9344 | 51 | −12.4 | 12.6 | −5.11 | −5.55 | 5.10 |
| L anterior insula | 13 | 5824 | 33.2 | 20.5 | 4.7 | 6.45 | 6.90 | 6.36 |
| L superior temporal | 22 | 4672 | −55.5 | −8 | 0.3 | −4.79 | −6.16 | 6.16 |
| R inferior parietal | 39 | 3008 | 49.7 | −58.6 | 27.8 | −4.14 | −4.86 | 3.10 |
| DMPFC | 9 | 2880 | −6 | 49.5 | 28.1 | −5.18 | −4.90 | 6.02 |
| L temporal pole | 38 | 2688 | −44.6 | 8.6- | 24.8 | −4.27 | −6.98 | 2.44 |
| DACC | 32 | 2368 | 1.1 | 24 | 31.3 | 5.75 | 4.79 | 3.27 |
| L posterior insula | 13 | 2240 | −30.4 | 23.2 | 4.8 | 5.66 | 4.88 | 3.18 |
| L DLPFC | 9 | 1920 | −41.5 | 6.2 | 33.6 | 7.31 | 3.01 | 3.97 |
| L inferior parietal | 39 | 1408 | −59.5 | −60.5 | 26.9 | −3.62 | −4.66 | 3.63 |
| L middle frontal | 8 | 1344 | −24.2 | 15.5 | 36.9 | −4.34 | −5.82 | 2.75 |
| Medial parietal | 3 | 1280 | 6 | −33.7 | 61.8 | −2.88 | −5.45 | 1.09 |
| R superior frontal | 6 | 1216 | 21.5 | 29.1 | 53.8 | −4.49 | −3.36 | 2.20 |
| L posterior insula | 13 | 1088 | −38 | −21.2 | 22.8 | −4.17 | −3.16 | 3.10 |
| R temporal pole | 38 | 1024 | 50.2 | 10.1 | −18.7 | −3.57 | −3.57 | 7.48 |
| R superior frontal | 6 | 896 | −1.1 | 8 | 61.4 | 6.59 | 4.13 | 3.29 |
| (C) Strength-only (High > Low) | ||||||||
| L inferior parietal | 40 | 14400 | −48.6 | −44.9 | 22.3 | 5.25 | −2.32 | 17.35 |
| R superior parietal | 40 | 7168 | 38.3 | −38.4 | 56.2 | 3.61 | −2.80 | 10.98 |
| R superior temporal | 22 | 4480 | 50.5 | −44.6 | 14.2 | 3.47 | −2.09 | 12.78 |
| L medial frontal | 6 | 3904 | −11.8 | −18.1 | 52.9 | 3.20 | −2.14 | 11.26 |
| R inferior parietal | 39 | 3840 | 34.9 | −70.6 | 23.1 | 5.42 | 2.44 | 7.06 |
| L superior parietal | 7 | 1408 | −23 | −47.8 | 54.1 | 3.92 | −2.11 | 10.48 |
| Strength-only (Low > High) | ||||||||
| L medial frontal | 8 | 6464 | −1.4 | 28 | 46.9 | 5.95 | 2.09 | 22.27 |
| L DLPFC | 10 | 3648 | −37.4 | 45.4 | 8.9 | 4.58 | −2.08 | 9.86 |
| L DLPFC | 46 | 3328 | −45.3 | 17.5 | 26.6 | 6.80 | 2.10 | 11.44 |
| L middle frontal | 6 | 1088 | −33.3 | 5.7 | 58.9 | 5.53 | −1.92 | 6.68 |
| L fusiform | 20 | 832 | −45.5 | −12.9 | −21.2 | 3.33 | −1.74 | 6.00 |
Regions more active for remembered and forgotten trials than classify trials and modulated by study level (A), more active for remembered and forgotten trials than classify trials with no effect of study level (p <.05; B), and modulated by study level with no significant difference (p < .05) between forgotten and classify trials (C) are presented. Only cortical clusters including at least 13 voxels are presented. Talairach coordinates (x, y, and z) correspond to the center of mass for each cluster. Maximum t or F values are presented for each comparison. BA = Brodmann’s area; L = left; R = right; Max = maximum.
