Abstract
Aging purportedly diminishes the ability of the skeleton to respond to mechanical loading, but recent data show that old age did not impair loading-induced accrual of bone in BALB/c mice. Here, we hypothesized that aging limits the response of the tibia to axial compression over a range of adult ages in the commonly used C57BL/6. We subjected the right tibia of old (22 month), middle-aged (12 month) and young-adult (5 month) female C57BL/6 mice to peak periosteal strains (measured near the mid-diaphysis) of –2200 με and –3000 με (n=12-15/age/strain) via axial tibial compression (4 Hz, 1200 cycles/day, 5 days/week, 2 weeks). The left tibia served as a non-loaded, contralateral control. In mice of every age, tibial compression that engendered a peak strain of –2200 με did not alter cortical bone volume but loading to a peak strain of –3000 με increased cortical bone volume due in part to woven bone formation. Both loading magnitudes increased total volume, medullary volume and periosteal bone formation parameters (MS/BS, BFR/BS) near the cortical midshaft. Compared to the increase in total volume and bone formation parameters of 5-month mice, increases were less in 12– and 22-month mice by 45-63%. Moreover, woven bone incidence was greatest in 5-month mice. Similarly, tibial loading at –3000 με increased trabecular BV/TV of 5-month mice by 18% (from 0.085 mm3/mm3), but trabecular BV/TV did not change in 12– or 22-months mice, perhaps due to low initial BV/TV (0.032 and 0.038 mm3/mm3, respectively). In conclusion, these data show that while young-adult C57BL/6 mice had greater periosteal bone formation following loading than middle-aged or old mice, aging did not eliminate the ability of the tibia to accrue cortical bone.
Keywords: Mouse, Aging, Tibial Compression, MicroCT, Bone Formation
INTRODUCTION
Aging ostensibly reduces the responsiveness of the skeleton to mechanical loading. In humans [1] and mice [2, 3], age-related loss of bone mass is associated with reduced bone formation, governed by the altered activity and recruitment of osteoblasts [1, 4]. Mechanical loading might offset such reduction, and exercise studies in humans and rodents support this view. In humans, exercise partially attenuates bone loss from aging [5] and inactivity [6], and maintains bone density in early post-menopausal women [7]. Most studies of old mice and rats report no detrimental effect of old age on the responsiveness of bone to exercise [8-10]. An advantage of exercise approaches in animals (e.g. jump training, treadmill running) is that they can engender mechanical strains greater than habitual loading by involving the intrinsic contractile forces of muscles. The main disadvantage is that the applied loading to specific bone sites is difficult to control or measure [11]. Thus, exercise protocols do not provide for tight control of all the factors that might influence the bone adaptive response including peak strain, strain rate, frequency and rest insertion. Moreover, because the exercise is completed on an incentive basis it may also induce physiological stress.
Direct (extrinsic) skeletal in-vivo loading is a well-established approach to increase bone mass and strength in pre-clinical studies, while allowing for precise control over the local mechanical environment at the site of interest [11]. Several studies have reported greatly reduced or even negligible responses to skeletal loading in older animals, although these studies were limited to cortical bone and were done using invasive methods [12] or directly via application of loads in a non-physiological direction [13, 14]. To overcome these limitations, axial tibial compression was developed as a non-invasive loading method that applies loads in a physiological direction, and can stimulate cortical and cancellous bone accrual [15, 16]. In contrast to most direct loading studies [12–14], tibial compression of aged (22 month), male BALB/c mice induced greater endocortical bone formation and equivalent periosteal bone formation compared to young-adult (7 month) mice [17]. On the other hand, accrual of cortical bone volume was greater in 4-month old female BALB/c mice than in 7– and 12–month-old mice loaded by tibial compression [18]. The basis underlying these age effects in BALB/c mice is unclear but while BALB/c mice lose a modest amount of bone with advanced aging [3], it may be more appropriate to use C57BL/6 mice, which display greater cortical and trabecular bone loss with aging [19-21]. Studies challenging C57BL/6 mice at different ages to increase bone formation with tibial compression are limited. When compared to growing (2-3 month) mice, young-adult (5-6 month) C57Bl/6 female mice consistently produce less trabecular bone [15, 22, 23] although cortical results are mixed, with one study finding diminished response in adult mice [22] but others finding no difference between growing and adult cortical responsiveness [15,23]. It is unclear if diminished adaptation to tibial loading occurs from young-adult to old age in C57BL/6 mice. In summary, prior studies of aging and loading in rodents have been limited by the loading method used, by the choice of mouse strain or by failure to include truly old animals.
To address these limitations, we applied non-invasive tibial compression in order to assess the mechano-responsiveness of C57Bl/6 mice (an accepted model for skeletal aging) across a range of adult ages. We subjected 5-, 12– and 22-month-old female mice to two loading magnitudes and evaluated the tibial response using in vivo micro Computed Tomography and dynamic histomorphometry. We hypothesized that response to loading would diminish with aging. Results indicate that ‘truly old’ (22 months) and middle-aged (12 months) C57Bl/6 mice have reduced cortical and cancellous adaptation to tibial compression compared to young-adult mice (5 months).
METHODS
Animals
Mice were purchased from the National Institute of Aging (NIA, Bethesda, MD, USA) aged mouse colony (female, C57Bl/6). Ages represented three stages in life, as defined by Flurkey et al. [24]: 5 month (young-adult), 12 month (middle-aged), and 22 month (old). These stages correspond approximately to 20-30, 38-47 and 56-69 years in humans, respectively [24]. For the young-adult group, 5 months was selected because it is past the period of rapid bone accrual that occurs with growth [20, 21, 25], and near the age of ‘peak bone mass’ for female C57Bl/6 mice based on measures of total bone BMD [20, 26], size and strength [25]. In C57Bl/6J mice, 50% survival occurs at ~30 months of age [27] and old age begins at 18 months [24]. Therefore, we chose 12 and 22 months to represent before and after the beginning of old age. We recently assessed bone morphology at the tibial diaphysis in these three ages, and reported that total cross-sectional area did not change with age, whereas cortical bone area and thickness declined from 5 to 12, and from 12 to 22 months due to medullary expansion [28]. Mice were housed 4-5 per cage under standard conditions for 1-2 weeks before they were used in experiments. The work in this study involving animals is in compliance with all applicable agency policies and procedures. The study was approved by the Washington University Animal Studies Committee.
