Abstract
Neurologists increasingly recognize that critically ill patients are at high risk for seizures - particularly non-convulsive seizures - and that neuromonitoring is a useful tool for diagnosing seizures and assessing brain function in these patients. Amplitude-integrated electroencephalography (aEEG) is a simplified bedside neurophysiology tool that has become widely used in neonates over the past decade. Despite widespread interest by both neurologists and neonatologists in continuous brain monitoring, aEEG has been largely ignored by neurologists, forcing neonatologists to “go it alone” when interpreting data from this bedside tool. Although aEEG cannot replace conventional EEG for background monitoring and detection of seizures, it remains a useful instrument that complements conventional EEG, is being widely adopted by neonatologists, and should be supported by neonatal neurologists.
Keywords: Seizure, electroencephalogram, neonatal seizures, encephalopathy, hypoxic-ischemic encephalopathy, newborn, infant, status epilepticus, neurocritical care, neurointensive care, epilepsy
Introduction
Neurologists increasingly recognize that critically ill patients of all ages are at high risk for seizures - particularly non-convulsive seizures - and that neuromonitoring can provide useful information about brain function for assessment and prognostication. With the advent of effective neuroprotective therapy and advances in safe neuroimaging methods for neonates, neonatologists have increasingly incorporated continuous, real-time, bedside monitoring of neonatal brain function into their management strategies.
The gold standard for neonatal brain monitoring is continuous video-electroencephalograpy (cEEG), with electrodes placed according to the international 10–20 system, modified for neonates1. However, there are barriers to implementation of this technology: recording and interpretation require specialized training, it is expensive, and access to equipment is variable, often depending on the time of day or day of the week. Amplitude-integrated electroencephalography (aEEG) is a bedside neurophysiology tool that uses a limited number of channels to record raw EEG signal that is then filtered, rectified, processed, and displayed on a semilogarithmic amplitude and time-compressed scale (Figure 1). In most instances, neonatologists, nurses, or other Intensive Care Nursery (ICN) staff apply the electrodes and interpret the aEEG recording independently, without input from a neurologist. Due to its ease of application and interpretation, as well as the obstacles to conventional EEG monitoring, aEEG has been in widespread use in the ICNs of European centers for more than two decades, and has been increasingly used in North American ICNs over the past several years2–4.
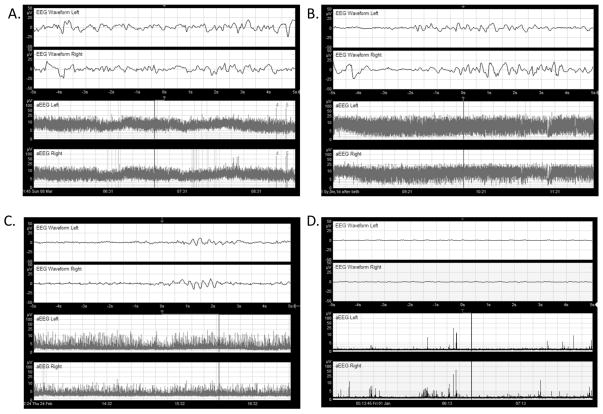
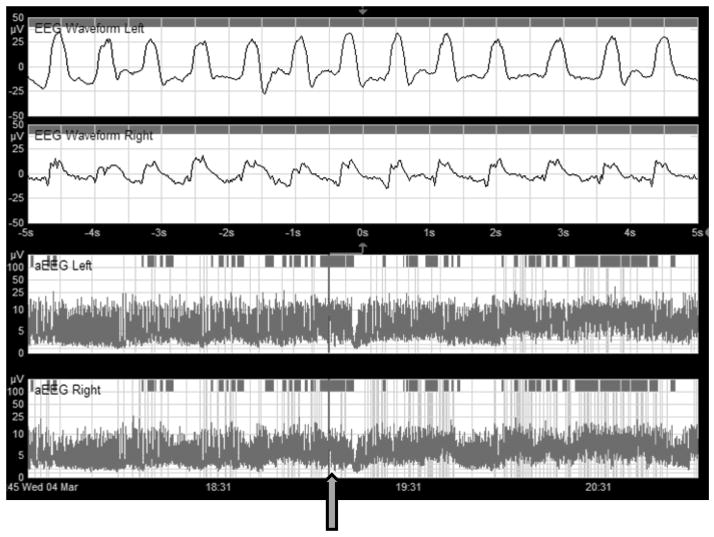
Figure 1.