Search Only
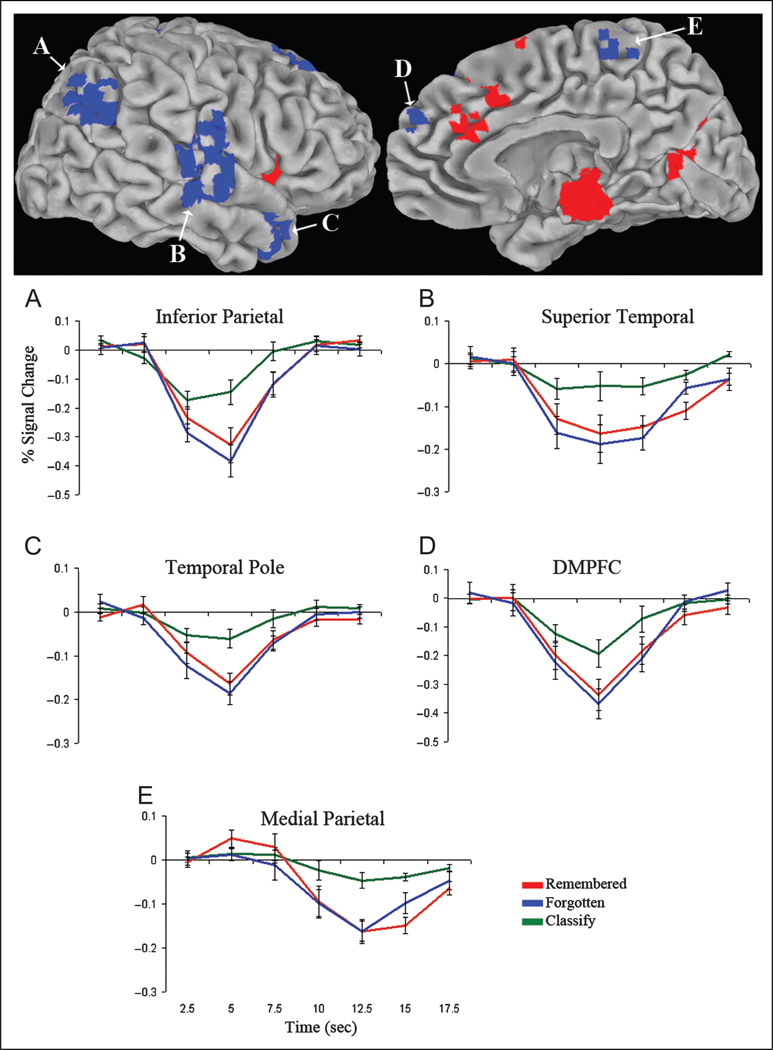
Responses associated with search but not modulated by memory strength or response time (Methods, Analysis 2: Search only; p < .05) were observed in bilateral DMPFC, temporal pole, superior temporal, medial parietal, and inferior parietal cortex (Figure 4; Table 2B), a subset of the default network. Impulse response curves from these regions illustrated greater negative deflection from baseline during both remembered and forgotten relative to classify trials. Because no hemispheric differences were found in inferior parietal cortex (p = .41), superior temporal cortex (p = .57), or temporal pole (p = .55), left and right impulse response curves for these clusters were averaged for display. A task effect in these clusters (F(2, 32) = 34.08, p < .001) was driven by greater deactivation for remembered and forgotten trials than classify trials (ps < .001), with no difference between remembered and forgotten trials (p = .09). There was no effect of study level in these regions (p = .60).
Figure 4.