In Vivo Loading
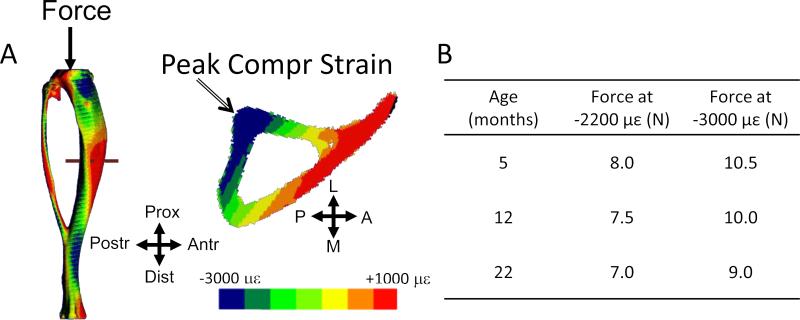
Mice were anesthetized (2-3% isofluorane) and their right tibiae subjected to axial compression for 1200 cycles/day (4 Hz triangle waveform with 0.1 sec rest-insertion after each cycle) using a materials testing system (Electropulse 1000, Instron, Norwood, MA, USA). This protocol has been shown to be anabolic for cortical and trabecular bone in young-adult mice [22, 29, 30]. Based on a recent strain gauge analysis [28], we selected age-specific peak forces in order to generate peak periosteal strains of –2200 με and –3000 με at the cortical mid-shaft (Fig. 1). These peak compressive values occur at the postero-lateral apex of the tibial cross-section (5 mm proximal of the tibio-fibular junction), with corresponding peak tensile values on the opposite surface of 1200 and 1600 με, respectively [28]. Throughout the manuscript we use the labels “–2200 µε” and “–3000 με” for purposes of describing the two loading magnitudes. We note that other locations within the cross section experience strains below these peak levels, and we emphasize that the nominal peak strain does not describe the applied strain over the entire tibia or within the cancellous compartment. Mice (n = 12–15 for each force and age group) were loaded 5 days/week for 2 weeks (study days 1-5 and 8-12). After each loading session, mice received buprenorphine (0.1 mg/kg i.m.) to mitigate any pain associated with knee joint injury [31], and were returned to their cages where they were observed to resume unrestricted activity. The left tibia served as a non-loaded, contralateral control.
Figure 1.
(A) Contour plots of axial strain engendered by tibial compression (adapted from Patel et al., 2014, with permission). Shown is a 5-month sample at a force of 10 N. The peak tibial strain magnitude is compressive and occurs at the postero-lateral apex of the cross-section (arrow). The force magnitudes required to generate peak strains of –2200 and –3000 microstrain (με) in female C57Bl/6 mice were determined from strain gauge measurements [28] and are shown in the (B) table. L: Lateral, A: Anterior, M: Medial, P: Posterior.
Micro Computed Tomography
Right and left tibiae were scanned in vivo at the beginning of the study (“0 week”, 3 or 4 days prior to loading) and immediately before sacrifice (“2 weeks”, day 15 or 16) by microCT (VivaCT 40, Scanco, Brüttisellen, Switzerland) to determine bone morphology and mineral density. Scan resolution was 21 μm (70 kV, 114 μA, 100 ms integration time). This scanning protocol has proven sensitive enough to detect longitudinal bone changes [17, 18], and represents a compromise that sacrifices optimal image quality in order to reduce radiation exposure. Negative radiation side effects have been noted by others who scanned at a higher resolution (10 μm) and more frequently (every 5 days) [23]. Cortical parameters were assessed at a region of interest (hereafter referred to as the mid-diaphysis) centered 5 mm proximal to the distal tibiofibular junction (TFJ), spanning 34 slices (0.7 mm). This is the mid-point of the region bounded by the proximal and distal TFJs and is the location where strain values (Fig. 1) were measured [28]. It is proximal to the 50% midpoint of the tibia, but corresponds approximately to the location of peak strain along the tibial length [28, 32, 33]. Trabecular parameters were assessed at the proximal metaphysis, distal of the growth plate, and spanned 100 slices (2.1 mm), of which 30 slices, following disappearance of the growth plate, were analyzed. Automatic contouring was used to analyze all slices with a lower/upper threshold of 180/1000 (330 mg HA/cm3, selected based on visual inspection) to segment bone from other tissue. The outcomes for cortical bone included bone volume (Ct.BV), total volume (Ct.TV), medullary volume (Me.V), and tissue mineral density (TMD). The outcomes for trabecular bone included bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and volumetric bone mineral density (vBMD).
Dynamic Histomorphometry
Fluorochromes were injected intraperitoneally on days 5 (calcein green, 10 mg/kg; Sigma, Saint Louis, MO, USA) and 12 (alizarin complexone, 30 mg/kg; Sigma), and mice were killed on day 17. Dynamic histomorphometric analysis was performed on 30 μm thick sections taken at the same mid-diaphyseal location used for cortical microCT analysis, using commercial software (Osteo II, BIOQUANT, Nashville, TN, USA) to determine measures of bone formation. Those measures included standard lamellar indices: single- and double-labeled surface per bone surface (sLS/BS, dLS/BS), mineralizing surface (MS/BS), mineral apposition rate (MAR), and bone-formation rate (BFR/BS) [34]. For each section, we analyzed the entire endocortical (Ec) and periosteal (Ps) surfaces separately. To account for the presence of woven bone formation in some samples, MS/BS+, MAR+ and BFR/BS+ were modified (+) to include both woven and lamellar values. MS/BS+ was the addition of lamellar MS/BS and woven mineralizing surface, the latter equal to the percentage of woven perimeter (Wo.S/BS). MAR+ was the weighted interlabel width per day for both lamellar and woven bone and was represented by equation (Ir.Wi · dL.Pm + Mean.Wo.Wi · Wo.Pm)/ (5 · (dL.Pm + Wo.Pm)), where Ir.Wi is lamellar interlabel width, dL.Pm is double label perimeter, Mean.Wo.Pm is average woven width, Wo.Pm is woven perimeter and 5 represents the number of days between labeling injections. This is analogous to the approach of de Souza et al. [15] who reported interlabel area on samples showing a mix of lamellar and woven formation. BFR/BS+ was the product of MS/BS+ and MAR+. When there was no woven bone, these measures simplify to the traditional lamellar indices from Dempster et al. [34].
Statistics
Paired t-tests compared tibial structure longitudinally (0 vs. 2 wk) for each tibia, and bone formation indices between loaded tibiae and the time-referent control tibiae from the same animal (Right vs. Left). Chi-square tests compared the incidence of woven bone between left and right tibiae, and between ages. To address our primary question of age effects, one-way ANOVAs with post-hoc Tukey tests compared the relative change in tibial structure over two weeks and the bone formation indices between ages (5 vs. 12 vs. 22 month), with loaded and control tibiae analyzed separately. To address the effect of age on loading level, a two-way ANOVA (age and loading level [–2200 με vs –3000 με]) was performed, again with loaded and control tibiae analyzed separately, followed by post-hoc Tukey tests to compare outcomes between loading levels for each age. Relative (Loaded-Control) outcomes using dynamic histomorphometric parameters were avoided because non-detectable values (i.e., absent double-labels) would under-power several comparisons. Importantly, one- and two-way ANOVAs using relative Ps.MS/BS yielded similar results to those using loaded only values. Statistical significance was considered at p < 0.05 (StatView 5.0, SAS Institute, Cary, NC, USA).