Two channel (four-electrode) aEEG display. The upper two panels display single-channel EEG recorded from the left (top) and right (second from top) centro-parietal leads. The y-axis shows amplitude on a logarithmic scale; the y-axis shows time in seconds. The bottom two panels show the corresponding aEEG for each side. Note the rectification of voltages (with all values >0) and the time compression on the x-axis (this screen shows 3.5 hours of aEEG). The vertical bar indicates the segment of EEG displayed across the aEEG panels.
(A) Normal or Continuous Normal Voltage (CNV): upper margin >10 μV and a lower margin >5 μV.
(B) Moderately abnormal or Discontinuous Normal Voltage (DNV): upper margin >10 μV, lower margin <5 μV. The tracing is discontinuous and there is no sleep-wake cycling.
(C) Burst Suppression (BS) is a pattern of mixed inactivity with bursts of higher amplitude.
(D) Suppressed activity or Flat Tracing (FT) is defined by a low voltage/inactive aEEG with amplitude <5 μV; may be called called Continuous Low Voltage (CLV) when the background is around 5 μV.5, 6
The dramatic increase in aEEG use has largely occurred independent of pediatric neurologists. Despite shared interest in continuous brain monitoring among both neurologists and neonatologists, aEEG has primarily been adopted by neonatologists. Pediatric neurologists have been less enthusiastic (and in some cases reluctant) to accept its use due to aEEG’s limited sensitivity for seizure detection, and the lack of evidence that such monitoring improves outcomes4.
As pediatric neurologists, we advocate that aEEG is a useful tool that can complement, although not replace, cEEG. Neurologists working in the ICN should become familiar with the advantages and limitations of this technology, and be prepared to interpret bedside aEEG recordings. The particular expertise of the pediatric neurologist in aEEG interpretation adds important data to management decisions. Skill in the use of aEEG benefits neurologists in many ways: aEEG can be used to evaluate changes in encephalopathy over time, and provides useful prognostic information for many neonates, in addition to identifying many seizures. Neurologists will find aEEG fairly easy to interpret at the bedside, even without formal training in neurophysiology. Finally, neurology involvement in aEEG use and bedside interpretation can improve communication and coordination of care with the neonatology team.
Evaluation of aEEG Background
aEEG background is categorized by features similar to those used in conventional neonatal EEG interpretation: amplitude criteria, continuity and discontinuity, and presence of sleep wake cycling. The two most widely used classification schemes are illustrated in Figure 1. The system described by al Naqeeb and colleagues classifies aEEG as Normal, Moderately Abnormal, or Suppressed using a voltage-based criteria5. An alternate system, proposed by Toet and colleagues, relies on pattern recognition in combination with voltage criteria to distinguish between five categories: Continuous Normal Voltage (CNV), Discontinuous Normal Voltage (DNV), Burst Suppression (BS), Continuous Low Voltage (CLV), and Flat Trace (FT)6. A study comparing inter-rater reliability of the two aEEG classification systems found more consistent interpretation with the simple voltage criteria system than the pattern recognition system, and both systems had equivalent agreement with conventional EEG background classification criteria 7.
Overall, aEEG background has been shown to have fair to good agreement with EEG background classification when studied in term newborns with encephalopathy7–10. Given the prognostic value of EEG background in neonates with encephalopathy 11–13, there has been particular interest in how aEEG background correlates directly with clinical outcomes.
Prior to the advent of therapeutic hypothermia for neonates with HIE, abnormal aEEG background pattern during the first 3–6 postnatal hours was reported to be highly predictive of adverse outcome, and the combination of aEEG background assessment with the clinical neurological examination was more predictive than either alone5, 6, 14. aEEG background remains predictive of outcome in neonates treated with therapeutic hypothermia. Although abnormal aEEG in the first 6 hours of life in a cooled patient has a lower positive predictive value for poor outcome than in the pre-hypothermia therapy era, normal early aEEG is reassuring for high likelihood of good outcome, and delayed recovery beyond 48 hours is highly predictive of later neurodevelopmental disability (PPV ~90% 15). Failure to develop sleep wake cycling in the first 72 hours of life is also strongly predictive of later disability15.