Regions activated by search but not by memory strength. Statistical activation map displaying the conjunction of regions more (red) or less (blue) active during remembered and forgotten trials than classify trials (p < .05, corrected for multiple comparisons), with an exclusion mask of regions in which activity differed (p < .05) between low-, medium-, and high-study recall conditions. Clusters are overlaid on the right pial surface of the Talairach and Tournoux N27 average brain. Graphs depict the time course of the percent signal change (± SE) in bilateral inferior parietal cortex (A), superior temporal cortex (B), temporal pole (C), DMPFC (D), and medial parietal cortex (E), illustrating greater negative deflection from baseline during remembered and forgotten trials relative to classify trials.
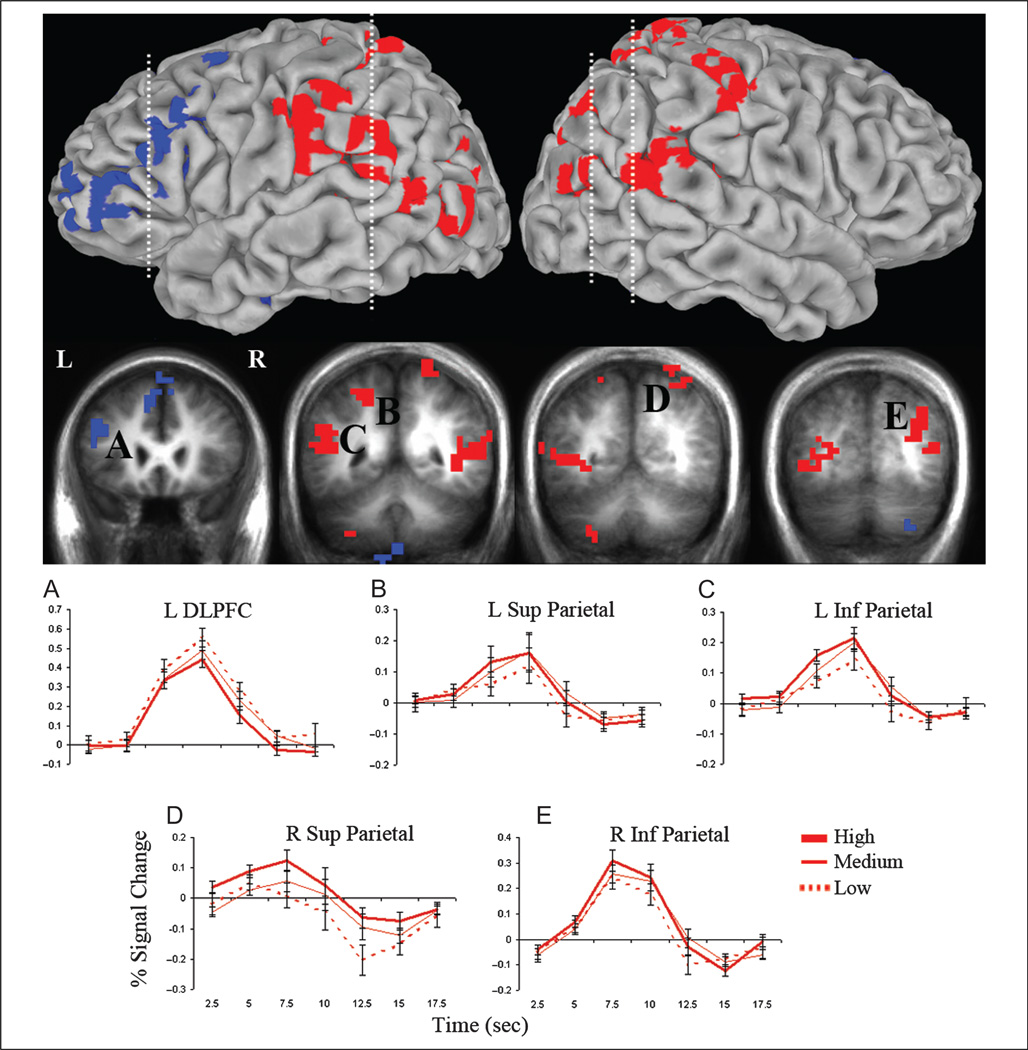
Strength Only
Regions showing a study-level effect but not strongly activated by search nor modulated by response time (Methods, Analysis 3: Strength only; p < .05) included left dorsolateral PFC (DLPFC) and bilateral superior and inferior parietal cortex (Figure 5; Table 2C). Parietal impulse response curves showed a stepwise increase in activity from low- to medium- to high-study recall conditions and greater activity during remembered trials than both forgotten and classify trials. An effect of study level (F(2, 32) = 20.34, p < .001) and a Study level × Region interaction (F(6, 96) = 3.42, p < .01) reflected greater activation for high-than low-study recall (ps < .001) and medium- than low-study recall (ps < .01) in all parietal regions and for high- than medium-study recall in right superior parietal cortex (p < .01). Left DLPFC demonstrated an inverse strength effect, with increasing activity from high- to medium- to low-study conditions. An effect of study level (F(2, 32) = 8.77, p < .001) confirmed greater activation for the low- than high-study (p < .01) and medium- than high-study (p < .01) recall conditions. Activity in parietal regions (F(2, 32) = 13.64, p < .001) and left DLPFC (F(2, 32) = 37.21, p < .001) showed task effects, driven by greater activation for remembered trials than both forgotten (ps < .001) and classify (parietal, p < .01; DLPFC, p < .001) trials, with no difference between forgotten and classify trials (ps > .05).
Figure 5.