RESULTS
Tibial Compression Increased Periosteal Bone Formation in All Ages but was Greatest in the Young-Adult Mice
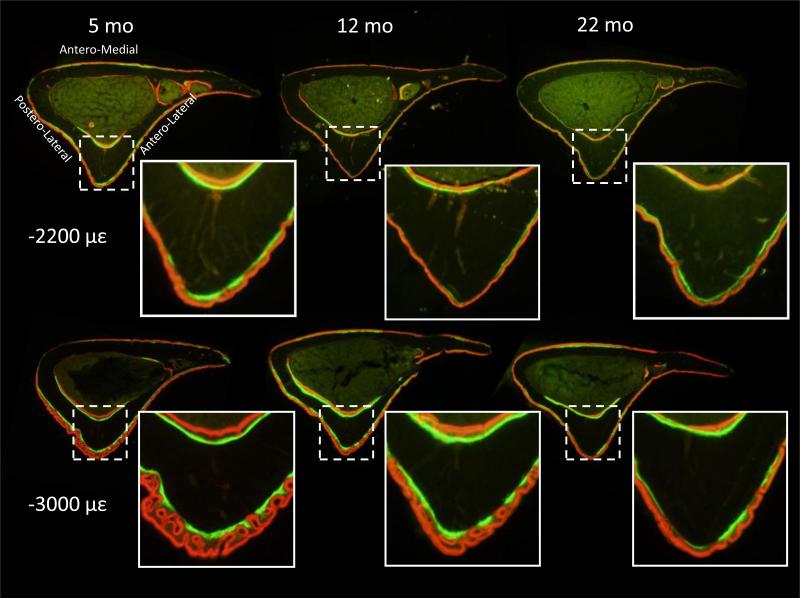
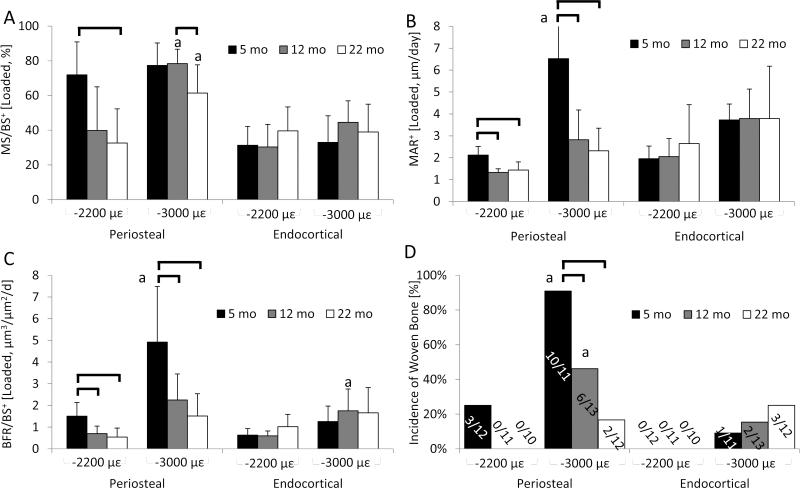
In agreement with our hypothesis, dynamic histomorphometric and microCT measures at the mid-diaphysis indicated that aging attenuated the response of cortical bone to 2 weeks of tibial compression at two levels of mechanical strain (–2200 and –3000 με). Both loading levels induced increased double-labeling on the periosteal and endocortical surfaces of tibiae in mice from all ages, while compression at –3000 με also initiated some periosteal woven bone (Fig. 2). On the periosteal surface, tibiae loaded at –2200 με had at least 12–fold more mineralizing surface than the control tibia at all ages from increases in both single- and double-labeling (Table 1). Bone formation rate was 7–fold greater in the loaded tibia than the control in 5-month old mice, but not different between loaded and control in 12– or 22-month mice because loading did not increase mineral apposition in older mice. Between ages, MS/BS+, MAR+ and BFR/BS+ of loaded tibiae were greater in 5-month-old mice than 12– and 22- month mice by 50-180%, with no differences between 12– and 22-month mice (Fig. 3). Loading at –3000 με induced a similar pattern of changes to periosteal bone formation indices as well as increased woven bone incidence, which was greatest in 5-month animals and declined with age (Table 1 and Fig. 3). Further, the larger tibial compression magnitude engendered a larger periosteal response in most of the bone formation indices of 5-month tibiae, but there were fewer magnitude-dependent increases in older tibiae. On the endocortical surface, some bone formation indices were elevated in the tibiae of 5-month mice loaded at –2200 με and in all tibiae loaded at –3000 με, but were not different between ages at either strain level. Reduced bone formation with aging was evident in several parameters in the control tibiae; here, periosteal MS/BS+ was greater in 5-month mice than in 22-month mice in the –2200 με group, and endocortical MS/BS+ was greater in 5-month mice than in 12–month mice in the –3000 με group.
Figure 2.
Dynamic histomorphometry images of tibiae (right) from a range of adult mice subjected to tibial compression at –2200 με and –3000 με peak periosteal strain. Lamellar double-labeling was evident in all ages and both loading magnitudes, but loading at –3000 µε also induced woven bone formation.
Table 1.