As with cEEG in the setting of hypothermia, aEEG is most useful in showing evolution of the background over several days. The compressed time scale of the aEEG display is ideal for evaluating the evolution of the background quickly and accurately at the bedside, since several hours of monitoring data can be viewed on a single screen.
Because of the high sensitivity and specificity of early aEEG to predict outcome after neonatal encephalopathy, two large trials of therapeutic hypothermia used abnormal aEEG background as an inclusion criterion “to improve the specificity of case selection [and] to control for severity of injury” beyond what was considered possible with clinical neurological examination alone16, 17. Following that precedent, many ICNs use aEEG to determine degree of encephalopathy when selecting patients for therapeutic hypothermia. Similarly, aEEG is widely used in prognostication for neonates with encephalopathy. Given these high stakes, it is particularly important that the neurologist provides perspective on aEEG, particularly to help avoid common aEEG pitfalls.
For example, just as in EEG, artifacts can contaminate the aEEG background, leading to potential for misinterpretation(Figure 2). In one study of 200 hours of aEEG, 12% of recordings were significantly affected by artifact18. Preliminary evidence shows artifact may be even more common in aEEG of preterm infants19. Such artifacts are commonly recognized by those familiar with EEG, but may not be immediately obvious if relying only on the aEEG trace. Electrocardiogram (EKG) artifact can elevate the aEEG’s voltage and make aEEG background appear falsely normal (Figure 2). Similarly, in some tracings, electrographic seizures can falsely elevate the overall voltage of the aEEG background. Both of these types of artifact can lead inexperienced users to mistakenly read the aEEG as “normal,” which could exclude an infant from hypothermia therapy20(Figure 3). The neurologist’s expertise in identifying seizures, both clinically and on EEG, and knowledge of common extracerebral EEG artifacts, can prevent such errors, with potentially significant treatment implications.
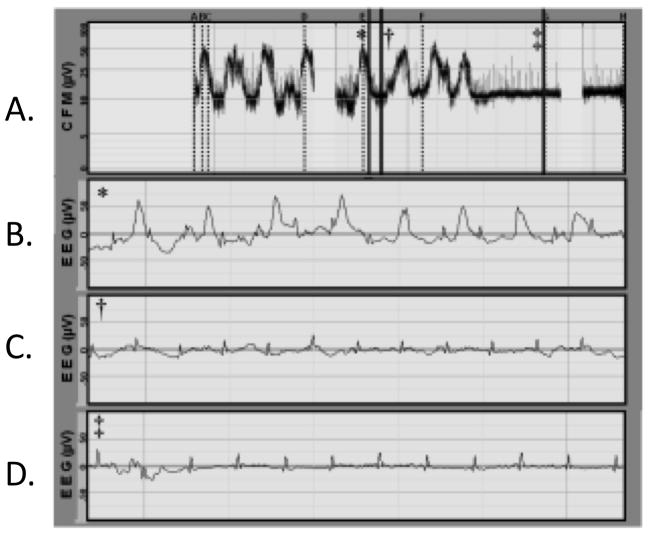
Figure 2.
The figure shows: aEEG voltage band (single channel C3–C4, panel A), and 3 segments of raw EEG corresponding to the vertical bar markers on aEEG (panels C, D, and E). At first glance, the aEEG voltage appears at or above 10 μV throughout (panel A). Review of raw EEG (panels C, D, and E), however, shows the overall aEEG background amplitude is elevated by artifact and seizure. Panel B shows the raw EEG with a seizure and concomitant electrocardiogram (EKG) artifact. Panel C shows EKG artifact during what might be an episode of post-ictal suppression. Panel D reveals a very suppressed background, apart from the EKG artifact, which is up-shifting the aEEG’s narrow voltage band by about 10 μV.
Figure 3.