Regions modulated by memory strength but not significantly activated by search. Statistical activation map showing areas with increasing (red) or decreasing (blue) activity with increasing study level during recall (p < .05, corrected for multiple comparisons), with an exclusion mask of regions in which activity differed (p < .05) between the forgotten and classify trials. Clusters are overlaid on the lateral pial surface of the Talairach and Tournoux N27 average brain and coronal cross-sections (indicated with dashed lines) of the mean anatomical image of all participants. Graphs display the time course of the percent signal change (±SE) in the left DLPFC (A) and left and right superior (B, D) and inferior (C, E) parietal cortex for low-, medium-, and high-study recall conditions.
DISCUSSION
This study identified distinct sets of brain regions in which BOLD signals were differentially regulated by the attempt to retrieve an episodic memory and the strength of a recalled memory. Although behavioral measures of mental search and memory strength may be highly correlated, these findings indicate that these separable components of memory retrieval evoke dissociable brain activity. Areas of the default network, including medial and inferior lateral parietal cortex, DMPFC, superior temporal cortex, and temporal pole, were more strongly deactivated during task conditions that required retrieval attempts than during a nonmemory task but were not modulated by memory strength. In contrast, activations in DLPFC and regions of superior and inferior parietal cortex depended on the strength of a recalled memory but were not differentially modulated by retrieval attempt. Search- and strength-driven responses overlapped in DACC and anterior insula, which were both activated during attempted retrieval and modulated by memory strength.
Dissociating Retrieval Strength from Search
The cascade of neural processes required for recollection may be initiated by control or attentional mechanisms that guide sequential search processes necessary for any nonspontaneous, effortful recall attempt (Nobel & Shiffrin, 2001). The extent to which brain regions subserving mental search are engaged during successful recall may be modulated in part by the strength of the recalled memory; however, strength should contribute minimally, if at all, to search-driven signals when a memory is not retrieved. Although memory strength is expected to increase parametrically with increasing study repetitions (de Zubicaray et al., 2010), the degree of mental search required to retrieve a studied association may not necessarily follow an identical parametric modulation but may be influenced by alternative factors.