Dynamic histomorphometry of varying age cortical bone subjected to two different tibial compression loading magnitudes
| Outcomes | −2200 με | −3000 με | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mo (n=12) | 12 mo (n=11) | 22 mo (n=11) | 5 mo (n=11) | 12 mo (n=13) | 22 mo (n=12) | |||||||
| Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | |
| Ps.sLS/BS [%] | 10 ± 9 | 28 ± 15* | 4 ± 6 | 45 ± 29* | 2 ± 2 | 41 ± 21* | 27 ± 20 | 16 ± 11 | 21 ± 18c | 25 ± 13 | 13 ± 14 | 37 ± 16* |
| Ps.dLS/BS [%] | 0 ± 1 | 54 ± 24* | 0 ± 1 | 18 ± 20*a | 0 ± 0 | 12 ± 19a | 0 ± 0 | 48 ± 18* | 0 ± 0 | 61 ± 14* | 0 ± 0 | 41 ± 19*b |
| Ps.MS/BS+ [%] | 6 ± 6 | 69 ± 17* | 3 ± 4 | 40 ± 25* | 1 ± 1a | 33 ± 20*a | 14 ± 10 | 77 ± 13* | 11 ± 9c | 78 ± 8* | 8 ± 7 | 61 ± 16*b |
| Ps.MAR+ [μm/d] | 1.2 ± NA | 2.1 ± 0.4 | 1.2 ± NA | 1.3 ± 0.2a | NA | 1.4 ± 0.4a | 1.3 ± 0.5 | 6.5 ± 3.9* | 0.7 ± NA | 2.8 ± 1.4a | 1.1 ± 0.1 | 2.3 ± 1.0a |
| Ps.BFR/BS+ [μm/d] | 0.21 ± NA | 1.51 ± 0.63* | 0.15 ± NA | 0.70 ± 0.35a | NA | 0.54 ± 0.42a | 0.17 ± 0.17 | 4.9 ± 2.6* | 0.12 ± NA | 2.3 ± 1.2a | 0.05 ± 0.01 | 1.5 ± 1.0a |
| Ps.Wo.S/BS [%] | 0 ± 0 | 3 ± 9 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 21 ± 16* | 0 ± 0 | 5 ± 8* | 0 ± 0 | 1 ± 2 |
| Ps.Woven Area [mm2] | NA | 0.04 ± 0.05 | NA | NA | NA | NA | NA | 0.12 ± 0.09 | NA | 0.05 ± 0.04 | NA | 0.05 ± 0.04 |
| Ps.Incidence of Woven | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 10* | 0 | 6*a | 0 | 2a |
| Ec.sL/BS [%] | 69 ± 26 | 23 ± 12* | 55 ± 19 | 34 ± 11* | 50 ± 12 | 41 ± 11 | 54 ± 16 | 15 ± 8* | 42 ± 14 | 11 ± 5* | 56 ± 11 | 26 ± 15* |
| Ec.dL/BS [%] | 3 ± 3 | 20 ± 9* | 8 ± 6 | 13 ± 11 | 7 ± 6 | 19 ± 12* | 5 ± 7 | 29 ± 12* | 5 ± 5 | 40 ± 14* | 6 ± 8 | 30 ± 12* |
| Ec.MS/BS+ [%] | 37 ± 12 | 31 ± 11* | 35 ± 13 | 30 ± 13 | 33 ± 9 | 40 ± 14 | 32 ± 8 | 36 ± 11 | 26 ± 8a | 45 ± 12 | 34 ± 8b | 43 ± 11* |
| Ec.MAR+ [μm/d] | 1.6 ± 0.93 | 2.0 ± 0.6* | 2.1 ± 0.78 | 2.1 ± 0.8 | 1.9 ± 1.08 | 2.6 ± 1.8 | 2.0 ± 0.7 | 3.7 ± 0.7* | 2.4 ± 1.4 | 3.8 ± 1.4* | 2.1 ± 0.5 | 3.8 ± 2.4 |
| Ec.BFR/BS+ [μm/d] | 0.53 ± 0.23 | 0.63 ± 0.3* | 0.72 ± 0.22 | 0.60 ± 0.22 | 0.61 ± 0.31 | 1.02 ± 0.56 | 0.65 ± 0.31 | 1.4 ± 0.6* | 0.54 ± 0.16 | 1.8 ± 1.0* | 0.79 ± 0.21 | 1.7 ± 1.2* |
| Ec.Wo.S/BS [%] | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0* | 0 ± 1 | 0 ± 0 | 2 ± 4 | 0 ± 0 | 3 ± 5 |
| Ec.Woven Area [mm2] | NA | NA | NA | NA | NA | NA | NA | 0.01 ± NA | NA | 0.02 ± 0.01 | NA | 0.03 ± 0.01 |
| Ec.Incidence of Woven | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 3 |
p<0.05, Control vs. Loaded
: significant difference compared to 5 month of respective load
: significant difference compared to 12 month of respective load
: significant difference compared to −2200 με (Control) , p<0.05.
NA: No samples with a non-zero value
Value ±NA: less than three samples with a non-zero value
Data are represented as mean±SD
MS/BS+, MAR/BS+, BFR/BS+ represent the combination of lamellar and woven values
Figure 3.
a) MS/BS+, (b) MAR+, (c) BFR/BS+ and (d) incidence of woven bone (number of mice per group showing load-induced woven bone) of the periosteal and endocortical envelopes from a range of adult tibiae (only right tibiae) subjected to compression at –2200 με and –3000 με. Aging reduced the periosteal bone formation by tibial compression at –2200 με with minimal differences in bone formation outcomes between middle-aged and old tibiae and no endocortical changes between ages. Loading at –3000 με induced similar changes but enhanced periosteal bone formation of 5-month tibiae by inducing woven bone. Data are represented as mean±SD. Black bracket: significant difference between ages; “a”: significant difference between loading magnitudes of respective age. +: Represents the combination of woven and lamellar values; p<0.05.
Tibial Compression Increased Cortical Total Volume in an Age-Dependent Manner
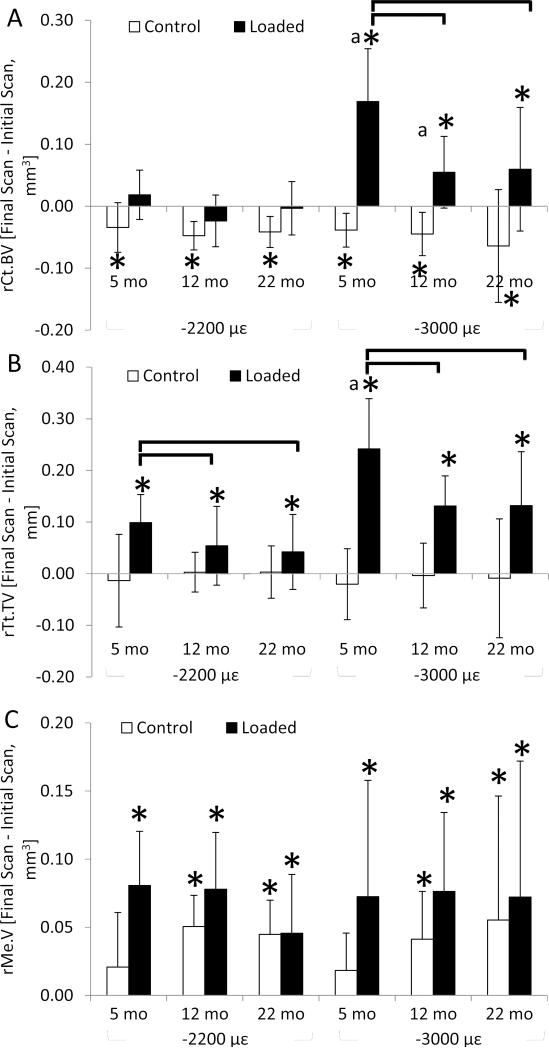
Dynamic compression of –2200 με for 2 weeks increased total volume and medullary volume of the cortical (diaphyseal) region in loaded tibiae of all ages (Table 2, Fig. 4), indicating periosteal apposition but endosteal bone loss despite enhanced endocortical bone formation indices (described above). These two effects led to no net change in cortical bone volume at –2200 με. Age effects were evident in the relative change in total volume, which was greater in 5-month tibiae than 12– or 22-month tibiae by 45% and 57%, respectively. Similar to compression at –2200 με, loading at –3000 με increased total volume in an age-dependent manner and also increased medullary volume. By contrast, loading at –3000 με increased cortical bone volume in all ages, indicating that periosteal apposition outpaced endocortical bone loss, with the greatest increase occurring in 5-month tibiae. This finding is likely driven by the woven bone incidence which was greatest in the 5-month, –3000 με group. Compression at –3000 με increased TMD in 5- and 12–month tibiae albeit by only 1%. Between loading levels, tibial compression at the higher strain magnitude induced a greater expansion of total volume by 2.3-fold and cortical bone volume by 9.3-fold in 5-month mice. Similarly, change in cortical bone volume was greater in the tibiae of 12–month mice loaded at –3000 με than at –2200 με. In the control tibiae, total volume did not change in any animals and medullary volume increased in 12– and 22-month tibiae of both loading levels, resulting in similar cortical bone loss (approximately 6%) across ages and loading levels.