The top panel shows five hours of aEEG from a term neonate with encephalopathy. Note that in the first hour, the lower margin of the activity band is consistently above 5 μV, and thus might be misinterpreted as normal. However, review of the accompanying EEG below shows clear continuous seizures, which have elevated the overall amplitude on aEEG. The latter half of the aEEG shows the true background voltage after resolution of seizures; this aEEG is consistent with severe encephalopathy.
Neonatologists recognize the challenges of aEEG interpretation, and many report limited confidence in being the sole reader of aEEG4. The neurologist familiar with aEEG background can better anticipate early concerns (or early positive signs) that the neonatologist may already have identified based on this technique. At the same time, the neurologist can complement this single indicator with a comprehensive clinical evaluation and review of cEEG and neuroimaging data. Overall, aEEG has proven utility in rapidly identifying normal and abnormal background patterns, with defined validity for prognostication. Neurologists can take advantage of this utility, while also providing a complimentary perspective to optimize aEEG background interpretation.
aEEG for seizure detection
In contrast to seizures among older children and adults, the majority of neonatal seizures are subclinical – they have no external manifestations. Many authors have described this phenomenon, with rates of subclinical seizures typically exceeding 50% and often approaching 80% or more of all neonatal seizures.21–23 Conversely, many unusual neonatal movements have no electrographic correlate, but based on beside observation they may easily be misdiagnosed as seizures24. Therefore, clinical observation alone is inadequate for the diagnosis, quantification, and management of neonatal seizures. For these reasons, continuous bedside monitoring is recommended for all neonates with clinical suspicion and/or high risk for seizures25.
While there is considerable debate about the use of aEEG for seizure detection, as detailed below, scientific evidence and clinical experience suggest that aEEG is useful to detect many seizures and can lead to improved real-time diagnosis and treatment (Figure 4).
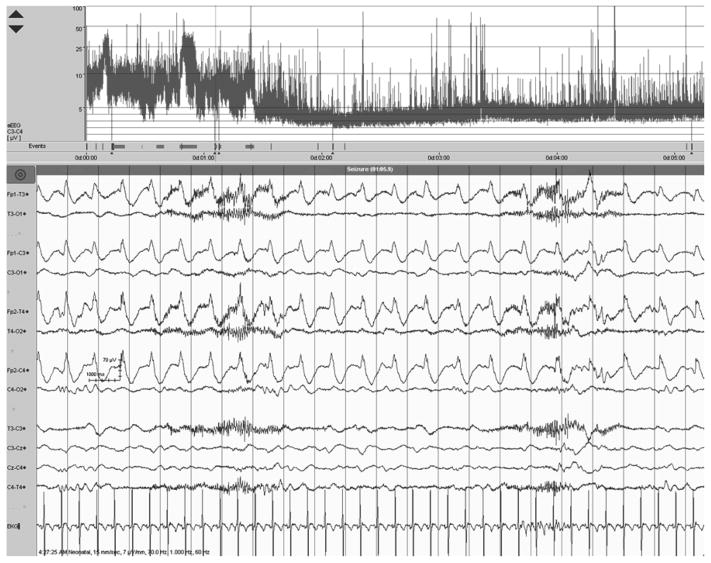
Figure 4.
The top 3 panels display left, right, and crosscerebral aEEG, respectively. The bottom panel shows corresponding EEG signal at the time indicated by the arrow. Seizures are visible on aEEG as abrupt increases in amplitude, repeated throughout the first half of the aEEG. Seizures are confirmed by review of the corresponding EEG.
cEEG remains the gold standard for neonatal seizure detection and quantification, with electrodes placed according to the international 10–20 system, modified for neonates1. An electrographic seizure, as seen on conventional EEG, must have a sudden, evolving, repetitive, and stereotyped electrographic pattern with clear beginning, middle, and ending, and a minimum duration of 10 seconds. The advantages of conventional EEG include its detailed information about the interictal background, seizures, seizure burden, and response to anticonvulsant medication, as well as differential diagnosis of paroxysmal clinical events, many of which are found not to be seizures.