This study developed distinct operational definitions of search and recall strength to dissociate (1) activations related to retrieval attempt that do not vary according to memory strength from (2) responses that depend on the strength of a recalled memory but are not strongly modulated by retrieval attempt. In the current study, directed search for an episodic memory should not occur during the classify task, which should only require semantic search processes, but is expected to be engaged during the recall task regardless of retrieval success. Therefore, search-related activity was operationalized as a greater response during both remembered and forgotten recall trials than classify trials. The subset of activations related to retrieval strength, which might be weakly present in these contrasts (Table 1), was excluded by identifying effects of study repetition on the activity.
Differences in retrieval strength were identified by comparing successful recall of word pairs recently encountered with varying repetition. Critically, because these conditions did not differ according to recall success, effects should be predominantly driven by the variable strengths of the retrieved associations. To better isolate differences associated with recollection strength from confounding effects of search associated with unsuccessful retrieval, only remembered trials were included in the study-level comparison and an exclusion mask of the forgotten versus classify contrast was applied. Nevertheless, because of the inherent correlation between search and strength, this definition cannot comprehensively capture all strength-related activity while purely excluding search; rather than perfectly isolating strength-driven responses, it more likely reflects above-threshold strength signals that are minimally contaminated by search processes.
Default Network Deactivates during Effortful Retrieval Attempts
Task conditions that selectively required memory search deactivated several regions traditionally associated with the default network. This finding is consistent with prior research that default network activity is reduced during the performance of attentionally demanding, goal-directed tasks (Buckner, Andrews-Hanna, & Schacter, 2008; McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003; Raichle et al., 2001; Shulman et al., 1997), such as the effortful mental search required in this cued recall task. Activity in the default network positively correlates with medial temporal memory regions, negatively correlates with regions subserving attention and working memory (Newton, Morgan, Rogers, & Gore, 2010; Fox et al., 2005; Greicius et al., 2003, 2004), and is regulated by retrieval effort, success, or memory strength (Gimbel & Brewer, 2011; Kim, 2010; Daselaar et al., 2009; Henson et al., 2005). Prior studies have shown correlated activity in the hippocampus and default network during attempted recall, which is most strongly deactivated for poorly remembered associations (Reas et al., 2011). In this study, although the hippocampal response during successful recall was below threshold, the hippocampal response during failed recall was robustly deactivated, consistent with Reas et al. (2011). Despite evidence for default network activations during memory retrieval, which are generally attributed to autobiographical or self-referential task conditions (Spreng & Grady, 2010; Maguire, 2001; Andreasen et al., 1995), these results provide further evidence for task-negative responses in these regions during effortful episodic memory retrieval (Gimbel & Brewer, 2011; Israel, Seibert, Black, & Brewer, 2010), which may be driven by mental search processes (Reas et al., 2011). Furthermore, they expand on prior studies, which did not simultaneously assess effects of search and associative memory strength, to reveal that default network deactivations are more likely attributable to search than retrieval strength differences.
BOLD signal magnitude during retrieval can correlate with factors linked to response time, including the temporal duration of memory search or linear summation of the physiological response to time-on-task (Yarkoni, Barch, Gray, Conturo, & Braver, 2009). The primary search and strength analyses therefore controlled for this potential confound by including response time as an independent regressor. However, because more demanding, extended search efforts are expected to delay responses, this study also examined how retrieval response times modulate BOLD signal amplitude. Subregions of the default network demonstrated a negative correlation with response time during episodic retrieval attempt. This correlation was not as strong during the nonmemory classification task; however, the dynamic range of RT was smaller for this task, and so, one cannot conclude that default network activity is uniquely modulated by episodic memory search. Nevertheless, together with results from the primary search analysis, these findings support the interpretation that default network suppression is regulated to some degree by episodic memory search, including and beyond its effects on RT.