Table 2.
Structural morphology of varying aged cortical bone subjected to two tibial compression loading magnitudes
| Outcomes | Time [weeks] |
−2200 με | −3000 με | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mo (n=13) | 12 mo (n=12) | 22 mo | (n=12) | 5 mo (n=15) | 12 mo (n=15) | 22 mo (n=14) | |||||||
| Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | ||
| Ct.BV [mm3] | 0 week | 0.94 ± 0.05 | 0.91 ± 0.06 | 0.89 ± 0.09 | 0.87 ± 0.10 | 0.74 ± 0.07 | 0.71 ± 0.04 | 0.95 ± 0.05 | 0.94 ± 0.04 | 0.87 ± 0.06 | 0.85 ± 0.06 | 0.78 ± 0.14 | 0.74 ± 0.06 |
| 2 weeks | 0.90 ± 0.05* | 0.95 ± 0.07 | 0.84 ± 0.10* | 0.85 ± 0.11 | 0.70 ± 0.09* | 0.70 ± 0.06 | 0.91 ± 0.05* | 1.10 ± 0.08* | 0.82 ± 0.05* | 0.90 ± 0.08* | 0.71 ± 0.08* | 0.80 ± 0.09* | |
| Ct.TV [mm3] | 0 week | 1.38 ± 0.10 | 1.34 ± 0.12 | 1.41 ± 0.12 | 1.37 ± 0.12 | 1.41 ± 0.10 | 1.41 ± 0.07 | 1.47 ± 0.09 | 1.46 ± 0.09 | 1.42 ± 0.12 | 1.42 ± 0.11 | 1.52 ± 0.24 | 1.49 ± 0.12 |
| 2 weeks | 1.36 ± 0.12 | 1.48 ± 0.16* | 1.43 ± 0.13 | 1.42 ± 0.13* | 1.41 ± 0.09 | 1.45 ± 0.10* | 1.45 ± 0.07 | 1.70 ± 0.08* | 1.42 ± 0.09 | 1.55 ± 0.12* | 1.51 ± 0.15 | 1.63 ± 0.09* | |
| Me.V [mm3] | 0 week | 0.44 ± 0.06 | 0.43 ± 0.07 | 0.52 ± 0.11 | 0.49 ± 0.11 | 0.67 ± 0.06 | 0.70 ± 0.07 | 0.52 ± 0.05 | 0.52 ± 0.05 | 0.55 ± 0.07 | 0.57 ± 0.08 | 0.75 ± 0.12 | 0.75 ± 0.09 |
| 2 weeks | 0.46 ± 0.07 | 0.53 ± 0.10* | 0.58 ± 0.14* | 0.57 ± 0.13* | 0.71 ± 0.07 | 0.75 ± 0.08* | 0.54 ± 0.03 | 0.60 ± 0.06* | 0.59 ± 0.05* | 0.65 ± 0.09* | 0.80 ± 0.11* | 0.83 ± 0.11* | |
| TMD [mg HA/cm3] | 0 week | 1054 ± 22 | 1057 ± 13 | 1079 ± 28 | 1074 ± 28 | 1048 ± 61 | 1065 ± 27 | 1050 ± 16 | 1056 ± 13 | 1079 ± 22 | 1088 ± 15 | 1035 ± 30 | 1069 ± 22 |
| 2 weeks | 1041 ± 29* | 1059 ± 13 | 1066 ± 26 | 1078 ± 19 | 1031 ± 50 | 1068 ± 26 | 1044 ± 25 | 1066 ± 12* | 1074 ± 22 | 1098 ± 16* | 1031 ± 35 | 1076 ± 19 | |
p<0.05, 0 week vs. 2 weeks
Figure 4.
Relative change in (a) cortical bone volume, (b) total volume and (c) medullary volume over 2 weeks of tibial compression at –2200 με and –3000 με. Tibial compression at –2200 με increased total volume in an age-dependent manner but also increased medullary volume, resulting in no net change in cortical bone. By contrast, tibial compression at –3000 με increased total volume and cortical bone volume in an age-dependent manner, despite increased medullary volume. All control tibiae lost cortical bone volume. Data are represented as mean±SD. *: significant difference between final and initial value; Black bracket: significant difference between ages; “a”: significant difference between loading magnitudes of respective age; p<0.05.
Tibial Compression Increased Trabecular Bone in Young-Adult but not in Middle-Aged or Old Mice
Similar to the cortical bone findings, loading increased trabecular bone parameters in an age-dependent manner. Compression at –2200 με imparted few changes to the trabecular bone of the loaded tibia, except for modest trabecular thickening (+11%) in 5-month tibia (Table 3). By contrast, tibial compression at –3000 με of the 5-month mice increased BV/TV by 18% via trabecular thickening of 31%. In the older animals, BV/TV was not changed in the loaded tibiae but trabecular thickness was greater by 16% in the tibiae of 12–month mice following compression at –3000 με. Similarly, vBMD was greater in the loaded tibiae of 5- and 22-month mice. In the control tibiae, 5-month mice subjected to both loading magnitudes lost BV/TV and vBMD.
Table 3.