Several studies have evaluated the accuracy of aEEG for seizure detection, as summarized in Table 1. A typical neonatal seizure is of low-to-moderate amplitude (which may be less obvious on aEEG display) and quite brief – 60% are less than 90 seconds in duration (90 seconds corresponds to ~1.4mm on a typical aEEG display)26. Neonatal seizures are also often of very low frequency, and might be filtered out of the aEEG trace (which filters frequencies <2 Hz and >15Hz). These inherent features of neonatal seizures can render them difficult to detect with precision on aEEG (Figure 5).
Table 1.
Single-Channel Amplitude-integrated EEG for Neonatal Seizure Detection
| Author | Patients with seizures (N) | Controls without seizures (N) | Individual Seizures (N) | aEEG reader experience | Sensitivity for individual seizures | Sensitivity for seizure-positive records | Specificity |
|---|---|---|---|---|---|---|---|
| Rennie[32] | 19 | 21 | Not reported | Novice | 38% | 4/19 correctly identified by all 4 observers | 92% |
| Shellhaas[27] | 125 | 19 | 851 | Varied | 25.5% ± 10.6% | 40.3% ± 16.8% | Not reported |
| Shah[29]* | 7 | 14 | 41 | Expert | 27–56% | 85% | Not reported |
| Frenkel[31] | 10 | 28 | 41 | Varied | 71–84%** | 68–84% | 0.39–0.96 |
Adding a second aEEG channel, with corresponding raw EEG, improved sensitivity in this study (but without the raw EEG, sensitivity remained poor).
Used single-channel EEG with access to raw single-channel EEG.
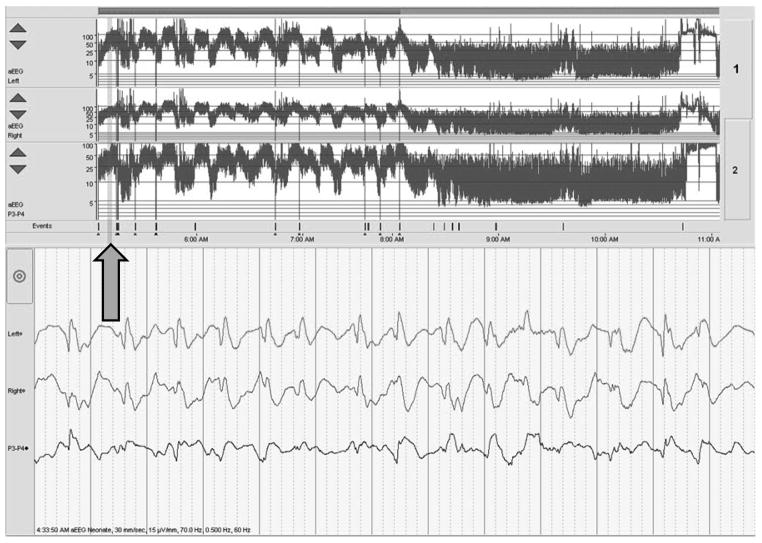
Figure 5.
Brief seizures can be difficult to detect on aEEG, but they may be revealed upon review of the raw EEG. The arrow points to the aEEG at the time of the seizure displayed above, in the raw EEG channels.
When reviewing the aEEG literature, it is critical to distinguish the sensitivity reported for individual seizure detection from the sensitivity for seizure-positive aEEG records. The former applies to each individual seizure, while the latter indicates only that at least one seizure was detected on the aEEG record. Multivariate analyses indicated that neonatologists’ expertise with aEEG interpretation, increasing individual seizure duration, higher seizure amplitude, and larger number of seizures per hour improve the odds of seizures detection on single channel aEEG.27 Electrode location is also important, as frontal or forehead electrode positioning is likely to decrease sensitivity for seizure detection, because these electrodes are subject to myogenic and extracerebral artifact28, and neonatal seizures rarely originate in the frontal lobes.26 Shah, et al29, demonstrated that the use of dual-channel aEEG in addition to availability of the raw EEG from which the aEEG was derived (a feature available on most modern aEEG monitors), improved sensitivity for seizure detection significantly. Neurologists should note that the “raw” EEG trace is not identical to a conventional single-channel EEG, since many devices still subject the “raw” trace to the aEEG filters and integration.