Parietal Cortex and DLPFC are Modulated by Memory Strength
Although the parietal cortex is known to serve an essential role in visuospatial attention, working memory, and sensory association (Corbetta & Shulman, 2002; Posner & Petersen, 1990), parietal subregions are also engaged during memory retrieval. However, whether parietal involvement is necessary versus auxiliary for memory retrieval remains unresolved. Imaging studies report increased BOLD responses and ERP amplitudes during recognition of previously studied items (Kahn et al., 2004; Konishi, Wheeler, Donaldson, & Buckner, 2000; McDermott, Jones, Petersen, Lageman, & Roediger, 2000; Donaldson & Rugg, 1998) as well as signal modulation by recognition confidence level, memory strength, perceived oldness, or recollection versus familiarity (Montaldi et al., 2006; Yonelinas et al., 2005; Shannon & Buckner, 2004; Wheeler & Buckner, 2003, 2004; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Rugg et al., 1998; Wilding & Rugg, 1996; Smith, 1993). However, inconsistent reports of episodic memory deficits following parietal lesions, and that any impairments are generally mild, suggest that parietal regions indirectly support memory retrieval. In accordance with prior research, this study confirmed that subregions of superior and inferior parietal cortex are regulated by the strength of a recalled memory and further demonstrated that this modulation was not significantly associated with the attempt to retrieve.
fMRI studies have identified regions of superior parietal cortex that are sensitive to strength but are also engaged by search. For example, activity in the intraparietal sulcus is regulated by retrieval confidence (Kim & Cabeza, 2007; Montaldi et al., 2006; Moritz et al., 2006; Yonelinas et al., 2005) and is more active for familiarity than recollection. This same region has been implicated in visual and memory search and in directing attention for strategic retrieval (Sestieri, Shulman, & Corbetta, 2010; Shulman, Ollinger, Linenweber, Petersen, &Corbetta, 2001; Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000) and demonstrates an early electrophysiological response during episodic memory recall associated with preretrieval search processes (Seibert et al., 2011). Notably, the strength-specific parietal activations in this study did not directly overlap with previously reported attention-related responses in intraparietal sulcus (Seibert et al., 2011; Shulman et al., 2001; Corbetta et al., 2000), consistent with evidence that lateral parietal cortex includes multiple submodules that perform distinct supportive roles during memory retrieval (Nelson et al., 2010). Although superior parietal regions might be expected to be engaged by recalling weaker memories or by more effortful retrieval attempts, in the present cued recall task, superior parietal responses showed greater activity for the successful retrieval of stronger memories. The diverse functions performed by superior parietal cortex may account for discrepant reports of its activation by search, familiarity, and recall of stronger memories (Seibert et al., 2011; Kim & Cabeza, 2007; Moritz et al., 2006; Wheeler & Buckner, 2003). These regions have been implicated in various operations such as allocating attention to task-relevant features, guiding retrieval mode, or performing postretrieval evaluation (Donaldson, Wheeler, & Petersen, 2010; Cabeza, 2008; Ciaramelli et al., 2008; Vilberg & Rugg, 2008; Dosenbach et al., 2007; Buckner, 2003), processes that may be highly engaged during recollection of a strong memory.
Inferior parietal regions are activated during recollection and recognition of more deeply encoded memories (Iidaka, Matsumoto, Nogawa, Yamamoto, & Sadato, 2006; Henson et al., 2005; Yonelinas et al., 2005; Shannon & Buckner, 2004; Wheeler & Buckner, 2004) but are not modulated by familiarity, and inferior parietal lesions selectively impair spontaneous recall while sparing guided retrieval (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007). Consistent with these reports, in this study, inferior parietal subregions were regulated by memory strength, demonstrating greater BOLD signal during recall of stronger associations. Critically, these findings expand on evidence that recollection activates inferior parietal cortex to reveal that, even within recollection, the magnitude of this activation depends on the strength of the recalled memory. These strength-sensitive parietal regions overlapped with the supramarginal and angular gyri of the TPJ, areas implicated in multiple convergent cognitive functions involved in attentional shifts during retrieval (Cabeza, Ciaramelli, & Moscovitch, 2012). Inferior parietal regions may subserve the spontaneous detection of task-relevant stimuli or may revert attention from the environment to an internal stimulus (Cabeza et al., 2012; Ciaramelli et al., 2008; Astafiev, Shulman, & Corbetta, 2006), processes that may be more strongly engaged by the attentional capture of a more deeply encoded memory.