Structural morphology of varying age trabecular bone subjected to two tibial compression loading magnitudes (n=13-15)
| Outcomes | Time [weeks] |
−2200 με | −3000 με | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 month | 12 month | 22 month | 5 month | 12 month | 22 month | ||||||||
| Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | Control | Loaded | ||
| BV/TV [mm3/mm3] | 0 week | 0.11 ± 0.03 | 0.12 ± 0.03 | 0.06 ± 0.02 | 0.05 ± 0.03 | 0.07 ± 0.05 | 0.10 ± 0.06 | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.05 | 0.04 ± 0.02 |
| 2 weeks | 0.09 ± 0.04* | 0.12 ± 0.04 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.07 ± 0.04 | 0.09 ± 0.06 | 0.07 ± 0.02* | 0.10 ± 0.03* | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 | |
| Tb.Th [μm] | 0 week | 56 ± 5 | 55 ± 5 | 67 ± 10 | 65 ± 5 | 73 ± 10 | 68 ± 8 | 52 ± 4 | 52 ± 3 | 61 ± 10 | 56 ± 8 | 66 ± 15 | 66 ± 14 |
| 2 weeks | 53 ± 3* | 61 ± 3* | 61 ± 8* | 61 ± 11 | 73 ± 9 | 69 ± 8 | 53 ± 4 | 68 ± 7* | 57 ± 10 | 65 ± 10* | 67 ± 11 | 70 ± 17 | |
| Tb.N [1/mm] | 0 week | 3.9 ± 0.4 | 4.0 ± 0.4 | 2.9 ± 0.2 | 2.8 ± 0.4 | 3.5 ± 1.0 | 4.0 ± 1.1 | 3.7 ± 0.5 | 3.9 ± 0.4 | 2.9 ± 0.4 | 2.8 ± 0.3 | 3.3 ± 0.8 | 3.7 ± 0.7 |
| 2 weeks | 3.8 ± 0.5 | 4.0 ± 0.4 | 2.9 ± 0.3 | 2.8 ± 0.3 | 4.0 ± 1.5 | 4.2 ± 1.6 | 3.6 ± 0.4 | 3.9 ± 0.6 | 2.8 ± 0.4 | 2.9 ± 0.3 | 3.2 ± 0.7 | 3.6 ± 1.2 | |
| vBMD [mg HA/cm3] | 0 week | 127 ± 26 | 133 ± 19 | 79 ± 26 | 69 ± 26 | 89 ± 34 | 99 ± 43 | 108 ± 17 | 106 ± 17 | 62 ± 17 | 53 ± 23 | 65 ± 42 | 54 ± 22 |
| 2 weeks | 115 ± 27* | 130 ± 26 | 81 ± 26 | 75 ± 37 | 88 ± 32 | 99 ± 44 | 102 ± 17* | 117 ± 24* | 63 ± 14 | 61 ± 21 | 63 ± 22 | 70 ± 22* | |
p<0.05, 0 week vs. 2 weeks
Data are represented as mean±SD.
DISCUSSION
The non-invasive application of axial tibial compression increased periosteal bone formation of the middiaphysis in all age groups, albeit in an age-dependent manner. The periosteal response of young-adult mice was greater than that of middle-aged or old mice, and there was no difference between middle-aged and old mice. Subjecting the tibiae to a greater peak mechanical strain of –3000 με increased the periosteal response, especially in younger animals, but it also increased the incidence of woven bone formation. These data suggest that tibial compression at a peak of –2200 με may be near the limit for load-induced lamellar bone formation for young-adult mice under the current loading parameters, as these loaded tibiae exhibited high MS/BS (> 70%) and MAR (>2 μm/day). The induction of endocortical bone formation by loading was also observed, but this was not different between ages. Longitudinal comparisons of structure by microCT corroborated the dynamic histomorphometric measures. Loading-induced accrual of total volume was less with age, medullary volume increased in all ages and applying greater mechanical strain further enhanced total volume in young-adult mice. Similarly, all tibiae loaded at –3000 με gained cortical bone volume, with young-adult mice attaining the greatest response. Finally, trabecular responses to loading were also observed to be age- and magnitude-dependent, with greatest responses in young-adult tibiae driven by increased trabecular thickness. Together, these data demonstrate a reduction in the bone anabolic response to mechanical loading in middle-aged and old C57BL/6 mice compared to young-adult mice.
We examined mouse ages that correspond to young-adult, middle-aged and old phases of life, analogous to approximately 25, 45 and 65 years in humans, respectively [24]. Across this age range, the tibia in C57Bl/6 females exhibits age-related changes similar to those described in humans [35], namely reduced cortical bone area and thickness, increased medullary area and decreased cancellous bone mass. We chose not to include younger mice (e.g., 2-4 months), as they are rapidly accruing bone mass [20, 21, 25] and our focus was on skeletal aging not growth.
Our finding that tibiae from C57Bl/6 mice have reduced cortical mechano-responsiveness with aging is consistent with several previous studies of direct loading using old animals, although the degree of the age decline we observed is less than the dramatic declines reported in two early studies. Ulnae from 3-year old turkeys had a negligible response to compressive loading at –3000 με whereas the same loading protocol was strongly anabolic in 1-year old animals [12]. Similarly, tibiae from 19-month-old rats had a bone formation response an order of magnitude lower than 9-month animals when both were subjected to four-point bending [14]. The notion that old animals are essentially non-responsive to loading was challenged by Srinivasan et al., who applied rest-inserted cantilever tibial bending to 22-month-old female C57Bl/6 mice and observed a modest periosteal response at 1200 με, albeit the bone formation rate was a 60% less than in 4-month old animals [13]. Our findings, also in female C57Bl/6 mice but based on a different loading method, indicate a 65-70% lower periosteal bone formation rate in old mice compared to young-adult mice loaded at equivalent peak strain (either –2200 or –3000 με). Another consistent finding between these two studies is that the bone formation rate in loaded tibiae from old mice exceeds the rate in non-loaded bones from young-adult mice, suggesting that loading has the potential to overcome the normal age-related decline in periosteal apposition.
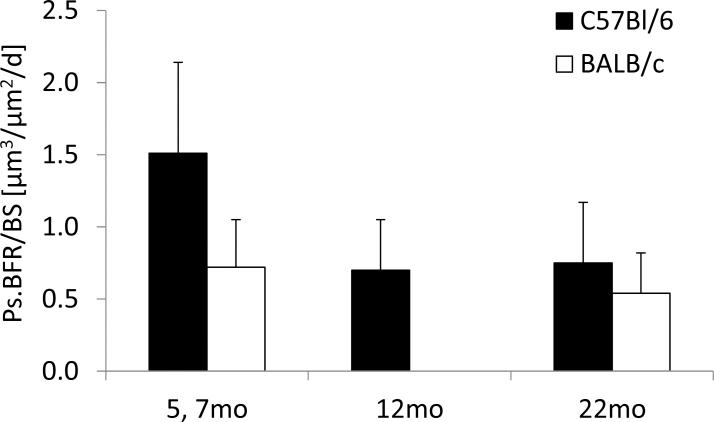
We have previously applied tibial compression to BALB/c mice across their life span [17, 18]. Our initial study reported no decline in periosteal mechano-responsiveness with aging when comparing 7– to 22-month-old male BALB/c mice [17], a finding that was unexpected and at odds with prior reports in humans, rats and C57Bl/6 mice [5, 8]. A re-examination of these findings in the context of our current results suggests that the lack of a relative age decline in the BALB/c mice was due to the modest response in 7–month mice rather than an unusually large response in 22-month mice (Fig. 5). In fact, the periosteal bone formation rates in loaded tibiae from 22-month BALB/c and C57Bl/6 mice are comparable, indicating that old mice from the two strains have a similar rate of bone formation when loaded to the same peak strain. By contrast, the rate in 7–month BALB/c mice is one-half that in 5-month-old C57Bl/6, suggesting either a mouse strain effect or an age effect from 5 to 7 months. Our second study of BALB/c mice supports the latter. We observed less accrual of cortical bone volume after 6 weeks of tibial compression when comparing 4 and 7 months mice (4 mo: +13% increased bone volume, 7 mo: +4%, 12 mo: +6%) [18]. Taken together, our results indicate that mice at 4-5 months age are approximately twice as responsive to anabolic bone loading than mice aged 12 months and older, and that the age decline may begin as soon as 5 to 7 months age.