Given concern about adverse effects of anticonvulsant medications30, neurologists also have concerns about the possibility of over-treating neonates for seizures based on inaccurate interpretation of aEEG. It is reassuring that most of the published studies report fairly high specificity for seizure-detection with aEEG,29, 31, 32 although this is not a universal finding.33 Overall, the literature suggests that if a seizure is identified by aEEG, the diagnosis is correct. False positives are relatively rare. Reasons for false positive seizure detection include the ubiquitous extracerebral artifact in the ICN. For example, rocking or patting a neonate, or using a bag for assisted ventilation, can create rhythmic artifact that is easily mistaken for seizures. Mistaking patting for an ictal discharge is rare when time-locked video is recorded with the cEEG, but video is not available with most aEEG machines. Electrode malfunction and EKG artifact are also very common and can be difficult to distinguish from abnormal (cerebral) EEG or aEEG patterns.
Because of the potential for over-diagnosis (based on artifacts on the aEEG or raw EEG trace) and under-diagnosis (due to the time-compressed aEEG trace, and inherent features of neonatal seizures), cEEG remains the gold standard for neonatal seizure detection. However, aEEG - especially when rapidly applied and reviewed by an experienced clinician - can provide reassurance that paroxysmal clinical events are not seizures and can identify many seizures, allowing prompt administration of antiseizure medications. Expedited cEEG monitoring, to confirm a seizure diagnosis, quantify the seizure burden, and guide treatment, must back up the use of aEEG for seizure detection.
Optimizing Use of Concurrent aEEG/EEG
aEEG has been traditionally recorded on a separate bedside machine often called a cerebral function monitor (CFM). Several bedside monitors with similar features are commercially available: most include standardized aEEG display for one or more channels and have the ability to annotate events, such as clinically-apparent seizures or medication administration. While these machines have the advantage of being easy to use, we have found that having both an aEEG machine and a separate cEEG machine at the bedside is clumsy, costly, and can lead to challenges in communication between the bedside team and neonatal neurologist as they look at different tracings.
Bedside display of aEEG with concurrent cEEG recording is a potentially good solution (Figure 6). Using this system, the same machine is used to record both aEEG (with limited electrodes placed at the time of admission or of initial clinical concern by the bedside nurse or neonatologist and displayed as aEEG at the bedside), followed by full electrode placement for conventional EEG when possible (and displayed remotely as cEEG for the neurologist and neurophysiologist). With this system, the bedside ICN clinician can monitor for changes on the aEEG, and the neonatal EEG reader, who has access to the cEEG, can review any areas of concern upon request, as well as according to guidelines for care25.
Figure 6.
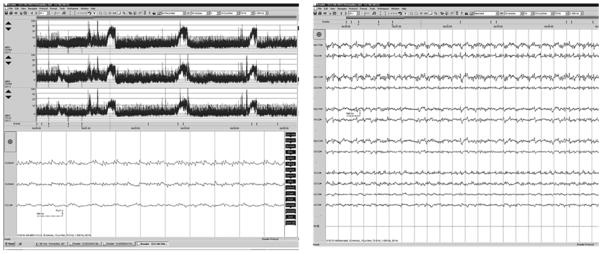
In this example, a single machine is used to acquire the same data, which are displayed in differently for different users, optimizing the advantages of both aEEG and EEG. The bedside team sees the display at left, showing overall trends and allowing quick review of suspicious segments of EEG. The neurophysiologist can confirm these abnormalities through review of conventional, neonatal montage EEG.
The advantages to this system are many. First, the hospital supports and maintains a single type of machine that can be used both in the intensive care setting and the neurophysiology laboratory. Second, while most hospitals support remote access for cEEG from the ICU, many aEEG stand-alone systems do not have this capacity. Third, annotated events (either at the bedside or in the neurophysiology laboratory) are displayed for all viewers. For example, the neonatologist can see that the neurologist has reviewed a section of tracing, and the neurologist can see where the bedside nurse has noted a suspicious clinical event or administered a medication. Finally, we have found that communication between the neurologist and neonatologist is improved and education is facilitated when all are looking at the same tracing.