Although both DLPFC and parietal cortex were sensitive to memory strength, these effects were inverted between regions, such that DLPFC was more active during weaker recall. DLPFC is functionally connected with superior parietal regions (Nelson et al., 2010; Dosenbach et al., 2007; Seeley et al., 2007) and may interact with these areas to guide retrieval mode or perform strategic monitoring during retrieval (Donaldson et al., 2010; Ciaramelli et al., 2008; Rugg, Henson, & Robb, 2003; Henson et al., 1999). A reversal of strength effects in DLPFC supports previous interpretations that, during retrieval, parietal responses signal retrieval success whereas frontal regions may perform error monitoring processes (Donaldson et al., 2010) that would be enhanced during retrieval of poorer memories. It is possible that differences associated with performing postretrieval classification may have contributed to differences between strength conditions. However, this is unlikely to be the predominant source of the observed strength effects, given prior reports that the same pre-frontal and parietal regions are engaged during retrieval tasks that do not involve semantic classification. Collectively, these findings suggest that lateral prefrontal and parietal regions integrate distinct retrieval-related attention and cognitive control processes that depend on the strength of the retrieval event.
Dissociable Networks with Overlapping Nodes Subserve Retrieval Strength and Search
Although search- and strength-driven responses were largely dissociable, DACC and anterior insula were both responsive to memory search and more active during recall of weaker associations. This is consistent with evidence that these areas are involved in the execution of various cognitive control processes that may indirectly support episodic memory retrieval such as goal-directed cognition, stimulus salience processing, and task set maintenance and may mediate these functions by integrating information from external and internal sources or across multiple domains such as attention or working memory. Activation of these regions by both retrieval effort and memory strength provides support for their role in multidomain control processing and is consistent with reports that these regions subserve functions as diverse as working memory, personal salience assessment, and autobiographical or spatial planning (Spreng et al., 2010; Vincent et al., 2008; Seeley et al., 2007).
Furthermore, DACC and anterior insula have been identified as nodes of a centralized control center, or frontoparietal control network, that integrates widespread signals from distinct, interactive neural networks. The functional–anatomical correlates of strength and search identified in this study correspond well with these intersecting attention and default networks. Prior studies have reported that these networks are anti-correlated or are engaged by tasks demanding attention or externally directed thought on the one hand and passive or internally directed processing on the other (Kim, 2010; Spreng et al., 2010; Vincent et al., 2008; Dosenbach et al., 2007; Seeley et al., 2007). Regions of these networks functionally dissociated during performance of this associative recall task, demonstrating differential sensitivities to retrieval effort and memory strength.
Conclusions
Multiple interactive neurocognitive processes may underlie brain activations during guided episodic memory retrieval. The present investigation reveals that, although highly correlated, retrieval effort and recollection strength mediate distinct responses in dissociable sets of brain regions. The finding of separable but overlapping search and strength areas, which correspond anatomically with three previously identified cortical networks, advances our understanding of the functional role of these default, attention, and cognitive control networks in episodic memory retrieval.
Acknowledgments
This work was supported by NINDS K02 NS067427, the General Electric Medical Foundation, and the Department of Neurosciences, University of California, San Diego.