Figure 5.
Periosteal bone formation rates for tibiae loaded at a peak periosteal strain of –2200 με from our previous study in BALB/c mice [17] and current results in C57Bl/6. Data are represented as mean±SD.
A limitation in synthesizing the results of our two previous studies and the current one is that they used different loading waveforms and mouse strains. To address this potential issue, we recently evaluated the response to tibial compression of young-adult BALB/c and C57Bl/6 mice and compared two previously published protocols [30]. The first protocol (which we labeled ‘WashU’) corresponds to the one used in our two prior BALB/c studies [17, 18], while the second protocol (which we labeled ‘Cornell/HSS’ based on work by others [22, 29]) corresponds to the one we used in the present study. The differences in responses between loading protocols were most pronounced in trabecular bone; the greater trabecular response induced by the Cornell/HSS protocol prompted us to adopt it for the current study. On the other hand, there were no differences between protocols for cortical bone accrual after 6 weeks. Moreover, both loading protocols were anabolic for BALB/c as well as C57Bl/6 mice, with similar cortical bone accrual in the two strains. Thus, we suggest that differences between loading protocols and mouse strains do not greatly influence the conclusions we make here about age effects and loading based on results of past [17, 18] and present studies from our lab.
Similar to the age-related decline in lamellar bone formation with aging, tibial compression at a higher mechanical strain engendered a greater incidence of periosteal woven bone formation in young-adult mice than in old mice. This finding is consistent with results of Turner et al. [14] who reported a lower incidence of woven bone with increasing age in rats subjected to tibial bending. Further, unlike the minimal amount of woven bone formed by tibial compression of –3000 με in 7–month BALB/c mice [17], woven bone was consistently created in C57Bl/6 loaded at –3000 με, suggesting an age- and/or mouse strain-dependence. The current findings are also consistent with a study by Turner et al. [36] showing a load magnitude dependent transition from lamellar to woven bone formation using the tibial four-point bending model. The magnitude of mechanical strain where the transition occurred in their study (~ 1900 με [36]) was lower than in our study most likely because the axial tibial compression method avoids the confounding effects of local periosteal compression engendered by four-point tibial bending. In addition, when woven bone was observed in their study it was “all or nothing”, whereas we find that woven bone can occur in modest amounts over only a part of the cross-section. Another important observation from our study is that woven bone that was obvious on histological examination was undetectable by microCT imaging of the same samples (data not shown). This highlights the importance of high resolution dynamic histomorphometry in discerning the type of bone formation induced by loading.
Few changes were noted in trabecular bone structure following tibial compression, although there was evidence for magnitude effects and potentially age-related effects. Loading increased trabecular BV/TV only in 5-month-old mice at the higher strain level (–3000 με; 10.5 N). Trabecular thickness increased in 5-month-old mice loaded at –2200 and –3000 με and in 12–month-old mice loaded at –3000 με. These findings are in line with previous reports on young-adult mice. Tibial compression can increase trabecular bone volume fraction in 4-month C57Bl/6 and BALB/c mice after 6 weeks of alternate-day loading (10 N force) [30]. Similarly, tibial compression on alternate days for 4 weeks increases trabecular BV/TV and thickness in 4-month C57BL/6 mice (9 N force) [37]. Tibial compression daily for 2 weeks increases trabecular BV/TV in 6-month-old C57Bl/6 mice at peak force of 11 N but not at 6 N [22]. Therefore, while we did not stimulate changes in trabecular bone structure in old mice, it is possible that more bouts and/or greater force would be effective. However, the applied loads in each age group were selected based upon peak mechanical strain in the cortical bone (near the mid-diaphysis) and the engendered strains in the metaphyseal cancellous bone were unknown for each age. Without determination of the cancellous strains, it is not possible to distinguish whether the lack of response of the cancellous compartment in older tibiae was due to a disparate application of localized strain between age groups or a biological attenuation in mechano-responsiveness. Lastly, none of the loaded tibiae in the current study or in a previous study [30] using the current loading protocol lost cancellous bone. However, several studies [31, 38] show that a single, unilateral tibial compression (9-12 N) in C57BL/6 mice leads to damage of the anterior cruciate ligament and articular cartilage, and to acute epiphyseal trabecular loss and then partial recovery over 8 weeks. It is possible that loading-induced increases in metaphyseal trabecular bone were confounded by differences in the degree of knee trauma between experiments, contributing to the decreased response in aged animals at both loading levels. Further, cage activity was not measured quantitatively, so as to determine if the ambulation of the animals returned to normal following loading, but the animals appeared to do so within an hour.
There were a few changes in the loaded and contralateral tibiae that warrant further comment. Firstly, we did not detect an increase in cortical bone volume of tibiae loaded at –2200 με, despite increased total volume. It is possible that the resolution of the microCT scans could not capture small changes in cortical bone structure after only 2 weeks of tibial compression. A more likely explanation is that the accrual of cortical bone by load-induced periosteal expansion was offset by medullary expansion. On the other hand, compression of –3000 με did increase cortical bone volume due to greater periosteal expansion and a similar amount of medullary expansion as occurs at –2200 με. Expansion of the medullary cavity may be a common occurrence with tibial compression [18, 37], which suggests that bone resorption should be examined on this surface. Nevertheless, loading prevented loss of cortical bone volume in the contralateral tibiae of all groups and the loss in trabecular BV/TV of 5-month old tibiae. Bone loss in the contralateral tibia is a longitudinal outcome that appears in other studies applying tibial compression to mature mice [17, 18]. However, these losses may be age-related rather than a systemic response to loading; studies with sham and age-matched controls show that losses in the contralateral tibiae of loaded animals match those in the controls, with no effect from administration of the anesthetic Buprenorphine [23, 30].