Conclusions
An editorial in 2011 noted that aEEG has “become the standard technology” for brain monitoring in term neonates: “many neonatologists would like to have continuous access to EEG monitoring but often this is not achievable as neurophysiology departments may not provide an ‘out of hours service.’” 4, 34 A recent survey of specialists primarily in Europe and North America found that 65% used aEEG in their ICN, either alone or in conjunction with EEG4. Eighty percent of those surveyed reported that neonatologist alone - without neurologist or neurophysiologist input - interpreted that aEEG. When neurologists fail to support aEEG, ICN staff have no alternative but to “go it alone.”4
Pediatric neurologists will find that their perspective is uniquely valuable in assessing aEEG, and that neonatology colleagues are usually appreciative of the support. Indeed, in Boylan’s survey regarding aEEG use, only 28% of respondents felt confident in their own skills in aEEG interpretation4.
aEEG is here to stay, with the benefit of bringing convenient and timely neuromonitoring to the forefront of ICN care. For too long, infants’ brain function has not been a focus in the ICN. Rather than resisting change, we encourage neurologists to embrace aEEG, learn to interpret the aEEG background, and use aEEG as a springboard to optimal neuromonitoring and improved collaboration in the care of neonates at high risk for cerebral dysfunction.
Acknowledgments
Amy J. Markowitz, JD, provided editorial support. The authors thank Dr. John Barks for his assistance with figures.
Funding
NIH/NINDS 5K23NS066137 and the Neonatal Brain Research Institute at UCSF support Dr. Glass. NIH/NICHD 5K23HD068402 supports Dr. Shellhaas.
Footnotes
Author Contributions
Hannah C. Glass – Drafted portions of the manuscript, edited the manuscript and approved the final draft.
Courtney Wusthoff – Drafted portions of the manuscript, edited the manuscript and approved the final draft.
Renée Shellhaas – Drafted portions of the manuscript, edited the manuscript and approved the final draft.
Declaration of conflicting interests
None
Ethical Approval
Not applicable
References
- 1.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28(6):611–7. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 2.de Vries LS, Hellstrom-Westas L. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005 May;90(3):F201–7. doi: 10.1136/adc.2004.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponnusamy V, Nath P, Bissett L, et al. Current availability of cerebral function monitoring and hypothermia therapy in UK neonatal units. Arch Dis Child Fetal Neonatal Ed. 2010 Sep;95(5):F383–4. doi: 10.1136/adc.2009.181578. [DOI] [PubMed] [Google Scholar]
- 4.Boylan G, Burgoyne L, Moore C, et al. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010 Aug;99(8):1150–5. doi: 10.1111/j.1651-2227.2010.01809.x. [DOI] [PubMed] [Google Scholar]
- 5.al Naqeeb N, Edwards AD, Cowan FM, et al. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999 Jun;103(6 Pt 1):1263–71. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- 6.Toet MC, Hellstrom-Westas L, Groenendaal F, et al. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999 Jul;81(1):F19–23. doi: 10.1136/fn.81.1.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shellhaas RA, Gallagher PR, Clancy RR. Assessment of neonatal electroencephalography (EEG) background by conventional and two amplitude-integrated EEG classification systems. J Pediatr. 2008 Sep;153(3):369–74. doi: 10.1016/j.jpeds.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom-Westas L. Comparison between tape-recorded and amplitude-integrated EEG monitoring in sick newborn infants. Acta Paediatr. 1992 Oct;81(10):812–9. doi: 10.1111/j.1651-2227.1992.tb12109.x. [DOI] [PubMed] [Google Scholar]
- 9.Toet MC, van der Meij W, de Vries LS, et al. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics. 2002 May;109(5):772–9. doi: 10.1542/peds.109.5.772. [DOI] [PubMed] [Google Scholar]
- 10.Evans E, Koh S, Lerner J, et al. Accuracy of amplitude integrated EEG in a neonatal cohort. Arch Dis Child Fetal Neonatal Ed. 2010 May;95(3):F169–73. doi: 10.1136/adc.2009.165969. [DOI] [PubMed] [Google Scholar]
- 11.Biagioni E, Mercuri E, Rutherford M, et al. Combined use of electroencephalogram and magnetic resonance imaging in full-term neonates with acute encephalopathy. Pediatrics. 2001 Mar;107(3):461–8. doi: 10.1542/peds.107.3.461. [DOI] [PubMed] [Google Scholar]