REFERENCES
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, et al. Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. European Journal of Neuroscience. 2006;23:591–596. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Functional-anatomic correlates of control processes in memory. Journal of Neuroscience. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI: I. Retrieval effort versus retrieval success. Neuroimage. 1998;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends in Cognitive Sciences. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera BS, Amdur RJ, Patel P, Mendenhall WM. A radiation oncologist’s guide to contouring the hippocampus. American Journal of Clinical Oncology. 2009;32:20–22. doi: 10.1097/COC.0b013e318178e4e8. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers in Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Frontiers in Human Neuroscience. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray GI, McMahon KL, Dennis S, Dunn JC. Memory strength effects in fMRI studies: A matter of confidence. Journal of Cognitive Neuroscience. 2010;23:2324–2335. doi: 10.1162/jocn.2010.21601. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Rugg MD. Recognition memory for new associations: Electrophysiological evidence for the role of recollection. Neuropsychologia. 1998;36:377–395. doi: 10.1016/s0028-3932(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Wheeler ME, Petersen SE. Remember the source: Dissociating frontal and parietal contributions to episodic memory. Journal of Cognitive Neuroscience. 2010;22:377–391. doi: 10.1162/jocn.2009.21242. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gimbel SI, Brewer JB. Reaction time, memory strength, and fMRI activity during memory retrieval: Hippocampus and default network are differentially responsive during recollection and familiarity judgments. Cognitive Neuroscience. 2011;2:19–23. doi: 10.1080/17588928.2010.513770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Quarterly Journal of Experimental Psychology B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson RN, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: An fMRI study. Journal of Cognitive Neuroscience. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: Dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N. Frontoparietal network involved in successful retrieval from episodic memory. Spatial and temporal analyses using fMRI and ERP. Cerebral Cortex. 2006;16:1349–1360. doi: 10.1093/cercor/bhl040. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Israel SL, Seibert TM, Black ML, Brewer JB. Going their separate ways: Dissociation of hippocampal and dorsolateral prefrontal activation during episodic retrieval and post-retrieval processing. Journal of Cognitive Neuroscience. 2010;22:513–525. doi: 10.1162/jocn.2009.21198. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: Implications for models of recognition memory. Journal of Neuroscience. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: A PET study. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50:1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: Dissociating the neural correlates of confidence in veridical versus illusory memories. Journal of Neuroscience. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Human Brain Mapping. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. Journal of Neuroscience. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL., III Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event-related fMRI study. Journal of Cognitive Neuroscience. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Moritz S, Glascher J, Sommer T, Buchel C, Braus DF. Neural correlates of memory confidence. Neuroimage. 2006;33:1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory— A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, et al. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Human Brain Mapping. 2010;32:1649–1659. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PA, Shiffrin RM. Retrieval processes in recognition and cued recall. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:384–413. doi: 10.1037/0278-7393.27.2.384. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas ET, Gimbel SI, Hales JB, Brewer JB. Search-related suppression of hippocampus and default network activity during associative memory retrieval. Frontiers in Human Neuroscience. 2011;5:112. doi: 10.3389/fnhum.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert TM, Gimbel SI, Hagler DJ, Jr, Brewer JB. Parietal activity in episodic retrieval measured by fMRI and MEG. Neuroimage. 2011;55:788–793. doi: 10.1016/j.neuroimage.2010.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: Functional specialization and dynamic competition in human posterior parietal cortex. Journal of Neuroscience. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: 2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Linenweber M, Petersen SE, Corbetta M. Multiple neural correlates of detection in the human brain. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:313–318. doi: 10.1073/pnas.021381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgments. Journal of Cognitive Neuroscience. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Okado Y. Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. Journal of Neuroscience. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system: An approach to cerebral imaging. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- Tulving E. Precis of Tulving elements of episodic memory (Oxford-University-Press, 1983) Behavioral and Brain Sciences. 1984;7:223–238. [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wais PE. Hippocampal signals for strong memory when associative memory is available and when it is not. Hippocampus. 2011;21:9–21. doi: 10.1002/hipo.20716. [DOI] [PubMed] [Google Scholar]
- Wais PE, Squire LR, Wixted JT. In search of recollection and familiarity signals in the hippocampus. Journal of Cognitive Neuroscience. 2010;22:109–123. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: Control, perceived oldness, and content. Journal of Neuroscience. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS One. 2009;4:e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]