In conclusion, tibial compression of female C57BL/6 mice at a force that engenders a peak periosteal strain of –2200 με induced less periosteal bone formation in 12– and 22-month mice than 5-month mice. Increasing the magnitude of loading to engender a peak of –3000 με stimulated periosteal woven bone formation in 5- and 12–month mice, not in 22-month mice. There were few differences in the adaptation of cortical or trabecular bone to mechanical loading between 12– and 22-month-old mice. Kassem and Marie [4] extensively discuss the age-related changes to the osteoblast (e.g., attenuated proliferative capacity, reduced differentiation, functional impairment, competitive-enhancement of adipogenesis, etc.), which may contribute to the decline in responsiveness. In addition, osteoblastic and osteocytic apoptosis is enhanced with aging and may also deter mechanical adaptation [39]. Aging in mice may also increase osteoclast-derived levels of sclerostin [40], a negative regulator of bone formation primarily produced by osteocytes and required to decrease for an anabolic response to loading [41]. Loading of bone may alter resorption [42] and resorptive function [43] but, in a previous manuscript [18], we show that the expression of Rankl/Opg and Ctsk, while reduced by murine aging, were unchanged by tibial compression at any age. The mechanism(s) underlying the attenuation of bone formation in mice older than middle-age during tibial compression remains unclear [44], but these data model reduced formation in humans and provide a platform to undertake these questions.
Highlights.
Tibial compression increased periosteal cortical bone in a range of adult C57Bl/6 mice.
Aging above 12 months of age reduced the periosteal cortical bone response to loading.
Woven bone incidence at –3000 was greatest in 5-month mice.
These data provide a platform to determine the mechanisms of reduced bone formation with aging.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (R01 AR047867, P30 AR057235, T32 AR060719).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors state that they have no conflicts of interest.
REFERENCES
- 1.Parfitt AM. Bone remodeling in type I osteoporosis. J Bone Miner Res. 1991;6:95–7. doi: 10.1002/jbmr.5650060115. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–98. doi: 10.1016/s8756-3282(03)00199-6. [DOI] [PubMed] [Google Scholar]
- 3.Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int. 2010;86:470–83. doi: 10.1007/s00223-010-9359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10:191–7. doi: 10.1111/j.1474-9726.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- 5.Rianon NJ, Lang TF, Sigurdsson G, Eiriksdottir G, Sigurdsson S, Garcia M, Pajala S, Koster A, Yu B, Selwyn BJ, Taylor WC, Kapadia AS, Gudnason V, Launer LJ, Harris TB. Lifelong physical activity in maintaining bone strength in older men and women of the Age, Gene/Environment Susceptibility-Reykjavik Study. Osteoporos Int. 2012;23:2303–12. doi: 10.1007/s00198-011-1874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–29. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 7.Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA. Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int. 2006;17:133–42. doi: 10.1007/s00198-005-1938-9. [DOI] [PubMed] [Google Scholar]
- 8.Kotiya AA, Silva MJ. The Effect of Aging on Skeletal Mechanoresponsiveness: Animal Studies. In: Silva MJ, editor. Skeletal Aging and Osteoporosis. Springer; Berlin Heidelberg: 2013. pp. 191–216. [Google Scholar]
- 9.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12:1480–5. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]
- 10.Leppanen OV, Sievanen H, Jokihaara J, Pajamaki I, Kannus P, Jarvinen TL. Pathogenesis of age-related osteoporosis: impaired mechano-responsiveness of bone is not the culprit. PLoS One. 2008;3:e2540. doi: 10.1371/journal.pone.0002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact. 2001;1:249–62. [PubMed] [Google Scholar]
- 12.Rubin CT, Bain SD, McCleod KJ. Suppression of osteogenic response in the aging skeleton. Calcif. Tiss. Int. 1992;50:306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33:946–55. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10:1544–9. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- 15.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25:2006–15. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One. 2012;7:e34980. doi: 10.1371/journal.pone.0034980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17:1044–50. doi: 10.1359/jbmr.2002.17.6.1044. [DOI] [PubMed] [Google Scholar]
- 20.Buie HR, Moore CP, Boyd SK. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res. 2008;23:2048–59. doi: 10.1359/jbmr.080808. [DOI] [PubMed] [Google Scholar]
- 21.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 22.Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, van der Meulen MC. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49:439–46. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willie BM, Birkhold AI, Razi H, Thiele T, Aido M, Kruck B, Schill A, Checa S, Main RP, Duda GN. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone. 2013;55:335–46. doi: 10.1016/j.bone.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. 2nd ed. Academic Press; San Diego: 2007. [Google Scholar]
- 25.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14:2159–66. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 26.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 27.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–87. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel TK, Brodt MD, Silva MJ. J Biomech. 2014;Experimental and finite element analysis of strains induced by axial tibial compression in young-adult and old female C57Bl/6 mice.47:451–7. doi: 10.1016/j.jbiomech.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, van der Meulen MC. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–91. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of Tibial Structure and Strength to Axial Compression Depends on Loading History in Both C57BL/6 and BALB/c Mice. Calcif Tissue Int. 2013;93:211–21. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early response of mouse joint tissues to noninvasive knee injury suggests treatment targets. Arthritis Rheumatol. 2014 doi: 10.1002/art.38375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christiansen BA, Bayly PV, Silva MJ. Constrained tibial vibration in mice: a method for studying the effects of vibrational loading of bone. J Biomech Eng. 2008;130:044502. doi: 10.1115/1.2917435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stadelmann VA, Hocke J, Verhelle J, Forster V, Merlini F, Terrier A, Pioletti DP. 3D strain map of axially loaded mouse tibia: a numerical analysis validated by experimental measurements. Comput Methods Biomech Biomed Engin. 2009;12:95–100. doi: 10.1080/10255840903077287. [DOI] [PubMed] [Google Scholar]
- 34.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M.J. S, K.J. J. Age-Related Changes in Whole-Bone Structure and Strength. In: MJ S, editor. Skeletal Aging and Osteoporosis: Biomechanics and Mechanobiology. Springer-Verlag; Berlin Heidelberg: 2013. pp. 1–30. [Google Scholar]
- 36.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 37.Weatherholt AM, Fuchs RK, Warden SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone. 2013;52:372–9. doi: 10.1016/j.bone.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–82. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9:851–67. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ. Sclerostin is expressed in osteoclasts from aged mice and reduces osteoclast-mediated stimulation of mineralization. J Cell Biochem. 2013;114:1901–7. doi: 10.1002/jcb.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–17. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T, Nakamura T. Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res. 2003;18:2002–9. doi: 10.1359/jbmr.2003.18.11.2002. [DOI] [PubMed] [Google Scholar]
- 43.Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI. Mechanical loading modulates glutamate receptor subunit expression in bone. Bone. 2005;37:63–73. doi: 10.1016/j.bone.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Almeida M. Aging mechanisms in bone. Bonekey Rep. 2012:1. doi: 10.1038/bonekey.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]