- 12.van Lieshout HB, Jacobs JW, Rotteveel JJ, et al. The prognostic value of the EEG in asphyxiated newborns. Acta Neurol Scand. 1995 Mar;91(3):203–7. doi: 10.1111/j.1600-0404.1995.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmes GL, Lombroso CT. Prognostic value of background patterns in the neonatal EEG. J Clin Neurophysiol. 1993 Jul;10(3):323–52. doi: 10.1097/00004691-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Shalak LF, Laptook AR, Velaphi SC, et al. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003 Feb;111(2):351–7. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- 15.Thoresen M, Hellstrom-Westas L, Liu X, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010 Jul;126(1):e131–9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19–25;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 17.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009 Oct 1;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 18.Hagmann CF, Robertson NJ, Azzopardi D. Artifacts on electroencephalograms may influence the amplitude-integrated EEG classification: a qualitative analysis in neonatal encephalopathy. Pediatrics. 2006 Dec;118(6):2552–4. doi: 10.1542/peds.2006-2519. [DOI] [PubMed] [Google Scholar]
- 19.Suk D, Krauss AN, Engel M, et al. Amplitude-integrated electroencephalography in the NICU: frequent artifacts in premature infants may limit its utility as a monitoring device. Pediatrics. 2009 Feb;123(2):e328–32. doi: 10.1542/peds.2008-2850. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar S, Barks JD, Donn SM. Should amplitude-integrated electroencephalography be used to identify infants suitable for hypothermic neuroprotection? J Perinatol. 2008 Feb;28(2):117–22. doi: 10.1038/sj.jp.7211882. [DOI] [PubMed] [Google Scholar]
- 21.Clancy RR, Legido ADL. Occult neonatal seizures. Epilepsia. 1988;29:256–61. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence R, Mathur A, Tich SNT, et al. A Pilot study of continuous limited-channel aEEG in term infants with encephalopathy. Journal of Pediatrics. 2009;154:835–41. doi: 10.1016/j.jpeds.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Scher MS, Aso K, Beggarly ME, et al. Electrographic seizures in preterm and full-term neonates: clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics. 1993;91:128–34. [PubMed] [Google Scholar]
- 24.Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–91. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 25.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011 Dec;28(6):611–7. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 26.Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional and single channel EEG. Clinical Neurophysiology. 2007;118:2156–61. doi: 10.1016/j.clinph.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 27.Shellhaas RA, Saoita AI, Clancy RR. The Sensitivity of amplitude-integrated EEG for neonatal seizure detection. Pediatrics. 2007;120(4):770–7. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- 28.Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. Journal of Perinatology. 2009;29(3):237–42. doi: 10.1038/jp.2008.195. [DOI] [PubMed] [Google Scholar]
- 29.Shah DK, Mackay MT, Lavery S, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54. doi: 10.1542/peds.2007-1839. [DOI] [PubMed] [Google Scholar]
- 30.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99(23):15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frenkel N, Friger M, Meledin I, et al. Neonatal seizure recognition - comparative study of continuous amplitude-integrated EEG versus short conventional EEG recordings. Clinical Neurophysiology. 2011;122:1091–7. doi: 10.1016/j.clinph.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Rennie JM, Chorley G, Boylan GB, et al. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed. 2004;89:F37–F40. doi: 10.1136/fn.89.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans E, Koh S, Lerner JT, et al. Accuracy of amplitude integrated EEG in a neonatal cohort. Arch Dis Child Fetal Neonatal Ed. 2010;95:F169–F73. doi: 10.1136/adc.2009.165969. [DOI] [PubMed] [Google Scholar]
- 34.Boylan GB. EEG monitoring in the neonatal intensive care unit: a critical juncture. Clin Neurophysiol. 2011 Oct;122(10):1905–7. doi: 10.1016/j.clinph.2011.03.015. [DOI] [PubMed] [Google Scholar]